Abstract
Identifying the origins of insect vectors collected after community-wide residual insecticide applications is a relevant challenge in the Gran Chaco region where the main vector of Chagas disease Triatoma infestans usually reinfests human dwellings. Wing geometric morphometry was used to compare the right wings of 63 males and 54 females collected at 4 months post-spraying (MPS) with those from 165 males and 111 females collected before full-coverage spraying with pyrethroids in a well-defined rural area in northeastern Argentina. Male and female wing centroid size resulted significantly larger at 4 MPS than before interventions, but no significant changes in shape were detected. Metric disparity (variance of shape) varied significantly in males but not in females. Using shape variables, a relatively large fraction of post-spraying males (70%) and females (54%) could not be differentiated from those collected at the same source house or at the nearest infested house before interventions. Bugs collected at 4 and 8 MPS in a persistently infested house were mainly assigned to the source house. These results support the hypothesis of persistent bug populations that survived the insecticide application at local spatial scales, and are consistent with the occurrence of vector control failures most likely related to moderate pyrethroid resistance. Wing geometric morphometry is a useful tool for identifying sources of reinfestation, but it is limited by the spatial structure found in the reference populations. Combined with field and genetic data, this approach may contribute to the understanding of the reinfestation process and improvement of vector control strategies.
Keywords: Triatoma infestans, geometric morphometry, reinfestation, pyrethroid insecticide resistance
1. Introduction
Vector-borne transmission of Trypanosoma cruzi –the etiologic agent of Chagas disease– still represents a public health problem in several Latin American countries, especially in the Gran Chaco region of Argentina, Bolivia and Paraguay where the major vector Triatoma infestans (Klug) persists (Gürtler et al., 2005, 2007; WHO, 2007). One of the main obstacles to the success of some of the ongoing elimination programs of domestic Triatominae vectors of T. cruzi is the reinfestation of houses after insecticide spraying (Cecere et al., 2006, in press; Dujardin et al., 1997; Dumonteil et al., 2004; Gorla et al., 2009; Gurevitz et al., 2012; Gürtler et al., 2007). It is often unclear whether the vectors found after control interventions are derived from residual subpopulations that either survived treatment or re-invaded the area from external sources such as untreated communities or sylvatic foci (Dujardin et al., 1997; Romaña 1963; Schofield, 1988). Distinguishing between these two main hypotheses may have important consequences for the design and implementation of more effective vector control strategies adapted to local settings.
Identifying the source of reinfestant insects usually involves the comparison of specimens captured locally and in sylvatic or neighboring areas before and after control interventions, regardless of whether genetic or phenetic characters are used for such comparison (Dujardin et al., 2007). The aim of this approach is to test the hypothesis that the pre-intervention population is the parental generation of reinfestant insects. The first step in that direction is to assess the level of spatial structuring of vector populations before interventions. Both genetic and phenetic techniques were used to study the spatial structuring of major vector species within Triatominae at widely variable scales (Dumonteil et al., 2007; Gaspe et al., 2012; Hernández et al., 2010; Marcet et al., 2008; Monroy et al., 2003; Pérez de Rosas et al., 2008; Schachter-Broide et al., 2004). Moreover, isoenzyme, microsatellite- and mtDNA-based analyses that sought to identify the putative origins of triatomine bugs collected after insecticide spraying revealed various patterns (Dujardin et al., 1996; Dumonteil et al., 2007; Fitzpatrick et al., 2008; Marcet et al., 2008; Pérez de Rosas et al., 2007).
Traditional head morphometry suggested that T. infestans bugs collected after insecticide spraying were a residual population that survived insecticide treatment in Bolivia (Dujardin et al., 1997). Geometric morphometry has been proposed as an alternative, less costly method based on the assumption that an insect is more similar to its parents than to other conspecifics and is affected by microhabitat features (Dujardin et al., 2007). Using wing geometric morphometry, insectary-reared specimens of Triatoma protracta (Uhler) were assigned to its original parental line with high probabilities (Dujardin et al., 2007), and reinfestant specimens of domestic Rhodnius prolixus Stäl in Venezuela most likely originated from nearby palm trees or from a residual population (Feliciangeli et al., 2007). Microsatellite and mtDNA markers combined with wing geometric morphometry indicated that sylvatic foci of T. infestans in northwestern Argentina were closely related to local domestic or peridomestic populations (Ceballos et al., 2011). To the best of our knowledge, no previous study applied wing geometric morphometry to compare T. infestans populations collected before and after insecticide spraying campaigns at a small geographic scale.
Of the wide variety of peridomestic structures frequently infested with T. infestans, goat and pig corrals were identified as key ecotopes and sources of reinfestant insects in the dry Argentine Chaco (Cecere et al., 2004; Gorla et al., 2009; Gürtler et al., 2004). In contrast, domiciles, kitchens, storerooms and other peridomestic ecotopes used by chickens were the structures most frequently infested before and after insecticide spraying in Pampa del Indio, in the humid Argentine Chaco (Gurevitz et al., 2011, 2012). Wing geometric morphometry analyses detected the existence of spatial structuring of T. infestans populations within 4 km before control interventions (Gaspe et al., 2012). Early house reinfestation with T. infestans after spraying was partially attributed to moderate levels of pyrethroid resistance (Gurevitz et al., 2012), thereby suggesting that post-spraying specimens were survivors of local bug populations. As part of a multi-site study on the eco-epidemiology and control of Chagas disease in the Gran Chaco, here we used wing geometric morphometry to test the main hypotheses expressed above on the putative origins of T. infestans specimens collected after insecticide spraying in a well-defined rural area in Pampa del Indio. Our study, conceived before field bug collections and observed reinfestation patterns, gives further support to the hypothesis that post-spraying specimens mainly originated from local residual foci that survived insecticide treatment.
