Abstract
A novel antimicrobial peptide was isolated from the saliva of the Lyme disease tick vector, Ixodes scapularis, henceforth designated as ISAMP (Ixodes scapularis Anti Microbial Peptide). ISAMP was purified using a sequential method including ultra filtration, gel filtration and reverse-phase high performance liquid chromatography. The purified peak had a molecular weight of 5.3 kDa by MALDI/TOF-MS and its amino acid sequence, determined by Edman degradation was PDxGxPxxVKAGRxPxxSI. A BLASTP search revealed that the protein is a putative 5.3 kDa secreted protein (AAM93656) from I. scapularis. The predicted protein is composed of 69 amino acids with no conserved domain motifs. Purified ISAMP was found to have antimicrobial activities against bacteria. Gene expression studies were carried out to observe ISAMP expression in different tick tissues. RT-PCR results indicated that the gene was expressed in hemocytes, fat body and salivary gland but virtually no expression was observed in the midgut. ISAMP is only similar to other Ixodid tick proteins, thus it is a member of a unique family.
Keywords: Ticks, Saliva, Ixodes scapularis, antimicrobial peptide, innate immunity
Introduction
The black-legged tick, Ixodes scapularis (Acari: Ixodidae), is an important vector of microbial pathogens affecting the health of humans and domestic animals. I. scapularis ticks transmit Borrelia burgdorferi, the bacterial pathogen causing Lyme disease [1] as well as the agents causing human anaplasmosis (Anaplasma phagocytophilum) [2], human babesiosis (Babesia microti) [3], and an encephalitis-like virus [4]. While specific vector competence mechanisms in ticks have yet to be discovered and confirmed, reports have indicated that ticks possess innate processes for controlling various microbial infections when challenged [5].
Antimicrobial peptides (AMPs) are essential components of host defenses against infectious microorganisms. In chelicerate organisms they have been implicated in three alternative defensive systems: one is defined by the immediate up-regulation of genes encoding AMPs, another is characterized by the inducible systemic release of AMPs from cellular reservoirs, and the third involves systemic constitutive production of AMPs [6]. Known antimicrobial peptides can be divided into several groups that include linear peptides which form amphipathic and hydrophobic helices, cyclic peptides with unique amino acid compositions, cyclic peptides with thio-ether groups in the ring, and lipopeptides terminating in an amino alcohol, and macrocyclic knotted peptides [7]. Recent examples of two novel antimicrobial peptides from ticks, Ixosin [8] and Ixosin –B [9] were described from I. sinesis salivary glands, while a cationic defensin (varisin) was identified from the hemocytes of the hard tick, D. variabilis (Say) [5,10]. Defensin-like molecules or homologous genes also have been identified in the ticks A. americanum and I. ricinus [11, 12]. Functional responses for specific AMPs largely remain to be further characterized.
Ticks and other arthropods lack any adaptive immunity, and they apparently live harmoniously with certain microbes. However, they do appear to rely on an ability to induce AMPs [13] and other molecular and cellular responses such as a phenoloxidase system and hemocyte phagocytosis [14] for certain microbial defenses. Knowledge of innate immune responses in ticks, and characterizing specific AMPs, may help explain how ticks are able to survive in a microbe-rich world. Greater understanding of specific activity and efficiency in innate immune responses of different tick species also is likely to help provide clues to vector competence and vector efficiency for transmitting the diverse array of microbes associated with ticks [15]. However, the question of why some ticks are competent vectors of pathogens and others are not will probably be more complicated than elucidating the presence or absence of specific peptides as constituents of any given tick’s antimicrobial defenses.
Antimicrobial peptides may help ticks overcome host defensive responses, or keep ingested blood sterile in the tick body [8]. The function of each tissue involved (i.e. salivary gland, midgut, fat body, hemocytes) in the innate response may be to provide complementary protection from deleterious infection. In the case of ticks, the first line of defense likely starts in the saliva, leading to successful blood ingestion. In the course of characterizing I. scapularis salivary molecules whose genes were specifically up-regulated in B. burgdorferi-infected nymphs [16], we identified and characterized a novel AMP from this tick.
Materials and methods
Restriction enzymes, Taq DNA polymerase and polymerase chain reaction (PCR) product purification kits, Block-iT T7 TOPO linker were purchased from Invitrogen (Carlsbad, CA, USA) and Qiagen (Valencia, CA, USA). Proteo Mass peptide and the Protein MALDI-MS calibration kit was purchased from Sigma, USA.
