Abstract
Aims: Heme oxygenase-1 (HO-1, HMOX1) can prevent tumor initiation; while in various tumors, it has been demonstrated to promote growth, angiogenesis, and metastasis. Here, we investigated whether HMOX1 can modulate microRNAs (miRNAs) and regulate human non-small cell lung carcinoma (NSCLC) development. Results: Stable HMOX1 overexpression in NSCLC NCI-H292 cells up-regulated tumor-suppressive miRNAs, whereas it significantly diminished the expression of oncomirs and angiomirs. The most potently down-regulated was miR-378. HMOX1 also up-regulated p53, down-regulated angiopoietin-1 (Ang-1) and mucin-5AC (MUC5AC), reduced proliferation, migration, and diminished angiogenic potential. Carbon monoxide was a mediator of HMOX1 effects on proliferation, migration, and miR-378 expression. In contrast, stable miR-378 overexpression decreased HMOX1 and p53; while enhanced expression of MUC5AC, vascular endothelial growth factor (VEGF), interleukin-8 (IL-8), and Ang-1, and consequently increased proliferation, migration, and stimulation of endothelial cells. Adenoviral delivery of HMOX1 reversed miR-378 effect on the proliferation and migration of cancer cells. In vivo, HMOX1 overexpressing tumors were smaller, less vascularized and oxygenated, and less metastatic. Overexpression of miR-378 exerted opposite effects. Accordingly, in patients with NSCLC, HMOX1 expression was lower in metastases to lymph nodes than in primary tumors. Innovation and Conclusion: In vitro and in vivo data indicate that the interplay between HMOX1 and miR-378 significantly modulates NSCLC progression and angiogenesis, suggesting miR-378 as a new therapeutic target. Rebound Track: This work was rejected during standard peer review and rescued by Rebound Peer Review (Antioxid Redox Signal 16, 293–296, 2012) with the following serving as open reviewers: James F. George, Mahin D. Maines, Justin C. Mason, and Yasufumi Sato. Antioxid. Redox Signal. 19, 644–660.
Introduction
Heme oxygenase-1 (HMOX1, HO-1) is an inducible enzyme that degrades heme to carbon monoxide, ferrous ions, and biliverdin (33). HMOX1 may modulate tumor growth through the regulation of apoptosis, angiogenesis, and inflammatory response (18). Treatment such as chemotherapy, radiation, or photodynamic therapy may increase HMOX1 level in cancer cells (18, 40). Moreover, in comparison to the surrounding healthy tissues, HMOX1 expression is increased in different types of tumors, such as melanoma (47), glioblastoma (6), Kaposi sarcoma (36), or squamous cell carcinoma (SCC) (25, 49). Conversely, diminished HMOX1 expression was shown in human early-stage tumor specimens of non-small cell lung carcinoma (NSCLC) (5), suggesting an opposite HMOX1 role in different neoplasms. That is why mechanisms involved in the effect of HMOX1 on tumorigenesis still require elucidation.
We have recently demonstrated that HMOX1 may act in a similar manner to oncogene and increase the risk of hyperplastic growth of myogenic precursors through the regulation of microRNAs (miRNAs) (20). miRNAs are a class of small noncoding RNAs, which can very potently regulate the expression of genes and translation of proteins, interfering with ribosomal machinery. They commonly target 3′ untranslated regions (3′UTRs) of mRNAs, decreasing their stability and suppressing translation. However, they can also activate other genes (50). MiRNAs can affect various biological processes, such as proliferation, migration, and angiogenesis, which may lead to tumor progression. Down-regulation of miRNAs is a common feature of human malignancies (32). Aberrant expression of miRNAs may contribute to tumorigenesis by inhibiting the expression of tumor suppressor genes or by promoting the expression of proto-oncogenes and angiomirs. Accordingly, it has been suggested that miRNA expression profile in lung cancer can help predict survival of NSCLC patients (23).
An interesting example of the known oncomir and angiomir is miR-378. hsa-miR-378 precursor gives rise to two mature strands: hsa-miR-378 (hsa-miR-378a-3p) and hsa-miR-378* (hsa-miR-378a-5p). miR-378 promotes cell survival and tumor growth of glioblastoma by targeting two tumor suppressors: Sufu and Fus-1 (24). Its pro-tumorigenic and pro-angiogenic role was also demonstrated in A549 adenocarcinoma cells (4). miR-378 was also identified as a novel target of the c-Myc oncoprotein that is able to cooperate with activated Ras or human epidermal growth factor receptor 2 (HER2) in tumor development (9). Furthermore, miR-378* can induce molecular switch that is involved in the orchestration of the Warburg effect in breast cancer cells (8).
Based on the previously demonstrated effects of HMOX1, we hypothesized that it can influence human NSCLC growth, metastasis, and angiogenesis through modulation of miRNAs. Surprisingly, both in vitro and in vivo in an animal model, HMOX1 attenuated tumor cells proliferation and migration and decreased tumor growth, significantly affecting miRNA pathway. Interestingly, reciprocal interplay between HMOX1 and oncomir miR-378 influenced NSCLC in opposite ways. This interaction may be of significance for the tumor growth, angiogenesis, and metastasis.
Rebound Track.
This work was rejected during standard peer review and rescued by Rebound Peer Review (Antioxid Redox Signal 16: 293–296, 2012) with the following serving as open reviewers: James F. George, Mahin D. Maines, Justin C. Mason, and Yasufumi Sato. Comments by these reviewers supporting the rescue are listed next:
Mahin D. Maines (mahin_maines@urmc.rochester.edu): I am a qualified reviewer (per Antioxid Redox Signal 16: 293–296, 2012) and move to rescue this article that was rejected during the regular peer review process after reviewing all versions of the article and detailed reviewer comments. I differ from their assessment of this article. Currently, interactions between heme oxygenase‐1 (HO‐1) and miR‐378 and the mechanisms by which the miR affects non‐small cell lung carcinoma (NSCLC) growth, angiogenesis, and potentially metastasis have been analyzed using state‐of‐art techniques. Of particular interest is the demonstration that the level of HO‐1 in the cellular model is comparable to what is observed in the clinical samples. This indicates that data are of pathophysiological relevance; and, as such, can be considered a novel and a major step in advancing the field. By showing that treatment with N‐acetyl‐L‐cysteine (NAC) mimicked HO‐1 overexpression, the authors have provided a direct link between oxidative stress and HO‐1 activity. Furthermore, it has been convincingly demonstrated that miR‐378 affects HO‐1 expression by targeting its mRNA. The request of the reviewers to examine other microRNA (miRNA) is interesting but, in my opinion, it may preclude from publishing the article in a timely manner. Notably, regulation of gene expression by miR is a new frontier in HO research. My opinion is further supported by requests such as performing an Nrf2 study in additional cell lines and additional human samples. In my experience, these are classical comments, when there is a covert desire to suppress the publication of an article. I question whether the reviewers have evidence that HO‐1 mRNA differs in different human cell lines. I believe that the current version of this article is well done; reports a significant and timely finding; and, as such, merits publication. Therefore, in the interest of science, I take full responsibility to rescue this work from rejection.
Yasufumi Sato (y-sato@idac.tohoku.ac.jp): I am a qualified reviewer (per Antioxid Redox Signal 16: 293–296, 2012) and move to rescue this article that was rejected during the regular peer review process after reviewing all versions of the article and detailed reviewer comments. Comments to the Author: In this article, the authors investigated the possible involvement of miRNAs in the effect of HMOX1, and showed for the first time the interplay between HMOX1 and miR‐378 in NCI‐H292 cells and tissues from human NSCLC. This reviewer admits that the article would provide novel and important information on the mechanism of how HMOX1 exhibits its effect by modulating miR‐378, but has the following comments. 1. HMOX1‐mediated decrease of the expression of miR‐378 needs to be shown in an additional NSCLC cell line. 2. The activity of HMOX1 needs to be determined in the HMOX1 overexpressed cells. 3. The discussion should be more concise. Limited papers have reported the involvement of miRNAs such as miR‐122 in the regulation of HMOX1 expression. In fact, this is the first study demonstrating that HMOX1 decreases the expression of miR‐378. The results are rather circumstantial, but they are enough to show the relationship between HMOX1 and miR‐378. Therefore, in the interest of science, I take full responsibility to rescue this work from rejection.
Justin C. Mason (justin.mason@imperial.ac.uk): I am a qualified reviewer (per Antioxid Redox Signal 16: 293–296, 2012) and move to rescue this article that was rejected during the regular peer review process after reviewing all versions of the article and detailed reviewer comments. This novel and challenging study reports that HMOX1 exhibits a negative effect on NSCLC growth and metastasis, mediated in part through miR‐378. This finding advances the field and in its current form, leads to important new research questions to be addressed with regard to the value of therapeutic manipulation of HMOX1 in NSCLC. The authors explore the mechanisms underlying the actions of HMOX1, and whether they are related to its enzymatic products and/or reduced levels of oxidative stress. The answer, at least in part, lies within the revised article, which demonstrates that CO reduces expression of miR‐378, and that N‐acetyl‐L‐cysteine reproduces the effect of HMOX1 overexpression. The mechanistic approach taken is thorough, as exemplified by experiments demonstrating effects of on miR‐378 levels and NCI‐H292 proliferation and migration using gain of function, siRNA‐mediated loss of function, and HMOX1 overexpression reversal experiments. In vitro data are supported by a murine in vivo model and patient‐derived tissue samples. Questions with regard to the role of the other miRNAs shown to be regulated by HMOX1 are important. However, this work is beyond the scope of an initial manuscript. In my view, the data presented in various forms are convincing of an important influence of miR‐378. Although the data would be strengthened by the use of additional NSCLC lines, this is also beyond the scope of a study that has chosen to focus on one line and to adopt a detailed combined in vitro, in vivo, and ex vivo approach. Therefore, in the interest of science, I take full responsibility to rescue this work from rejection.