2. Materials and Methods
2.1. Study area
Fieldwork was conducted in Pampa del Indio (25° 55’S 56° 58’W), Chaco Province, northeastern Argentina. The study area included 353 houses and public buildings grouped in 13 rural villages (Gurevitz et al., 2011). House compounds were composed of human habitations (domiciles or domestic sites) and various peridomestic structures housing domestic animals (ecotopes); a given ecotope at a house compound may have one or more sites. Most domiciles were mud-and-thatch huts with roofs of metal or tarred-cardboard sheets, and occasionally had bricked walls plastered with cement. The district was last treated with residual insecticides by vector control programs approximately in 1996.
Vector collection surveys were conducted at baseline (September-November 2007), immediately after spraying with pyrethroid insecticides all houses (December 2007), and at 4 and 8 MPS as described elsewhere (Gurevitz et al., 2011, 2012). The initial insecticide spraying campaign covered nearly 99% of all houses, both inhabited and uninhabited ones, and all identified sites within the domestic/peridomestic area (Gurevitz et al., 2012). All domestic and peridomestic sites were georeferenced and skilled bug collectors searched them for triatomine bugs using timed manual catches with a dislodging aerosol. In several houses bugs were also collected after the stipulated search time, during insecticide applications, and by householders a few days after the surveys. These additional bug collections were used solely as a qualitative measure of infestation. All foci detected after initial control actions were selectively sprayed with insecticides immediately after the 8 MPS survey. The house-specific prevalence of infestation with T. infestans declined from 46.9% at baseline to 12.0% at 4 MPS (April 2008) and 6.7% at 8 MPS (August 2008) (Gurevitz et al., 2012).
2.2. Insects
Pre-intervention collection sites with at least 10 adult T. infestans of the same sex were selected for analysis of the spatial structuring of bug populations using wing geometric morphometry (Gaspe et al., 2012). For present purposes we included males and females collected at >88% of infested houses containing adults at 4 MPS. In practice, the analyses were stratified according to baseline infestation levels (as determined by any method): a) apparently non-infested houses (no T. infestans collected); b) low-density infestations (with <10 nymphs or adult bugs collected), and c) high-density infestations (≥10 nymphs or adults collected).
One special case study was a house (CT09) found highly infested at baseline, 4 and 8 MPS. A sample of the bugs collected at a single site (a dilapidated small chapel used as a chicken coop) was included in the pre-spraying sample. All males and females collected at 4 and 8 MPS at different collection sites within the house compound were also analyzed, and the percentage of post-spraying insects assigned to the corresponding reference group computed.
2.3. Metric data
The wings were mounted between microscope slides and cover slips as described in Schachter-Broide et al. (2004). Photographs of each pair of wings were taken using a digital camera (Sony MVC-CD300, US) and a stereo-microscope (Zeiss SV11, Germany). We used the 10 type-I landmarks (vein intersections) identified for T. infestans wings, according to Bookstein (1990), as described elsewhere (Schachter-Broide et al., 2004). Only right wings were included in the analyses to avoid pseudoreplication.
2.4. Size variation
Centroid size variations between pre- and post-spraying samples were tested using non-parametric tests (1000 permutations).
2.5. Shape variation
Shape variables (partial warps, PW) were obtained using the Generalized Procrustes Analysis superimposition algorithm (Rohlf, 1996). To avoid having small sample sizes relative to the number of variables, we used a restricted representation of shape (i.e., a set of principal components known as relative warps) derived from shape variables.
