Abstract
AIM: To investigate the incidence of neoplasms in inflammatory bowel disease (IBD) patients and the potential causative role of thiopurines.
METHODS: We performed an observational descriptive study comparing the incidence of malignancies in IBD patients treated with thiopurines and patients not treated with these drugs. We included 812 patients which were divided in two groups depending on whether they have received thiopurines or not. We have studied basal characteristics of both groups (age when the disease was diagnosed, sex, type of IBD, etc.) and treatments received (Azathioprine, mercaptopurine, infliximab, adalimumab or other immunomodulators), as well as neoplasms incidence. Univariate analysis was performed with the student t test, χ2 test or Wilcoxon exact test as appropriate. A logistic regression analysis was performed as multivariate analysis. Statistical significance was establish at P values of less than 0.05, and 95%CI were used for the odds ratios.
RESULTS: Among 812 patients included, 429 (52.83%) have received thiopurines: 79.5% azathioprine, 14% mercaptopurine and 6.5% both drugs. 44.76% of patients treated with thiopurines and 46, 48% of patients who did not receive this treatment were women (P > 0.05). The proportion of ulcerative colitis patients treated with thiopurines was 30.3% compare to 66. 67% of patients not treated (P < 0.001). Mean azathioprine dose was 123.79 ± 36.5 mg/d (range: 50-250 mg/d), mean usage time was 72.16 ± 55.7 mo (range: 1-300 mo) and the accumulated dose along this time was 274.32 ± 233.5 g (1.5-1350 g). With respect to mercaptopurine, mean dose was 74.7 ± 23.9 mg/d (range: 25-150 mg/d), mean usage time of 23.37 ± 27.6 mo (range: 1-118 mo), and the accumulated dose along this time was 52.2 ± 63.5 g (range: 1.5-243 g). Thiopurine S-methyltransferase activity was tested in 66% of patients treated with thiopurines, among which 98.2% had an intermediate or high activity. Among the patients treated with thiopurines, 27.27% (112 patients) and 11.66% (50 patients) received treatment with Infliximab and Adalimumab respectively, but only 1.83% (7 patients) and 0.78% (3 patients) received these drugs in the group of patients who did not received thiopurines (P < 0.001 and P < 0.001 respectively). Finally, 6.8% (29 patients) among those treated with thiopurines have received other immunesupresants (Methotrexate, Tacrolimus, Cyclosporin), compare to 1% (4 patients) of patients not treated with thiopurines (P < 0.001). Among patients treated with thiopurines, 3.97% developed a malignancy, and among those not treated neoplasms presented in 8.1% (P = 0.013). The most frequent neoplasms were colorectal ones (12 cases in patients not treated with thiopurines but none in treated, P < 0.001) followed by non-melanoma skin cancer (8 patients in treated with thiopurines and 6 in not treated, P > 0.05).
CONCLUSION: In our experience, thiopurine therapy did not increase malignancies development in IBD patients, and was an efective and safe treatment for these diseases.
Keywords: Malignancy, Neoplasm, Inflammatory bowel disease, Crohn’s disease, Ulcerative colitis, Thiopurines, Azathioprine, Mercaptopurine
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic disorder which is characterised by episodes of inflammatory activity alternated with episodes of remission of this inflammation. Ethiology is mostly unknown, and there is no curative treatment, being the goal of current therapies to maintain patients in remission.
Among the drugs used for the treatment of this disease there are corticoids, immunomodulators (azathioprine, mercaptopurine, cyclosporine or tacrolimus) and biological therapies (infliximab and adalimumab) which interfere in the altered inflammatory and immunological process of these patients in order to induce and maintain clinical remission. Given the usefulness of these drugs for treating this disease, their relative safety with regards to side effects and the tendency to be more strict in symptoms control (top-down vs set-up tendency), immunomodulators and biological therapies have become drugs extensively used in this field. However, these treatments imply new challenges, as their hypothetical ability to induce neoplastic diseases, determined by their interference with the immune response which could limit its ability to control dysplastic cells, favouring the development of tumours. Indeed, Azathioprine has been classified as carcinogenic by the International Agency for Research on Cancer. Regarding this relationship between thiopurines and neoplastic diseases, several studies dealing with this adverse effect[1] have been published.
Thiopurines are drugs known to be antiproliferative given that their main effect is that they prevent the proliferation of T lymphocytes, promoting the incorporation of thiopurine analogues into DNA, which prevents the synthesis of purine nucleotides[2], as well as blocking several enzymes involved in the synthesis of DNA, RNA and proteins. All of those mechanisms block the proliferation and functions of the lymphocytes, inhibiting the synthesis of antibodies, and reducing the number of circulating monocytes and granulocytes, which is more evident in tissues and situations with a high cellular turnover.
The presence of thiopurine analogues in the DNA not only alters its structure by preventing cellular proliferation, but also increases the risk of mutagenesis[3-6]. This theoretic possibility becomes more evident in recent studies which describe an increased number of somatic mutations in circulating leukocytes in patients treated with thiopurines in comparison to patients who are not treated[7], more so, the number of mutations is proportional to the dose and duration of the treatment with thiopurines.
Also, this structurally altered DNA becomes more sensitive to radiation, especially ultraviolet radiation (UVA), which creates reactive oxygen species with the potential to modify genetic material and nearby proteins[2,8-11]. Hypothetically, those events determine an increased susceptibility neoplasms. For instance, we find the highest number of non-melanoma skin cancer cases in patients with thiopurines prescribed as immunosuppressants in organ transplant[12]. Other studies have suggested a relation between the development of malignancies and the total thiopurine doses, its metabolite levels and thiopurine S-methyltransferase (TPMT) mutations[13-17].
Therefore, with the aim of review the incidence of thiopurine induced neoplasms in our IBD patients and treated with thiopurines at the University Hospital Virgen de las Nieves (Granada, Spain), we designed a retrospective observational study, comparing our results with previously published papers.
MATERIALS AND METHODS
An observational, retrospective study, analysing data from patients with a confirmed IBD diagnosis from 1996 to the present was designed. Data were collected from the electronic clinical charts of each patient, as well as from our local database. We included demographical variables and specific data such as TPMT activity, use of thiopurines (azathioprine and mercaptopurine), as well as other immunosuppressants and biological agents. We also studied the appearance of neoplastic diseases, age of diagnosis and type of disease.
Statistical analysis
Resulting variables were analysed by means of SPSS 18 software (Chicago, IL, United States). Univariate analysis was performed with the χ2 test, Fisher exact test, Student t test or Wilcoxon as appropriate, we used 95%CI for de odds ratios and statistical signification was considered when P < 0.05.