James F. George (jgeorge@uab.edu): I am a qualified reviewer (per Antioxid Redox Signal 16: 293–296, 2012) and move to rescue this article that was rejected during the regular peer review process after reviewing all versions of the article and detailed reviewer comments. This article contains a relatively large amount of data with the stated goal of determining the extent to which HO‐1 affects miRNA expression and how this might affect tumor growth. HO‐1 is known to provide cytoprotection via a number of mechanisms, particularly the anti‐oxidant effects of the products of heme degradation. The cytoprotective effects of HO‐1 have led some to conclude that induction of HO‐1 in tumors would be inimical to chemotherapy. This article convincingly shows in multiple experiments using different modes of induction and knockdown that, in fact, the role of HO‐1 with regard to cellular regulation in general and tumor growth in particular is highly complex. Even more interesting is the discovery of a bidirectional relationship between HO‐1 and the induction of miRNA expression. The authors show that there is a link between the induction of specific miRNAs, most notably miRNA‐378, and tumor growth/angiogenesis. Many of the observed effects can also be induced using CO, linking them with the products of HO‐1 activity. Evidence is provided for the possibility that the observations are applicable in humans in vivo by noting reduced expression of HO‐1 in primary NSCLC tumors versus secondary tumors. Therefore, the article provides multiple significant observations as well as data to support a plausible mechanism. These are unique observations. Therefore, in the interest of science, I take full responsibility to rescue this work from rejection.
Results
HO-1 modulates miRNA transcriptome in NCI-H292 NSCLC
To assess the effect of HO-1 on tumorigenic and angiogenic capabilities of NSCLC, we generated NCI-H292 cells stably overexpressing HMOX1 after transfection with plasmid (NCI-pcDNA-HO-1) and transduction with retroviral vectors (NCI-HO-1). NCI-H292 cells modified with pcDNA3.1 plasmid vector (NCI-pcDNA) and retroviral empty vector (NCI-EV-ctrl) served as controls. The main advantage of NCI-H292 cell line is its low basal level of HMOX1 in comparison to another commonly used model: A549 cells (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/ars). Transfection with plasmids allowed obtaining 41.4±5.4 more HMOX1 mRNA than in control cell line (Supplementary Fig. S1B), whereas transduction with retroviral vectors resulted in 2544.27±301.47 higher HMOX1 level than in EV-ctrl cell line (Supplementary Fig. S1C), as estimated with quantitative polymerase chain reaction (qPCR). HMOX1 overexpression in cells modified with retroviral vectors was also confirmed at protein level (Supplementary Fig. S1D). The cells overexpressing HMOX1 displayed higher HMOX1 activity, which was reversed by tin protoporphyrin-IX (SnPPIX) treatment (Supplementary Fig. S1E). HMOX1 level turned out to be comparable with HMOX1 expression that was induced pharmacologically with cobalt protoporphyrin-IX (CoPPIX), hemin, and with HMOX1 level in NSCLC samples from patients (Supplementary Fig. S1F, G). Therefore, this model was selected for the majority of our studies.
First, we demonstrated that HMOX1 overexpression slightly, but significantly, enhanced the expression of DiGeorge syndrome critical region-8 (DGCR8), Drosha, and Dicer1 mRNA (Fig. 1A–C), the genes that are responsible for miRNA processing. Accordingly, Drosha and DGCR8 proteins were elevated in HMOX1 overexpressing cells and in control cells with pharmacologically increased HMOX1 level by CoPPIX treatment (Supplementary Fig. S2A–C). Concomitantly, a global amount of small RNA in range 19–25 nt (miRNA) was increased in comparison to all <250 nt small RNA (Fig. 1D) and total RNA (Fig. 1E); whereas the amount of <250 nt small RNA compared with total RNA was unaffected (Fig. 1F).
FIG. 1.
HMOX1 modulates miRNA transcriptome in NCI-H292 cells. HMOX1 overexpression in NCI-H292 up-regulates DGCR8 (A), Drosha (B), Dicer1 (C) (qPCR, n=4). HMOX1 enhances global amount of 19–25 nt small RNA (mature miRNAs) compared with <250 nt small RNA (D), total RNA (E); whereas amount of <250 nt small RNA compared with total RNA is unaffected (F), (Bioanalyzer analysis, n=3). miRCURY™ LNA array analysis (Exiqon) of RNA: miRNAs down-regulated (G) and up-regulated (H) by HMOX1 (n=3, p≤0.05 in paired t test and fold of induction ≥1.15 or ≤0.85). *p<0.05. DGCR8, DiGeorge syndrome critical region-8; EF2, elongation factor 2; EV-ctrl, control cell line modified with empty retroviral vector; HO-1, heme oxygenase-1; miRNA, microRNA; qPCR, quantitative polymerase chain reaction.
Subsequently, miRCURY™ LNA array analysis revealed that elevated HMOX1 level significantly modulated the expression of miRNAs that were potentially involved in tumor growth and vascularization. Expression of known oncomirs (members of miR-17–92 cluster, miR-20b, miR-135a, miR-210, and miR-378) and angiomirs (miR-17–92, miR-210, and miR-378) was down-regulated; whereas several tumor-suppressive miRNAs (miR-22, miR-24, miR-129-3p, miR-130a, miR-181a, miR-193b, miR-424, and miR-1246) and anti-angiomirs (miR-424) were up-regulated (Fig. 1G, H). The most potently down-regulated was miR-378; so, we selected it for further investigations. Moreover, with qPCR, we also confirmed the expression pattern of the selected miRNAs involved in tumor growth and angiogenesis in the second stable model, with lower HMOX1 overexpression level after transfection with pcDNA3.1 plasmid vectors (Supplementary Fig. S3). Importantly, miR-378 was also down-regulated in cells overexpressing HMOX1 after plasmid transfection (Supplementary Fig. S3).
HMOX1 diminishes and miR-378 enhances proliferation, migration, and angiogenic capabilities of NCI-H292 cells in vitro
Since miRNAs expression profile in HMOX1 overexpressing NCI-H292 cells suggested diminished tumorigenic and angiogenic capabilities, we investigated them further in vitro. HMOX1 overexpression increased the number of cells with a high level of p53, a known tumor suppressor (Supplementary Fig. S4A, B). Moreover, we observed a transition of cell cycle to G1 phase (Supplementary Fig. S4C), which was concomitant with a significant attenuation in proliferation of HMOX1 overexpressing cells (Fig. 2A), as well as we demonstrated a significant decrease in migration that was estimated as an inhibition of cell motility in scratch assay (Fig. 2B). Furthermore, the effects of HMOX1 on the proliferation of NCI-H292 cells were mimicked by carbon monoxide, one of HMOX1 activity products (Fig. 2A, B). Cellular parameters were also verified in NCI-H292 cells with a stable lower HMOX1 overexpression level after transfection with pcDNA3.1 plasmid vectors. We again observed a slight, but significant attenuation in proliferation (Fig. 2C) and a significant decrease in cell migration (Fig. 2D). Accordingly, HMOX1 silencing with siRNA (Supplementary Fig. S5A, B) enhanced proliferation (Fig. 2E) and migration (Fig. 2F) in both control and HMOX1 overexpressing cells.
FIG. 2.
HMOX1 and carbon monoxide diminish proliferation of NCI-H292 cells. Proliferation estimated with BrdU incorporation assay (n=6) (A) and migration estimated by scratch assay after 18 h from the scratch (n=3) (B) of NCI-H292 cells stably overexpressing HMOX1 and in EV-ctrl cells, either nontreated or treated with 10 μM CO-releasing molecule (CORM) or 10 μM inactive CORM (iCORM). Proliferation (n=3) (C) and migration after 18 h from the scratch (n=3) (D) of NCI-H292 cells with lower HMOX1 overexpression level after transfection with pcDNA plasmids. Proliferation (n=3) (E) and migration (n=3) after 24 h from the scratch (F) of NCI-H292 cells transfected with HMOX1 siRNA (siHO-1) and scrambled siRNA (siSCR). *p<0.05, **p<0.01, ***p<0.001. BrdU, 5-bromo-2′-deoxyuridine.
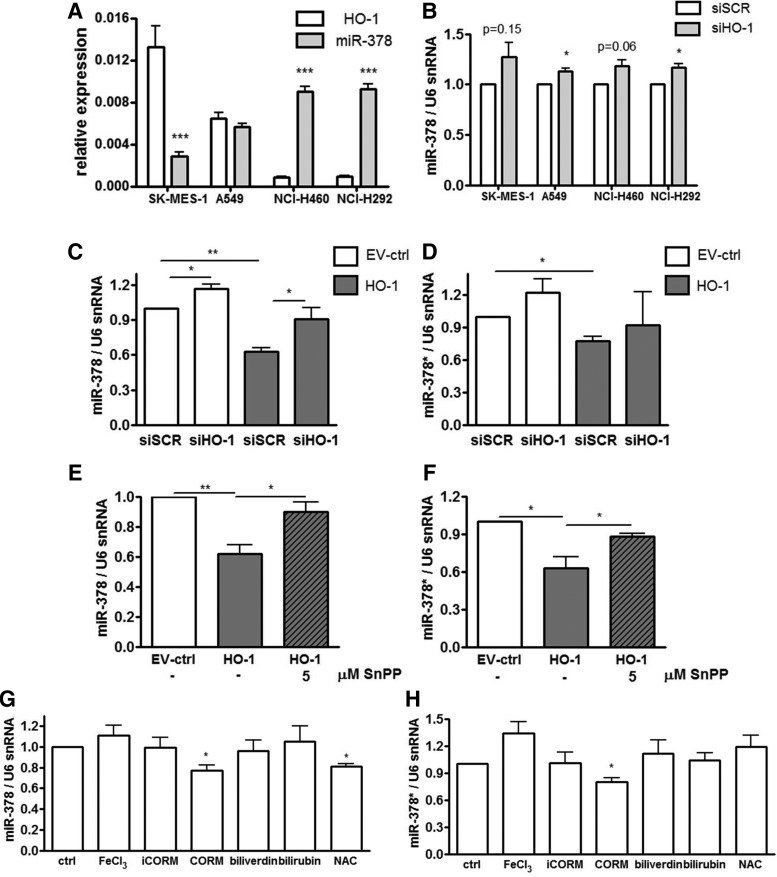
We suspected that miR-378 might be one of the crucial mediators of the observed effects. Interplay between HMOX1 and miR-378 was then demonstrated in different NSCLC cell lines: SK-MES-1, A549, NCI-H460, and NCI-H292 and cell lines with a higher HMOX1 basal level displayed lower miR-378 expression (Fig. 3A). Moreover, silencing of HMOX1 with siRNA (Supplementary Fig. 5C) up-regulated or showed a tendency to up-regulate miR-378 level in those cell lines (Fig. 3B).
FIG. 3.