Source of Ticks
Ixodes scapularis ticks were collected from forested sites in southern Rhode Island. For some experiments, adult ticks were allowed to feed on New Zealand white rabbits under controlled conditions [17]. A restraining collar was placed around the neck of each rabbit, and their ears were covered with cotton stockinette prior to tick exposure. For these experiments, ticks were allowed to blood feed for up to 120 hrs. All animal studies were approved by the University of Rhode Island Institutional Animal Care and Use Committee (protocol number AN01-12-014).
Tick Saliva Collection
We used the pilocarpine induction method to induce ticks to salivate [18]. Ticks were permitted to engorge for 4–5 days on the ear of a rabbit, after which they were removed by traction. Ticks weighing 200 – 300 μg were used for tick saliva extraction. Upon harvesting, ticks were rinsed in distilled water and were immediately fixed to glass slides with double-sided tape, and a sterile glass micropipette was placed around the hypostome to collect saliva. Salivation was induced by the application of 2 μl of pliocarpine (50 μg/ml) in 95% ethanol to the scutum of the tick. Additional 1 μl volumes of pilocarpine were applied at 20 minute intervals when little salivation was observed. Ticks were incubated at 35°C in a humid chamber until salivation ceased (2 to 3 hours). Micropipettes were removed from the ticks, and the amount of saliva collected was determined. Typically, volumes ranging from 15–30 μl per tick were collected. The saliva was pooled and stored at −70°C until being analyzed.
Peptide purification
Tick saliva (200–350 μl) was ultracentrifuged at 4°C using YM-10 and YM-3 membranes (MWCO 10 and 3 kDa: Amicon Co, MA, USA), and the fractions checked for antimicrobial activity [8]. The pooled, concentrated antimicrobial fractions were subjected to consecutive chromatographic separations using a Superdex Peptide 10/300GL column, and a reverse-phase HPLC (Waters Co., Ireland). All purification experiments were carried out in triplicate using different pools of tick saliva.
The peptide solution was loaded into a Superdex Peptide 10/300 GL column (10 mm x 300 mm; GE Health care, USA) pre-equilibrated with 30% acetonitrile in methanol and 0.1% formic acid, and was eluted at a flow rate of 0.25 ml/min which was collected with a fraction collector. The eluate was monitored by its absorbance at 215 nm. All of the peaks were concentrated using an Eppendorf vacufuge, and concentrated samples were checked for antimicrobial activity using the method of Yu et al. [8].
The highest antimicrobial fraction in the gel filtration chromatography was subjected to further purification on HPLC reverse-phase column system (Hitachi Analytical HPLC system, CA). HPLC purification was achieved with a 0.3 mm × 150 mm C8 Magic column (Michrom Bioresources, Inc., CA) perfused at 2 or 5 μl/min using an ABI 140D pump and 785A UV detector from Applied Biosystems (Foster City, CA). Solution A contained water and 0.1% formic acid (FA), and solution B contained 30% acetonitrile in methanol and 0.1% FA. After injecting the sample (previously equilibrated with 10% methanol and containing 0.1% FA) into the column, a gradient from 10% to 95% B was imposed for 20 min at a flow rate of 5 μl/min. After this time, the flow rate was decreased to 2 μl/min. Fractions were collected using the Gilson 203B fraction collector (Middleton, WI, USA) at 1-min intervals. Protein values for each of the fractions were measured using Nano Drop UV/Vis Spectrometer, and antimicrobial activity was determined as described [8].
Mass Spectrometry
Molecular mass of the peptide was determined by MALDI/TOF-MS analyses. The experiments were conducted on Ciphergen ProteinChip® System (USA) using the matrix solution of α-cyano-4-hydroxycinnamic acid (Sigma, USA) suspended in 100% acetonitrile.
Amino Acid Sequencing
N-terminal amino acid sequencing of the isolated peptide was obtained by automated Edman degradation. Phenylthiohydantoin-derivatives were detected using a pulse liquid automatic sequencer (Applied Biosystems, CA, USA). The resulting sequences were analyzed using the Expert Protein Analysis System (ExPASy) server of the Swiss Institute of Bioinformatics (SIB). Similarity searches were performed using BLASTP and NR databases of the National Center for Biotechnology Information (NCBI).
