Introduction
Within the recent epidemic of oropharyngeal squamous cell carcinoma (OPSCC),1 HPV has been found to play a pivotal role in defining a subset of patients with distinct carcinogenesis,2, 3 risk factors,4 clinical presentation,5 and prognosis.6,7, 8 HPV-related OPSCC appears to be a wholly different disease than that classically described for HPV-negative tumors, which are typically driven by the carcinogenic effects of tobacco and alcohol exposure. Although much effort has been put into describing the subset of HPV-positive patients, research has not yet translated into therapies that address the different biology of HPV-related OPSCC and its associated better outcomes. The identification of biomarkers to aid in prognostic and therapeutic decisions, the potential for de-escalation of therapy, and the incorporation of therapies targeted to relevant HPV-related pathways are areas that are actively being evaluated in clinical trials. In this review we address the implications of these findings in clinical care.
[FOR SEO and INDEX: Oropharyngeal cancer, HPV, OPSCC, implications in clinical care]
HPV Basics
The causal role of HPV in carcinogenesis was first described in cervical cancer, but the virus has also been implicated in oropharyngeal, penile, anal, vaginal, and vulvar cancer.9 It is estimated that 5.2% of all cancers worldwide are attributable to HPV, and the burden of incidence and costs is increasing for non-cervical HPV-related cancers, especially in the oropharynx.9 The incidence of OPSCC increased from 1988 to 2004, mainly driven by an increase in HPV-positive OPSCC of 225% while HPV-negative disease has declined by 50%.1 HPV now accounts for 45 to 90% of cases of OPSCC in developed countries, and over 90% of these are caused by the HPV16 subtype.10,11 HPV status has important implications in OPSCC tumor biology, clinical presentation, prognosis, and potential treatment options, but its importance in other head and neck squamous cell carcinoma (HNSCC) subsites is unclear. HPV has been detected in laryngeal (6%), hypopharyngeal (3%), oral cavity (4%) and paranasal (14%) cancers,4,12 but the significantly lower rates of HPV-positivity and inconsistent findings among the studies suggest that HPV may not be playing a causative role in these subsites.13
HPV and Carcinogenesis
Human papillomaviruses are small, non-enveloped, double-stranded DNA viruses that exhibit tropism for squamous epithelium.3 They are classified into high risk (HR: HPV16, HPV18, HPV51, HPV53) and low risk (LR: HPV6, HPV11) based on the ability of the virus to promote progression to cancer.14 The process of HPV carcinogenesis is well characterized in cervical cancer, where infection is established in the basal cell layer of the epithelium and leads to either a subclinical infection or a benign or malignant lesion.15 Although most women will have a cervical HPV infection over their lifetime, about 10% of these become persistent and only a minority may progress to cancer.15 For OPSCC it is now known that 1% of the population has an oral HPV16 infection, and that this confers a 50-fold increased risk for HPV-positive OPSCC,16 but the intermediate steps in progression have not been described. The cryptic epithelium that covers the tonsil and tongue base (BOT) serves as a viral reservoir and facilitates infection through increased access to its basal layer,17,18 with an apparent predilection of this anatomic site to transformation by HPV similar to the cervical transformation zone.18
The process of malignant transformation arises from the continued function of the E6 and E7 oncoproteins expressed by HR-HPV.3 These target several critical cellular pathways, providing multiple simultaneous oncogenic hits and leading to deregulation of proliferation, evasion of apoptosis, and induction of invasive and metastatic properties.3 As a result, HPV infection reduces the number of subsequent mutations needed to develop invasive carcinoma. The difference in oncogenic potential between HR- and LR-HPV may rely in more efficient disruption of the biologic activities of E6 and E7 proteins.3, 19 Specifically, E7 inhibits the retinoblastoma tumor suppressor protein (pRb) and targets it for degradation, while E6 inactivates the p53 tumor suppressor.3,19 Inhibition of pRb function by viral proteins allows cells to continue dividing despite signals for cell cycle arrest due to oncogenic stress, such as those mediated by p53 or p16.20 HR-HPV E6 also induces the expression of vascular endothelial growth factor (VEGF) and activates telomerase, an essential step in immortalization.3,19 The combined effects of E6 and E7 on the pRb/p53 pathways create an environment of genomic instability which is highly conducive to cancer development.3,19
Carcinogenesis in HPV-positive OPSCC is a process of cell-cycle deregulation mediated by viral oncoproteins which is specific to the epithelium under transformation.2 In contrast, HPV-negative OPSCC usually results from exposure to environmental carcinogens such as tobacco and alcohol, leading to disruption of those same cancer-promoting signaling pathways targeted by HPV. This occurs via the process of field cancerization, or the progressive accumulation of mutations over large regions of aerodigestive mucosa, and leads to a higher probability of developing additional primary tumors.5 The highly localized carcinogenesis in HPV-positive OPSCC represents a profound difference in tumor biology.
[For SEO and INDEX: Oropharyngeal cancer, HPV carcinogenesis, E6, E7, p53, pRb]
Clinical Implications of HPV-positive OPSCC
The distinct carcinogenesis of HPV-positive OPSCC leads to important differences in the population at risk for this disease, their clinical presentation and prognosis. From the physician’s standpoint, this information is highly relevant in patient education and counseling. In the future, this information may soon translate into differential workup and tailored treatments for this patient population.
Clinical Presentation
Clinical presentation of HPV-positive OPSCC is different than that of HPV-negative patients. Patients tend to be younger and are more likely to be white, married and college educated, and typically present without a history of smoking or drinking.4,16 Given that HPV is a sexually-transmitted disease, factors that increase oral or genital HPV exposure increase the risk of OPSCC, such as increasing age, increasing number of lifetime vaginal or oral sexual partners, ever having participated in casual sex, infrequent use of barriers during vaginal or oral sex and ever having had a sexually transmitted disease. Oral HPV infection increases the risk for HPV-positive OPSCC4 and this risk is higher among individuals who first performed oral sex at 18 years or younger or with increasing number of cigarettes smoked per day.16 Other risk factors for HPV-positive OPSCC include immunosuppression, seropositivity for HR-HPV, history of an HPV–associated malignancy, and being the husband of a woman with cervical cancer.4, 5,16 Patients tend to present with low T and high N stage tumors on the American Joint Committee on Cancer TNM staging system,11,21,22 and histologically, these are usually non-keratinizing, poorly-differentiated, and of basaloid morphology.6,17,23
HPV Status Determination
There is currently no general consensus on which method for diagnosing HPV-positive cancer should be used in HNSCC as the techniques differ in sensitivity, specificity and other technical considerations.24 Real-time PCR (RT-PCR) performed on microdissected tumors can detect very small quantities of HPV DNA and describe the subtype, but it gives no information on host cell integration or activity, which are critical for carcinogenesis, and is not available in most clinical laboratories.24,25,26 In situ hybridization (ISH) localizes HPV DNA integrated into the host cell genome with high specificity, indicating viral presence and activity, but is less sensitive and more time-consuming than RT-PCR and is only available at selected centers.24,25 Immunohistochemistry (IHC) of p16 has been suggested as a surrogate marker for HPV infection due to the simplicity, low cost, high sensitivity and good correlation to HPV RT-PCR and ISH.25,26, 27 However, as p16 has been reported to be constitutively expressed in tonsillar epithelium, can rarely be overexpressed in HPV-negative tumors, and the practice and reporting of IHC varies, its clinical application as a single assay may occasionally be misleading.2,26,20 With these considerations, an expert panel recommends a cost-efficient algorithm for HPV detection. Initial testing should include p16 IHC with HPV16 ISH performed concurrently or after a positive IHC for confirmation. In case of discrepancy, a consensus ISH probe that detects an extended panel of HPV types or RT-PCR can be used to determine HPV status.28 However, to allow for standardized detection and robust clinical investigations, a true consensus will have to be reached on the method of HPV detection in OPSCC
Prevention
The distinct pathogenesis of HPV-positive OPSCC raises important public health considerations aiming to decrease the rising incidence of this disease through health promotion and primary prevention strategies. The prevalence of oral HPV16 infection is 1% in the U.S. and has been associated with a 50-fold increased risk for HPV-positive OPSCC.4, 16 Infection is more common in smokers, and its increasing incidence has been related to the changing sexual behaviors among the population.16 In the primary care setting, efforts towards the modification of risk factors should be directed to reducing high-risk sexual behavior, decreasing oral HPV infection, and smoking cessation.