HMOX1 and carbon monoxide diminish the level of pro-oncogenic and pro-angiogenic miR-378. HMOX1 mRNA and miR-378 basal level (n=4) in different NSCLC cell lines: SK-MES-1, A549, NCI-H460, and NCI-H292 (A). The effect of HMOX1 silencing with siRNA on miR-378 level in different NSCLC cell lines (n=4) (B). miR-378 (n=4) (C) and miR-378* level (n=4) (D) in NCI-H292 cells transfected with HMOX1 siRNA (siHO-1) and scrambled siRNA (siSCR). miR-378 (n=4) (E) and miR-378* (n=3) (F) level in NCI-H292 cells overexpressing HMOX1 treated with 5 μM SnPPIX. miR-378 (G) and miR-378* (H) level in NCI-H292 cells treated for 24 h with 10 μM FeCl3, 10 μM CORM, 10 μM iCORM (negative control), 10 μM biliverdin, 10 μM bilirubin, and 0.5 mM NAC. Inhibition of HMOX1 by siRNA or SnPPIX restored the level of miR-378, while CO or NAC down-regulated it (n=4, for NAC: n=3). *p<0.05, **p<0.01, ***p<0.001. NAC, N-acetyl-L-cysteine; NSCLC, non-small cell lung carcinoma; SnPPIX, tin protoporphyrin-IX.
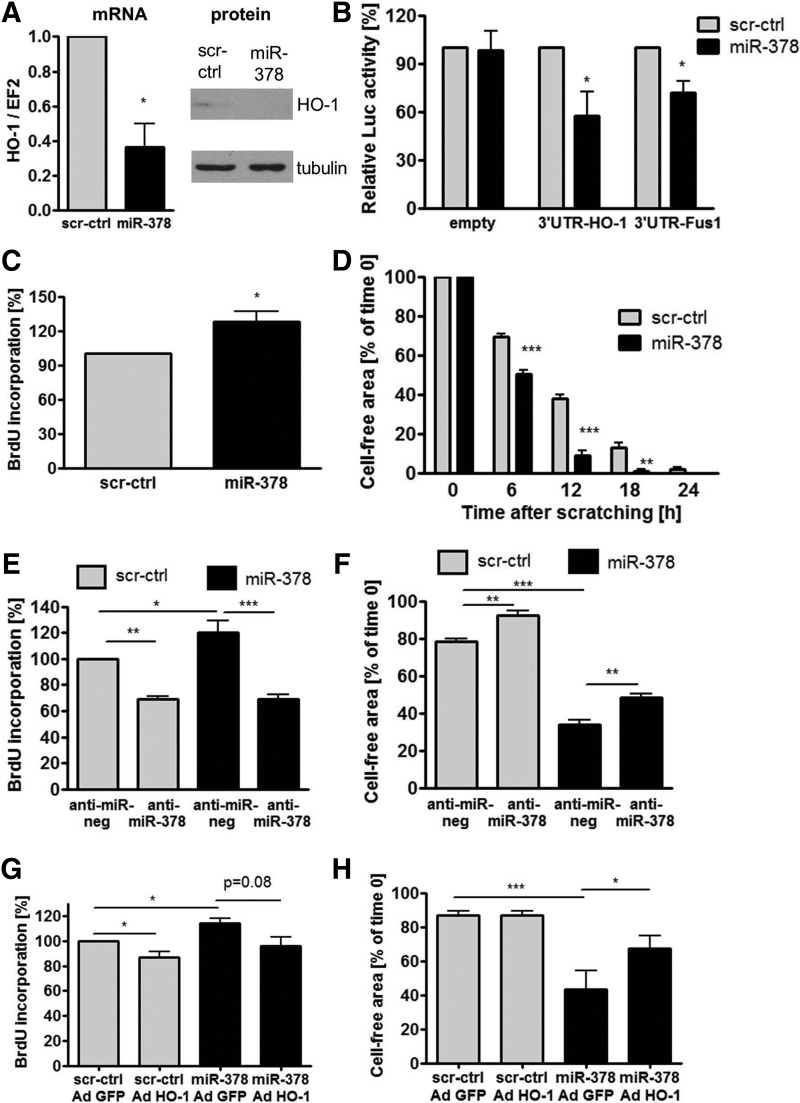
Of note, miR-378 (hsa-miR-378a-3p) is expressed along with the second strand miR-378* (has-miR-378a-5p), which was not detected in microarrays, but with real-time PCR we managed to evaluate its expression (Fig. 3C–H). Silencing of HMOX1 with siRNA in NCI-H292 cells partially but significantly reversed the influence of HMOX1 on miR-378 expression (Fig. 3C) and seemed to affect miR-378* (Fig. 3D). Importantly, the pharmacological inhibition of HMOX1 with SnPPIX also reversed the effects of HMOX1 on miR-378 (Fig. 3E) and miR-378* (Fig. 3F). To determine which product of HMOX1 activity might be responsible for miR-378 and miR-378* down-regulation, we treated NCI-H292 cells with FeCl3, CO-releasing molecule (CORM), inactive CORM (iCORM, negative control), biliverdin, bilirubin, and with a known reactive oxygen species (ROS) scavenger: N-acetyl-L-cysteine (NAC). It turned out that the effect of HMOX1 on miR-378/378* level can be mimicked by only one of its products: carbon monoxide (Fig. 3G, H). Furthermore, NAC down-regulated miR-378 (Fig. 3G), but not miR-378* level (Fig. 3H). Interestingly, restoration of miR-378 level by transfection with miRNA precursors in HMOX1 overexpressing cells did not reverse HMOX1 effects on proliferation (Supplementary Fig. S5D) and migration (Supplementary Fig. S5E), which was presumably due to the high expression of HMOX1. However, control NCI-H292 cells transfected with pre-miR-378 tended to behave in an opposite manner to HMOX1 overexpressing cells.
The data indicated that higher expression of miR-378 may be required to overcome the effect of HMOX1. Accordingly, stable miR-378 precursor overexpressing cells were obtained using lentiviral vectors with feline immunodeficiency viral vector (FIV) or human immunodeficiency viral vector (HIV) backbones. Control cell lines were transduced with vectors harboring a scrambled sequence. Transduction resulted in overexpression of miR-378 and miR-378* (Supplementary Fig. S6A–D). Accordingly, and as expected, mRNA expression levels of known miR-378 targets, namely Sufu, Fus-1 (24) and cytochrome P450 2E1 (CYP2E1) (39), were decreased in miR-378/378* overexpressing cells (Supplementary Fig. S6E–G).
Interestingly, interplay between HMOX1 and miR-378 was evidenced, as miR-378 precursor overexpression down-regulated HMOX1 mRNA and protein (Fig. 4A and Supplementary Fig. S6H). miR-378 also reduced luciferase activity after transfection with constructs harboring Renilla luciferase with 3′UTR of HMOX1 (pSL-3′UTR-HO-1) and TUSC2 (pSL-3′UTR-Fus1) in comparison to empty constructs with luciferase (pSL-empty), suggesting that miR-378 may directly bind to 3′UTR region of HMOX1 mRNA (Fig. 4B). miR-378 overexpression also decreased the number of NCI-H292 cells with high p53 level (Supplementary Fig. S7A, B), diminished the percentage of cells in G1 phase, and showed a tendency to increase the percentage of cells in S and G2/M phases (Supplementary Fig. S7C), displaying enhanced proliferation (Fig. 4C) and migration (Fig. 4D). The effects were opposite to those observed in HMOX1 overexpressing cells (Fig. 2A–D). Accordingly, blocking of miR-378 with anti-miR-378 reversed the effects of miR-378 overexpression on proliferation (Fig. 4E) and partially on migration (Fig. 4F). To determine whether HMOX1 can reverse the effect of miR-378 on proliferation and migration of lung cancer, NCI-H292 cells overexpressing miR-378 were transduced with adenoviral vectors harboring HMOX1 (AdHO-1) or GFP (AdGFP) as a control. Temporal HMOX1 overexpression with adenoviral vectors tended to reverse the effect of miR-378 on proliferation (Fig. 4G) and restored migration (Fig. 4H).
FIG. 4.
miR-378 down-regulates HMOX1 and enhances proliferation and migration of NCI-H292 cells. HMOX1 mRNA (qPCR, n=3) and protein level (Western blotting) in NCI-H292 cells stably overexpressing miR-378 (A). Luciferase activity in NCI-H292 cells overexpressing miR-378 after transfection with constructs harboring Renilla luciferase with 3′UTR of HMOX1 (pSL-3′UTR-HO-1) and TUSC2 (pSL-3′UTR-Fus1) in comparison to empty constructs with luciferase (pSL-empty) (n=3) (B). Proliferation estimated with BrdU incorporation assay (n=3) (C) and migration estimated with scratch assay (n=3) (D) of NCI-H292 cells stably overexpressing miR-378. Proliferation (n=3) (E) and migration (n=3) (F) of NCI-H292 cells transfected with anti-miR-378 and anti-miR-negative control. Proliferation (n=3) (G) and migration (n=4) (H) of NCI-H292 cells overexpressing miR-378 transduced with adenoviral vectors harboring HMOX1. *p<0.05, **p<0.01, and ***p<0.001. UTR, untranslated region.
Since HMOX1 and miR-378 were previously demonstrated to influence angiogenesis, we first estimated the production of pro-angiogenic factors. stromal cell-derived factor-1 (SDF-1) was expressed at the limit of detection. HMOX1 overexpression did not significantly modify vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) secretion (Supplementary Fig. S8A), whereas stable miR-378 overexpression significantly up-regulated both of them (Supplementary Fig. S8B). Subsequently, expression of other pro-angiogenic and pro-oncogenic mediators was evaluated with real-time PCR. It revealed that angiopoietin-1 (Ang-1) and mucin-5AC (MUC5AC) were down-regulated by HMOX1 (Fig. 5A); whereas Ang-1, Ang-2, and MUC5AC were up-regulated by miR-378 (Fig. 5B).
FIG. 5.
HMOX1 diminishes and miR-378 enhances angiogenic potential of NCI-H292 cells in vitro. MUC5AC, Ang-1, and Ang-2 level in NCI-H292 cells overexpressing HMOX1 (qPCR, n=4) (A) and miR-378 (qPCR, n=3) (B). miR-378 level in exosomes produced by NCI-H292 cells overexpressing HMOX1 (qPCR, n=5) (C) and miR-378 (qPCR, n=3) (D). Formation of capillary-like structures in Matrigel assay from lung microvascular endothelial cells treated with conditioned media from NCI-H292 cells overexpressing HMOX1 (E, F) and miR-378 (E, G) (n=3). *p<0.05. Ang, angiopoietin; MUC5AC, mucin-5AC.