Antimicrobial Assays
Standard bacterial strains were used in antimicrobial assays [8]. Gram-positive bacteria B. cereus, B. subtilis and S. aureus; Gram-negative, E. coli Edl 933 and E. coli MG/655 were obtained from laboratories at the University of Rhode Island. Bacteria were grown in Luria-Bertain (LB) media to an OD (600 nm) of 0.8. A 10 μl aliquot of the bacteria was taken and added to 8 ml of fresh LB media with 0.7% agar and poured over a 90 mm Petri dish containing 25ml of 1.5 % agar in LB media. After the top agar hardened, 20 μl of each test sample was dropped onto the surface of the top agar and completely dried before being incubated overnight at 37° C. The antimicrobial activity was identified by a clear zone formed on the surface of the top agar representing inhibition of bacterial growth. Minimal inhibitory concentration (MIC) was determined in the liquid LB medium by incubating the bacteria with variable amounts of the test sample [8]. The MIC was defined as the minimum concentration of sample at which no visible bacterial growth occurred. The protein concentration was quantified by UV absorbance at 215 nm and 225 nm using the formula: concentration (mg/ml) (A215nm – A225nm) x 0.144. Three independent experiments were performed for each sample.
Hemolysis Assays
Hemolysis assays were undertaken using rabbit red blood cells in liquid medium [19]. Serial dilutions of the peptides were used, and after incubation at 37°C for 30 min, the cells were centrifuged and the absorbance in the supernatant was measured at 595nm. Maximum hemolysis was determined by adding 1% Triton X-100 to a sample of cells.
Tissue expression profile analysis
Gene specific primers were designed from the tick-specific EST sequence for ISAMP (Gene accession number AF483734) to amplify a cDNA fragment from different tick tissues (fat body, hemocytes, salivary glands and midguts) dissected from partially-fed (~ 48–72 hrs) adult I. scapularis. Total RNA was isolated from each tick tissue using an RNAqueous@ total RNA isolation kit (Ambion). Concentration of total RNA was determined spectrometrically at 260 and 280nm, and aliquots were stored at −80°C until use. Total RNA was reverse transcribed using Maloney marine leukemia virus (M-MLV) reverse transcriptase according to an Invitrogen kit protocol. For each set, cDNA was PCR amplified using GSPs for ISAMP(Forward 5′ AAGGTACTTCCTAATAGCCCGCCA 3′ and Reverse 5′ AGCCATTATTGCAACGTGAGCAGG –3′), Na+K+ ATPase (Forward 5′ ACGAAACTGCCGAGAGCGACATTA 3′ and Reverse 5′ ATCCTGAGACCTTTGTCCATGCCT 3′) as a control (Stratagene, USA) by using the PCR program of 94°C for 4min, 35 cycles of 94°C for 1min, 49°C for 1 min, and 72°C for 1 min respectively.
Results
Purification of antimicrobial peptides
Tick saliva was divided into three parts after passing through the Amicon filters by ultra filtration, namely Fraction I (> MW 10 kDa), Fraction II (MW 3–10 kDa) and Fraction III (< MW 3 kDa), respectively. Each fraction was subjected to an antimicrobial activity assay, and Fraction II showed the highest antimicrobial activity level of about 3.5 MIC μg/ml (Table 1). Fraction II from separate samples of tick saliva were pooled and concentrated using an Eppendorf vacufuge.
Table 1.
Antimicrobial activity of the fractionated saliva by ultrafiltration membrane system
| Fractions | Antimicrobial activity (MICμg/ml) |
|---|---|
| Fraction I (>MW 10 kDa) | 30.4 ± 0.34 |
| Fraction II (MW 3–10 kDa) | 3.5 ± 0.05 |
| Fraction III (<MW 3 kDa) | 8.5 ± 0.10 |
MIC, Minimal peptide concentration required for total inhibition of cell growth (E.coli Edl 933) in liquid medium. This concentration represents mean values of three independent experiments performed in duplicates.