As HPV-positive OPSCC is related to only a few HR-HPV subtypes,10,16 there is great potential for the prevention of this disease through vaccination targeting those subtypes. Vaccination is effective only before infection is established, as it induces neutralizing antibodies that prevent virion entry but do not halt the progression of existing lesions.15,19 The two U.S. Food and Drug Administration-approved vaccines (HPV bivalent29 and quadrivalent30 vaccines) prevent persistent cervical HPV16 infection.15 The bivalent vaccine is indicated for the prevention of cervical cancer in females, while the quadrivalent vaccine has additionally been approved for the prevention of genital warts and genital cancers in both sexes through age 26. Vaccination may have higher impact in OPSCC than in cervical cancer given that, unlike cervical cancer, there is no screening strategy for OPSCC and incidence is estimated to surpass that of cervical cancer by 2020.1 Parents of children of both sexes should be informed that vaccination is available and, though currently not approved for this indication, may reduce the risk of other HPV-related cancers including OPSCC.
[For SEO and INDEX: Oropharyngeal cancer, clinical implications, diagnosis of HPV-positive cancer, unknown primary]
Implications of HPV for Treatment and Outcomes
Survival in HPV-positive OPSCC
Over the last few decades, there has been improved survival in OPSCC and a resultant move towards organ preservation therapy as the primary treatment choice for these patients. Older studies looking at the effect of HPV on survival had mixed results, but the data which has accrued over the intervening years is increasingly convincing that HPV-positive OPSCC has a more favorable prognosis.31 A recent historical demographic analysis of OPSCC patients at a single institution by Dahlstrom et al. shows that, after 1995, patients with OPSCC were more likely to be male, white, never- or former smokers, and have low T and high N stage tumors.32 These characteristics are now known to be closely associated with HPV-positive tumors. Thus, it appears that the changing demographics are due to the rise in the proportion of HPV-positive OPSCC, and that survival also improved in their cohort of patients. However, the external validity of these results is limited by the inability to control for confounders, such as treatment regimens, in this historical analysis.
Randomization to treatment regimens
Only recently have clinical trials begun to include HPV status in their patient stratification, but retrospective subgroup analyses have been performed in several phase III, multicenter trials involving the prospective randomization of HNSCC patients to different treatment regimens. In each, a post hoc analysis has been performed to investigate the effect of HPV status on patient survival. Ang et al. investigated the RTOG 0219 trial, which randomized patients to concurrent chemoradiation with either standard fractionation or accelerated fractionation RT.7 The subgroup of OPSCC patients had an increase in OS and PFS for HPV-positive as compared to HPV-negative OPSCC (Table 1). Rischin et al. had similar results in their analysis of the HeadSTART trial, which compared the effect of the addition of tirapazamine to CRT on outcome in OPSCC.33 They also noted improved survival at 2-years in the HPV-positive subgroup as well as lower rates of loco-regional failure compared to the HPV-negative subgroup (Table 1). More recently, Posner et al. looked at HPV-related outcomes in the TAX 324 trial, which compared the addition of docetaxel to a standard induction chemotherapy (IC) regimen.22 Their results demonstrate increased OS and PFS in HPV-positive as compared to HPV-negative OPSCC, along with a decreased risk of death and a significant reduction in loco-regional failure rates (Table 1). Overall, these retrospective analyses of prospectively-treated patients strongly suggest that there is a survival benefit in HPV-positive OPSCC. However, the generalizability of these results is limited by their retrospective nature and post hoc analysis; in addition, the ability of all three studies to determine HPV status was limited by tissue availability.
Table 1.
Selected studies evaluating the effect of HPV status on survival. Studies or presented results limited to previously untreated OPSCC except as noted.
| Study | Study Design | Results | ||
|---|---|---|---|---|
| HR (95% CI) or % | p | |||
| Dahlstrom et al. 2012 | Retrospective study comparing pre and post 1995 patients in one center. N=3891 HR: Overall survival analysis with the earlier cohort as reference |
Tumor subsite | ||
| BOT | 0.6 (0.5–0.8) | 0.001 | ||
| Tonsil | 0.6 (0.5–0.8) | < 0.001 | ||
| CRT | 0.4 (0.3–0.6) | < 0.001 | ||
| N status | ||||
| N1 | 0.5 (0.4–0.8) | 0.001 | ||
| N2 | 0.5 (0.4–0.6) | < 0.001 | ||
| TNM stage | ||||
| Stage III | 0.5 (0.4–0.8) | < 0.001 | ||
| Stage IV | 0.5 (0.4–0.6) | < 0.001 | ||
|
| ||||
| Ang et al. 2010 | Retrospective analysis (RTOG 0129 trial: accelerated vs. standard fractionation RT each with concurrent cisplatin) N= 323 patients with HPV data |
OS at 3 years | ||
| HPV+ | 82% | |||
| HPV− | 57.1% | |||
| 0.42 (0.27 to 0.66) | <0.001 | |||
| PFS at 3 years | ||||
| HPV+ | 73.7% | |||
| HPV− | 43.4% | |||
| 0.49 (0.33–0.74) | <0.001 | |||
| LR recurrence | ||||
| HPV+ | 13.6% | |||
| HPV− | 35.1% | <0.001 | ||
| 2nd primary tumors | ||||
| HPV+ | 5.9% | |||
| HPV− | 14.6% | 0.02 | ||
|
| ||||
| Rischin et al. 2010 | Retrospective analysis (TROG 02.02 trial. CRT with or without tirapazamine) N= 185 patients with HPV data |
OS at 2 years | ||
| HPV+ | 91% | |||
| HPV− | 74% | |||
| 0.36 (0.17–0.74) | 0.004 | |||
| PFS at 2 years | ||||
| HPV+ | 87% | |||
| HPV− | 72% | |||
| 0.39 (0.2–0.74) | 0.003 | |||
| LR recurrence | ||||
| HPV+ | 93% | |||
| HPV− | 86% | |||
| 0.43 (0.17–1.11) | 0.091 | |||
|
| ||||
| Posner et al. 2011 | Retrospective analysis (TAX 324 trial: IC TPF vs. PF, both followed by CRT) N= 111 patients with HPV data |
OS at 5 years | ||
| HPV+ | 82% | |||
| HPV− | 35% | <0.0001 | ||
| PFS at 5 years | ||||
| HPV+ | 78% | |||
| HPV− | 28% | <0.0001 | ||
| LR recurrence | ||||
| HPV+ | 13% | |||
| HPV− | 42% | 0.0006 | ||
|
| ||||
| Fakhry et al. 2008 | Prospective analysis (ECOG 2399 trial. Sequential therapy to CRT or surgery) N= 101, includes larynx cancer |
Response to IC | ||
| HPV+ | 82% | |||
| HPV− | 55% | 0.01 | ||
| Response to CRT | ||||
| HPV+ | 84% | |||
| HPV− | 57% | 0.007 | ||
| OS at 2 years | ||||
| HPV+ | 95% | |||
| HPV− | 62% | |||
| 0.36 (0.15–0.85) | 0.02 | |||
| PFS at 2 years | ||||
| HPV+ | 86% | |||
| HPV− | 53% | |||
| 0.27 (0.10–0.75) | 0.01 | |||
|
| ||||
| O’Rorke et al. 2012 | Meta-analysis N= 42 studies, 4,834 patients |
OS | 0.47 (0.35–0.62) | 0.08 |
| PFS | 0.48 (0.33–0.69) | 0.87 | ||
Abbreviations: HPV: Human papillomavirus, HPV+: HPV-positive, HPV-: HPV-negative, HR: Hazard ratio HPV+ vs. HPV− unless as noted, BOT: Base of tongue, CRT: Chemoradiation, RT: Radiotherapy, OS: Overall survival, PFS: Progression free survival, LR: Loco-regional, IC: Induction chemotherapy, OPSCC: Oropharyngeal cancer, TPF: Docetaxel+cisplatin+fluorouracil, PF: Cisplatin+fluorouracil
Oropharyngeal and laryngeal cancer
Published data on the effect of HPV on survival has been provided in a prospective fashion in a single study. In 2008, Fakhry et al. reported the results of a sub-study in ECOG 2399, a phase II trial on the use of sequential therapy for organ preservation in resectable advanced stage oropharyngeal and laryngeal cancer.6 HPV status was determined prospectively in both subsites, and none of the laryngeal tumors were HPV-positive. They found that HPV-positive patients had higher response rates to induction chemotherapy (IC) and chemo-radiotherapy (CRT), as well as improved OS and PFS (Table 1). However, this effect did not reach statistical significance for the oropharyngeal subsite alone as it was underpowered for subgroup analysis. At the time of the study design, it was thought that HPV was an etiologic factor in laryngeal cancer as well, so those patients were included in the trial. By the time of the data analysis, this hypothesis had largely been disproven, but the number of oropharyngeal patients alone was insufficient for the results of the multivariate analysis to remain statistically significant.