It has been suggested that miRNA can be transferred between cells. Accordingly, here we demonstrate that angiomir miR-378 is exported from NCI-H292 cells in exosomes, and HMOX1 could down-regulate not only expression of miR-378, but also its exosomal export (Fig. 5C). Inversely, cells overexpressing miR-378 secreted more miR-378 in exosomes (Fig. 5D). Therefore, we wanted to determine whether the secretion of miR-378 can affect the endothelial cells (EC). Indeed, lung microvascular EC treated with conditioned media harvested from NCI-H292 cells proliferated more potently than those cultured with unconditioned media, evidencing pro-angiogenic properties of the cancer cells. NCI-HO-1 conditioned media slightly, but significantly, inhibited proliferation of EC (Supplementary Fig. S8C); however, NCI-miR-378-conditioned media did not exert significant effects (Supplementary Fig. S8D). Furthermore, EC treated with media harvested from NCI-HO-1 formed less tubule-like structures on Matrigel (Fig. 5E, F); whereas the effect of media on NCI-miR-378 tended to be opposite (Fig. 5E, G). The effect of conditioned media harvested from NCI-H292 cells on the formation of tubule-like structures was also confirmed using human umbilical vein endothelial cells (Supplementary Fig. S8E–G).
HMOX1 diminishes and miR-378 enhances NSCLC growth, vascularization, oxygenation, and distal metastasis in vivo
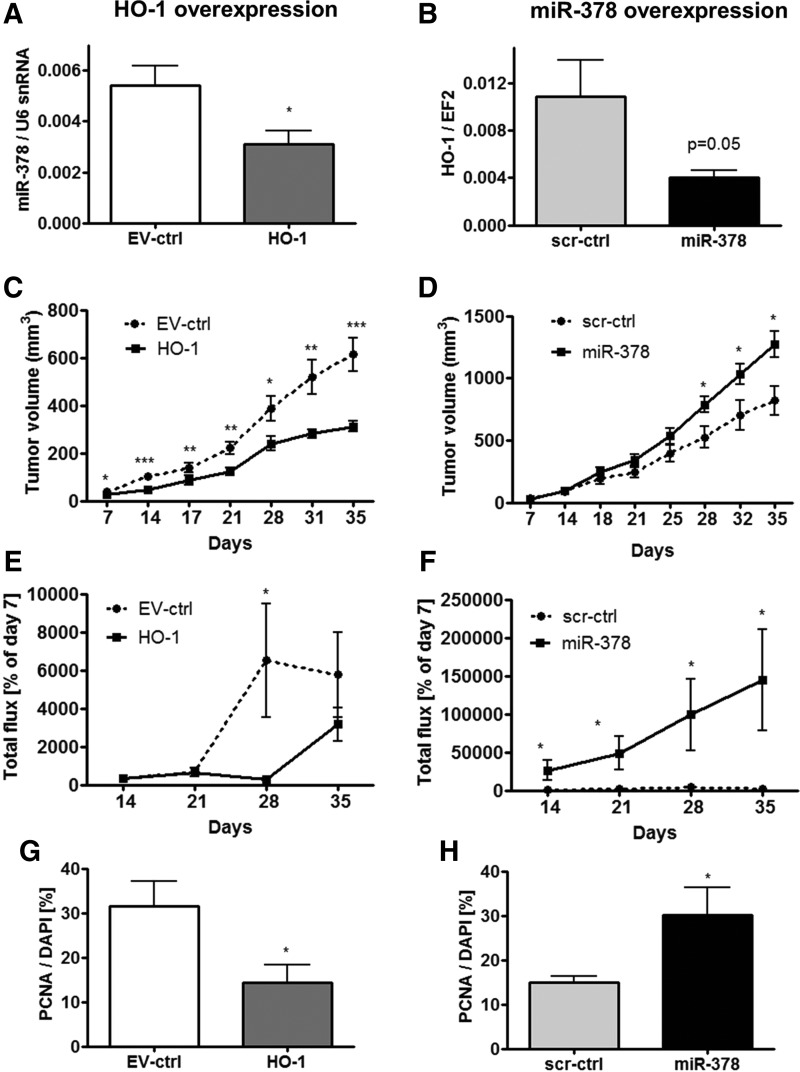
For in vivo experiments we utilized NCI-H292-Luc cells in subcutaneous xenografts in a Swiss nude immunodeficient murine model. We confirmed the interplay between HMOX1 and miR-378/miR-378* in vivo, showing the mutual regulation of the expression of each other (Fig. 6A, B and Supplementary Fig. S9A–D). HMOX1 overexpression significantly inhibited NCI-H292 xenograft growth (Fig. 6C). On the contrary, miR-378 overexpression enhanced tumor growth (Fig. 6D). Measurements of luciferase activity by IVIS showed slightly diminished total flux signal in tumors overexpressing HMOX1 at 28 days after implantation (Fig. 6E) and enhanced luminescence in tumors overexpressing miR-378 starting from 14 day (Fig. 6F), confirming tumor growth estimated with caliper (Fig. 6C, D). Attenuated proliferation of cells overexpressing HMOX1 and enhanced proliferation of cells overexpressing miR-378 was demonstrated by proliferating cell nuclear antigen (PCNA) staining in tumor cryosections (Fig. 6G, H, see also Supplementary Fig. S9E, F). Similarly, plasmid-transfected cells overexpressing HMOX1 at a lower level were utilized in the xenograft NOD-SCID murine model. HMOX1 overexpression again resulted in significant inhibition of tumor growth rate (Supplementary Fig. S9G).
FIG. 6.
HMOX1 diminishes and miR-378 enhances NSCLC subcutaneous xenograft growth in Swiss nude mice. miR-378 level in NSCLC subcutaneous xenografts overexpressing HMOX1 in Swiss nude mice, n=7 (A). HMOX1 level in NSCLC subcutaneous xenografts overexpressing miR-378 (n=7) (B). Tumor volume in HMOX1 (C) and miR-378 (D) overexpressing xenografts estimated by caliper (n=9). Luciferase activity detected with IVIS in tumors after implantation of NCI-H292-Luc cells overexpressing HMOX1 (E) and miR-378 (F) (percentage of day 7 after implantation, n=5–8). PCNA-positive cell ratio to DAPI detected by immunofluorescent staining in cryosections of tumors overexpressing HMOX1 (G) and miR-378 (H) five weeks after implantation (n=4–5). *p<0.05, **p<0.01, ***p<0.001. DAPI, 4′,6-diamidino-2-phenylindole; PCNA, proliferating cell nuclear antigen.
Since the tumor growth is highly dependent on angiogenesis, we determined the effect of transgenes in NCI-H292 cells on tissue vascularization. Nodules formed by HMOX1 overexpressing cells were smaller, and no eye-visible necrosis was detected (Supplementary Fig. S10A). On the contrary, tumors overexpressing miR-378 were bigger with traces of edema and were better vascularized (Supplementary Fig. S10B). Staining for cluster of differentiation 31 (CD31) revealed the presence of vessels inside the nodules and indicated the lower number of capillaries in tumors overexpressing HMOX1 (Fig. 7A and Supplementary Fig. S10C), but higher in tumors overexpressing miR-378 (Fig. 7B and Supplementary Fig. S10D). Concomitantly, oxygen partial pressure, estimated in vivo near the tumor surface by ruthenium fluorescence quenching measurement, was diminished in tumors overexpressing HMOX1 (Fig. 7C) and enhanced in tumors overexpressing miR-378 (Fig. 7D). Moreover, mice with xenografts overexpressing HMOX1 tended to have diminished miR-378 level in blood-circulating exosomes (Supplementary Fig. S10E), whereas this tended to increase in the case of miR-378 overexpression (Supplementary Fig. S10F), supporting the results from in vitro measurements (Fig. 5C, D). Then, we evaluated the modulation of genes regulating angiogenesis and metastasis. SDF-1 protein in tumors was down-regulated by HMOX1 (Fig. 7E) and up-regulated by miR-378 (Fig. 7F). Moreover, the high level of HMOX1 led to a decrease in MUC5AC, Ang-1, and matrix metalloproteinase 12 (MMP12) and an increase in tumor necrosis factor-alpha (TNF-α) and interleukin-1beta (IL-1β) (Fig. 7G and Supplementary Fig. S10G). Opposite effects were visible in tumors overexpressing miR-378 (Fig. 7H and Supplementary Fig. S10H).
FIG. 7.
HMOX1 and miR-378 oppositely regulate vascularization, oxygenation, and gene expression in NSCLC subcutaneous xenografts in Swiss nude mice. Number of capillaries calculated after staining for CD31 and DAPI in cryosections of tumors overexpressing HMOX1 (n=8) (A) and miR-378 (n=8) (B). Partial pressure of oxygen in xenografts overexpressing HMOX1 (n=8) (C) and miR-378 (n=8) (D). SDF-1 protein level in tumors overexpressing HMOX1 (n=9) (E) and miR-378 (n=9) (F). Ang-1 (n=5) and MUC5AC (n=7–8) expression in tumors overexpressing HMOX1 (G) and miR-378 (H) (qPCR, n=5–10). *p<0.05, **p<0.01. CD31, cluster of differentiation 31; SDF-1, stromal cell-derived factor-1.
Metastatic potential of tumor cells was measured as an amount of human ubiquitin c transcripts in different organs of the host. HMOX1 showed a tendency to diminish distal metastasis to the brain (Fig. 8A). On the contrary, miR-378 enhanced distal metastasis to the lungs, seemed to nonsignificantly favor metastasis to the brain, and did not affect the local, right-limb bone marrow (Fig. 8B). No metastasis was detected in left-limb bone marrow, liver, and adrenal glands (Fig. 8A, B). Ex-vivo bioluminescence imaging allowed for detecting the luciferase signal in the excised right inguinal lymph nodes, the closest nodes to the xenograft implantation, in all experimental groups (Supplementary Fig. S11A, B). Number of platelets, modulating tumor angiogenesis and metastasis, was decreased in mice-bearing tumors overexpressing HMOX1 (Fig. 8C) and increased in case of miR-378 overexpression (Fig. 8D) compared with mice with control tumors and healthy animals.
FIG. 8.
HMOX1 diminishes and miR-378 enhances distal metastasis of NSCLC. Metastasis in organs collected five weeks after subcutaneous implantation of 5×105 NCI-H292 cells overexpressing HMOX1 (A) and miR-378 (B) to Swiss immunodeficient mice. Metastasis was calculated as the level of human Ubc normalized to total EF2 (qPCR, ΔCt, n=5–6). Number of platelets in blood of mice with xenografts overexpressing HMOX1 (C) and miR-378 (D) (n=7–9, PBS group: n=3). HMOX1 (E) and miR-378 (F) expression level in NSCLC primary tumors and metastases in patients suffering from lung cancer (qPCR, n=13–24). *p<0.05, **p<0.01, ***p<0.001. hUbc, human ubiquitin c; BMR, bone marrow right; BML, bone marrow left; AG, adrenal glands.