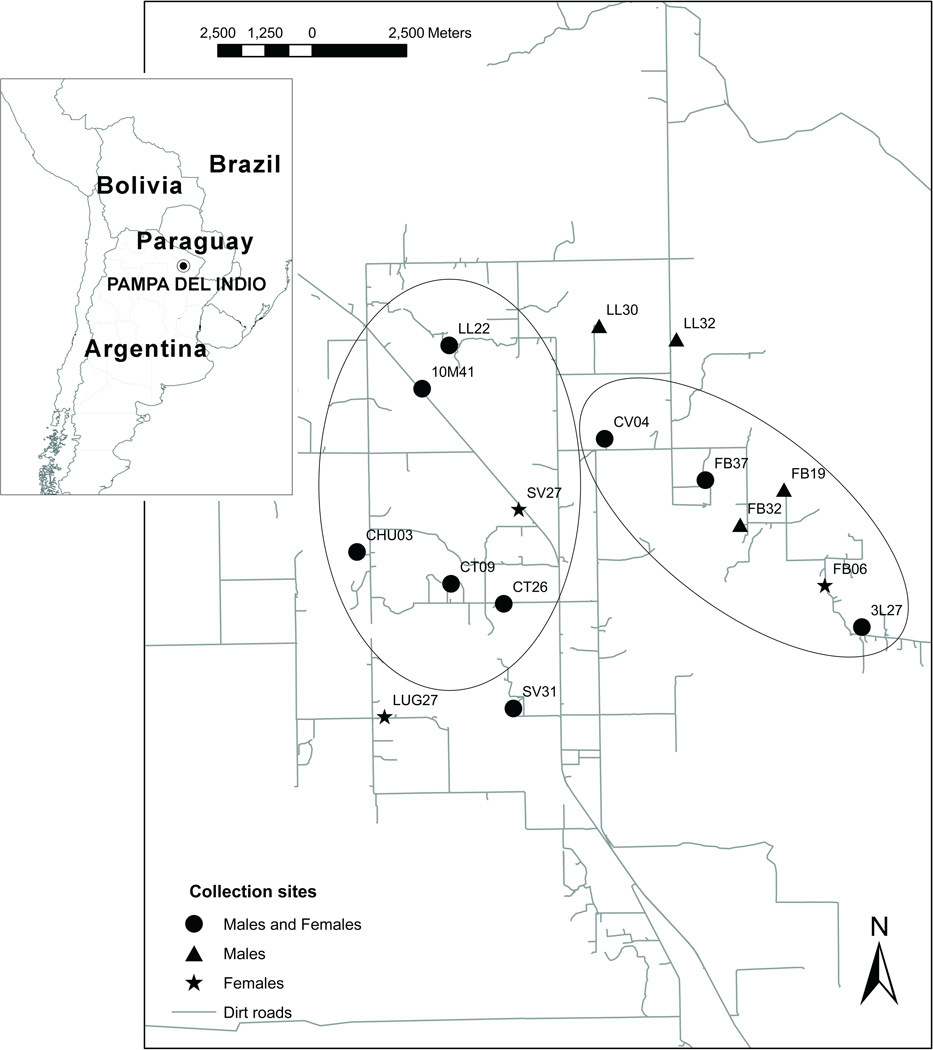
The analyses of pre-spraying bug populations showed the occurrence of two groups that included collections sites located less than 4 km apart. Collection sites within each group presented no significant differences in Mahalanobis distances (in males) whereas shape variables differed between groups (Gaspe et al., 2012) (Fig. 1). These groups included 165 males and 111 females, and were used as reference groups to assign post-spraying specimens. To verify the quality of the reclassification analysis and to assess quantitatively the confidence levels of post-spraying assignments, a crosschecked classification of males and females in both reference groups was performed: each individual was removed, treated as external data, and then reassigned to one of the groups. The percentage of correctly-assigned specimens was computed for both reference groups.
Figure 1.
Pre-spraying reference groups. Map of the study area showing the pre-spraying groups of T. infestans identified with wing geometric morphometry. These reference groups were used for the assignment of post-intervention specimens. The letters and numbers in the map refer to the ID of the house were the insects analyzed were collected. Black circles: houses with males and females included in the analyses. Black triangles: houses with only males included in the analyses. Black stars: houses with only females included in the analyses.
Post-spraying specimens were entered one by one to the discriminant analysis of pre-spraying samples and assigned to the reference group with which they had the shortest Mahalanobis distance. Each individual classification was performed on shape variables computed from the total of reference specimens plus the individual to be classified; thus, for each classification, shape variables of the reference specimens were recomputed after adding only one post-spraying individual (Dujardin et al., 2010; Dujardin & Kitthawee, 2012). When partial warps are used, the one-by-one procedure avoids distorting shape variations between reference groups. If many unknown individuals were entered at once, their influence on the consensus is increased and shape discrimination between reference groups may be distorted. We estimated the percentage of specimens assigned to the reference group in which its house was included. When post-spraying bugs occurred in houses that had not been included in the pre-spraying sample, the nearest infested house was taken as the reference group. Metric disparity was computed as an alternative way to compare pre- and post-spraying samples.
2.6. Allometry
The influence of size on shape was verified through multivariate regression and permutation tests for statistical significance (Good, 2000). When the allometric residue was found significant, the common allometric model hypothesis was investigated via a MANCOVA based on Wilk's statistic (Dujardin et al., 2007).
2.7. Software
TPSdig2 (version 2.09) was used for landmark digitalization; MOG for Procrustes superimposition, generation of PW, assignment of “unknown specimens”, and validated reclassification tests; VAR for non-parametric comparisons of centroid size; COV for examination of residual allometry within shape variables, common-allometry model tests, and metric disparity analysis. TpsDig2 was developed by F. J. Rohlf and is available at www.life.bio.sunysb/morpho. The modules MOG, VAR, COV developed by J.P.D. are included in the CLIC package available at mome-clic.com
3. Results
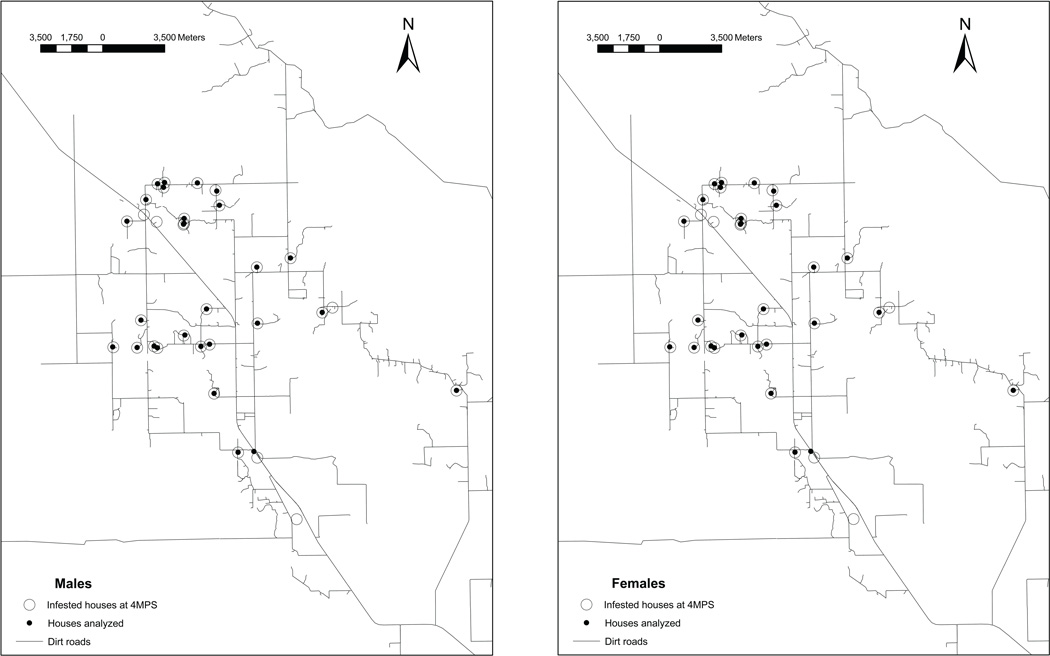
A total of 63 males (from 24 sites) and 54 females (from 33 sites) collected after full-coverage insecticide spraying were included in wing geometric morphometry analyses (Table 1). These bugs had been collected in 88% (21 of 24) and 91% (30 of 33) of all the houses infested at 4 MPS that had adult bugs captured, respectively (Fig. 2). More than 60% of all males and females collected at 4 MPS were analyzed for wing geometric morphometry.