Multivariable logistic-regression analyses involving treatment regimen and prespecified baseline characteristics, which were the ones with statistical significance in univariate analyses and also the factors with a potential or already recognized influence on neoplasm development, were performed to evaluate the risk of malignancies. A stepwise procedure was used to identify independent risk factors for neoplasm development (with P = 0.05 as the threshold level for variables to be entered into the model and retained in the final model).
An online pubmed search was performed with the terms “thiopurines”, “azathiopurine”, “mercaptopurine”, “neoplasm”, “malignancy”, “inflammatory bowel disease”, “ulcerous colitis (UC)” and “Crohn’s disease (CD)”, in order to review published data on thiopurines, neoplasms and IBD.
RESULTS
Eight hundred and twelve patients with confirmed IBD diagnosis were included; 48.4% had UC and 45.6% were women (Table 1). The average age of diagnosis was 35.23 ± 16.5 years (range: 5-85 years), 34.99 ± 16.7 years for females (range: 5-85 years) and 35.43 ± 16.4 years (range: 5-82 years) for males.
Table 1.
Patients' characteristics n (%)
| Thiopurines (n = 429) | No thiopurines (n = 383) | P value | |
| Age of diagnosis of IBD (yr) | 30.85 | 40.13 | < 0.001 |
| Sex | |||
| Female | 192 | 178 | > 0.050 |
| Male | 237 | 205 | > 0.050 |
| Ulcerative colitis1 | n = 130 | n = 263 | < 0.001 |
| Extension | |||
| E1 | 5 (3.85) | 51 (19.4) | |
| E2 | 42 (32.3) | 103 (39.2) | |
| E3 | 83 (63.85) | 109 (41.4) | |
| Severity | |||
| S1 | 51 (39.2) | 150 (57) | |
| S2 | 58 (44.6) | 102 (38.8) | |
| S3 | 21 (16.2) | 11 (4.2) | |
| Crohn's disease2 | n = 299 | n = 120 | < 0.001 |
| Age of diagnosis (yr) | |||
| A1 | 33 (11) | 6 (5) | |
| A2 | 214 (71.6) | 68 (56.7) | |
| A3 | 52 (17.4) | 46 (38.3) | |
| Location | |||
| L1 | 137 (45.8) | 46 (38.3) | |
| L2 | 54 (18) | 34 (28.3) | |
| L3 | 96 (32.1) | 39 (32.5) | |
| L4 | 12 (4.1) | 1 (0.8) | |
| Behaviour | |||
| B1 | 155 (51.8) | 105 (87.5) | |
| B2 | 55 (18.4) | 10 (8.3) | |
| B3 | 89 (29.8) | 5 (4.2) | |
| P | 73 (24.4) | 15 (12.5) | |
| Neoplasms | n = 17 | n = 31 | 0.013 |
Patients with ulcerative colitis treated with thiopurines had more severity and disease extension;
Patients with Chron's disease treated with thiopurines more usually had ileal or ileocholic location and an inflammatory or penetrating behaviour; Patients under thiopurine treatment had a lower age at diagnosis. IBD: Inflammatory bowel disease.
Among CD patients (Table 1), 67.3% had been diagnosed between 16 to 40 years (A2 in Montreal classification), the most frequent location (43.7%) was ileal (L3 of Montreal classification) (Table 2)[18] and the most usual pattern (62.1%) was inflammatory (B1 of Montreal). In UC (Table 1), 48.9% had pancolitis (E3 in Montreal classification) and 51.1% presented as a mild inflammatory bout (S1 of Montreal).
Table 2.
Montreal classification of inflammatory bowel disease
| IBD | Montreal classification |
| Crohn disease | |
| Age of diagnosis | A1 below 16 yr |
| A2 between 17 and 40 yr | |
| A3 above 40 yr | |
| Location | L1 ileal |
| L2 colonic | |
| L3 ileocolonic | |
| L4 isolated upper disease | |
| Behaviour | B1 non-stricturing, non-penetrating |
| B2 stricturing | |
| B3 penetrating | |
| P perianal disease | |
| Ulcerative colitis | |
| Extent | E1 |
| Ulcerative proctitis (distal to the rectosigmoid junction) | |
| E2 | |
| Left sided UC (distal to the splenic flexure) | |
| E3 | |
| Extensive UC (proximal to the splenic flexure) | |
| Severity | S0 |
| Clinical remission (asymptomatic) | |
| S1 | |
| Mild UC: Passage of four or fewer stools/d with or without blood, absence of any systemic illness, and normal inflammatory markers | |
| S2 | |
| Moderate UC: Passage of more than four stools per day but with minimal signs of systemic toxicity | |
| S3 | |
| Severe UC: Passage of at least six bloody stools daily, pulse rate of at least 90 beats/min, temperature of at least 37.5 °C, haemoglobin of less than 10.5 g/100 mL, and ESR of at least 30 mm/h | |
IBD: Inflammatory bowel disease; ESR: Erythrocyte sedimentation rate; UC: Ulcerative colitis.
With regards to the treatments used (Table 3), more than a half of the patients (52.8%) have been treated with thiopurines (mostly with azathiopurine) at some point during their illness; other treatments such as biological ones and immunosuppressants (methotrexate, cyclosporine, tacrolimus) have been used less frequently (25.2%). In general, the treatment with thiopurines was more frequently used in CD patients.
Table 3.
Treatments n (%)
| Thiopurines (n = 429) | No thiopurines (n = 383) | P value | |
| TPMT activity | 283 (66) | - | |
| Low | 5 (1.8) | - | |
| Intermediate | 43 (15.2) | - | |
| High | 235 (83) | - | |
| Azathioprine | 341 (79.5) | - | |
| Mercaptopurine | 60 (14) | - | |
| Azathioprine + Mercaptopurine | 28 (6.5) | - | |
| Infliximab | 112 (26.1) | 7 (1.8) | < 0.001 |
| Adalimub | 50 (11.7) | 3 (0.8) | < 0.001 |
| Other immunosuppressants | 29 (6.8) | 4 (1) | < 0.001 |
Patients (98.2%) treated with thiopurines have intermediate or high activity. Most of the patients treated with thiopurines take azathioprine. TPMT: Thiopurine S-methyltransferase.