To extrapolate results from mice to patients suffering from NSCLC, we evaluated the expression of HMOX1 and miR-378 in primary NSCLC tumors and in metastases to lymph nodes. Quantitative real-time PCR revealed a higher HMOX1 levels in primary NSCLC than in metastases (Fig. 8E), whereas miR-378 expression seemed to be the opposite (Fig. 8F).
HMOX1 diminishes while miR-378 does not modulate resistance of cells to chemotherapeutics and oxidative stress
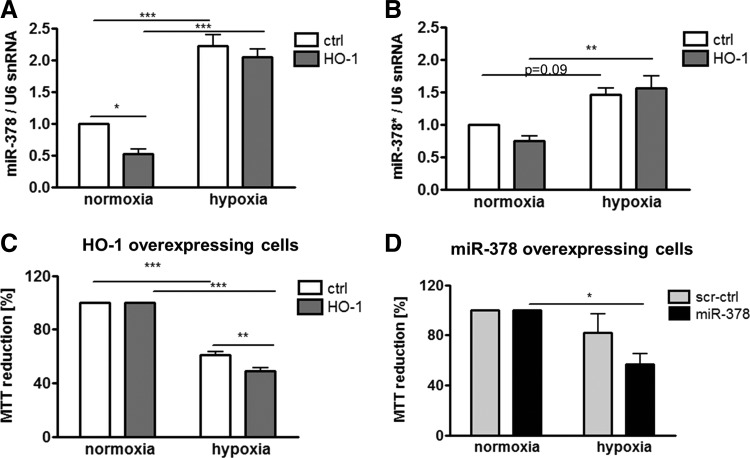
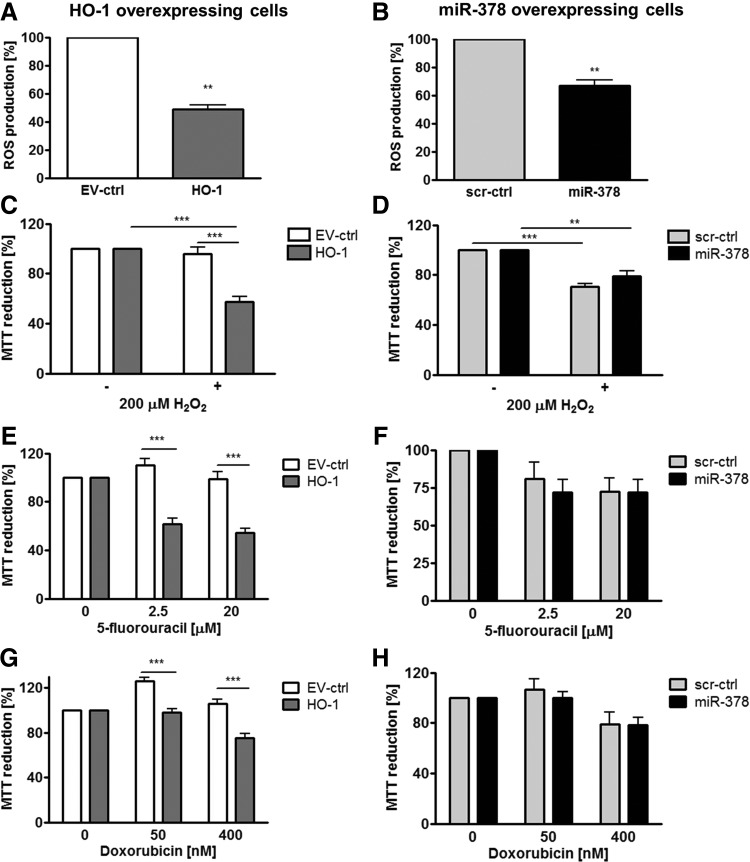
In many studies, HMOX1 has been considered a cytoprotective enzyme, which is up-regulated in stressful conditions and protects cells against oxidative stress injury (53). Therefore, we evaluated the role of HMOX1 and miR-378 in the resistance of cancer cells to stress. First, we have shown that hypoxia up-regulated miR-378 and miR-378* in control and HMOX1 overexpressing cell lines (Fig. 9A, B). Nevertheless, in hypoxia, both HMOX1 and miR-378 overexpressing cells displayed reduced viability (Fig. 9C, D). Interestingly, both HMOX1 and miR-378 displayed diminished production of ROS (Fig. 10A, B). However, viability of HMOX1 overexpressing cells was diminished after treatment with H2O2, doxorubicin, and 5-fluorouracil; whereas miR-378 overexpression did not modify it significantly (Fig. 10C–H).
FIG. 9.
Hypoxia increases miR-378 in NCI-H292 cells and modulates viability of the cells. miR-378 (A) and miR-378* (B) level after 24 h of hypoxia (0.5%–1% of oxygen) in NCI-H292 cells, (qPCR, ΔΔCt, n=3). Viability of NCI-H292 cells overexpressing HMOX1 (C) and miR-378 (D) estimated with MTT assay after 24 h of hypoxia in comparison to normoxia (n=4). *p<0.05, **p<0.01 ***p<0.001. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide.
FIG. 10.
HMOX1 overexpression in NCI-H292 cells diminishes and miR-378 does not modulate resistance to chemotherapeutics and oxidative stress. ROS production in NCI-H292 cells overexpressing HMOX1 (A) and miR-378 (B) estimated with 2′,7′-dichlorodihydrofluorescein diacetate by fluorescent measurement (n=3–4). Viability of NCI-H292 cells overexpressing HMOX1 and miR-378 estimated by MTT assay after 18 h of treatment with 200 μM H2O2 (n=3) (C, D) and after 72 h of treatment with 2.5 and 20 μM 5-fluorouracil (n=4–8) (E, F), 50 and 400 nM doxorubicin (n=4–8) (G, H). **p<0.01, ***p<0.001. ROS, reactive oxygen species.
Discussion
The salient finding of the present study is the demonstration of mutual interactions between HMOX1 and miR-378 in NSCLC cells in which a high level of HMOX1 correlates with a low expression of mirR-378. Investigating the mechanisms of that interplay in a model of NCI-H292 cell line, with low basal HMOX1 expression, we showed that HMOX1 overexpressing NCI-H292 cells displayed reduced proliferation and migration. Furthermore, growth of the HMOX1-overexpressing cells in Swiss nude mice was impaired, tumors were less vascularized and revealed decreased expression of SDF-1 and Ang-1. Distal metastasis to the brain tended to be attenuated by HMOX1 overexpression and was accompanied by down-regulation of MMP12 and MUC5AC and up-regulation of TNF-α and IL-1β. Interestingly, HMOX1 overexpression in NCI-H292 cells increased total pool of miRNAs with some suppressor miRNAs up-regulated and oncogenic ones decreased. The most strongly inhibited was miR-378, a known oncomir, with pro-angiogenic properties. Further studies revealed that carbon monoxide, one of HMOX1 by-products, significantly decreased miR-378 expression, and the effect was mimicked by NAC, indicating the redox-dependent regulation. On the other hand, when miR-378 was overexpressed by lentiviral vectors transduction in NCI-H292 cells, it inhibited the expression of its known target genes, such as Sufu, Fus-1, and CYP2E1 and also targeted mRNA of HMOX1. Overexpression of miR-378 affected NSCLC properties in an opposite manner to HMOX1: proliferation and migration of tumor cells were increased, tumor growth was accelerated, with augmented tumor vascularization and oxygenation, distal metastasis to lungs appeared to be increased, and angiogenic or inflammatory gene expressions were regulated by miR-378 inversely than in HMOX1 overexpressing cells.
Both this and our other recent study (20) indicate that HMOX1 is a potent regulator of miRNAs biogenesis and function, acting in a cell-type specific manner. Indeed, certain miRNAs that are up-regulated by HMOX1 in NCI-H292 cells were previously described as tumor suppressors inhibiting either proliferation or migration of cancer cells. This group consists of miR-181a—tumor suppressor in NSCLC (11); miR-193b—inhibitor of invasion of breast cancer (29); miR-424, which arrests cancer cells in G1 phase (43) and inhibits angiogenesis (3); miR-22—inhibitor of invasion of ovarian cancer (28); miR-24—inhibitor of cancer cell proliferation (38); miR-129-3p whose low expression was associated with poor clinicopathological features of primary gastric cancer patients (48); miR-130a—inhibitor of migration of NSCLC cells (1); and miR-1246, which can induce apoptosis (57).
Accordingly, the expression of known oncomirs was down-regulated by HMOX1. For example, miR-378 promoted glioma growth (24) and NSCLC invasion (4). Members of miR-17–92 cluster, such as miR-17*, −18a, −18b, −19b, and −20a, were previously associated with carcinogenesis and were up-regulated in tumors, including lung cancer (17). miR-210 and miR-135a also augmented tumor growth and metastasis (31, 41), while miR-20b displayed an oncogenic role in normoxia (27). Similarly, angiomirs, miR-17–92 cluster, miR-378, miR-210, and miR-135, were down-regulated (7, 24, 30, 35).
In humans, HMOX1 promoter displays (GT)n repeat polymorphism, and short S allele leads to a higher basal HMOX1 level (45). Longer (GT)n repeats may be associated with the development of lung adenocarcinoma in Japanese male smokers (19). This suggests that lower HMOX1 expression may favor lung cancer development, which fits our results. Similarly, HMOX1 also inhibited the development of breast cancer (14) and tongue SCC (55), but it promoted the growth of melanoma (52), pancreatic cancer (44), and sarcoma (34).
We next hypothesized that miR-378, as the oncomir and angiomir being most potently down-regulated by HMOX1 in comparison to other miRNAs, might mediate HMOX1 effects in NCI-H292. Transient restoration of its level did not reverse the effects of HMOX1 overexpression in NCI-H292 cells, probably because HMOX1 regulates tumor growth through many miRNAs. However, we observed that both temporal and stable miR-378 overexpression stimulates proliferation and migration in NCI-H292 cells. Similarly, miR-378 promoted cell survival and tumor growth of glioblastoma by targeting Sufu and Fus-1 (24); facilitated the c-Myc-mediated transformation of breast cancer (9); and favored the NSCLC invasion to the brain (4). Regulation of Sufu and Fus-1, as well as a tendency to augmented brain metastasis, was also observed in our studies. Moreover, miR-378* was identified as a molecular switch involved in the orchestration of the Warburg effect in human breast cancer (8).