Table 1.
Percentage of post-spraying T. infestans bugs assigned to the reference group that included the source house of each individual bug or the nearest infested house, according to bug stage and pre-spraying house infestation level.
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Pre- spraying infestation |
N° of houses |
N° of insects analyzed |
% of insects assigned |
N° of houses |
N° of insects analyzed |
% of insects assigned |
| Negative | 4 | 4 | 75 | 4 | 4 | 50 |
| Low abundance |
5 | 8 | 75 | 5 | 7 | 29 |
| High abundance |
12 | 51 | 69 | 21 | 43 | 58 |
| Totals |
21 | 63 | 70 | 30 | 54 | 54 |
Figure 2.
Post-spraying infested houses. Map of the study area showing houses infested with T. infestans at 4MPS. White circles: infested houses with presence of adult bugs. Black circles: houses included in post-spraying analyses. A. Males. B. Females.
3.1. Size variation
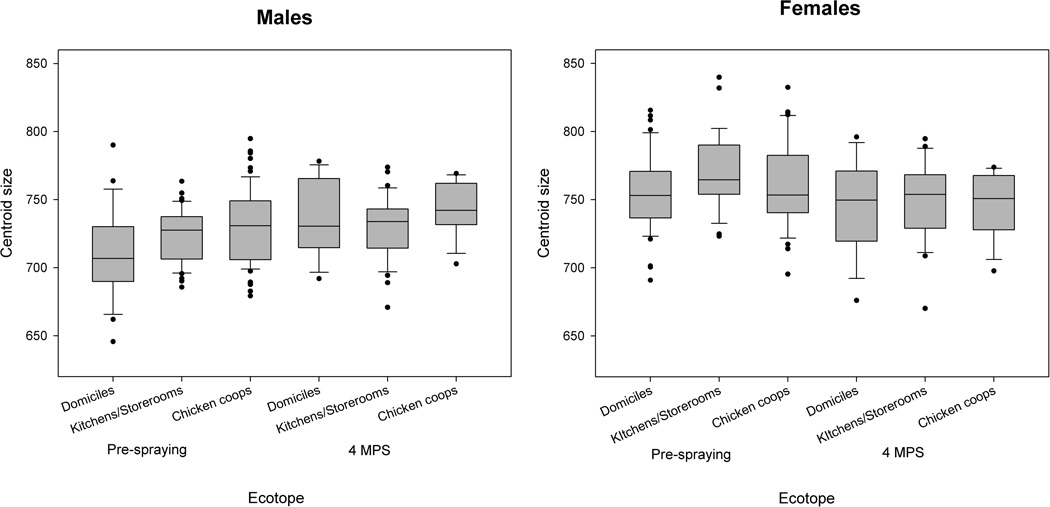
Pre-spraying males and females had significantly smaller CS than those collected at 4 MPS (P = 0.03, P = 0.007, respectively), whereas its variance did not differ significantly between surveys (P > 0.1). When comparisons were stratified by ecotope, males collected at 4 MPS had significantly larger wings than pre-spraying males only in domestic sites (P = 0.01) (Fig. 3).
Figure 3.
Size variation. Variation of wing centroid size of male and female T. infestans between ecotopes and collection dates in Pampa del Indio. Each box shows the median and the first and third quartiles.
In house CT09, males collected at 4 MPS had significantly larger wing centroid size than those collected at baseline and at 8 MPS. No significant differences were found in wing size between males collected at 8 MPS and pre-spraying samples, nor were they found in female centroid size.
3.2. Shape variation
The cross-checked classification of pre-intervention specimens in both reference groups showed that 73–77% of males and 65–67% of females were correctly reclassified. Similar percentages of reclassification were obtained when both shape and size variables were included in the cross-checked classification.
Comparison of pre-spraying and 4 MPS samples showed that, on average, 70% of the males examined could not be differentiated from males collected at houses from the reference group where either the same (source) house or the nearest infested house before interventions were included (Table 1). The percentage of assignment varied slightly with the intensity of house infestation before interventions. More than 80% of the post-spraying males analyzed originated from highly infested houses, and 55% of them came from house CT09 (see below). We found no apparent association between unassigned individuals and bug collection ecotope.
On average, 54% of the female bugs analyzed were assigned to the reference group in which either the source house or the nearest infested house were included (Table 1). The majority (80%) of post-spraying females examined were collected from houses that highly infested before interventions.
Metric disparity was similar in all female bug populations (P > 0.05), whereas males collected at 4 MPS were significantly more disparate than pre-spraying males (P = 0.039).