Survival disparity
Awareness of the increase in incidence of HPV-positive OPSCC has also solved one of the survival conundrums in OPSCC. Multiple studies had reported a disparity in survival rates between African American and white patients, despite controlling for tumor site, age, and other risk factors.34,35 However, using data from the TAX 324 trial, Settle et al. concluded that the disparity in survival between African American and white OPSCC patients was entirely due to significant differences in rates of HPV infection between the two groups.12 A more recent report confirms that the shorter PFS in African American OPSCC patients may be due to HPV status, treatment type, and higher T stage at presentation but is not due to race.36
In summary, HPV-positive OPSCC has improved survival, lower rate of disease progression, and lower chance of loco-regional recurrence when compared to HPV-negative OPSCC. Interestingly, there have not been significant differences in the rates of distant failure, which may or may not be related to insufficient power to detect a difference.6,7,22,33 Furthermore, a recent meta-analysis by O’Rorke et al., the most comprehensive to date, reports a 53% better overall and 74% better disease-specific survival for HPV-positive OPSCC, as well as statistically significant improvement in PFS and DFS.31 Prospective data is limited; however, there are ongoing clinical trials which include HPV status stratification in their trial design (Table 2). Lastly, several of the recent trends observed in OPSCC - improved survival, change in patient demographics, and suspected racial outcome disparities - are likely due to differences in incidence of HPV-positive OPSCC.
Table 2.
Summary of selected OPSCC HPV-related trials. Source: www.clinicaltrials.gov.
| Trial | Study Design | Population† | HPV Assay | Primary Outcome | Status and Projected End Date§ | Relevance |
|---|---|---|---|---|---|---|
|
| ||||||
| NCT01598792 | REALISTIC: Dose escalation trial of recombinant listeria monocytogenes (Lm)-based vaccine encoding HPV-16 target antigens (ADXS11-001) Phase I |
In remission after standard treatment | p16 IHC | Systemic or local adverse events | 10/2014 | Novel immune modulation strategy |
| NCT01064921 | Dose escalation study of vorinostat administered with CRT (standard RT+P) Phase I |
Advanced, unresectable | HPV tested, method not reported | Maximum tolerated dose and toxicity | 01/2013 | Novel targeted therapy |
| NCT01088802 | De-escalation CRT (IMRT: 70 Gy, 63 Gy and 58.1 Gy to primary and neck. CT: P/CP) Phase I/II |
Resectable | HPV tested, method not reported | 2 year toxicity and locoregional control | 02/2015 | Decrease morbidity |
| NCT01585428 | Lymphodepletion Followed by autologous tumor-infiltrating lymphocytes and high-dose aldesleukin Phase II |
M1 or locally advanced, refractory or recurrent HPVAC | HPV ISH PCR | Objective tumor response and duration | 04/2014 | Novel immune modulation strategy |
| NCT01590355 | ORATOR: TORS + ND vs. RT/CRT ± surgical salvage. Phase II |
Early stage | HPV tested, method not reported | Quality of life 1 year post treatment | 06/2021 | Novel surgical technique, decrease morbidity. Attempt to identify genetic markers of treatment failure and toxicity. |
| NCT01530997 | De-escalation CRT (RT:v54-60 Gy, P:30 mg/m2) followed by surgery (salvage for residual primary, SND for pre-treatment nodal disease) Phase II |
p16 IHC | CR after de- escalated CRT | 01/2014 | Decrease morbidity | |
| NCT01525927 | IC (T, CP, FU) followed by reduced dose RT/CRT for responders or standard CRT (EBRT/IMRT ± P/Carbo) for non responders Phase II |
p16 IHC HPV ISH | Response 3 months post- therapy (CR+PR) | 07/2013 | Decrease morbidity. Determine efficacy of sequential therapy in OPSCC. | |
| NCT01084083 | ECOG-E1308: IC (PT+P+cetuximab) followed by cetuximab+low/standard dose IMRT Phase II |
Advanced, resectable | HPV ISH p16 IHC | 2 year PFS | Active, not recruiting 03/2015 | Decrease morbidity. Determine efficacy of sequential therapy in OPSCC. |
| NCT01221753 | IC (T+P+FU) followed by CRT (low vs. standard dose IMRT with CP+cetuximab) Phase II |
Locally advanced | HPV ISH p16 IHC | Loco-regional control at 2 and 5 years | 09/2015 | Decrease morbidity. Determine efficacy of sequential therapy in OPSCC. |
| NCT01302834 | RTOG-1016: IMRT+cetuximab vs. IMRT+P Phase III |
p16 IHC | 5 year OS | 01/2015 | Decrease morbidity. Direct comparison cetuximab vs. cisplatin for CRT. | |
Primary untreated HPV+ OPSCC except as noted.
Trials are active and recruiting except as noted, with estimated primary completion date as reported on www.clinicaltrials.gov.
Abbreviations: +: And, /: Or, T: Docetaxel, PT: Paclitaxel P: Cisplatin, FU: 5-Flourouracil, CP: Carboplatin, ±: with or without, EBRT: External beam radiation therapy, CR: Complete response, PR: Partial response, ND: Neck dissection, SND: Selective neck dissection, IC: Induction chemotherapy, TORS: Transoral robotic surgery, HPVAC: HPV-associated cancer (cervical, oropharyngeal, vaginal, anal or penile cancer), APC: Antigen-presenting cells.