HMOX1 was not predicted to be a typical miR-378 target with bioinformatics tools. However, in our analysis, one of the methods called RNA22 (37) predicted such probability, what was supported by experiments, where HMOX1 was down-regulated in response to miR-378 overexpression. Alternatively miR-378 may regulate HMOX1 indirectly. For example, CYP2E1, a direct target of miR-378 (39), may influence HMOX1 expression (46). In our study, CYP2E1 was also down-regulated in miR-378 overexpressing cells.
Importantly, miR-378 overexpressing cells with down-regulated HMOX1 behaved oppositely to HMOX1 overexpressing cells with down-regulated miR-378. Enhanced proliferation of NCI-miR-378 cells and diminished proliferation of NCI-HO-1 cells in vitro may translate for mode of tumor progression in vivo. Tumor growth rate may be also explained by transition of cell cycle from G1 to S and G2/M phase or partial growth arrest in G1 phase in NCI-miR-378 or NCI-HO-1, respectively. Furthermore, HMOX1 silencing with siRNA and inhibition of miR-378 with anti-miR reversed the effect of HMOX1 and miR-378 on proliferation of cancer cells. Similarly, A549, another adenocarcinoma cell line, exhibited growth arrest and increased cells' number in G0/G1 phase when overexpressing HMOX1 (26); whereas A549 cells overexpressing miR-378 proliferated faster (4). Diminished proliferation of cancer cells with elevated HMOX1 expression was also demonstrated for breast (13) and prostate cancer (14).
In our hands, HMOX1 overexpression tended to attenuate distal metastasis, while miR-378 enhanced lung metastasis. These observations are supported by an analysis of NSCLC clinical samples in which HMOX1 expression was lower in lymph node metastasis than in primary tumors, while miR-378 tended to be nonsignificantly higher in metastasis. Other studies also demonstrated the association of miR-378 with brain metastasis and enhanced migratory capabilities of A549 adenocarcinoma cells overexpressing miR-378 (4). In regulation of metastatic properties, the MMP12 or MUC5AC, differently regulated by HMOX1 and miR-378 in vivo, may be involved. MMP12 expression correlated with local recurrence and metastatic disease in NSCLC patients (15), and MUC5 overexpression was associated with early postoperative metastasis in NSCLC (56). Platelets may enhance NSCLC metastasis (21) and here, tumors overexpressing miR-378 were also associated with increased number of platelets in blood, whereas HMOX1 exerted the opposite effect.
The metastatic potential is affected by tumor vascularization. In many studies, HMOX1 has been shown as a pro-angiogenic enzyme up-regulating VEGF (18). There is only one study demonstrating the anti-angiogenic effect of HMOX1 in prostate cancer (10). Here, we demonstrated diminished angiogenic potential of lung cancer cells overexpressing HMOX1. However, no direct relation with VEGF or IL-8 level was established, although Ang-1 and SDF-1 were inhibited by HMOX1. In contrast, miR-378 overexpression increased VEGF, IL-8, Ang-1, and SDF-1, indicating the role of those factors in miR-378-enhanced angiogenesis in NSCLC. miR-378 can promote VEGF expression by competing with miR-125a for the same seed region in the VEGF 3′-UTR (16) or through Sufu–sonic hedgehog homolog (Shh) pathway (51).
Importantly, enhancement of tumor growth by miR-378 and its inhibition by HMOX1 could also partially result from the regulation of tumor suppressors, such as p53 (22). Finally, we demonstrated that HMOX1 overexpressing cells were more sensitive to hypoxia, oxidative stress caused by H2O2, and chemotherapeutics: 5-fluorouracil or doxorubicin. The potentially puzzling higher sensitivity of HMOX1 overexpressing cells can be explained by other studies indicating that higher levels of HMOX1 can be associated with significant oxygen cytotoxicity, due to accumulation of reactive iron (2).
To conclude, we demonstrated for the first time that HMOX1 overexpression inhibits tumorigenic and angiogenic capabilities of human NCI-H292 NSCLC cells. Mechanistically, this involves the regulation of miRNAs. Of particular significance, there is an interplay between HMOX1 and miR-378, which is reflected by reciprocal regulation of their expression in several NSCLC cell lines and by the opposite effects on lung cancer growth, vascularization, oxygenation, and progression (Supplementary Fig. S12). Importantly, the described cross-talk may be of clinical significance, as evidenced by the lower expression of HMOX1 and the tendency of higher miR-378 in lymph node metastasis in NSCLC patients.
Materials and Methods
Cell line
NSCLC cell lines NCI-H292, NCI-H460, A549, and SK-MES-1 were purchased from the American Type Culture Collection (ATCC). The NCI-H292 cell line purity was authenticated by a short tandem repeat analysis at LGC Standards Ltd company by PowerPlex® 16HS 16 Loci Service. NCI-H292 cells were grown in RPMI 1640 medium (PAA or Lonza) with 10% FBS (PAA), penicillin (100 U/ml), and streptomycin (0.1 mg/ml; Invitrogen). Other cell lines were cultured as described in Supplementary Materials and Methods.
Generation of stable cell line overexpressing HMOX1 and miR-378/378*
Retroviral vectors were produced using Phoenix-Ampho HEK293 packaging cell line with MSCV luciferase PGK-hygro (Addgene), pLNCX2, or pLNCX2-HO-1 plasmids and then, NCI-H292 cells were transduced, as previously described (54), and subsequently selected with antibiotics: G418 (800 μg/ml; Cytogen) for pLNCX2 (NCI-EV-ctrl cells) or pLNCX2-HO-1 (NCI-HO-1 cells) backbones, and hygromycin (Invitrogen; 600 μg/ml) for MSCV luciferase PGK-hygro backbone (NCI-Luc, NCI-Luc-EV-ctrl, and NCI-Luc-HO-1 cells for in vivo experiments).
Lentiviral vectors particles (pEZX-MR01, FIV backbone, or pEZX-MR03, HIV backbone), harboring miR-378 precursor or control scrambled sequence, were purchased from Gene Copoeia. Since in vitro results were analogous for both vector backbones, for subsequent in vivo experiments, only cells transduced with pEZX-MR03 backbone were used, as they maintained higher luciferase activity.
Characterization of cell lines
Details relevant to characterization of cell lines and angiogenic assays are provided in Supplementary Materials and Methods.
Microarray profiling
MicroRNA Array Profiling was performed by Exiqon Company using miRCURY™ LNA array version 11.0. Results of at least a 1.15-fold difference and p≤0.05, estimated in paired t test using MeV program—TM4 Microarray Software Suite (42)—were considered significant. Microarray data are deposited in Gene Expression Omnibus (GEO) under accession number GSE37779.
Animal studies with NCI-H292 cells transduced with viral vectors
All animal work was approved by the Local Ethical Committee for Animal Research in Orleans in France. All manipulations on animals were performed under anesthesia with inhaled isoflurane. 6-week-old Swiss nude females were injected subcutaneously with 5×106 empty vector control (EV-ctrl) or HMOX1 overexpressing cells or scrambled vector control (scr-ctrl) or miR-378/378* overexpressing NCI-H292-Luc cells in PBS. Tumor size was monitored weekly with caliper (V=D×d2×0.5; V, tumor volume; D, the biggest dimension; d, the smallest dimension) and with IVIS® Lumina II Imaging System (Caliper Life Science) after intraperitoneal injections of luciferin (150 μl, 5 mg/ml). Tumor tissue pO2 was estimated with fiber-optic oxygen-sensing device (OxyLite™; Oxford Optronix). For hematological analysis of blood, 150 μl of noncoagulated blood was taken from the eye using Pasteur pipette to a tube with EDTA. It was analyzed with a digital computer analyzer of hematology MS9-5 V (Melet Schloesing Laboratoires). The animals were sacrificed 5 weeks after xenografting, and tumor, lungs, brain, bone marrow, liver, adrenal glands samples, and blood from the heart were collected. Tissue specimens for staining were frozen in TissueTEK® O.C.T. compound. Cryosections were stained for CD31 and PCNA as previously described (12, 54).
Human studies
Human studies were approved by the Local Ethical Committee of the Collegium Medicum of the Jagiellonian University in Krakow, Poland. Biopsies of primary lung adenocarcinoma and lymph nodes metastases were analyzed.
Statistical analysis
Data are presented as means±SEM of at least three independent experiments. Data were analyzed with unpaired or paired Student's t-test or one-way ANOVA with Tukey's post-test or two-way ANOVA with Bonferroni post-test; p<0.05 was considered significant. Statistical analysis was performed using GraphPad Prism 5 software (trial version).
Innovation
Here, we have shown for the first time that in contrast to its known pro-tumorigenic effect in many cancers, HMOX1 in human NSCLC NCI-H292 cells attenuates cell proliferation, angiogenesis, and metastasis, significantly affecting miRNAs. An oncogenic miR-378 is the most strongly inhibited by HMOX1 and reciprocally, miR-378 overexpression decreases HMOX1 but increases pro-angiogenic genes enhancing tumor growth. In vitro and in vivo data indicate that the interplay between HMOX1 and miR-378 significantly modulates NSCLC progression and angiogenesis.