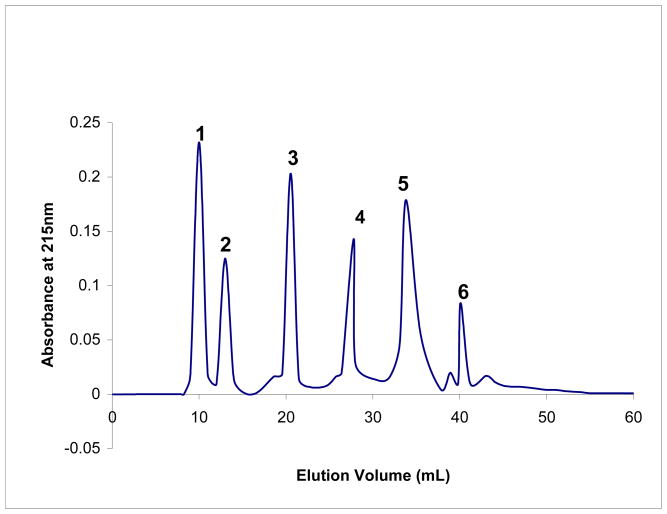
The pooled fraction II was loaded onto the gel filtration Superdex Peptide 10/300GL column using 30% acetonitrile in methanol and 0.1% formic acid. Fractions representing each peak were subjected to an antimicrobial activity assay, and two peaks (3 and 5) exhibited antimicrobial activity. The 3rd peak exhibited the highest degree of antimicrobial activity at about 3.3 ± 0.02 MIC μg/ml and all further studies were carried out using this fraction (Fig. 1). Further experiments are in progress to purify and characterize the antimicrobial activity in Peak 5.
Fig. 1.
Gel filtration chromatography of fraction II obtained after ultrafiltration. Fraction II from Table I was subjected to separation on a Superdex Peptide 10/300 GL column using 30% acetonitrile in methanol and 0.1% formic acid. The eluate was monitored by absorbance at 215nm. All peaks were concentrated through vacufuge and checked for their antimicrobial activities.
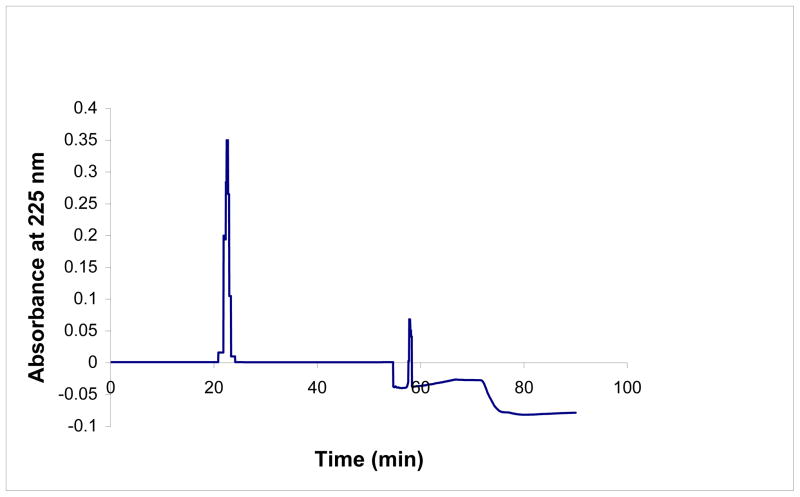
Peak 3 from the gel filtration chromatography (Superdex peptide 10/300GL column) was applied to a HPLC reverse-phase C-18 column, fractions were obtained and collected, and the peak fraction was checked for antimicrobial activity (Fig. 2). This peak showed an antimicrobial activity of about 3.2 ± 0.02 MIC μg/ml (Table 2) and this fraction was collected and pooled.
Fig. 2.
High performance liquid chromatography of fractions obtained after gel filtration chromatography. Fraction 3 from Superdex Peptide 10/300 GL was subjected to separation on a HPLC-Reverse C18 column using a linear gradient of acetonitrile (10–95%). The eluate was monitored at 225nm. All fractions were concentrated through vacufuge and checked for antimicrobial activity.
Table 2.
Antimicrobial activity of ISAMP for Gram positive and Gram negative bacteria.
| Microorganisms | Antimicrobial activity (MIC μg/ml) |
|---|---|
| B.cereus | 5.8 ± 0.05 |
| B.subtilis | 12.3 ± 0.1 |
| S.aureus | 10.4 ± 0.08 |
| E.coli Edl 933 | 3.2 ± 0.02 |
| E.coli MG/655 | 4.2 ± 0.03 |
MIC, Minimal peptide concentration required for total inhibition of cell growth in liquid medium. This concentration represents mean values of three independent experiments performed in duplicates.