Possible Mechanisms Underlying the Survival Benefit in HPV-positive OPSCC
The unique carcinogenesis of HPV-positive OPSCC provides several possible explanations for the survival benefit seen in these patients. For instance, the lack of field cancerization in HPV-positive OPSCC patients may explain the improved loco-regional control, reduced risk of recurrence and reduced risk of second primary tumors as compared to HPV-negative cancers.5,7,37,38 Others have proposed an increased sensitivity to RT and CT in HPV–positive cancers as an explanation for improved survival.39,38,40 In vitro data from cervical cancer cell lines showed that cisplatin treatment induced apoptosis by both p53–dependent and –independent pathways,39 suggesting that p53 could be reactivated and enable the cell to respond to DNA damage after CT-mediated repression of viral E6 and E7. HPV-positive OPSCC has also shown better survival and higher local control rates after treatment with radiotherapy (RT, risk ratio for local treatment failure of 0.33), that may result from persistent, functional p53.38
Genomic differences
Another mechanism for improved survival may be genomic differences between HPV-positive and HPV-negative tumors. Sequencing of HNSCC reveals fewer mutations in protein-encoding genes in HPV-positive cancers, suggesting that genetic instability is less pronounced.41,42 There may also be a difference in the degree of intratumor heterogeneity between HPV-positive and HPV-negative OPSCC. Intratumor heterogeneity refers to the number of subpopulations of tumor cells within a single tumor that have different genotypes. Considering that therapy can select for subpopulations of cells that resist that particular treatment,43 high intratumor heterogeneity is increasingly recognized as a risk for treatment failure or recurrence. A measure of genomic intratumor heterogeneity based on next-generation DNA sequencing indicates that HPV-positive tumors have less intratumor heterogeneity than do HPV-negative tumors, and thus may be more likely to respond to therapy without recurrence.44
Immune system
The immune system may also contribute to the increased survival observed in HPV-positive OPSCC. A robust cytotoxic lymphocytic response related to HPV-positive tumors is associated with better response to therapy and increased survival.40,45,46 Pre-clinical models suggest that improved treatment response in HPV-positive tumors results from the combination of treatment effect and immune response, rather than to an intrinsic sensitivity of the cells to therapy.47 In the absence of the immune system, HPV-positive cell lines were more resistant to therapy, while in vivo, an immunocompetent environment was required to achieve complete tumor clearance.47 However, the authors did not evaluate the role of the immune system in response to therapy in the HPV-negative setting, so it is unclear if they were measuring a general antitumor response or one specific to HPV. Clinically, several mechanisms have been described to suggest that the improved response of HPV-positive tumors to RT may be related to enhancement of the immune response.40 As yet, the proposed mechanisms underlying the improved survival in HPV-positive OPSCC have not been clinically validated, but it is likely that they all contribute with varying degrees.
Current Treatment Implications for HPV-positive OPSCC and Unknown Primary
As HPV delineates a distinct type of OPSCC, HPV status should be routinely tested in all patients presenting with OPSCC or an unknown primary. HPV-positivity in OPSCC will offer more accurate prognostic information than that given by TNM staging alone,37,32 as nodal involvement leads to over-staging of disease that does not represent the same risk when compared to other head and neck cancers with the same stage.37 In the setting of an unknown primary, studies have shown that HPV positivity in a lymph node biopsy may be used to localize the primary with high specificity to the oropharynx, providing diagnostic information which may change radiotherapy planning.48 For an HPV-positive unknown primary, RT might be limited to the oropharynx and neck as opposed to a much wider field potentially from the nasopharynx down to the larynx depending on neck disease localization, thus significantly decreasing treatment-related morbidity and complications.49
De-escalation of Treatment and New Targets
Knowledge that HPV-positive OPSCC may result in better response to therapy, improved loco-regional control, and better survival may open an avenue to specialized treatment regimens that achieve the same oncologic outcomes with decreased morbidity. Currently available treatment modalities - CRT, sequential therapy or surgery-49 achieve comparable survival, but differ in associated risks and complications. Surgical approaches carry risks common to invasive procedures, while current acute and late complications associated to RT-based modalities negatively impact patient quality of life.50 Additionally, the long-term side effects of cancer treatment seen in survivors of other malignancies will likely increase in this patient population. RT-induced cardiovascular disease has become the leading non-cancer cause of mortality among survivors of some cancers, and significant trends in RT-induced malignancies have been reported among survivors of adult-onset cancers for several malignancies.51 Risk of stroke and occlusive carotid artery disease is particularly increased in HNSCC patients.51 The HPV-positive population is younger, higher-functioning, has fewer comorbidities and less smoking and alcohol exposure with subsequent decreased cardiovascular risk.4,16 They are also likely to achieve better survival regardless of treatment modality.7 We are facing a subgroup of patients who will likely achieve complete response and outlive their cancer by 10 to 30 years and will have to endure the side-effects of cancer treatment for their lifetime. Thus, evaluating the possibility of treatment de-escalation to optimize long-term quality of life is of paramount importance.
Several trials are underway evaluating different de-escalation regimens for HPV-positive OPSCC. Strategies include modifying existing treatment modalities, evaluating novel targeted therapies or inducing immunologic responses to HPV-positive tumors (Table 2). An initial approach is to reduce the radiation dose of CRT regimens. A phase I/II trial is evaluating reduced radiation doses to the primary tumor and neck with concurrent platinum therapy in resectable OPSCC patients (Table 2). Another phase II trial is evaluating reduced dosing of CRT followed by surgery in previously untreated OPSCC (Table 2). These studies will address whether reduced CT or RT dosing schemes result in equivalent survival with decreased morbidity in CRT regimens.
Induction chemotherapy
The role of IC in treatment of HNSCC remains unclear. Induction chemotherapy has not shown a survival advantage in HNSCC, but some evidence suggests that it may contribute to improved survival in OPSCC.52 Three phase II trials are currently active to address this question. One study uses IC to select patients to reduced-dose RT or standard CRT; a second trial evaluates IC followed by cetuximab with reduced or standard RT; and a third trial administers IC followed by reduced or standard RT with concurrent platinum and cetuximab. These trials will determine if sequential therapy is effective in OPSCC, and may provide insight into the relative efficacy of IC followed by platinum-only, cetuximab-only or combined platinum-cetuximab regimens concurrent to standard or reduced dose RT, and their differential effects on morbidity and oncolologic outcomes (Table 2).
Surgical alternatives
New surgical alternatives in the treatment of OPSCC include transoral laser microsurgery (TLM) and transoral robotic surgery (TORS), and offer an additional pathway to organ preservation. TLM has become one standard of care for early laryngeal cancer with utility in the oropharynx.53 TLM uses surgical tools available in most hospitals, maximizes conservation of normal mucosa, and achieves adequate outcomes.53 The line-of-sight limitation posed by the laser while accessing the oropharynx has been partially addressed by the development of fiberoptic delivery systems.53 However, as tumor removal is typically achieved by piecemeal resection, novel pathological techniques are often required to adequately assess margin status. Alternatively, TORS provides improved surgical access to the oropharynx and enhanced visual evaluation of margins due to improved infield optics, 3-dimensional imagery, tremor filtration and high-precision movements, although at increased cost.54,55 TORS shows promising oncologic outcomes demonstrated by the 1– and 2–year OS rates of 90% and 80–90%, respectively, in the initial retrospective studies on highly selected patients.54 TORS offers the additional benefit of complete pathologic staging information via en bloc resection with markedly decreased morbidity compared to CRT regimens.54, 56 Additionally, successful control of the primary tumor burden opens avenues for de-escalation regimens that could decrease or avoid adjuvant treatment requirements.54 This surgical approach is currently under investigation as a de-escalation modality in OPSCC, where patients with resectable OPSCC will be randomized to TORS with neck dissection or RT plus or minus CT with surgical salvage for persistent disease. This study will offer a direct comparison of TORS to RT or CRT, and determine if TORS achieves better functional outcomes in early stage OPSCC (Table 2).