In house CT09, a large percentage of the specimens collected after interventions (67% of females and 82% of males) was assigned to the reference group that included the source house CT09 (Table 2). These percentages were higher at 4 MPS than at 8 MPS, and in males than in females. Regarding post-spraying bugs collected at a single collection site (small chapel), the fraction of males assigned to the reference group at 4 MPS (67%) was higher than at 8 MPS (48%). Moreover, shape variables were significantly different only among pre-spraying and 8 MPS samples.
Table 2.
Percentage of male and female T. infestans from house CT09 assigned to the (pre-spraying) reference group where the source house was included, according to time elapsed after insecticide spraying and bug collection site.
| Males | Females | ||||
|---|---|---|---|---|---|
| Months after spraying |
Collection Site |
N° of insects analyzed |
% of assigned insects |
N° of insects analyzed |
% of assigned insects |
| Four | Latrine | 13 | 100 | 2 | 100 |
| Chapel | 15 | 67 | 7 | 57 | |
| Total | 28 | 82 | 9 | 67 | |
| Eight | Latrine | 3 | 67 | 0 | − |
| Chapel | 11 | 27 | 6 | 50 | |
| Storeroom | 1 | 0 | 0 | − | |
| Kitchen | 5 | 100 | 1 | 100 | |
| Domicile | 1 | 0 | 4 | 25 | |
| Total | 21 | 48 | 11 | 45 | |
3.3. Allometry
Shape variation between pre-spraying and 4 MPS samples was significantly related to size variation both in females (P = 0.002) and males (P < 0.001). This allowed us to compare the allometric trends between samples collected before spraying and at 4 MPS for each sex. The model of a common allometric trend was not rejected (females: Wilks’ lambda = 0.85, P = 0.09; males, Wilks’ lambda = 0.93, P = 0.67). In house CT09, no residual allometry was found in males, implying that the analyses performed on shape variables were not influenced by any size effect.
4. Discussion
Our study gives further support to the hypothesis that reinfestant specimens mainly originated from local residual foci that survived insecticide treatment. The present study has unprecedented levels of spatial detail at bug collection site and extent up to the meso-scale, and included a large number of infested houses as part of a longitudinal study of >300 rural dwellings from a well-defined area. A relatively large fraction (70–82% of males and 54–67% of females) of the T. infestans bugs collected after full-coverage insecticide spraying could not be differentiated from bugs collected before interventions at the reference group that included the source house of the individual bug or the nearest infested house. Therefore, there is not sufficient evidence to reject the hypothesis that post-spraying specimens were survivors or the offspring of pre-spraying bug populations in a large fraction of the infested sites investigated, especially for males. Although we cannot discount that some of the post-spraying bugs assigned were immigrants from other houses included in the same reference group, they would be immigrant survivors from within the study area given the large spatial scale of control operations. However, bug populations decimated by the insecticidal sprays would contribute with much fewer dispersants. Moreover, if active bug dispersal occurred after initial interventions, it would have been restricted to the time period between insecticide spraying and 4 MPS because temperatures between 4 and 8 MPS (over the fall-winter period) were adverse or highly adverse for bug movement. The morphometry-based results are consistent with other pieces of evidence related to local reinfestation patterns (Gurevitz et al., 2012): i) the occurrence of rather large colonies of T. infestans including late-stage nymphs and adult bugs as early as 4 MPS (residual foci); ii) most (87%) of the houses infested at 4 MPS had also been infested before insecticide spraying; iii) laboratory-based evidence of moderate levels of pyrethroid resistance causing vector control failures as determined by discriminant dose assays, and iv) a field-based experiment demonstrating that an enclosed bug colony survived multiple pyrethroid sprays (with standard and double dose) and finally was suppressed after treatment with malathion. In addition, pyrethroid insecticides have much lower effectiveness in peridomestic sites than in domestic sites, and therefore are more prone to leave peridomestic residual foci in the Argentine Chaco (Cecere et al., 2013).
Both male and female reinfestant specimens from houses apparently not infested before interventions showed high percentages of assignment to the most immediate reference group. This suggests that these bugs may have invaded from neighboring houses or were survivors from undetected, very low-density bug populations at the source house. Conversely, specimens that were not assigned to the reference group in which the source house was included may have originated from houses included in the other reference group; from houses outside the study area (i.e., not included in the baseline sample), or from sylvatic foci. Although sylvatic foci of T. infestans have not been detected in Pampa del Indio yet (Alvarado-Otegui et al., 2012), they occur elsewhere in Bolivia, Argentina, Paraguay and Chile (Bacigalupo et al., 2010; Ceballos et al., 2009, 2011; Dujardin et al., 1987; Noireau et al., 2005; Rolón et al., 2011). Current data do not allow an assessment of alternative sources, as there are no reference groups for external communities and sylvatic bug populations. Another alternative is that bugs not assigned to the reference group were true descendants of the source population and were “wrongly” classified, perhaps related to limitations in the resolution power of wing geometric morphometry. Our approach was not able to differentiate among these hypotheses at the fine spatial scale of the study, which included bug populations not subjected to periodic large perturbations such as professional insecticidal sprays.
The detailed analysis of a house (CT09) persistently infested up to 8 MPS was particularly meaningful, given the evidence on persistent residual foci. Wing geometric morphometry analyses also suggested that the specimens collected in this house after insecticide spraying most likely were survivors or the offspring of the pre-intervention bug population. A relatively large fraction of the adult bugs collected in this house (67% of females and 82% of males) was assigned to the corresponding reference group. The percentage of specimens assigned to the source house decreased from 4 to 8 MPS as a new generation of bugs developed. These differences can be more likely attributed to the effects of the time elapsed between bug collection dates rather than to seasonal effects which are expected to affect bug size more than shape (Schachter-Broide et al., 2009). Dujardin, Beard & Ryckmann (2007) showed that the assignment of the first generation of adult T. protracta to the parental (founder) generation was stronger than that displayed by successive generations reared in the insectary.