Structural characterization
The purified I. scapularis antimicrobial peptide fraction was subjected to amino acid sequence analysis by automated Edman degradation. The amino acid sequence of this fraction is PDxGxPxxVKAGRxPxxSI and is composed of 19 amino acid residues. The BLASTP search showed this protein to be a putative 5.3 kDa secreted protein (AAM93656) from I. scapularis. The predicted protein is composed of 69 amino acids with no conserved domain motifs. Apart from BLASTP search, this protein sequence exactly matched the sequence of a predicted secretory protein with molecular weight of 5.3kDa and pI of 8.68, categorized in a family of genes (AF483734.1) with unknown function [20]. The MALDI/TOF analysis in this study confirmed that this purified peptide, ISAMP has a molecular weight of 5300.5 Da (Fig 3).
Fig. 3.
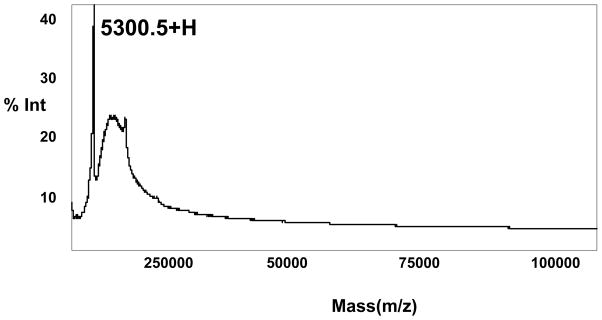
MALDI/TOF-MS analysis of purified antimicrobial peptide from RP-HPLC. Positive ion MALDI-TOF mass spectra are shown when the purified peptide was loaded onto α-cyano-4-hydroxycinnamic acid as a matrix. Spectra were analyzed to the most intense matrix peak at m/z 5300.5.
Antimicrobial activity
ISAMP exhibited antimicrobial activity against both Gram positive and Gram negative strains tested. The MICs of ISAMP for different bacteria are shown in Table 2, but two Gram negative E. coli strains were more susceptible to ISAMP.
Hemolytic activity
Some peptides with antimicrobial activity also exhibit hemolytic activities [7]. To test ISAMP for hemolytic activity, rabbit red blood cells were used. ISAMP exhibited insignificant hemolytic activity on red blood cells even with peptide concentrations up to 150 μg/ml.
Tissue expression profile
To estimate the gene expression level of ISAMP in different tissues of I. scapularis, RT-PCR experiments were performed (Fig. 4). Reactions were normalized using the intensity values of the tick Na+K+ ATPase gene as a control. ISAMP gene expression was observed in the fat body (FB), hemocytes (HE) and salivary glands (SG) of partially-fed female ticks. Virtually no ISAMP gene expression was observed in tick midguts (Fig. 4-Lane 5).
Fig. 4.
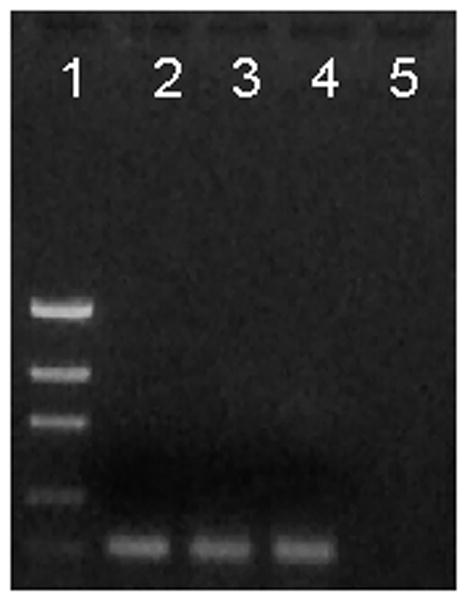
Gene expression of ISAMP in different tick tissues. RT-PCR was carried out using gene specific primers for ISAMP. Total RNA was extracted from different tissues dissected from 2–3 days fed female I. scapularis. All experiments were carried out in triplicate. The cDNA from different tissues were PCR amplified using a gene specific primer. Lanes: 1. Low mass DNA ladder; 2. Fat body; 3. Hemocytes; 4. Salivary glands; 5. Midguts.