Novel targeted therapies
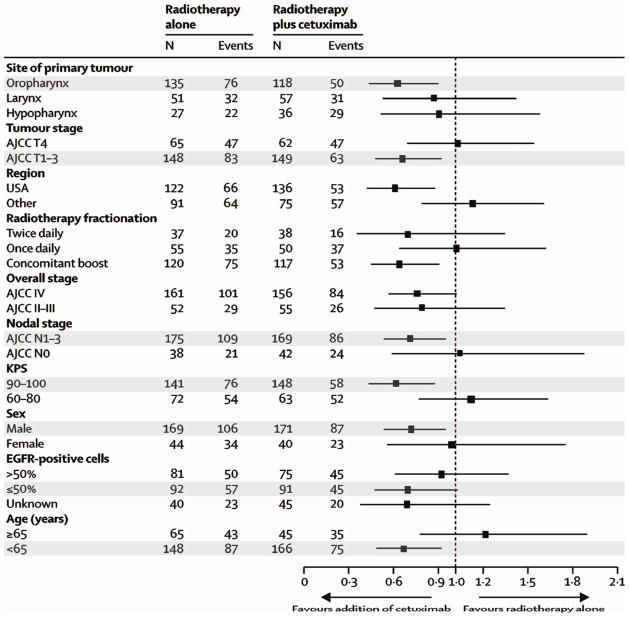
The increased understanding of molecular signaling pathways in HNSCC has led to studies of novel targeted therapies that may decrease the morbidity associated with conventional treatment of HNSCC. The first FDA–approved targeted therapy for HNSCC is cetuximab, a monoclonal antibody against EGFR. It showed a 5–year survival benefit when added to RT (RT: 36.4%, RT and cetuximab: 45.6%, HR: 0.73, p=0.018) with decreased morbidity and an improvement in quality of life although at a significantly higher cost.57,58 Of note, the survival benefit was restricted to patients with clinical and tumor characteristics associated with HPV-positive OPSCC (Figure 1).59 This may suggest that the benefit of cetuximab as initial treatment is limited to HPV-positive patients. However, these results may merely portray the known improved survival in HPV-positive patients, their better response to treatment, or a marked imbalance of HPV-positive patients between the intervention groups despite randomization.60
Figure 1.
Hazard ratios for overall survival based on patient pre-treatment characteristics. Marked categories represent characteristics associated with HPV-positive OPSCC. Adapted from Bonner et al.57 Reprinted with permission. © 2013 Elsevier. All rights reserved.
In contrast, panitumumab, another EGFR inhibitor, may only benefit HPV-negative patients in the recurrent/metastatic setting, according to an initial report.61 Differences in clinical setting (primary vs. recurrent/metastatic tumors) or treatment regimen (chimeric vs. humanized anti-EGFR antibody, radiation vs. chemotherapy) may explain these apparently contradictory findings.62 More studies are needed to determine how HPV status affects response to EGFR inhibitors in HNSCC (Table 2).
The RTOG-1016 trial is performing a direct comparison of RT with cisplatin or cetuximab in OPSCC patients with prospective HPV testing that will address the efficacy of cetuximab as single agent in CRT regimens, compare the toxicity profiles, and possibly the relative efficacy in HPV-positive and HPV-negative patients (Table 2).
Histone deacetylase inhibitors have emerged as another potential drug to reverse aberrant epigenetic changes associated with cancer.63 One of these drugs, vorinostat, is being evaluated for safety and maximum tolerated dose when administered concurrently with CRT in the treatment of OPSCC (Table 2). Several additional biochemical pathways such as VEGF and intracellular signaling pathways are being targeted for treatment with drugs currently in various stages of development for the treatment of HNSCC.58 These drugs may lead to new treatment alternatives for OPSCC.
Specific targeting of HPV-positive tumor cells may be achieved due to the unique expression of E6 and E7 oncoproteins by HPV-positive OPSCC. For example, suppression of cellular E6 and E7 protein levels by short hairpin RNA is able to restore p53 and pRb function and induce apoptosis in cell line studies.64 As a consequence, small molecule inhibitors that inhibit the protein-protein interaction of the viral oncoproteins E6 and E7 are actively being investigated, which may sensitize tumor cells to other therapies.58 Tumor expression of E6 and E7 may also provide the possibility to induce or enhance cell-mediated immunity against tumor cells. This strategy is being investigated in several phase I and II trials in HNSCC (NCT01493154, NCT00019110, and NCT01462838, available at www.clinicaltrials.gov). Specifically in OPSCC, one study is using a listeria monocytogenes-based vaccine to deliver HPV antigens for recognition by the immune system and elicit an immune response (Table 2).65 Another approach is harvesting, expanding and re-administering tumor-infiltrating lymphocytes to patients to enhance the cytotoxic anti-tumoral immune response as a treatment strategy in HPV-related cancers (Table 2). These approaches have the intrinsic benefit of using physiologic anti-tumoral responses as a treatment modality that theoretically carries decreased risk and morbidity. Further research will allow us to evaluate the safety and efficacy of novel treatment strategies and to gain understanding into the mechanisms involved in the response to treatment of HPV-positive and HPV-negative OPSCC.
Biomarkers and Risk Stratification for Patient Selection
There has also been a recent effort to use biomarkers to identify which patients will benefit from de-escalation of therapy and who will require standard treatments. Approaches using IC as a stratification marker to select patients for early surgical intervention should there be an insufficient response, have been unsatisfying.17 It is unclear whether IC achieves effective downstaging of the tumor making RT more effective, or more likely selects potentially curable patients.52 Furthermore, this selection method is time-consuming, carries additional costs and morbidity, and has not shown a survival advantage in HNSCC.17, 52
A second strategy is to identify biomarkers that can prospectively distinguish between those patients with a high probability to respond to treatment and achieve cure versus those likely to fail therapy and at high risk of recurrence and death. HPV status is one widely used biomarker and currently the most accepted means of stratification, but alone it is insufficient to direct therapy outside of clinical trials. There is also a need to find complementary biomarkers to further stratify HPV-positive patients, as the ever-increasing incidence of HPV-positive cases translates into approximately half of all OPSCC recurrences presenting in HPV-positive patients, despite the improved outcomes.37, 66
Approaches to stratifying HPV-positive patients
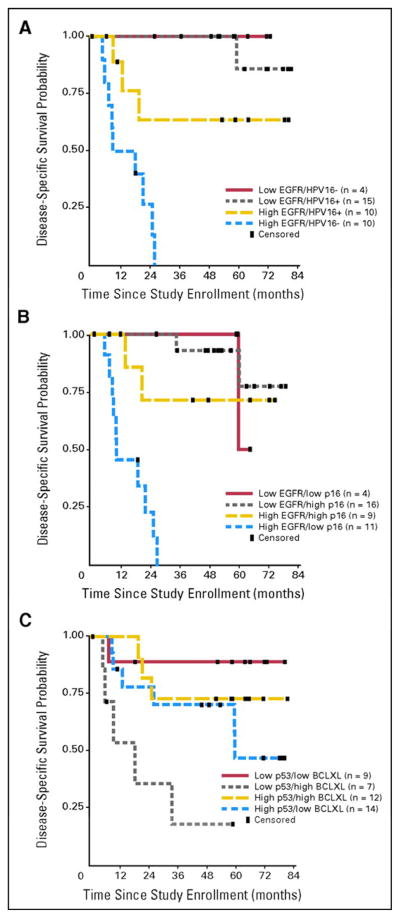
Several approaches have been proposed with this objective. Kumar et al.67 studied how HPV status and expression of EGFR, p16, p53, Bcl-xL and p53 mutations on pretreatment biopsies affected OS and DFS in patients with advanced OPSCC enrolled in an organ-sparing trial. Treatment consisted of IC followed by CRT and adjuvant paclitaxel or surgery and RT. Patients with favorable expression profiles (low EGFR and high HPV titer/p16, or low p53 with low Bcl-xL), showed significantly better OS and DFS (Figure 2).67 This study shows how in a group of homogeneously treated patients, the combination of HPV status and EGFR expression could accurately stratify survival. Moreover, p53 and Bcl-xL were found to be predictors of survival independent of HPV status, which points towards a molecular mechanism that may underlie these findings. Inhibition of apoptosis by high Bcl-xL favors DNA repair by the elevated p53, allowing the cells to continue to grow despite cisplatin-induced DNA damage.67 Furthermore, the percentage of HPV-positive patients who fail treatment is similar to the percentage of HPV-positive tumors reported to harbor p53 mutations,23,67,68 suggesting that mutant p53 may be used as a marker to further stratify the HPV-positive population.
Figure 2.