The average daily dose of azathiopurine was 123.79 ± 36.5 mg (range: 50-250 mg), with an average usage time 72.16 ± 55.7 mo (range: 1-300 mo), and the accumulated dose for this time was 274.32 ± 233.5 g (range: 1.5-1350 g). With regards to mercaptopurine, the average daily dose was 74.7 ± 23.9 mg (range: 25-150 mg), with an average time under this thiopurine treatment of 23.37 ± 27.6 mo (range: 1-118 mo), and a total accumulated dose of 52.2 ± 63.5 g (range: 1.5-243 g). TPMT activity (tested by high-performance liquid chromatography: low < 5 IU/mL, intermediate 5-20 IU/mL, high > 20 IU/mL) has been tested, before using thiopurines, for 66% of the patients treated with these drugs, of which 98.2% had intermediate or high activity. The average usage time for infliximab was 29.09 ± 25.8 mo (range: 1-112 mo) and for adalimumab 25.4 ± 20.4 mo (range: 2-79 mo).
Fifty-one point nine percent of the women received thiopurines, as well as 71.4% of patients with CD and 33.1% of the patients with UC P < 0.001 (OR = 5.04, 95%CI: 3.74-6.79).
We found a total of 74 neoplasms in 67 patients (7 patients had 2 neoplasms), 21 of those neoplasms appeared before the diagnosis of IBD, and so they were excluded from the analysis, leaving 53 neoplasms which appeared in 48 patients (5 of which had 2 neoplasms) (Figure 1). Of these neoplasms, 37 (69.8%) appeared after the IBD diagnosis but before the use of thiopurines, while 16 (30.2%) were identified after the treatment with thiopurines had started.
Figure 1.
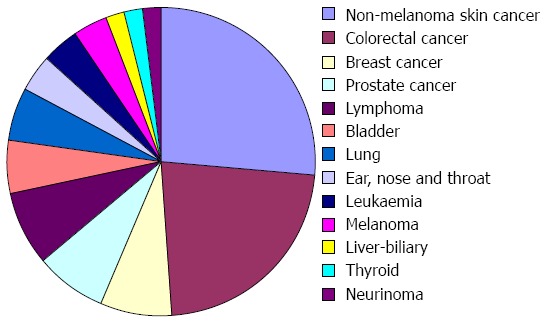
Types of neoplasms (n = 53). The most frequent neoplasms were non-melanoma skin cancer, colorectal cancer and haematological (lymphomas and leukaemia).
Mean time from IBD diagnosis to malignancy finding was 117 ± 91.8 mo (110.96 ± 99.6 mo in patients with no thiopurine treatment, and 129.21 ± 75.7 mo in treated patients, P > 0.05), with a mean time from IBD diagnosis to tiopurine treatment of 49.45 ± 57.05 mo. Mean time from thiopurine initiation to malignancy diagnosis was 67.05 ± 53.7 mo. Mean follow up time was 146.25 ± 91.2 mo, 151.56 ± 98 mo in patients who did not received thiopurines and 141.35 ± 84 mo in patients who did (P > 0.05).
The most frequent neoplasms were non-melanoma skin cancer, colorectal cancer and haematological (lymphomas and leukaemia). In general, the neoplasms were less frequent in the group of patients treated with thiopurines except for non-melanoma skin cancer, lymphoma and prostate cancer; however, these differences reached statistical significance only in the cases of breast and colorectal cancer, which had been more frequent in patients not treated with thiopurines (Figure 2).
Figure 2.
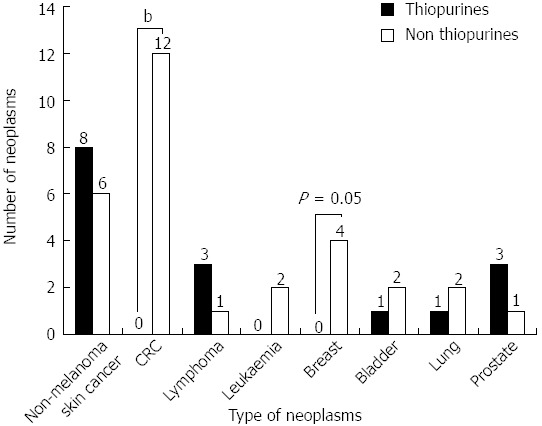
All the neoplasms, except for non-melanoma skin cancer, lymphomas and prostate, have been more common in patients not treated with thiopurines, however, significant differences have only been identified in colorectal and breast cancer. Non thiopurines vs thiopurines, bP value < 0.01 (OR = 0.96, 95%CI: 0.94-0.98); P value = 0.05 (OR = 0.99, 95%CI: 0.98-1).
In the univariate analysis, we identified a higher incidence of neoplasms among patients not treated thiopurines (8.1% vs 4%) (P = 0.013, OR = 0.469, 95%CI: 0.255-0.861). However, when considering the two types of thiopurines separately [azathioprine (AZA) or 6-mercaptopurine (6-MP)] we did not find these differences: in AZA group, 4.3% of those treated with it developed a neoplasm in comparison to 7.2% of those not treated with AZA (P > 0.05); in the 6-MP group, 2.3% of those treated with it developed a neoplasm, in comparison to 6.4% of those not treated with 6-MP (P > 0.05). Moreover, we did not observe differences in the incidence of neoplasms in relation to other treatments (infliximab, adalimumab or other non-thiopurine immunosuppressants).
With regard to sex, 4.3% of the women have developed a neoplasm in comparison to 7.2% of the men (P > 0.05); however, we observed a higher incidence of neoplasms among women not taking thiopurines compared to the ones treated with this drugs; P = 0.039 (OR = 0.294, 95%CI: 0.093-0.930), these differences are not seen among men (P > 0.05).
With respect to the type of IBD, we have identified more neoplasms among patients with UC (7.9%) than among patients with CD (4.1%), P = 0.021 (OR = 0.494, 95%CI: 0.269-0.907). However, when we studied the emergence of neoplasms in relation to the treatment with thiopurines in each type of IBD (UC and CD), we found no differences in any of the groups (P > 0.05).
In patients with CD, after stratifying patients by age of diagnosis, location and disease pattern, we noted a higher number of neoplasms among patients not treated with thiopurines in all groups, without reaching statistical significance (Figure 3A). This also occurred in patients with ulcerative colitis when stratifying them regarding the extension of the disease and its severity (Figure 3B).
Figure 3.
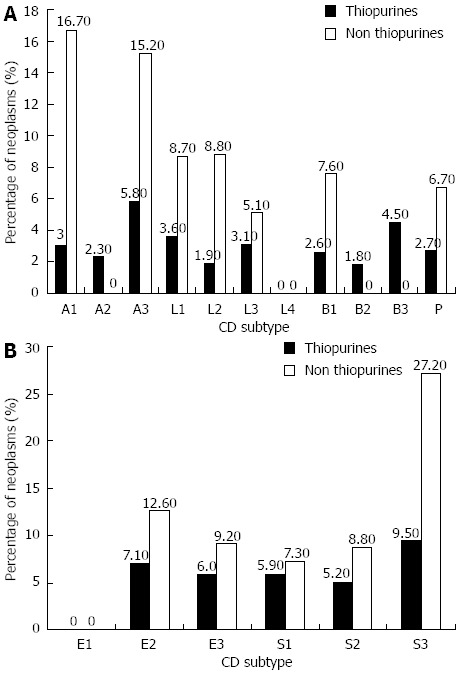
There seem to be no statistically significant differences in the appearance of neoplasms in any of the locations, behaviours or ages of diagnosis of the patients with Crohn’s disease and ulcerative colitis in relation to thiopurines intake. A: Percentage of neoplasms according to Crohn’s disease (CD) subtype; B: Percentage of neoplasms according to ulcerative colitis (UC) subtype.