Discussion
Due to their blood feeding habit, ticks have many opportunities for encountering a diversity of microbes. Knowledge of innate immune responses in ticks, particularly related to their antimicrobial peptides is important for understanding how and which microbes survive inside the tick. Growing evidence indicates the likely role of this innate response in determining tick survival following microbial exposure as well as vector competence [21]. In this study, we identified a novel antimicrobial peptide in the tick vector I. scapularis which is more active against Gram negative E. coli than Gram positive bacteria. Similarly, another antimicrobial peptide (Ixosin), from the tick I. sinensis [9], also was active against Gram negative bacteria. Defensins are the most widely characterized class of antimicrobial molecules occurring in arthropods, and various defensins or defensin-like molecules have been described in tick hemolymph, midgut, and fat body [5,10,11,12,22]. Defensins typically exhibit potent antimicrobial activity against Gram-positive bacteria [23], although these molecules also can attack Gram-negative bacteria, fungi and even protozoan parasites [24].
Differences in the repertoire, structure, and activity of antimicrobial peptides in insects likely have implications for vector competence to particular pathogens. For example, while American dog tick (D. variablis) defensin was found to contribute to eliminating Lyme disease spirochetes (B. burgdorferi), a defensin-like molecule in I. scapularis (a competent vector for the causative agent of Lyme disease) did not [5].
ISAMP differs from known defensin and hebraein antimicrobial peptides, as it has no cysteine residues and therefore, is presumed to be configured as a linear antimicrobial peptide. Defensins form a unique family of cysteine-rich cationic and structural polypeptides with three or four disulfide bridges, whereas, hebraein is composed of around one hundred amino acids, and contains 6 cysteines and multiple histidines [8,25,26]. ISAMP is the first presumed linear antimicrobial peptide reported from I. scapularis. By BLAST search, the gene did not show any similarity with other putative antimicrobial peptide genes, but it is part of an extensive gene family; the ISAMP gene along with other gene family members was shown to be up-regulated in ticks infected with B. burgdorferi [16]. The tissue expression profile shows that the gene is expressed mostly in tick salivary gland, hemocytes, and fat body but was not observed in midgut. This clearly differs from that of I. scapularis defensin which is expressed mostly in midguts and fat body [22]. In insects, expression of defensin is induced in the fat body following bacterial infection [23]. In Drosophila, septic injury alone, bacterial challenge with E. coli or M. luteus, or challenge with the fungus B. bassiana induced expression of defensin, between 0 and 3 hrs post-challenge [27].
Insects and arthropods rely on a suite of systemic responses, typically classified into two main types, to combat infection [28]. “Constitutive” defenses always are present and ready to act; they rely on the response of immune cells (hemocytes) and several rapidly activated enzyme cascades, such as phenoloxidase to defend against pathogens [29]. Coupled with this line of defense is an “induced” response, consisting mainly of a suite of antimicrobial peptides [30]. This latter component of antimicrobial response takes at least 1 to 3 hours to “activate” [31] and 12 to 48 hours to reach peak levels [32].
While the saliva of most organisms is one of the first lines of defense against ingested microbes, in ticks, saliva is critical for manipulating host hemostatic defenses [33]. We found active expression of the ISAMP gene in salivary glands, as well as hemocytes and fat body. Moreover, we used purified ISAMP to confirm that this molecule is secreted in saliva and triggers antibody production in tick-sensitized Guinea pigs (Data not shown). It is possible that ISAMP performs various functions in blacklegged ticks, perhaps aiding their blood feeding as well as helping to keep ingested blood sterile. The fact that ISAMP appears to be uniquely expressed in B. burgdorferi-infected ticks also suggests a possible role for this molecule in spirochete survival or transmission. The discovery of this tick antimicrobial peptide with its novel structure adds a new member to the increasing family of known antimicrobial peptides/proteins. Further studies seem warranted to identify its possible role in pathogen transmission (B. burgdorferi) or as a potential anti-tick or transmission-blocking vaccine target.
Typically, the best antimicrobials for pharmacological use are those exhibiting low hemolytic activity with high antimicrobial activity. ISAMP exhibited insignificant hemolytic activity against rabbit red blood cells, implying that it may be a relatively safe antimicrobial peptide for use in mammals if its activity warrants that. Additionally, the relatively small size and lack of cysteines makes this polypeptide amenable to chemical synthesis, without need of folding protocols.