Some of the CT09 bugs not assigned to the reference group perhaps may have invaded from other sources. However, the nearest house found infested at 4 MPS was 1 km apart (i.e., within the estimated flight range of T. infestans, which ranges up to 1.5-2.5 km), and the only female bug collected there was assigned to the same reference group as insects from house CT09. Another possible explanation for the imperfect assignment recorded is related to the composition of bug samples. All the adult bugs captured at 4 or 8 MPS from different collection sites of house CT09 were analyzed, whereas only 40–54% of the adults collected at baseline in a single site (old chapel) were included. If the latter sample had failed to capture all the existing variability in the bug populations from this specific house compound, some post-spraying bugs would not be assigned correctly to the reference group. Increasing bug sample sizes may provide enhanced discriminant power.
The fairly good results of the cross-checked classification of bugs in the reference groups support its use for assignment of post-spraying bugs. Over all occasions and intensities of house infestations investigated, the percentages of assignment were slightly higher for males than for females and cross-checked classifications were also higher in males. These patterns are consistent with male populations having stronger spatial structuring than females before interventions (Gaspe et al., 2012). Moreover, the significant differences in male wing centroid size and metric disparity between 4 MPS and pre-spraying samples were most likely associated; changes in metric disparity could be an allometric effect caused by significant size variations. The latter supports the occurrence of between-survey differences in male populations. In spite of these results, the lack of differentiation in the allometric model suggests the absence of isolation. Moreover, size differences found between 4 MPS samples (fall) and pre-intervention (spring) or 8 MPS (winter) samples at house CT09 support the hypothesis of seasonal effects on wing size. Similarly, another study conducted elsewhere in the dry Chaco also found significant differences in wing centroid size between seasons, with spring males (October) being smaller than end-of-summer males (March-April) (Schachter-Broide et al., 2009).
The ability of wing geometric morphometry to identify the putative origins of bugs collected after control interventions depends on the level of spatial structuring of the reference (pre-intervention) populations. In our study area, pre-spraying adult bugs from sites distant less than 4 km could not be differentiated through wing geometric morphometry (Gaspe et al., 2012). Elsewhere in northwestern Argentina, both wing geometric morphometry and microsatellite markers showed consistent within-village spatial structuring of bug populations (Marcet et al., 2008; Schachter-Broide et al., 2004), which coincided with the occurrence of two independent reinfestation patterns over a five-year period (Cecere et al., 2004). These study sites had been under recurrent insecticide sprays that generated highly structured vector populations, unlike in Pampa del Indio. Determining the exact source of reinfestant specimens may be more difficult when efforts are focused on small geographic areas and involve relatively undisturbed bug populations, as in the current study. More powerful genetic markers may be needed to identify the sources of reinfestant bugs at fine spatial scales.
4.1 Conclusions
Most of the specimens collected after interventions most likely were survivors of the insecticide spray at the source house (including the offspring of preexisting bugs) or came from houses located less than 4 km apart, or a combination of these processes operating at a local scale. These results indicate a local source of infestation in the majority of the infested sites investigated (i.e., not invasion from communities external to the treated study villages). Improved residual application of insecticides (i.e., type of insecticide, dose, spray gear, coverage), together with environmental management, housing improvement and community participation, are needed for improved vector and disease control in the Gran Chaco.
Highlights.
The putative origin of post-spraying bugs was assessed by wing geometric morphometry
Post-spraying specimens were mainly originated from local residual foci
These results are consistent with other evidence based on local reinfestation patterns
Wing geometric morphometry may contribute to the understanding of the reinfestation process and improvement of vector control strategies.
Acknowledgements