Kaplan-Meier curves for DFS based on risk stratification by EGFR, HPV-16, p16, p53 and Bcl-xL. From Kumar et al.67 Reprinted with permission. © 2013 American Society of Clinical Oncology. All rights reserved.
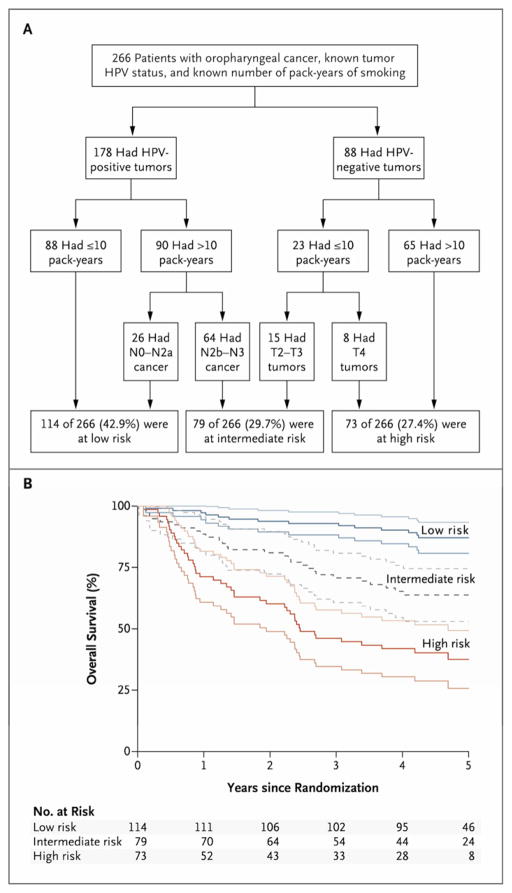
Ang and colleagues7 constructed an algorithm to classify patients into distinct risk categories following CRT. HPV status was the major determinant of OS followed by the pack years of smoking. These subgroups were further classified according to N stage for HPV-positive tumors and T stage for HPV-negative tumors. Patients were then classified into low, intermediate or high risk of death categories, which correlate with a 3-year OS of 93%, 70.8% and 46.2%, respectively (Figure 3).7 Classification into high- and intermediate-risk groups demonstrated a 7-fold and 4-fold higher risk of death than low risk patients.7 This is a simple method to estimate individual patient risk of death after treatment with CRT that is easily translatable to the clinic, since HPV status, TNM staging, and smoking history are readily available to clinicians.
Figure 3.
Risk classification scheme (A) and corresponding Kaplan-Meier curves for overall survival with their 95% CI (B). From Ang et al.7 Reprinted with permission. © 2013 Massachusetts Medical Society. All rights reserved.
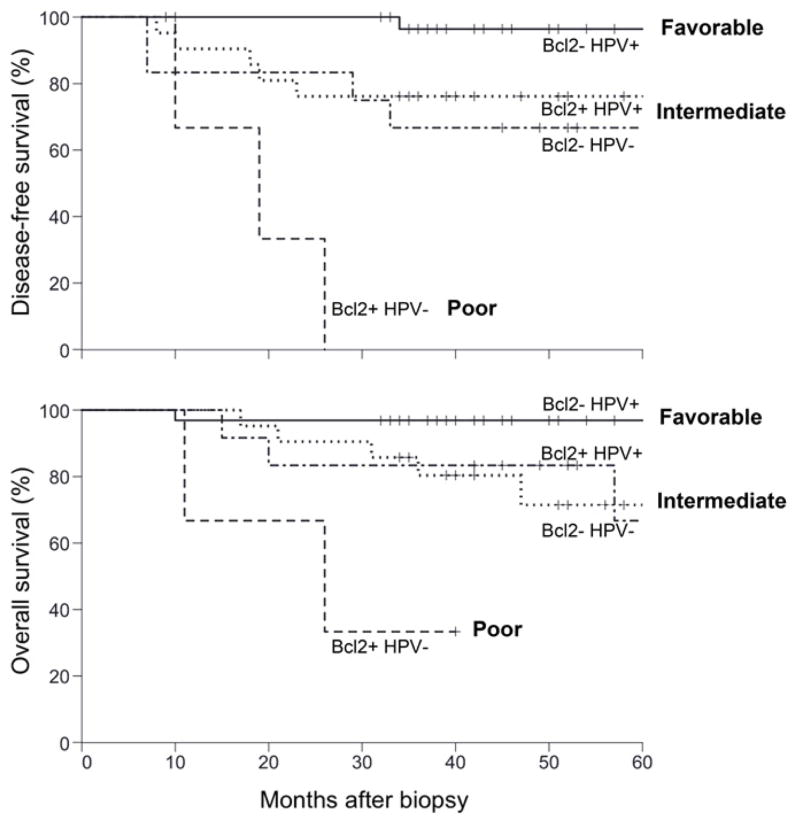
Nichols et al66 evaluated retrospectively whether Bcl-2 and HPV status could be used as markers for therapeutic response in a cohort of newly-diagnosed OPSCC patients for whom pre-treatment biopsies were available and had either a minimum 2-year follow up, death or recurrence. HPV infection and Bcl-2 were found to be independent predictors of improved DFS and OS. Patients with high Bcl-2 tumors had approximately a 7-fold increased risk of recurrence and death after adjusting for HPV status. The data also suggested that Bcl-2 was specifically associated with increased risk of distant metastasis with no relation to loco-regional recurrence. Based on HPV and Bcl-2, patients were segregated into 3 risk groups: those with 2 favorable markers showed excellent survival (HPV-positive and low Bcl-2); patients with one favorable and one unfavorable marker showed intermediate survival (HPV-positive and high Bcl-2, or HPV-negative and low Bcl-2); and those with both unfavorable markers showed poor survival (HPV-negative and high Bcl-2) (Figure 4).66 The high percentage of HPV-positive tumors that had high Bcl-2 (40%) suggests that this biomarker will become increasingly useful over time.66 Small molecule inhibitors of Bcl-2 are under investigation and may play an important role in defining new treatment regimens for OPSCC patients at high risk of recurrence and death. As Bcl-2 may also identify patients at higher risk for distant metastasis, it has the potential to be used as a marker for intensification of systemic treatment, such as the addition of IC before CRT rather than CRT alone.
Figure 4.
Kaplan-Meier curves for DFS and OS based on risk classification by HPV status and Bcl-2. Adapted from Nichols et al.66 Reprinted with permission. © 2013 American Association for Cancer Research. All rights reserved.
Several other markers have been studied with different degrees of reliability in HNSCC, and although their relation to HPV-positive OPSCC has not been established, may have future implications for risk stratification in this cancer.17,69 The studies presented were limited in some instances by their retrospective nature, and overall by the small sample sizes and limited generalizability given the different populations and variety of interventions studied. Nonetheless, these promising findings point the way towards future prospective studies in more general clinical scenarios. [Tags for SEO and INDEX: Oropharyngeal cancer, HPV, survival, mechanisms for improved survival, intratumor heterogeneity, de-escalation of therapy, biomarkers]
Summary
In the past decade, otolaryngologists and related specialists have seen the emergence and characterization of a new entity, HPV–positive oropharyngeal cancer, which has changed the way we understand and manage cancer of the head and neck. HPV-positive OPSCC has a distinct pathogenesis and develops in a localized environment of genomic instability and malignant transformation driven by the expression of the E6 and E7 viral oncoproteins. In contrast to HPV-negative OPSCC, it is a disease of younger patients with a distinct subset of risk factors related to sexual practices that have evolved over the past several decades. Fortunately, these patients show better oncologic outcomes than their historical cohort, as demonstrated by their favorable response to treatment and improved PFS and OS, although the exact mechanism underlying this benefit remains unknown.