Acknowledgments
We are particularly grateful to Drs. Marta Gomez-Chiarri, Roberta King, Shahid Karim, Assem Sayedahmed, and to Nathan Miller for helpful technical discussions. This work was supported by National Institute of Health grant AI 37230 (TNM), a subcontract to USDA Special Grant 2006-34438-17306 (TNM), and by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH (JMCR). Experiments were supported by the URI Proteomics Core facility, made available by the RI-BRIN and RI-INBRE (NIH award P20 RR16457). Because J.M.C.R. is a government employee and this is a government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the NIH reserves the right to provide the work to PubMedCentral for display and use by the public, and PubMedCentral may tag or modify the work consistent with its customary practices. You can establish rights outside of the U.S. subject to a government use license.
References
- 1.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis. Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 2.Telford SR, III, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mather TN, Telford SR, III, Moore SI, Spielman A. Borrelia burgdorferi and Babesia microti: efficiency of transmission from reservoirs to vector ticks (Ixodes dammini) Exp Parasitol. 1990;70:55–61. doi: 10.1016/0014-4894(90)90085-q. [DOI] [PubMed] [Google Scholar]
- 4.Telford SR, III, Armstrong PM, Katavolos P, Foppa I, Garcia O, Wilson ML, Spielman A. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis. 1997;3:165–170. doi: 10.3201/eid0302.970209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johns R, Soneshine DE, Hynes WL. Identification of defensin from the hemolymph of the American dog tick, Dermacentor variabilis. Insect Biochem Mol Biol. 2001;31:857–865. doi: 10.1016/s0965-1748(01)00031-5. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez de la Vega RC, Garcia BI, D’Ambrosio C, Diego-Garcia E, Scaloni A, Possani LD. Antimicrobial peptide induction in the haemolymph of the Mexican scorpion Centruroides limpidus in response to septic injury. Cell Mol Life Sci. 2004;61:1507–1519. doi: 10.1007/s00018-004-4096-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta. 1999;462:11–28. doi: 10.1016/s0005-2736(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 8.Yu D, Sheng Z, Xu X, Li J, Yang H, Liu Z, Huw RH, Lai R. A novel antimicrobial peptide from salivary glands of the hard tick, Ixodes sinensis. Peptides. 2006;27:31–35. doi: 10.1016/j.peptides.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Liu H, Liu X, Wu X. Purification and cloning of a novel antimicrobial peptide from salivary glands of the hard tick, Ixodes sinensis. Comparative Biochemistry and Physiology. 2008;149:557–561. doi: 10.1016/j.cbpb.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Ceraul SM, Soneshine DE, Ratzlaff RE, Hynes WL. An arthropod defensin expressed by the hemocytes of the American dog tick, Dermacentor variabilis (Acari: Ixodidae) Insect Biochem Mol Biol. 2003;33:1099–1103. doi: 10.1016/s0965-1748(03)00122-x. [DOI] [PubMed] [Google Scholar]
- 11.Todd SM, Sonenshine DE, Hynes WL. Tissue and life-stage distribution of a defensin gene in the Lone Star tick, Amblyomma americanum. Med Vet Entomol. 2007;2:141–147. doi: 10.1111/j.1365-2915.2007.00682.x. [DOI] [PubMed] [Google Scholar]
- 12.Rudenko N, Golovchenko M, Grubhoffer L. Gene organization of a novel defensin of Ixodes ricinus: first annotation of an intron/exon structure in a hard tick defensin gene and first evidence of the occurrence of two isoforms of one member of the arthropod defensin family. Insect Mol Biol. 2007;16:501–507. doi: 10.1111/j.1365-2583.2007.00745.x. [DOI] [PubMed] [Google Scholar]
- 13.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 14.Taylor DM. Innate immunity in Ticks: A review. J Acarol Soc Jpn. 2006;15:109–127. [Google Scholar]
- 15.Sonenshine DE. Biology of Ticks. Vol. 1. Oxford Univ Press; NY: 1991. [Google Scholar]