In the logistic regression analysis, we only identified the age of diagnosis of IBD as a risk factor (P = 0.03, OR = 1.34, 95%CI: 1.003-1.066).
DISCUSSION
For years, the aim of treating IBD has been to control the patient’s symptoms, but as we have access to new treatments for this disease, the goal is becoming more ambitious, even trying to obtain mucosal healing. This is the reason for international guidelines to propose the use of stronger treatments in earlier stages and, although with this form of treatment we improve our control of the disease and its complications, we also increase the number of patients with treatments with potentially significant side effects (immunosuppressants, biological therapies, etc.). One of these undesirable effects is the development of a neoplastic disease which could be increased by the use of immunosuppressants and immunomodulators, given that these drugs block the natural containment mechanisms of neoplastic diseases as has been previously described in solid organ transplantation receptors.
On the other hand, in IBD as a chronic inflammatory disorder, there is a higher risk of neoplasms (it is known that there is a higher risk of colorectal cancer especially in more severe a long-lasting forms of the condition) and although the use of immunosuppressants such as thiopurines may reduce this risk by controlling the underlying inflammatory process, it is hard to establish whether the increased risk of malignancies in patients with IBD treated with thiopurines is due to the disease itself or to the treatment. Aiming to clarify this matter, there is an increasing number of publications suggesting a causative relationship between neoplasms affecting IBD patients and the treatment provided[1].
In our study, the incidence of neoplasms has been double among patients not treated with thiopurines despite having used them for a prolonged period of time (an average of 72.16 mo for AZA and 23.37 mo for MP) and with high doses (average accumulated dose of 274.3 g of AZA and 52.2 g of MP), which could be due to an improved control of the inflammatory process preventing the appearance of dysplasia and its subsequent progression to neoplasm.
The most frequent neoplasms have been skin ones, excluding melanoma, of which we have identified 14 cases (8 in the thiopurines group and 6 in the non-thiopurines group, P > 0.05). Although there are few studies published, the risk of developing this type of neoplasm is estimated to be higher in patients who are treated with thiopurines, especially in relation to the duration of the treatment and the accumulated dose of thiopurines. In the Long et al[19] study published in 2010 a relation was established between the risk of developing skin cancer in 50000 patients with IBD, suggesting that there is an increased risk of non-melanoma skin cancer in patients with IBD (RR = 1.64, 95%CI: 1.51-1.78) and the use of thiopurines increases this risk in direct relation to the duration of the treatment (OR = 3.56, 95%CI: 2.81-4.5 at 90 d; OR = 4.27, 95%CI: 3.08-5.92 at 1 year). The risk with other biological treatments was also increased (OR = 2.18, 95%CI: 1.07-4.46). In another study[20] in which 9618 patients with IBD were enrolled, the risk of developing non-melanoma skin cancer was also higher among those who were treated with thiopurines. Furthermore, in a recent paper[21] including 1084 patients, the risk of developing non-melanoma skin cancer was estimated to be 5 times higher among those who were treated with thiopurines, especially in Caucasian patients (12 times higher). On the other hand, nearly all the studies refer to exposure to sunlight and UVA as a risk factor for this type of neoplasm, recommending to patients treated with thiopurines avoiding this exposure. Indeed, in our study, even though the absolute number of patients affected with this type of cancer is higher in the group of patients treated with thiopurines, there were no significant differences, which could be due to the low number of cases. Despite these data the risk/benefit balance is in favor of using thiopurines, given the low aggressiveness of these neoplasms. However, these patients must be advised to use sunscreen and avoid prolonged exposure to sunlight.
In our study, the second most common neoplasm was colorectal cancer, which was only identified in the group of patients which were not treated with thiopurines (and had not been treated with biological drugs either), of which most are men with UC. It is a well-known fact that patients with uncontrolled IBD have a higher risk of developing this type of neoplasm, especially in extensive UC with a long-term course. It is known that the use of treatments such as 5-aminosalicylates which help to control the inflammatory activity of the IBD reduce the incidence of colorectal cancer[22-26]. The effect of the thiopurines is hard to establish given that many studies refer to a neutral risk[27-31], however, in the 2009 study by Beaugerie et al[32] with 19438 patients included, they observed a seemingly protective effect derived of the use of thiopurines, an effect which has also been seen in another study published in the same year by Andrews et al[33]. Our results show a lower risk of colorectal cancer in patients with IBD treated with thiopurines (P < 0.001, OR = 0.961, 95%CI: 0.942-0.981), probably due to an improved control of the disease and a reduction of the sustained colonic inflammation. The fact that in our study colorectal cancer is more frequent in patients with UC, may be due to the fact that in these patients the inflammation of the colon increases its risk as well as the fact that CD patients received thiopurines more frequently, which would have played a protective role in relation to colorectal cancer by improving control over the inflammatory process.
Among the 812 patients, 5 lymphomas have been identified, of which 1 was diagnosed prior to the CD diagnosis, thus, it has been excluded from the analysis. Of the 4 remaining, there has been 1 case identified among the patients not treated with thiopurines and 3 among those treated with thiopurines, without significant differences. There are contradictory data regarding the appearance of lymphomas in patients with IBD among different studies[34-38]. In the paper by Beaugerie et al[39] published in Lancet in 2009, with 19486 patients, an increased risk of developing intestinal lymphoma was identified in patients with IBD, which suggests that this disease per se entails a higher risk of developing lymphoma. The authors observed an increased risk of lymphoma among the patients on thiopurines therapy (HR = 5.28, 95%CI: 2.01-13.9, P = 0.0007) which decreased to a similar risk in patients never treated with these drugs when they were suspended; however, in a subgroup of patients who stopped taking thiopurines, inflammatory activity was greater and the risk of lymphoma was lower, concluding that the risk was associated to thiopurines and not to inflammatory activity. In other studies[40], there are even cases describing lymphoma regression in CD after suspending treatment with Azathioprine. In a 2005 meta-analysis[41] there was a RR of 4 in the patients with IBD who received thiopurines in relation to those who did not take them. However, the absolute risk of lymphoma continues to be low and is estimated in 300-1400 cases-year of treatment, although the subgroup of elderly patients with IBD who would comprise a higher risk of this malignancy.