Supplementary Material
Abbreviations Used
- Ang
angiopoietin
- BCA
bicinchoninic acid protein assay
- BrdU
5-bromo-2′-deoxyuridine
- CD31
cluster of differentiation 31 (platelet endothelial cell adhesion molecule)
- CoPPIX
cobalt protoporphyrin-IX
- CORM
carbon monoxide releasing molecule
- CYP2E1
cytochrome P450 2E1
- DAPI
4′,6-diamidino-2-phenylindole
- DCFH-DA
2′,7′-dichlorodihydrofluorescein diacetate
- DGCR8
DiGeorge syndrome critical region-8
- EC
endothelial cells
- EF2
elongation factor 2
- EV-ctrl
control cell line modified with empty retroviral vector
- FIV
feline immunodeficiency viral vector
- HER2
human epidermal growth factor receptor 2
- HIV
human immunodeficiency viral vector
- HMOX1 (HO-1)
heme oxygenase-1
- hUbc
human ubiquitin c
- iCORM
inactive form of carbon monoxide releasing molecule
- IL-1β
interleukin-1beta
- IL-8
interleukin-8
- miRNA
microRNA
- MMP12
matrix metalloproteinase 12
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- MUC5AC
mucin-5AC
- NAC
N-acetyl-L-cysteine
- NSCLC
non-small cell lung carcinoma
- PCNA
proliferating cell nuclear antigen
- PI
propidium iodide
- qPCR
quantitative polymerase chain reaction
- ROS
reactive oxygen species
- RV
retroviral vectors
- SCC
squamous cell carcinoma
- scr-ctrl
control cell line modified with scrambled lentiviral vector
- SDF-1
stromal cell-derived factor-1
- Shh
sonic hedgehog homolog
- SnPPIX
tin protoporphyrin-IX
- TNF-α
tumor necrosis factor-alpha
- UTR
untranslated region
- VEGF
vascular endothelial growth factor
Acknowledgments
The authors thank Alain Le Pape, Stephanie Lerondel, Marilyne Le Mee, Stephanie Retif, and Julien Sobilo (Center for Small Animals Imaging, CIPA TAAM UPS44, CNRS, Orleans, France) for help with IVIS imaging; David Gosset (Cytometry Platform of CBM UPR4301, CNRS Orleans, France) for help with the flow cytometer; and Jacek Stepniewski from UJ for the amplification of pSL plasmids. This work was supported by grants 347/N-INCA/2008 and N301 144336 from the National Science Center and CNRS-INCA-MSHE Polish-French conv. 2009–2011 and by COST Action TD901 HypoxiaNet. Klaudia Skrzypek and Magdalena Tertil were supported by the Conseil Regional du Centre, stipends for co-tutorial PhD theses. Alicja Jozkowicz was an International Senior Research Fellow from Wellcome Trust. Agnieszka Loboda is supported by the Foundation for Polish Science–Parent-Bridge Program that is co-financed by the European Union within European Regional Development Fund (POMOST/2010-2/8), and she is the recipient of the Ministry of Science and Higher Education Scholarship. The Faculty of Biochemistry, Biophysics, and Biotechnology, Jagiellonian University, received structural funds POIG.02.01.00-12-064/08, 02.02.00-00-014/08, 01.01.02-00-109/09, and 01.01.02-00-069/09.
Author Disclosure Statement
All authors declare that no competing financial interests exist.
References
- 1.Acunzo M. Visone R. Romano G. Veronese A. Lovat F. Palmieri D. Bottoni A. Garofalo M. Gasparini P. Condorelli G. Chiariello M. Croce CM. miR-130a targets MET and induces TRAIL-sensitivity in NSCLC by downregulating miR-221 and 222. Oncogene. 2012;31:634–642. doi: 10.1038/onc.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexiou P. Vergoulis T. Gleditzsch M. Prekas G. Dalamagas T. Megraw M. Grosse I. Sellis T. Hatzigeorgiou AG. miRGen 2.0: a database of microRNA genomic information and regulation. Nucleic Acids Res. 2010;38:D137–D141. doi: 10.1093/nar/gkp888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamorro-Jorganes A. Araldi E. Penalva LO. Sandhu D. Fernandez-Hernando C. Suarez Y. MicroRNA-16 and microRNA-424 regulate cell-autonomous angiogenic functions in endothelial cells via targeting vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1. Arterioscler Thromb Vasc Biol. 2011;31:2595–2606. doi: 10.1161/ATVBAHA.111.236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen LT. Xu SD. Xu H. Zhang JF. Ning JF. Wang SF. MicroRNA-378 is associated with non-small cell lung cancer brain metastasis by promoting cell migration, invasion and tumor angiogenesis. Med Oncol. 2012;29:1673–1680. doi: 10.1007/s12032-011-0083-x. [DOI] [PubMed] [Google Scholar]
- 5.De Palma G. Mozzoni P. Acampa O. Internullo E. Carbognani P. Rusca M. Goldoni M. Corradi M. Tiseo M. Apostoli P. Mutti A. Expression levels of some antioxidant and epidermal growth factor receptor genes in patients with early-stage non-small cell lung cancer. J Nucleic Acids. 2010;2010:147528. doi: 10.4061/2010/147528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deininger MH. Meyermann R. Trautmann K. Duffner F. Grote EH. Wickboldt J. Schluesener HJ. Heme oxygenase (HO)-1 expressing macrophages/microglial cells accumulate during oligodendroglioma progression. Brain Res. 2000;882:1–8. doi: 10.1016/s0006-8993(00)02594-4. [DOI] [PubMed] [Google Scholar]
- 7.Dews M. Homayouni A. Yu D. Murphy D. Sevignani C. Wentzel E. Furth EE. Lee WM. Enders GH. Mendell JT. Thomas-Tikhonenko A. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichner LJ. Perry MC. Dufour CR. Bertos N. Park M. St-Pierre J. Giguere V. miR-378(*) mediates metabolic shift in breast cancer cells via the PGC-1beta/ERRgamma transcriptional pathway. Cell Metab. 2010;12:352–361. doi: 10.1016/j.cmet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Feng M. Li Z. Aau M. Wong CH. Yang X. Yu Q. Myc/miR-378/TOB2/cyclin D1 functional module regulates oncogenic transformation. Oncogene. 2011;30:2242–2251. doi: 10.1038/onc.2010.602. [DOI] [PubMed] [Google Scholar]
- 10.Ferrando M. Gueron G. Elguero B. Giudice J. Salles A. Leskow FC. Jares-Erijman EA. Colombo L. Meiss R. Navone N. De Siervi A. Vazquez E. Heme oxygenase 1 (HO-1) challenges the angiogenic switch in prostate cancer. Angiogenesis. 2011;14:467–479. doi: 10.1007/s10456-011-9230-4. [DOI] [PubMed] [Google Scholar]
- 11.Gao W. Yu Y. Cao H. Shen H. Li X. Pan S. Shu Y. Deregulated expression of miR-21, miR-143 and miR-181a in non small cell lung cancer is related to clinicopathologic characteristics or patient prognosis. Biomed Pharmacother. 2010;64:399–408. doi: 10.1016/j.biopha.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Grochot-Przeczek A. Lach R. Mis J. Skrzypek K. Gozdecka M. Sroczynska P. Dubiel M. Rutkowski A. Kozakowska M. Zagorska A. Walczynski J. Was H. Kotlinowski J. Drukala J. Kurowski K. Kieda C. Herault Y. Dulak J. Jozkowicz A. Heme oxygenase-1 accelerates cutaneous wound healing in mice. PLoS One. 2009;4:e5803. doi: 10.1371/journal.pone.0005803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gueron G. De Siervi A. Ferrando M. Salierno M. De Luca P. Elguero B. Meiss R. Navone N. Vazquez ES. Critical role of endogenous heme oxygenase 1 as a tuner of the invasive potential of prostate cancer cells. Mol Cancer Res. 2009;7:1745–1755. doi: 10.1158/1541-7786.MCR-08-0325. [DOI] [PubMed] [Google Scholar]
- 14.Hill M. Pereira V. Chauveau C. Zagani R. Remy S. Tesson L. Mazal D. Ubillos L. Brion R. Asghar K. Mashreghi MF. Kotsch K. Moffett J. Doebis C. Seifert M. Boczkowski J. Osinaga E. Anegon I. Heme oxygenase-1 inhibits rat and human breast cancer cell proliferation: mutual cross inhibition with indoleamine 2,3-dioxygenase. FASEB J. 2005;19:1957–1968. doi: 10.1096/fj.05-3875com. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann HS. Hansen G. Richter G. Taege C. Simm A. Silber RE. Burdach S. Matrix metalloproteinase-12 expression correlates with local recurrence and metastatic disease in non-small cell lung cancer patients. Clin Cancer Res. 2005;11:1086–1092. [PubMed] [Google Scholar]
- 16.Hua Z. Lv Q. Ye W. Wong CK. Cai G. Gu D. Ji Y. Zhao C. Wang J. Yang BB. Zhang Y. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One. 2006;1:e116. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jevnaker AM. Khuu C. Kjole E. Bryne M. Osmundsen H. Expression of members of the miRNA17-92 cluster during development and in carcinogenesis. J Cell Physiol. 2011;226:2257–2266. doi: 10.1002/jcp.22562. [DOI] [PubMed] [Google Scholar]
- 18.Jozkowicz A. Was H. Dulak J. Heme oxygenase-1 in tumors: is it a false friend? Antioxid Redox Signal. 2007;9:2099–2117. doi: 10.1089/ars.2007.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kikuchi A. Yamaya M. Suzuki S. Yasuda H. Kubo H. Nakayama K. Handa M. Sasaki T. Shibahara S. Sekizawa K. Sasaki H. Association of susceptibility to the development of lung adenocarcinoma with the heme oxygenase-1 gene promoter polymorphism. Hum Genet. 2005;116:354–360. doi: 10.1007/s00439-004-1162-2. [DOI] [PubMed] [Google Scholar]