We thank Fundación Mundo Sano for long-term support at the study site, Jorge Nasir, the Chagas Control Program of Chaco and the National Vector Control Coordination for support in field operations; and Judith Schachter-Broide, Ana Laura Carbajal de la Fuente, Romina Piccinali and Yael Provecho for helpful advice. This study was supported by awards from the International Development Research Center and Tropical Disease Research (UNICEF/PNUD/WB/WHO) to R.E.G., and by National Institutes of Health/National Science Foundation Ecology of Infectious Disease program award R01 TW05836 funded by the Fogarty International Center and the National Institute of Environmental Health Sciences to U.K., R.E.G., and Joel Cohen. This study was approved by IRB 00001678 (National Institutes of Health registered). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. RE.G. is member of the CONICET Researcher Career.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarado-Otegui JA, Ceballos LA, Orozco MM, Enríquez GF, Cardinal MV, Schijman AG, Kitron U, Gürtler RE. The sylvatic transmission cycle of Trypanosoma cruzi in the humid Chaco of Argentina. Acta Trop. 2012;124:79–86. doi: 10.1016/j.actatropica.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacigalupo A, Torres-Pérez F, Segovia V, García A, Correa JP. Sylvatic foci of the Chagas disease vector Triatoma infestans in Chile: description of a new focus and challenges for control programs. Mem. Inst. Oswaldo Cruz. 2010;105:633–641. doi: 10.1590/s0074-02762010000500006. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. Introduction to methods for landmark data. In: Rohlf FJ, Bookstein FL, editors. Proceedings of the Michigan Morphometrics Workshop, The University of Michigan Museum of Zoology, Special Publication No. 2. Ann Arbor: Michigan; 1990. pp. 216–225. [Google Scholar]
- Ceballos LA, Piccinali RV, Berkunsky I, Kitron U, Gürtler RE. First finding of melanic sylvatic Triatoma infestans (Hemiptera: Reduviidae) in the Argentine Chaco. J. Med. Entomol. 2009;46:1195–1202. doi: 10.1603/033.046.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos LA, Piccinali RV, Marcet PL, Vazquez-Prokopec GM, Cardinal MV, Schachter-Broide J, Dujardin JP, Schijman AG, Dotson EM, Kitron U, Gürtler RE. Hidden sylvatic foci of the main vector of Chagas disease Triatoma infestans: threats to the vector elimination campaign? PLoS Negl. Trop. Dis. 2011;5:e1349. doi: 10.1371/journal.pntd.0001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecere MC, Vazquez-Prokopec GM, Gürtler RE, Kitron U. Spatiotemporal analysis of reinfestation by Triatoma infestans (Hemiptera: Reduviidae) following insecticide spraying in a rural community in northwestern Argentina. Am. J. Trop. Med. Hyg. 2004;71:803–810. [PMC free article] [PubMed] [Google Scholar]
- Cecere MC, Vazquez-Prokopec GM, Gürtler RE, Kitron U. Reinfestation sources for Chagas disease vector, Triatoma infestans, Argentina. Emerg. Inf. Dis. 2006;12:1096–1102. doi: 10.3201/eid1207.051445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecere MC, Vazquez-Prokopec GM, Ceballos LA, Boragno S, Zárate JE, Kitron U, Gürtler RE. Improved chemical control of Chagas disease vectors in the dry Chaco region. J. Med. Entomol. 2013;50 doi: 10.1603/me12109. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin JP, Tibayrenc M, Venegas E, Maldonado L, Desjeux P, Ayala FJ. Isozyme evidence of lack of speciation between wild and domestic Triatoma infestans (Heteroptera: Reduviidae) in Bolivia. J. Med. Entomol. 1987;24:40–45. doi: 10.1093/jmedent/24.1.40. [DOI] [PubMed] [Google Scholar]
- Dujardin JP, Cardozo L, Schofield CJ. Genetic analysis of Triatoma infestans following insecticidal control interventions in central Bolivia. Acta Trop. 1996;61:263–266. doi: 10.1016/0001-706x(96)00008-3. [DOI] [PubMed] [Google Scholar]
- Dujardin JP, Bermúdez H, Schofield CJ. The use of morphometrics in entomological surveillance of sylvatic foci of Triatoma infestans in Bolivia. Acta Trop. 1997;66:145–153. doi: 10.1016/s0001-706x(97)00038-7. [DOI] [PubMed] [Google Scholar]
- Dujardin JP, Beard CB, Ryckman R. The relevance of wing geometry in entomological surveillance of Triatominae, vectors of Chagas disease. Inf. Gen. Evol. 2007;7:161–167. doi: 10.1016/j.meegid.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Dujardin JP, Kaba D, Henry AB. The exchangeability of shape. BMC Res Notes. 2010;22:266. doi: 10.1186/1756-0500-3-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin JP, Kitthawee S. Phenetic structure of two Bactrocera tau cryptic species (Diptera: Tephritidae) infesting Momordica cochinchinensis (Cucurbitaceae) in Thailand and Laos. Zoology (Jena) 2013 doi: 10.1016/j.zool.2012.07.004. in press. [DOI] [PubMed] [Google Scholar]
- Dumonteil E, Ruiz-Piña H, Rodríguez-Félix E, Barrera-Pérez M, Ramírez-Sierra MJ, Rabinovich JE, Menu F. Re-infestation of houses by Triatoma dimidiate after intradomicile insecticide application in the Yucatán peninsula, Mexico. Mem. Inst. Oswaldo Cruz. 2004;99:253–256. doi: 10.1590/s0074-02762004000300002. [DOI] [PubMed] [Google Scholar]
- Dumonteil E, Tripet F, Ramírez-Sierra MJ, Payet V, Lanzaro G, Menu F. Assessment of Triatoma dimidiata dispersal in the Yucatán peninsula of Mexico by morphometry and microsatellite markers. Am. J. Trop. Med. Hyg. 2007;79:930–937. [PubMed] [Google Scholar]
- Feliciangeli MD, Sanchez-Martin M, Marrero R, Davies C, Dujardin JP. Morphometric evidence for a possible role of Rhodnius prolixus from palm trees in house re-infestation in the State of Barinas (Venezuela) Acta Trop. 2007;101:169–177. doi: 10.1016/j.actatropica.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick S, Feliciangeli MD, Sanchez-Martin MJ, Monteiro FA, Miles MA. Molecular genetics reveal that silvatic Rhodnius prolixus do colonise rural houses. PLoS Negl. Trop. Dis. 2008;2:e210. doi: 10.1371/journal.pntd.0000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspe MS, Schachter-Broide J, Gurevitz JM, Kitron U, Gürtler RE, Dujardin JP. Microgeographic spatial structuring of Triatoma infestans (Hemiptera: Reduviidae) populations using wing geometric morphometry in the Argentine Chaco. J. Med. Entomol. 2012;49:504–514. doi: 10.1603/me11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorla DE, Porcasi X, Hrellac H, Catalá SS. Spatial stratification of house infestation by Triatoma infestans in La Rioja, Argentina. Am. J. Trop. Med. Hyg. 2009;80:405–409. [PubMed] [Google Scholar]