Moreover, there is a clear relationship, already known from other studies[42-46], between the Epstein-Barr virus (EBV) infection and the development of lymphoma. In our series only one patient had a positive serology to EBV.
T cells hepatosplenic lymphoma is a highly aggressive variant of lymphoma, described in patients who were co-treated with thiopurines and infliximab[47-50], although there are other published cases of patients treated with TNF inhibitor monotherapy[51-53] or azathioprine alone[54-57], from which we deduce that the increased risk of developing this special form of lymphoma may be due to the IBD itself, its treatment (monotherapy or combined treatment) or a combination of both. For thiopurines, the cases published occur after 1-2 years of treatment. In our study we have not observed any case of this nature due to its low incidence.
Another malignant haematological disorder frequently associated with IBD and thiopurines is acute myeloid leukaemia (AML). We have identified two cases of AML, both of which have been in patients not treated with thiopurines (neither have they been treated with Infliximab or Adalimumab) which entails a 0.5%. In the study by Caspi et al[58], the risk of presenting this entity for patients with IBD has been established at 5.3 times higher than in the general population, and it has been mainly related to the dose and the duration of the treatment[59,60], increasing when this exceeds 5 years or 600 g of accumulated AZA doses. On the other hand, in other studies there is a subgroup of patients in which the accumulated dose is low and the risk of AML continues to be high, this leads us to think of an increased susceptibility to this drug within a subgroup of patients, which could be explained by the TPMT activity, as was clarified in the paper by Relling et al[61] and Bo et al[62] in which the low TPMT activity was related to the AML risk and secondary myelodysplastic syndromes.
Therefore, despite the existence of contradictory data, there is a higher risk of malignant haematological disorders in patients with IBD and this risk would be higher, although only slightly, in patients treated under thiopurines therapy (depending on the total accumulated doses, duration of the treatment, TPMT activity and the aggressiveness of the treatment measured through metabolites or toxicity such as leucopenia); however, this risk is overcome by the benefit of its use. In our study we observed no significant differences in these neoplasms incidence. However, we observed all the cases of leukemia neither in patients treated with thiopurines nor with TNF inhibitors, and most of the lymphomas appeared in patients treated with thiopurines. These differences with regard to the published data could be explained by to the low number of cases observed and the high TPMT activity found. Although some studies establish a relationship between the risk of developing these neoplasms with the levels of active thiopurines metabolites, this measurement is unavailable in the University Hospital Virgen de las Nieves, (Granada-Spain), thus, we are unable to provide more data.
Another important neoplasm observed in our series is breast cancer, which appeared in 4 cases, all of which occurred in women not treated with thiopurines (P = 0.05, OR = 0.99, 95%CI: 0.98-1). There are little data regarding the occurrence of this neoplasm in patients treated with thiopurines or with IBD and they don’t seem to have a higher risk compare to the general population[63,64]. However, there are more data regarding the development of other gynecological neoplasms such as cervical cancer. As most of them are related to the infection by human papillomavirus types 16 and 18, we could hypothesize that the use of immunosuppressants would increase the risk of this type of neoplasms; however, this has not been proved in the initial studies[37,65]. Indeed, other studies showed an increase incidence in cervical dysplasia in patients with IBD[66,67] and subsequent papers showed a higher risk of cervical cancer in relation to the duration of the treatment with azathioprine, combined use of corticoids or the use of tobacco[68-71]. In conclussion, the risk of breast or cervical cancer in patients with IBD is similar to the general population, and it seems not affected by thiopurines.
Among the risk factors identified in other studies, the accumulated doses of thiopurines, the usage time, the TPMT activity and the use of tobacco have been recognized. None of those factors has been found as a risk factor for any of the neoplasms described in our series. Regarding tobacco, we are unable to provide data as this information is lacking in our database. However, it is an obvious risk factor for the development of neoplastic pathology and it might have played a role.
In conclusion, in our study neoplasms were more frequent among the patients who have not been treated with thiopurines, as well as in patients diagnosed with UC (the treatment with thiopurines was more usual among CD patients), regardless of the sex. Considering the type of neoplasm, we observed significant differences in colorectal and breast cancer, for which the use of thiopurines has a protective role. In the rest of neoplasms we did not observe any difference, but the lymphomas and non-melanoma skin cancer had a higher incidence among patients treated with thiopurines, as occurs in the previously published studies. Due to this, we can conclude that the benefit provided by thiopurines use surpasses the risk.
COMMENTS
Background
Inflammatory bowel disease (IBD) is a chronic condition with bouts of bowel inflammation, resulting in abdominal pain, diarrhea, weight loss, and bleeding among other symptoms. Pathogenesis is mostly unknown, but autoimmunity is involved, and immunosupressants like thiopurines (azathioprine and mercaptopurine) are one of the available therapeutic options. However, these drugs have the theoretical potential to facilitate the development of other diseases controlled by the immune system, as infections or neoplasms. Therefore, it is essential to determine whether thiopurines therapy has an influence on neoplasms development, and if this effect is more intense that the benefits for IBD.
Research frontiers
A variety of drugs are used for IBD therapy, and changes have to be done depending on disease control or adverse events. Because of this, it is very difficult to determine whether the risk of neoplasm is attributable to a given drug, its combination with other, the time under treatment, doses or the disease itself. Moreover, intervening diseases, therapies, smoking and the severity of the disease itself could also have a role in neoplasms development.
Innovations and breakthroughs
Early thiopurines therapy provides a better control of IBD symptoms and prevents its complications. Nevertheless, a higher risk of neoplasms has been suggested in some papers after an early immunosuppressants introduction, although in other studies addressing this same issue this effect is not clear. They present a group of 812 IBD patients, 52.8% under thiopurines treatment, in which they have studied malignancies development.
Applications
Their results suggest that thiopurine treatment does not increase the risk of neoplasms in IBD patients. They have shown to be safe and effective drugs for disease control.
Terminology
IBD is a chronic sistemic condition that affects chiefly the gastrointestinal tract. The disease consists in inflammatory changes that involve the target organs. Thiopurines (azathioprine and mercaptopurine) are drugs known as immunosupresants, with an effect on the immune system and the inflammatory changes triggered by it.
Peer review
In this paper, the authors describe a retrospective analysis of 812 patients with inflammatory bowel diseases who were treated in a single center in Spain since 1996. The authors mainly compare malignancy incidences between patients treated with thiopurines and those who never received thiopurines.