- 20.Kozakowska M. Ciesla M. Stefanska A. Skrzypek K. Was H. Jazwa A. Grochot-Przeczek A. Kotlinowski J. Szymula A. Bartelik A. Mazan M. Yagensky O. Florczyk U. Lemke K. Zebzda A. Dyduch G. Nowak W. Szade K. Stepniewski J. Majka M. Derlacz R. Loboda A. Dulak J. Jozkowicz A. Heme oxygenase-1 inhibits myoblast differentiation by targeting myomirs. Antioxid Redox Signal. 2012;16:113–127. doi: 10.1089/ars.2011.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labelle M. Begum S. Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lago CU. Sung HJ. Ma W. Wang PY. Hwang PM. p53, aerobic metabolism, and cancer. Antioxid Redox Signal. 2011;15:1739–1748. doi: 10.1089/ars.2010.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landi MT. Zhao Y. Rotunno M. Koshiol J. Liu H. Bergen AW. Rubagotti M. Goldstein AM. Linnoila I. Marincola FM. Tucker MA. Bertazzi PA. Pesatori AC. Caporaso NE. McShane LM. Wang E. MicroRNA expression differentiates histology and predicts survival of lung cancer. Clin Cancer Res. 2010;16:430–441. doi: 10.1158/1078-0432.CCR-09-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DY. Deng Z. Wang CH. Yang BB. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A. 2007;104:20350–20355. doi: 10.1073/pnas.0706901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J. Lee SK. Lee BU. Lee HJ. Cho NP. Yoon JH. Choi HR. Kim EC. Upregulation of heme oxygenase-1 in oral epithelial dysplasias. Int J Oral Maxillofac Surg. 2008;37:287–292. doi: 10.1016/j.ijom.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 26.Lee PJ. Alam J. Wiegand GW. Choi AM. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc Natl Acad Sci U S A. 1996;93:10393–10398. doi: 10.1073/pnas.93.19.10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei Z. Li B. Yang Z. Fang H. Zhang GM. Feng ZH. Huang B. Regulation of HIF-1alpha and VEGF by miR-20b tunes tumor cells to adapt to the alteration of oxygen concentration. PLoS One. 2009;4:e7629. doi: 10.1371/journal.pone.0007629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J. Liang S. Yu H. Zhang J. Ma D. Lu X. An inhibitory effect of miR-22 on cell migration and invasion in ovarian cancer. Gynecol Oncol. 2010;119:543–548. doi: 10.1016/j.ygyno.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 29.Li XF. Yan PJ. Shao ZM. Downregulation of miR-193b contributes to enhance urokinase-type plasminogen activator (uPA) expression and tumor progression and invasion in human breast cancer. Oncogene. 2009;28:3937–3948. doi: 10.1038/onc.2009.245. [DOI] [PubMed] [Google Scholar]
- 30.Liu F. Lou YL. Wu J. Ruan QF. Xie A. Guo F. Cui SP. Deng ZF. Wang Y. Upregulation of MicroRNA-210 Regulates Renal Angiogenesis Mediated by Activation of VEGF Signaling Pathway under Ischemia/Perfusion Injury in vivo and in vitro. Kidney Blood Press Res. 2011;35:182–191. doi: 10.1159/000331054. [DOI] [PubMed] [Google Scholar]
- 31.Liu S. Guo W. Shi J. Li N. Yu X. Xue J. Fu X. Chu K. Lu C. Zhao J. Xie D. Wu M. Cheng S. MicroRNA-135a contributes to the development of portal vein tumor thrombus by promoting metastasis in hepatocellular carcinoma. J Hepatol. 2012;56:389–396. doi: 10.1016/j.jhep.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Lu J. Getz G. Miska EA. Alvarez-Saavedra E. Lamb J. Peck D. Sweet-Cordero A. Ebert BL. Mak RH. Ferrando AA. Downing JR. Jacks T. Horvitz HR. Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 33.Maines MD. Trakshel GM. Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986;261:411–419. [PubMed] [Google Scholar]
- 34.Marinissen MJ. Tanos T. Bolos M. de Sagarra MR. Coso OA. Cuadrado A. Inhibition of heme oxygenase-1 interferes with the transforming activity of the Kaposi sarcoma herpesvirus-encoded G protein-coupled receptor. J Biol Chem. 2006;281:11332–11346. doi: 10.1074/jbc.M512199200. [DOI] [PubMed] [Google Scholar]
- 35.Matsuyama H. Suzuki HI. Nishimori H. Noguchi M. Yao T. Komatsu N. Mano H. Sugimoto K. Miyazono K. miR-135b mediates NPM-ALK-driven oncogenicity and renders IL-17-producing immunophenotype to anaplastic large cell lymphoma. Blood. 2011;118:6881–6892. doi: 10.1182/blood-2011-05-354654. [DOI] [PubMed] [Google Scholar]
- 36.McAllister SC. Hansen SG. Ruhl RA. Raggo CM. DeFilippis VR. Greenspan D. Fruh K. Moses AV. Kaposi sarcoma-associated herpesvirus (KSHV) induces heme oxygenase-1 expression and activity in KSHV-infected endothelial cells. Blood. 2004;103:3465–3473. doi: 10.1182/blood-2003-08-2781. [DOI] [PubMed] [Google Scholar]
- 37.Miranda KC. Huynh T. Tay Y. Ang YS. Tam WL. Thomson AM. Lim B. Rigoutsos I. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126:1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 38.Mishra PJ. Song B. Wang Y. Humeniuk R. Banerjee D. Merlino G. Ju J. Bertino JR. MiR-24 tumor suppressor activity is regulated independent of p53 and through a target site polymorphism. PLoS One. 2009;4:e8445. doi: 10.1371/journal.pone.0008445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohri T. Nakajima M. Fukami T. Takamiya M. Aoki Y. Yokoi T. Human CYP2E1 is regulated by miR-378. Biochem Pharmacol. 2010;79:1045–1052. doi: 10.1016/j.bcp.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Nowis D. Legat M. Grzela T. Niderla J. Wilczek E. Wilczynski GM. Glodkowska E. Mrowka P. Issat T. Dulak J. Jozkowicz A. Was H. Adamek M. Wrzosek A. Nazarewski S. Makowski M. Stoklosa T. Jakobisiak M. Golab J. Heme oxygenase-1 protects tumor cells against photodynamic therapy-mediated cytotoxicity. Oncogene. 2006;25:3365–3374. doi: 10.1038/sj.onc.1209378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothe F. Ignatiadis M. Chaboteaux C. Haibe-Kains B. Kheddoumi N. Majjaj S. Badran B. Fayyad-Kazan H. Desmedt C. Harris AL. Piccart M. Sotiriou C. Global microRNA expression profiling identifies MiR-210 associated with tumor proliferation, invasion and poor clinical outcome in breast cancer. PLoS One. 2011;6:e20980. doi: 10.1371/journal.pone.0020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saeed AI. Sharov V. White J. Li J. Liang W. Bhagabati N. Braisted J. Klapa M. Currier T. Thiagarajan M. Sturn A. Snuffin M. Rezantsev A. Popov D. Ryltsov A. Kostukovich E. Borisovsky I. Liu Z. Vinsavich A. Trush V. Quackenbush J. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 43.Sarkar S. Dey BK. Dutta A. MiR-322/424 and −503 are induced during muscle differentiation and promote cell cycle quiescence and differentiation by down-regulation of Cdc25A. Mol Biol Cell. 2010;21:2138–2149. doi: 10.1091/mbc.E10-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sunamura M. Duda DG. Ghattas MH. Lozonschi L. Motoi F. Yamauchi J. Matsuno S. Shibahara S. Abraham NG. Heme oxygenase-1 accelerates tumor angiogenesis of human pancreatic cancer. Angiogenesis. 2003;6:15–24. doi: 10.1023/a:1025803600840. [DOI] [PubMed] [Google Scholar]
- 45.Taha H. Skrzypek K. Guevara I. Nigisch A. Mustafa S. Grochot-Przeczek A. Ferdek P. Was H. Kotlinowski J. Kozakowska M. Balcerczyk A. Muchova L. Vitek L. Weigel G. Dulak J. Jozkowicz A. Role of heme oxygenase-1 in human endothelial cells: lesson from the promoter allelic variants. Arterioscler Thromb Vasc Biol. 2010;30:1634–1641. doi: 10.1161/ATVBAHA.110.207316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi S. Takahashi T. Mizobuchi S. Matsumi M. Yokoyama M. Morita K. Miyazaki M. Namba M. Akagi R. Sassa S. CYP2E1 overexpression up-regulates both non-specific delta-aminolevulinate synthase and heme oxygenase-1 in the human hepatoma cell line HLE/2E1. Int J Mol Med. 2003;11:57–62. [PubMed] [Google Scholar]
- 47.Torisu-Itakura H. Furue M. Kuwano M. Ono M. Co-expression of thymidine phosphorylase and heme oxygenase-1 in macrophages in human malignant vertical growth melanomas. Jpn J Cancer Res. 2000;91:906–910. doi: 10.1111/j.1349-7006.2000.tb01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai KW. Wu CW. Hu LY. Li SC. Liao YL. Lai CH. Kao HW. Fang WL. Huang KH. Chan WC. Lin WC. Epigenetic regulation of miR-34b and miR-129 expression in gastric cancer. Int J Cancer. 2011;129:2600–2610. doi: 10.1002/ijc.25919. [DOI] [PubMed] [Google Scholar]
- 49.Tsuji MH. Yanagawa T. Iwasa S. Tabuchi K. Onizawa K. Bannai S. Toyooka H. Yoshida H. Heme oxygenase-1 expression in oral squamous cell carcinoma as involved in lymph node metastasis. Cancer Lett. 1999;138:53–59. doi: 10.1016/s0304-3835(98)00372-3. [DOI] [PubMed] [Google Scholar]
- 50.Vasudevan S. Tong Y. Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 51.Wang S. Olson EN. AngiomiRs—key regulators of angiogenesis. Curr Opin Genet Dev. 2009;19:205–211. doi: 10.1016/j.gde.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Was H. Cichon T. Smolarczyk R. Rudnicka D. Stopa M. Chevalier C. Leger JJ. Lackowska B. Grochot A. Bojkowska K. Ratajska A. Kieda C. Szala S. Dulak J. Jozkowicz A. Overexpression of heme oxygenase-1 in murine melanoma: increased proliferation and viability of tumor cells, decreased survival of mice. Am J Pathol. 2006;169:2181–2198. doi: 10.2353/ajpath.2006.051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Was H. Dulak J. Jozkowicz A. Heme oxygenase-1 in tumor biology and therapy. Curr Drug Targets. 2010;11:1551–1570. doi: 10.2174/1389450111009011551. [DOI] [PubMed] [Google Scholar]
- 54.Was H. Sokolowska M. Sierpniowska A. Dominik P. Skrzypek K. Lackowska B. Pratnicki A. Grochot-Przeczek A. Taha H. Kotlinowski J. Kozakowska M. Mazan A. Nowak W. Muchova L. Vitek L. Ratajska A. Dulak J. Jozkowicz A. Effects of heme oxygenase-1 on induction and development of chemically induced squamous cell carcinoma in mice. Free Radic Biol Med. 2011;51:1717–1726. doi: 10.1016/j.freeradbiomed.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yanagawa T. Omura K. Harada H. Nakaso K. Iwasa S. Koyama Y. Onizawa K. Yusa H. Yoshida H. Heme oxygenase-1 expression predicts cervical lymph node metastasis of tongue squamous cell carcinomas. Oral Oncol. 2004;40:21–27. doi: 10.1016/s1368-8375(03)00128-3. [DOI] [PubMed] [Google Scholar]
- 56.Yu CJ. Yang PC. Shun CT. Lee YC. Kuo SH. Luh KT. Overexpression of MUC5 genes is associated with early post-operative metastasis in non-small-cell lung cancer. Int J Cancer. 1996;69:457–465. doi: 10.1002/(SICI)1097-0215(19961220)69:6<457::AID-IJC7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y. Liao JM. Zeng SX. Lu H. p53 downregulates Down syndrome-associated DYRK1A through miR-1246. EMBO Rep. 2011;12:811–817. doi: 10.1038/embor.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.