There are several important treatment implications. Could we identify OPSCC patients who will show complete response to treatment a priori and decrease the morbidity of treatment safely and with the same oncologic outcomes to achieve better quality of life? Can we also predict which patients will fail treatment, allowing us to test more intensified regimens in the population at risk to increase the probability of initial cure? What should these intensified and de-intensified regimens be? Which biomarkers will allow us to make these predictions to be able to offer personalized therapy? Does organ-preservation therapy work in the absence of HPV infection?
Different organ preservation regimens, surgical approaches, and novel targeted therapy strategies that address cancer-related pathways and HPV–specific targets are being studied to begin offering some insight into these challenging questions. Some changes to clinical practice have already been recommended, such as determining HPV status for all patients with oropharyngeal cancer or unknown primary. OPSCC patients should also be strongly encouraged to participate in clinical trials. The success of trials evaluating different treatment modalities as well as de-escalation of therapy will depend on adequate recruitment; thus we encourage active enrollment of patients to be able to determine new standards of care. There is great potential for the prevention of this disease through modification of risk factors and potentially with vaccination such that we may be able to stabilize or decrease the impact of this cancer epidemic.
Synopsis.
Oropharyngeal squamous cell carcinoma (OPSCC) originating from human papillomavirus (HPV) infection has emerged as a new entity in head and neck cancer, defining a subset of patients with distinct carcinogenesis, risk factors profiles and clinical presentation that show markedly improved survival than do patients with classic OPSCC. De-escalation of therapy and identification of relevant biomarkers to aid in patient selection are actively being investigated. This review addresses the implications of these findings in clinical care.
Key Points.
Human papillomavirus (HPV)-positivity defines a subset of oropharyngeal squamous cell carcinoma (OPSCC) patients with distinct carcinogenesis, risk factors, clinical presentation and prognosis, representing a different disease from other head and neck squamous cell carcinoma (HNSCC).
Cancer in these patients is mainly driven by the viral E6 and E7 oncoproteins, which interfere with p53 and pRb tumor-suppressor pathways.
Patients are typically younger, non-drinkers and non-smokers with risk factors associated with sexual exposure to HPV.
Patients with HPV-positive OPSCC show better response to treatment, overall survival (OS), and progression free survival (PFS) than those with HPV-negative tumors.
Reasons for improved survival are unknown. Current hypotheses include decreased field cancerization, decreased genetic instability and tumor heterogeneity, reactivation of p53 by chemotherapy (CT) and radiotherapy (RT), and improved immune response in HPV-positive cancers.
The improved outcomes found in HPV-positive OPSCC have confounded clinical trial results in the recent past. Ongoing trials need to include assessment of HPV status in their design.
Clinical trials are underway to determine whether de-escalation of therapy is possible in HPV-positive OPSCC patients to achieve similar survival with reduced short- and long-term morbidity.
Biomarkers that may direct different therapeutic approaches are actively being investigated.
Prevention should be focused on modification of risk factors with a special emphasis on HPV vaccination.
Acknowledgments
We are grateful to the Principles and Practice of Clinical Research Course and Latin American Initiative Program for training support to JBV.
Abbreviations: Impact of HPV on Oropharyngeal Cancer
- CRT
chemo-radiotherapy
- CT
chemotherapy
- HNSCC
head and neck squamous cell carcinoma
- HPV
human papillomavirus
- IC
induction chemotherapy
- ISH
In situ hybridization
- OPSCC
oropharyngeal squamous cell carcinoma
- OS
overall survival
- PFS
progression free survival
- RT
radiotherapy
- TLM
transoral laser microsurgery
- TORS
transoral robotic surgery
Footnotes
Conflict of Interest: None.
Disclosures: Funding sources and support: Flight Attendant Medical Research Institute; NIH NIDCR R01 DE022087; NCI R21 CA119591; NIDCD T32 DC000020; 2012 AAO-HNSF Resident Research Award; Principles and Practice of Clinical Research and Latin American Initiative program, Harvard Medical School.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Begum S, Cao D, Gillison M, et al. Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res. 2005;11(16):5694–5699. doi: 10.1158/1078-0432.CCR-05-0587. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin-Drubin ME, Munger K. Oncogenic activities of human papillomaviruses. Virus Res. 2009;143(2):195–208. doi: 10.1016/j.virusres.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 5.Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol. 2006;24(17):2606–2611. doi: 10.1200/JCO.2006.06.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 7.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health. 2010;46(4 Suppl):S20–26. doi: 10.1016/j.jadohealth.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 10.D’Souza G, Dempsey A. The role of HPV in head and neck cancer and review of the HPV vaccine. Prev Med. 2011;53 (Suppl 1):S5–S11. doi: 10.1016/j.ypmed.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marur S, D’Souza G, Westra WH, et al. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11(8):781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Settle K, Posner MR, Schumaker LM, et al. Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res (Phila) 2009;2(9):776–781. doi: 10.1158/1940-6207.CAPR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joseph AW, D’Souza G. Epidemiology of human papillomavirus-related head and neck cancer. Otolaryngol Clin North Am. 2012;45(4):739–764. doi: 10.1016/j.otc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Chow LT, Broker TR, Steinberg BM. The natural history of human papillomavirus infections of the mucosal epithelia. APMIS. 2010;118(6–7):422–449. doi: 10.1111/j.1600-0463.2010.02625.x. [DOI] [PubMed] [Google Scholar]
- 15.Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 16.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307(7):693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimminger CM, Danenberg PV. Update of prognostic and predictive biomarkers in oropharyngeal squamous cell carcinoma: a review. Eur Arch Otorhinolaryngol. 2011;268(1):5–16. doi: 10.1007/s00405-010-1369-x. [DOI] [PubMed] [Google Scholar]
- 18.Vidal L, Gillison ML. Human papillomavirus in HNSCC: recognition of a distinct disease type. Hematol Oncol Clin North Am. 2008;22(6):1125–1142. vii. doi: 10.1016/j.hoc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10(8):550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 20.Mroz EA, Baird AH, Michaud WA, et al. COOH-terminal binding protein regulates expression of the p16INK4A tumor suppressor and senescence in primary human cells. Cancer Res. 2008;68(15):6049–6053. doi: 10.1158/0008-5472.CAN-08-1279. [DOI] [PubMed] [Google Scholar]
- 21.Mellin H, Friesland S, Lewensohn R, et al. Human papillomavirus (HPV) DNA in tonsillar cancer: clinical correlates, risk of relapse, and survival. Int J Cancer. 2000;89(3):300–304. [PubMed] [Google Scholar]
- 22.Posner MR, Lorch JH, Goloubeva O, et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011;22(5):1071–1077. doi: 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 24.Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of Methods for Oropharyngeal Cancer HPV Status Determination in US Cooperative Group Trials. Am J Surg Pathol. 2012;36(7):945–954. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palka KT, Slebos RJ, Chung CH. Update on molecular diagnostic tests in head and neck cancer. Semin Oncol. 2008;35(3):198–210. doi: 10.1053/j.seminoncol.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34(4):459–461. doi: 10.1002/hed.21974. [DOI] [PubMed] [Google Scholar]
- 27.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116(9):2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 28.Adelstein DJ, Ridge JA, Gillison ML, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting, November 9–10, 2008, Washington, D. C Head Neck. 2009;31(11):1393–1422. doi: 10.1002/hed.21269. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Food and Drug Administration. [Accessed 7/23/2012, 2012];Approved Products > Cervarix. 2012 http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm186957.
- 30.U.S. Food and Drug Administration. [Accessed 7/23/2012, 2012];Approved Products > Gardasil. 2012 http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM094042.