Footnotes
P- Reviewers Guslandi M, Kullak-Ublick G S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Smith MA, Irving PM, Marinaki AM, Sanderson JD. Review article: malignancy on thiopurine treatment with special reference to inflammatory bowel disease. Aliment Pharmacol Ther. 2010;32:119–130. doi: 10.1111/j.1365-2036.2010.04330.x. [DOI] [PubMed] [Google Scholar]
- 2.Karran P. Thiopurines, DNA damage, DNA repair and therapy-related cancer. Br Med Bull. 2006;79-80:153–170. doi: 10.1093/bmb/ldl020. [DOI] [PubMed] [Google Scholar]
- 3.Ling YH, Chan JY, Beattie KL, Nelson JA. Consequences of 6-thioguanine incorporation into DNA on polymerase, ligase, and endonuclease reactions. Mol Pharmacol. 1992;42:802–807. [PubMed] [Google Scholar]
- 4.Somerville L, Krynetski EY, Krynetskaia NF, Beger RD, Zhang W, Marhefka CA, Evans WE, Kriwacki RW. Structure and dynamics of thioguanine-modified duplex DNA. J Biol Chem. 2003;278:1005–1011. doi: 10.1074/jbc.M204243200. [DOI] [PubMed] [Google Scholar]
- 5.Tidd DM, Paterson AR. A biochemical mechanism for the delayed cytotoxic reaction of 6-mercaptopurine. Cancer Res. 1974;34:738–746. [PubMed] [Google Scholar]
- 6.Krynetskaia NF, Cai X, Nitiss JL, Krynetski EY, Relling MV. Thioguanine substitution alters DNA cleavage mediated by topoisomerase II. FASEB J. 2000;14:2339–2344. doi: 10.1096/fj.00-0089com. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen T, Vacek PM, O’Neill P, Colletti RB, Finette BA. Mutagenicity and potential carcinogenicity of thiopurine treatment in patients with inflammatory bowel disease. Cancer Res. 2009;69:7004–7012. doi: 10.1158/0008-5472.CAN-09-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan B, Wang Y. Mutagenic and cytotoxic properties of 6-thioguanine, S6-methylthioguanine, and guanine-S6-sulfonic acid. J Biol Chem. 2008;283:23665–23670. doi: 10.1074/jbc.M804047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Donovan P, Perrett CM, Zhang X, Montaner B, Xu YZ, Harwood CA, McGregor JM, Walker SL, Hanaoka F, Karran P. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science. 2005;309:1871–1874. doi: 10.1126/science.1114233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brem R, Karran P. Multiple forms of DNA damage caused by UVA photoactivation of DNA 6-thioguanine. Photochem Photobiol. 2012;88:5–13. doi: 10.1111/j.1751-1097.2011.01043.x. [DOI] [PubMed] [Google Scholar]
- 11.Brem R, Li F, Karran P. Reactive oxygen species generated by thiopurine/UVA cause irreparable transcription-blocking DNA lesions. Nucleic Acids Res. 2009;37:1951–1961. doi: 10.1093/nar/gkp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attard NR, Karran P. UVA photosensitization of thiopurines and skin cancer in organ transplant recipients. Photochem Photobiol Sci. 2012;11:62–68. doi: 10.1039/c1pp05194f. [DOI] [PubMed] [Google Scholar]
- 13.Karran P, Attard N. Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nat Rev Cancer. 2008;8:24–36. doi: 10.1038/nrc2292. [DOI] [PubMed] [Google Scholar]
- 14.McLeod HL, Krynetski EY, Relling MV, Evans WE. Genetic polymorphism of thiopurine methyltransferase and its clinical relevance for childhood acute lymphoblastic leukemia. Leukemia. 2000;14:567–572. doi: 10.1038/sj.leu.2401723. [DOI] [PubMed] [Google Scholar]
- 15.Lennard L, Thomas S, Harrington CI, Maddocks JL. Skin cancer in renal transplant recipients is associated with increased concentrations of 6-thioguanine nucleotide in red blood cells. Br J Dermatol. 1985;113:723–729. doi: 10.1111/j.1365-2133.1985.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmiegelow K, Al-Modhwahi I, Andersen MK, Behrendtz M, Forestier E, Hasle H, Heyman M, Kristinsson J, Nersting J, Nygaard R, et al. Methotrexate/6-mercaptopurine maintenance therapy influences the risk of a second malignant neoplasm after childhood acute lymphoblastic leukemia: results from the NOPHO ALL-92 study. Blood. 2009;113:6077–6084. doi: 10.1182/blood-2008-11-187880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bo J, Schrøder H, Kristinsson J, Madsen B, Szumlanski C, Weinshilboum R, Andersen JB, Schmiegelow K. Possible carcinogenic effect of 6-mercaptopurine on bone marrow stem cells: relation to thiopurine metabolism. Cancer. 1999;86:1080–1086. doi: 10.1002/(sici)1097-0142(19990915)86:6<1080::aid-cncr26>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5–36. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 19.Long MD, Herfarth HH, Pipkin CA, Porter CQ, Sandler RS, Kappelman MD. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2010;8:268–274. doi: 10.1016/j.cgh.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh H, Nugent Z, Demers AA, Bernstein CN. Increased risk of nonmelanoma skin cancers among individuals with inflammatory bowel disease. Gastroenterology. 2011;141:1612–1620. doi: 10.1053/j.gastro.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 21.Setshedi M, Epstein D, Winter TA, Myer L, Watermeyer G, Hift R. Use of thiopurines in the treatment of inflammatory bowel disease is associated with an increased risk of non-melanoma skin cancer in an at-risk population: a cohort study. J Gastroenterol Hepatol. 2012;27:385–389. doi: 10.1111/j.1440-1746.2011.06865.x. [DOI] [PubMed] [Google Scholar]
- 22.Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol. 2008;14:3937–3947. doi: 10.3748/wjg.14.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinczowski D, Ekbom A, Baron J, Yuen J, Adami HO. Risk factors for colorectal cancer in patients with ulcerative colitis: a case-control study. Gastroenterology. 1994;107:117–120. doi: 10.1016/0016-5085(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 24.Moody GA, Jayanthi V, Probert CS, Mac Kay H, Mayberry JF. Long-term therapy with sulphasalazine protects against colorectal cancer in ulcerative colitis: a retrospective study of colorectal cancer risk and compliance with treatment in Leicestershire. Eur J Gastroenterol Hepatol. 1996;8:1179–1183. doi: 10.1097/00042737-199612000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther. 2000;14:145–153. doi: 10.1046/j.1365-2036.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 26.Bansal P, Sonnenberg A. Risk factors of colorectal cancer in inflammatory bowel disease. Am J Gastroenterol. 1996;91:44–48. [PubMed] [Google Scholar]