- 16.Ribeiro JM, Alarcon-Chaidez F, Francischetti IM, Mans BJ, Mather TN, Valenzuela JG, Wikel SK. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem Mol Biol. 2006;36:111–29. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Mather TN, Mather ME. Intrinsic competence of three ixodid ticks (Acari) as vectors for the Lyme disease spirochete. J Med Entomol. 1990;27:646–650. doi: 10.1093/jmedent/27.4.646. [DOI] [PubMed] [Google Scholar]
- 18.Higgs GA, Vane JR, Hart RJ, Potter C, Wilson RG. Prostaglandins in the saliva of the cattle tick Boophilus microplus (Canestrini) (Acarina, Ixodidae) Bull Entomol Res. 1976;66:665. [Google Scholar]
- 19.Biggnami GS. A rapid and sensitive hemolysis neutralization assay for palytoxin. Toxicon. 1993;31:817–820. doi: 10.1016/0041-0101(93)90389-z. [DOI] [PubMed] [Google Scholar]
- 20.Valenzuela JG, I, Francischetti MB, Pham VM, Garfield MK, Mather TN, Ribeiro JMC. Exploring the sialome of the tick Ixodes scapularis. J Exp Biol. 2002;205:2843–2864. doi: 10.1242/jeb.205.18.2843. [DOI] [PubMed] [Google Scholar]
- 21.Sonenshine DE, Hynes WL. Molecular characterization and related aspects of the innate immune response in ticks. Front Biosci. 2008;13:7046–7063. doi: 10.2741/3209. [DOI] [PubMed] [Google Scholar]
- 22.Hynes WL, Ceraul M, Todd SM, Seguin KC, Sonenshine DE. A defensin-like gene expressed in the black-legged tick, Ixodes scapularis. Med Vet Entomol. 2005;19:339–344. doi: 10.1111/j.1365-2915.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- 23.Gillespie JP, Kanost MR, Trenczek T. Biological mediators of insect immunity. Ann Rev Entomol. 1997;42:611–643. doi: 10.1146/annurev.ento.42.1.611. [DOI] [PubMed] [Google Scholar]
- 24.Shahabuddin M, Fields I, Bulet P, Hoffmann JA, Miller LH. Plasomidum gallinaceum: different killing of some mosquito stages of the parasite by insect defensin. Exp Parasitol. 1998;89:103–112. doi: 10.1006/expr.1998.4212. [DOI] [PubMed] [Google Scholar]
- 25.Lai R, Lomas LO, Jonczy J, Turner PC, Rees HH. Two novel non-cationic defensin like antimicrobial peptides from haemolymph of the female tick, Ambylomma hebraeum. Biochem J. 2004;379:681–685. doi: 10.1042/BJ20031429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai R, Takeuchi H, Lomas LO, Turner PC, Rees HH, Jonczy J. A new type of antimicrobial protein with multiple histidines from the hard tick, Amblyomma hebraeum. FASEB. 2004;18:1447–1449. doi: 10.1096/fj.03-1154fje. [DOI] [PubMed] [Google Scholar]
- 27.Lemaitre B, Reichhart JM, Hoffmann JA. Drosophila host defense: Differential induction of antimicrobial peptide genes after infection by various classes of microorganims. Proc Natl Acad Sci USA. 1997;94:14614–14619. doi: 10.1073/pnas.94.26.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cherry S, Silverman N. Host–pathogen interactions in Drosophila: new tricks from an old friend. Nat Immunol. 2006;7:911–917. doi: 10.1038/ni1388. [DOI] [PubMed] [Google Scholar]
- 29.Cerenius L, Lee BL, Söderhäll K. The proPOsystem:pros and cons for its role in invertebrate immunity. Trends Immunol. 2008;29:263–271. doi: 10.1016/j.it.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Iwanaga S, Lee B. Recent advances in the innate immunity of invertebrate animals. J Biochem Mol Biol. 2005;38:128–150. doi: 10.5483/bmbrep.2005.38.2.128. [DOI] [PubMed] [Google Scholar]
- 31.Lavine MD, Chen G, Strand MR. Immune challenge differentially affects transcript abundance of three antimicrobial peptides in hemocytes from the moth Pseudoplusia includens. Insect Biochem Mol Biol. 2005;35:1335–1346. doi: 10.1016/j.ibmb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Haine ER, Moret Y, Siva-Jothy MT, Rolff J. Antimicrobial defense and persistent infection in insects. Science. 2008;322:1257–1258. doi: 10.1126/science.1165265. [DOI] [PubMed] [Google Scholar]
- 33.Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM. The role of saliva in tick feeding. Front Biosci. 2009;14:2051–2088. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]