- 31.O’Rorke MA, Ellison MV, Murray LJ, et al. Human papillomavirus related head and neck cancer survival: A systematic review and meta-analysis. Oral Oncol. 2012 doi: 10.1016/j.oraloncology.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Dahlstrom KR, Calzada G, Hanby JD, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: A staging system in need of repair. Cancer. 2012 doi: 10.1002/cncr.27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02. 02 phase III trial. J Clin Oncol. 2010;28(27):4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodwin WJ, Thomas GR, Parker DF, et al. Unequal burden of head and neck cancer in the United States. Head Neck. 2008;30(3):358–371. doi: 10.1002/hed.20710. [DOI] [PubMed] [Google Scholar]
- 35.Settle K, Taylor R, Wolf J, et al. Race impacts outcome in stage III/IV squamous cell carcinomas of the head and neck after concurrent chemoradiation therapy. Cancer. 2009;115(8):1744–1752. doi: 10.1002/cncr.24168. [DOI] [PubMed] [Google Scholar]
- 36.Chernock RD, Zhang Q, El-Mofty SK, et al. Human papillomavirus-related squamous cell carcinoma of the oropharynx: a comparative study in whites and African Americans. Arch Otolaryngol Head Neck Surg. 2011;137(2):163–169. doi: 10.1001/archoto.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mroz EA, Forastiere AA, Rocco JW. Implications of the oropharyngeal cancer epidemic. J Clin Oncol. 2011;29(32):4222–4223. doi: 10.1200/JCO.2011.37.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24(36):5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 39.Butz K, Geisen C, Ullmann A, et al. Cellular responses of HPV-positive cancer cells to genotoxic anti-cancer agents: repression of E6/E7-oncogene expression and induction of apoptosis. Int J Cancer. 1996;68(4):506–513. doi: 10.1002/(SICI)1097-0215(19961115)68:4<506::AID-IJC17>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 40.Vu HL, Sikora AG, Fu S, et al. HPV-induced oropharyngeal cancer, immune response and response to therapy. Cancer Lett. 2010;288(2):149–155. doi: 10.1016/j.canlet.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 41.Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333(6046):1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salk JJ, Fox EJ, Loeb LA. Mutational heterogeneity in human cancers: origin and consequences. Annu Rev Pathol. 2010;5:51–75. doi: 10.1146/annurev-pathol-121808-102113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mroz EA, Rocco JW. Pending. [Google Scholar]
- 45.Wansom D, Light E, Worden F, et al. Correlation of cellular immunity with human papillomavirus 16 status and outcome in patients with advanced oropharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2010;136(12):1267–1273. doi: 10.1001/archoto.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wansom D, Light E, Thomas D, et al. Infiltrating lymphocytes and human papillomavirus-16--associated oropharyngeal cancer. Laryngoscope. 2012;122(1):121–127. [Google Scholar]
- 47.Spanos WC, Nowicki P, Lee DW, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135(11):1137–1146. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 48.Begum S, Gillison ML, Ansari-Lari MA, et al. Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res. 2003;9(17):6469–6475. [PubMed] [Google Scholar]
- 49.National Comprehensive Cancer Network. The NCCN Clinical Practice Guidelines in Oncology Head and Neck Cancers (Version 1.2012) [Accessed 6/12/2012]. [Google Scholar]
- 50.Wang X, Hu C, Eisbruch A. Organ-sparing radiation therapy for head and neck cancer. Nat Rev Clin Oncol. 2011;8(11):639–648. doi: 10.1038/nrclinonc.2011.106. [DOI] [PubMed] [Google Scholar]
- 51.Travis LB, Ng AK, Allan JM, et al. Second malignant neoplasms and cardiovascular disease following radiotherapy. J Natl Cancer Inst. 2012;104(5):357–370. doi: 10.1093/jnci/djr533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Licitra L, Vermorken JB. Is there still a role for neoadjuvant chemotherapy in head and neck cancer? Ann Oncol. 2004;15(1):7–11. doi: 10.1093/annonc/mdh001. [DOI] [PubMed] [Google Scholar]
- 53.Holsinger FC, Sweeney AD, Jantharapattana K, et al. The emergence of endoscopic head and neck surgery. Curr Oncol Rep. 2010;12(3):216–222. doi: 10.1007/s11912-010-0097-0. [DOI] [PubMed] [Google Scholar]
- 54.Dowthwaite SA, Franklin JH, Palma DA, et al. The role of transoral robotic surgery in the management of oropharyngeal cancer: a review of the literature. ISRN Oncol. 2012;2012:945162. doi: 10.5402/2012/945162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hartl DM, Ferlito A, Silver CE, et al. Minimally invasive techniques for head and neck malignancies: current indications, outcomes and future directions. Eur Arch Otorhinolaryngol. 2011;268(9):1249–1257. doi: 10.1007/s00405-011-1620-0. [DOI] [PubMed] [Google Scholar]
- 56.de Almeida JR, Genden EM. Robotic surgery for oropharynx cancer: promise, challenges, and future directions. Curr Oncol Rep. 2012;14(2):148–157. doi: 10.1007/s11912-012-0219-y. [DOI] [PubMed] [Google Scholar]
- 57.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 58.Fung C, Grandis JR. Emerging drugs to treat squamous cell carcinomas of the head and neck. Expert Opin Emerg Drugs. 2010;15(3):355–373. doi: 10.1517/14728214.2010.497754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brockstein BE, Vokes EE. Head and neck cancer in 2010: Maximizing survival and minimizing toxicity. Nat Rev Clin Oncol. 2011;8(2):72–74. doi: 10.1038/nrclinonc.2010.226. [DOI] [PubMed] [Google Scholar]
- 60.Eriksen JG, Lassen P, Overgaard J. Do all patients with head and neck cancer benefit from radiotherapy and concurrent cetuximab? Lancet Oncol. 2010;11(4):312–313. doi: 10.1016/S1470-2045(10)70035-8. [DOI] [PubMed] [Google Scholar]
- 61.Vermorken JSJ, Oliner K, Villanueva C, Foa P, Winquist E, Licitra L, Skladowski K, Pan Z, Bach BA. Safety and Efficacy of Panitumumab (pmab) in HPV Positive (+) and HPV Negative (−) Recurrent/metastatic (R/M) Squamous Cell Carcinoma of the Head and Neck (SCCHN): Analysis of the Phase 3 SPECTRUM Trial. Eur J Cancer. 2011;47(Supp 2):13. [Google Scholar]
- 62.Mroz EA, Rocco JW, Forastiere A. Reply to D.C. Gilbert et al. J Clin Oncol. 2012:891–892. [Google Scholar]
- 63.Iglesias-Linares A, Yanez-Vico RM, Gonzalez-Moles MA. Potential role of HDAC inhibitors in cancer therapy: insights into oral squamous cell carcinoma. Oral Oncol. 2010;46(5):323–329. doi: 10.1016/j.oraloncology.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 64.Rampias T, Sasaki C, Weinberger P, et al. E6 and e7 gene silencing and transformed phenotype of human papillomavirus 16-positive oropharyngeal cancer cells. J Natl Cancer Inst. 2009;101(6):412–423. doi: 10.1093/jnci/djp017. [DOI] [PubMed] [Google Scholar]
- 65.Shahabi V, Maciag PC, Rivera S, et al. Live, attenuated strains of Listeria and Salmonella as vaccine vectors in cancer treatment. Bioeng Bugs. 2010;1(4):235–243. doi: 10.4161/bbug.1.4.11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nichols AC, Finkelstein DM, Faquin WC, et al. Bcl2 and human papilloma virus 16 as predictors of outcome following concurrent chemoradiation for advanced oropharyngeal cancer. Clin Cancer Res. 2010;16(7):2138–2146. doi: 10.1158/1078-0432.CCR-09-3185. [DOI] [PubMed] [Google Scholar]
- 67.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26(19):3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Westra WH, Taube JM, Poeta ML, et al. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2008;14(2):366–369. doi: 10.1158/1078-0432.CCR-07-1402. [DOI] [PubMed] [Google Scholar]
- 69.Langer CJ. Exploring biomarkers in head and neck cancer. Cancer. 2012 doi: 10.1002/cncr.26718. [DOI] [PubMed] [Google Scholar]