- 27.Fraser AG, Orchard TR, Robinson EM, Jewell DP. Long-term risk of malignancy after treatment of inflammatory bowel disease with azathioprine. Aliment Pharmacol Ther. 2002;16:1225–1232. doi: 10.1046/j.1365-2036.2002.01297.x. [DOI] [PubMed] [Google Scholar]
- 28.Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634–1648. doi: 10.1053/j.gastro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Matula S, Croog V, Itzkowitz S, Harpaz N, Bodian C, Hossain S, Ullman T. Chemoprevention of colorectal neoplasia in ulcerative colitis: the effect of 6-mercaptopurine. Clin Gastroenterol Hepatol. 2005;3:1015–1021. doi: 10.1016/s1542-3565(05)00738-x. [DOI] [PubMed] [Google Scholar]
- 30.Levine JS, Burakoff R. Chemoprophylaxis of colorectal cancer in inflammatory bowel disease: current concepts. Inflamm Bowel Dis. 2007;13:1293–1298. doi: 10.1002/ibd.20186. [DOI] [PubMed] [Google Scholar]
- 31.Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Beaugerie L, Seksik P, Carrat F. Thiopurine therapy is associated with a three-fold decrease in the incidence of advanced colorectal neoplasia in IBD patients with longstanding extensive colitis: the CESAME prospective data. J Crohn’s and Colitis. 2009;3:S5–6. [Google Scholar]
- 33.Andrews JM, Travis SP, Gibson PR, Gasche C. Systematic review: does concurrent therapy with 5-ASA and immunomodulators in inflammatory bowel disease improve outcomes. Aliment Pharmacol Ther. 2009;29:459–469. doi: 10.1111/j.1365-2036.2008.03915.x. [DOI] [PubMed] [Google Scholar]
- 34.Loftus EV, Tremaine WJ, Habermann TM, Harmsen WS, Zinsmeister AR, Sandborn WJ. Risk of lymphoma in inflammatory bowel disease. Am J Gastroenterol. 2000;95:2308–2312. doi: 10.1111/j.1572-0241.2000.02316.x. [DOI] [PubMed] [Google Scholar]
- 35.Aithal GP, Mansfield JC. Review article: the risk of lymphoma associated with inflammatory bowel disease and immunosuppressive treatment. Aliment Pharmacol Ther. 2001;15:1101–1108. doi: 10.1046/j.1365-2036.2001.01023.x. [DOI] [PubMed] [Google Scholar]
- 36.Palli D, Trallori G, Bagnoli S, Saieva C, Tarantino O, Ceroti M, d’Albasio G, Pacini F, Amorosi A, Masala G. Hodgkin’s disease risk is increased in patients with ulcerative colitis. Gastroenterology. 2000;119:647–653. doi: 10.1053/gast.2000.16487. [DOI] [PubMed] [Google Scholar]
- 37.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 38.Farrell RJ, Ang Y, Kileen P, O’Briain DS, Kelleher D, Keeling PW, Weir DG. Increased incidence of non-Hodgkin’s lymphoma in inflammatory bowel disease patients on immunosuppressive therapy but overall risk is low. Gut. 2000;47:514–519. doi: 10.1136/gut.47.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, Hébuterne X, Cortot A, Bouhnik Y, Gendre JP, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617–1625. doi: 10.1016/S0140-6736(09)61302-7. [DOI] [PubMed] [Google Scholar]
- 40.Larvol L, Soule JC, Le Tourneau A. Reversible lymphoma in the setting of azathioprine therapy for Crohn’s disease. N Engl J Med. 1994;331:883–884. doi: 10.1056/NEJM199409293311321. [DOI] [PubMed] [Google Scholar]
- 41.Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121–1125. doi: 10.1136/gut.2004.049460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dayharsh GA, Loftus EV, Sandborn WJ, Tremaine WJ, Zinsmeister AR, Witzig TE, Macon WR, Burgart LJ. Epstein-Barr virus-positive lymphoma in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Gastroenterology. 2002;122:72–77. doi: 10.1053/gast.2002.30328. [DOI] [PubMed] [Google Scholar]
- 43.Kumar S, Fend F, Quintanilla-Martinez L, Kingma DW, Sorbara L, Raffeld M, Banks PM, Jaffe ES. Epstein-Barr virus-positive primary gastrointestinal Hodgkin’s disease: association with inflammatory bowel disease and immunosuppression. Am J Surg Pathol. 2000;24:66–73. doi: 10.1097/00000478-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Li S, Borowitz MJ. Primary Epstein-Barr virus-associated Hodgkin disease of the ileum complicating Crohn disease. Arch Pathol Lab Med. 2001;125:424–427. doi: 10.5858/2001-125-0424-PEBVAH. [DOI] [PubMed] [Google Scholar]
- 45.Schubert S, Renner C, Hammer M, Abdul-Khaliq H, Lehmkuhl HB, Berger F, Hetzer R, Reinke P. Relationship of immunosuppression to Epstein-Barr viral load and lymphoproliferative disease in pediatric heart transplant patients. J Heart Lung Transplant. 2008;27:100–105. doi: 10.1016/j.healun.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 46.Van Biervliet S, Velde SV, De Bruyne R, De Looze D, De Vos M, Van Winckel M. Epstein-Barr virus related lymphoma in inflammatory bowel disease. Acta Gastroenterol Belg. 2008;71:33–35. [PubMed] [Google Scholar]
- 47.Mackey AC, Green L, Liang LC, Dinndorf P, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:265–267. doi: 10.1097/MPG.0b013e31802f6424. [DOI] [PubMed] [Google Scholar]
- 48.Zeidan A, Sham R, Shapiro J, Baratta A, Kouides P. Hepatosplenic T-cell lymphoma in a patient with Crohn’s disease who received infliximab therapy. Leuk Lymphoma. 2007;48:1410–1413. doi: 10.1080/10428190701345433. [DOI] [PubMed] [Google Scholar]
- 49.Thayu M, Markowitz JE, Mamula P, Russo PA, Muinos WI, Baldassano RN. Hepatosplenic T-cell lymphoma in an adolescent patient after immunomodulator and biologic therapy for Crohn disease. J Pediatr Gastroenterol Nutr. 2005;40:220–222. doi: 10.1097/00005176-200502000-00026. [DOI] [PubMed] [Google Scholar]