- Good P. Permutation tests: a practical guide to resampling methods for testing hypotheses. New York: Springer; 2000. [Google Scholar]
- Gurevitz JM, Ceballos LA, Gaspe MS, Alvarado-Otegui JA, Enriquez GF, Kitron U, Gürtler RE. Factors affecting infestation by Triatoma infestans in a rural area of the humid Chaco in Argentina. PLoS Negl. Trop. Dis. 2011;5:e1365. doi: 10.1371/journal.pntd.0001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevitz JM, Gaspe MS, Enríquez GF, Vassena CV, Alvarado-Otegui JA, Provecho YM, Mougabure Cueto GA, Picollo MI, Kitron U, Gürtler RE. Unsuspected pyrethroid resistance and control failures of Chagas disease vector in Argentina. J. Med. Entomol. 2012;49:1379–1386. doi: 10.1603/me11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler RE, Canale DM, Spillman C, Stariolo R, Salomon OD, Blanco S. Effectiveness of residual spraying of peridomestic ecotopes with deltamethrin and permethrin on Triatoma infestans in rural western Argentina: a district-wide randomized trial. Bull. World Health Organ. 2004;82:196–205. [PMC free article] [PubMed] [Google Scholar]
- Gürtler RE, Cecere MC, Lauricella MA, Petersen RM, Canale D, Castañera MB, Chuit R, Segura EL, Cohen JE. Incidence of Trypanosoma cruzi infection among children following domestic reinfestation after insecticide spraying in rural northwestern Argentina. Am. J. Trop. Med. Hyg. 2005;73:95–103. [PMC free article] [PubMed] [Google Scholar]
- Gürtler RE, Kitron U, Cecere MC, Segura EL, Cohen JE. Sustainable vector control and management of Chagas disease in the Gran Chaco, Argentina. Proc. Natl. Acad. Sci. USA. 2007;104:16194–16199. doi: 10.1073/pnas.0700863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández ML, Abraham LB, Dujardin JP, Gorla DE, Catalá SS. Phenotypic variability and population structure of peridomestic Triatoma infestans in rural areas of the arid Chaco (western Argentina): spatial influence of macro- and microhabitats. Vector-borne Zoonotic Dis. 2010;11:503–513. doi: 10.1089/vbz.2009.0253. [DOI] [PubMed] [Google Scholar]
- Marcet PL, Mora MS, Cutrera AP, Jones L, Gürtler RE, Kitron U, Dotson EM. Genetic structure of Triatoma infestans populations in rural villages of Santiago del Estero, northern Argentina. Inf. Gen. Evol. 2008;8:835–846. doi: 10.1016/j.meegid.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy C, Bustamante DM, Rodas A, Rosales R, Mejía M, Tabaru Y. Geographic distribution and morphometric differentiation of Triatoma nitida Usinger 1939 (Hemiptera: Reduviidae: Triatominae) in Guatemala. Mem. Inst. Oswaldo Cruz. 2003;98:37–43. doi: 10.1590/s0074-02762003000100006. [DOI] [PubMed] [Google Scholar]
- Noireau F, Cortez MG, Monteiro F, Jansen AM, Torrico F. Can wild Triatoma infestans foci in Bolivia jeopardize Chagas disease control efforts? Trends Parasitol. 2005;21:7–10. doi: 10.1016/j.pt.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Pérez de Rosas AR, Segura EL, García BA. Microsatellite analysis of genetic structure in natural Triatoma infestans (Hemiptera: Reduviidae) populations from Argentina: its implication in assessing the effectiveness of Chagas’ disease vector control programmes. Mol. Ecol. 2007;16:1401–1412. doi: 10.1111/j.1365-294X.2007.03251.x. [DOI] [PubMed] [Google Scholar]
- Pérez de Rosas AR, Segura EL, Fichera L, García BA. Macrogeographic and microgeographic genetic structure of the Chagas’ disease vector Triatoma infestans (Hemiptera: Reduviidae) from Catamarca, Argentina. Genetica. 2008;133:247–260. doi: 10.1007/s10709-007-9208-8. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ. Morphometric spaces, shape components and the effects of linear transformations. In: Marcus LF, Corti M, Loy A, Naylor GJP, Slice D, editors. Advances in Morphometrics. New York: NATO ASI, Series A, Life Sciences, Plenum Publication; 1996. pp. 117–129. [Google Scholar]
- Rolón M, Vega MC, Román F, Gómez A, Rojas de Arias A. First report of colonies of sylvatic Triatoma infestans (Hemiptera: Reduviidae) in the Paraguayan Chaco, using a trained dog. PLoS Negl. Trop. Dis. 2011;5:e1026. doi: 10.1371/journal.pntd.0001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaña CA. In: Enfermedad de Chagas. López Libreros., editor. Buenos Aires: 1963. [Google Scholar]
- Schachter-Broide J, Dujardin JP, Kitron U, Gürtler RE. Spatial structuring of Triatoma infestans (Hemiptera, Reduviidae) populations from northwestern Argentina using wing geometric morphometry. J. Med. Entomol. 2004;41:643–649. doi: 10.1603/0022-2585-41.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter-Broide J, Gürtler RE, Kitron U, Dujardin JP. Temporal variations of wing size and shape of Triatoma infestans (Hemiptera: Reduviidae) populations from northwestern Argentina using geometric morphometry. J. Med. Entomol. 2009;46:994–1000. doi: 10.1603/033.046.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield CJ. The biosystematics of the Triatominae. In: Service MV, editor. Biosystematics of haematophagous insects. Oxford: Clarendon Press; 1988. pp. 284–312. [Google Scholar]
- WHO [World Health Organization] Reporte sobre la enfermedad de Chagas. 2007 TDR/SWG/09.