- 50.Rosh JR, Gross T, Mamula P, Griffiths A, Hyams J. Hepatosplenic T-cell lymphoma in adolescents and young adults with Crohn’s disease: a cautionary tale. Inflamm Bowel Dis. 2007;13:1024–1030. doi: 10.1002/ibd.20169. [DOI] [PubMed] [Google Scholar]
- 51.Mackey AC, Green L, Leptak C, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease: update. J Pediatr Gastroenterol Nutr. 2009;48:386–388. doi: 10.1097/mpg.0b013e3181957a11. [DOI] [PubMed] [Google Scholar]
- 52.Shale M, Kanfer E, Panaccione R, Ghosh S. Hepatosplenic T cell lymphoma in inflammatory bowel disease. Gut. 2008;57:1639–1641. doi: 10.1136/gut.2008.163279. [DOI] [PubMed] [Google Scholar]
- 53.Kotlyar D, Blonski W, Porter D, Mendizabal M, Lin MV, Lichtenstein GR. Hepatosplenic T-cell lymphoma (HSTCL) in inflammatory bowel disease (IBD): a rare complication after long-term thiopurine exposure; case report and systematic review of the literature. Gastroenterology. 2009;136 Suppl 1:A196–A197. [Google Scholar]
- 54.Navarro JT, Ribera JM, Mate JL, Granada I, Juncà J, Batlle M, Millá F, Feliu E. Hepatosplenic T-gammadelta lymphoma in a patient with Crohn’s disease treated with azathioprine. Leuk Lymphoma. 2003;44:531–533. doi: 10.1080/1042819021000035662. [DOI] [PubMed] [Google Scholar]
- 55.Mittal S, Milner BJ, Johnston PW, Culligan DJ. A case of hepatosplenic gamma-delta T-cell lymphoma with a transient response to fludarabine and alemtuzumab. Eur J Haematol. 2006;76:531–534. doi: 10.1111/j.1600-0609.2006.00646.x. [DOI] [PubMed] [Google Scholar]
- 56.Lemann M, de la Valussiere G, Bouhnik Y, Allez M, Touze Y, Bonnet J, Coffin B, Matuchansky C, Jian R, Colombel JF, et al. Intravenous cyclosporine for refractory attacks of crohn’s disease: long-term follow-up of patients. Gastroenterology. 1998;114 Suppl 1:A1020. [Google Scholar]
- 57.Moran G, Dillon J, Green J. Crohn’s disease, hepatosplenic T-cell lymphoma and no biological therapy: are we barking up the wrong tree. Inflamm Bowel Dis. 2009;15:1281–1282. doi: 10.1002/ibd.20802. [DOI] [PubMed] [Google Scholar]
- 58.Caspi O, Polliack A, Klar R, Ben-Yehuda D. The association of inflammatory bowel disease and leukemia--coincidence or not. Leuk Lymphoma. 1995;17:255–262. doi: 10.3109/10428199509056830. [DOI] [PubMed] [Google Scholar]
- 59.Knipp S, Hildebrandt B, Richter J, Haas R, Germing U, Gattermann N. Secondary myelodysplastic syndromes following treatment with azathioprine are associated with aberrations of chromosome 7. Haematologica. 2005;90:691–693. [PubMed] [Google Scholar]
- 60.Confavreux C, Saddier P, Grimaud J, Moreau T, Adeleine P, Aimard G. Risk of cancer from azathioprine therapy in multiple sclerosis: a case-control study. Neurology. 1996;46:1607–1612. doi: 10.1212/wnl.46.6.1607. [DOI] [PubMed] [Google Scholar]
- 61.Relling MV, Yanishevski Y, Nemec J, Evans WE, Boyett JM, Behm FG, Pui CH. Etoposide and antimetabolite pharmacology in patients who develop secondary acute myeloid leukemia. Leukemia. 1998;12:346–352. doi: 10.1038/sj.leu.2400928. [DOI] [PubMed] [Google Scholar]
- 62.Bo J, Schrøder H, Kristinsson J, Madsen B, Szumlanski C, Weinshilboum R, Andersen JB, Schmiegelow K. Possible carcinogenic effect of 6-mercaptopurine on bone marrow stem cells: relation to thiopurine metabolism. Cancer. 1999;86:1080–1086. doi: 10.1002/(sici)1097-0142(19990915)86:6<1080::aid-cncr26>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 63.Singh H, Nugent Z, Demers AA, Bernstein CN. Screening for cervical and breast cancer among women with inflammatory bowel disease: a population-based study. Inflamm Bowel Dis. 2011;17:1741–1750. doi: 10.1002/ibd.21567. [DOI] [PubMed] [Google Scholar]
- 64.Søgaard KK, Cronin-Fenton DP, Pedersen L, Sørensen HT, Lash TL. Survival in Danish patients with breast cancer and inflammatory bowel disease: a nationwide cohort study. Inflamm Bowel Dis. 2008;14:519–525. doi: 10.1002/ibd.20341. [DOI] [PubMed] [Google Scholar]
- 65.Hutfless S, Fireman B, Kane S, Herrinton LJ. Screening differences and risk of cervical cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2008;28:598–605. doi: 10.1111/j.1365-2036.2008.03766.x. [DOI] [PubMed] [Google Scholar]
- 66.Kane S, Khatibi B, Reddy D. Higher incidence of abnormal Pap smears in women with inflammatory bowel disease. Am J Gastroenterol. 2008;103:631–636. doi: 10.1111/j.1572-0241.2007.01582.x. [DOI] [PubMed] [Google Scholar]
- 67.Bhatia J, Bratcher J, Korelitz B, Vakher K, Mannor S, Shevchuk M, Panagopoulos G, Ofer A, Tamas E, Kotsali P, et al. Abnormalities of uterine cervix in women with inflammatory bowel disease. World J Gastroenterol. 2006;12:6167–6171. doi: 10.3748/wjg.v12.i38.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lees C, Critchley J, Chee N, Shand AG, Arnott ID, Satsangi J. Cervical dysplasia and IBD: no effect of disease status or immunosuppressants on analysis of 2199 smear records. Gastroenterology. 2008;134 Suppl 1:A143–A144. [Google Scholar]
- 69.Lyles T, Oster R, Gutierrez A. Prevalence of abnormal PAP smears in patients with IBD on immune modulator therapy. Gastroenterology. 2008;134 Suppl 1:A143. [Google Scholar]
- 70.Singh H, Demers AA, Nugent Z, Mahmud SM, Kliewer EV, Bernstein CN. Risk of cervical abnormalities in women with inflammatory bowel disease: a population-based nested case-control study. Gastroenterology. 2009;136:451–458. doi: 10.1053/j.gastro.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 71.Lees CW, Critchley J, Chee N, Beez T, Gailer RE, Williams AR, Shand AG, Arnott ID, Satsangi J. Lack of association between cervical dysplasia and IBD: a large case-control study. Inflamm Bowel Dis. 2009;15:1621–1629. doi: 10.1002/ibd.20959. [DOI] [PubMed] [Google Scholar]


