Abstract
AIM: To determine the magnetic resonance cholangiopancreatography (MRCP) and magnetic resonance imaging (MRI) features of pancreatitis with pancreas divisum (PD) and the differences vs pancreatitis without divisum.
METHODS: Institutional review board approval was obtained and the informed consent requirement was waived for this HIPAA-compliant study. During one year period, 1439 consecutive patients underwent successful MRCP without injection of secretin and abdominal MRI studies for a variety of clinical indications using a 1.5 T magnetic resonance scanner. Two experienced radiologists retrospectively reviewed all the studies in consensus. Disputes were resolved via consultation with a third experienced radiologist. The assessment included presence and the imaging findings of PD, pancreatitis, and distribution of abnormalities. The pancreatitis with divisum constituted the study group while the pancreatitis without divisum served as the control group. MRCP and MRI findings were correlated with final diagnosis. Fisher exact tests and Pearson × 2 tests were performed.
RESULTS: Pancreatitis was demonstrated at MRCP and MRI in 173 cases (38 cases with and 135 cases without divisum) among the 1439 consecutive cases. The recurrent acute pancreatitis accounted for 55.26% (21 of 38) in pancreatitis patients associated with PD, which was higher than 6.67% (9 of 135) in the control group, whereas the chronic pancreatitis was a dominant type in the control group (85.19%, 115 of 135) when compared to the study group (42.11%, 16 of 38) (χ2 = 40.494, P < 0.0001). In cases of pancreatitis with PD, the dorsal pancreatitis accounted for a much higher percentage than that in pancreatitis without PD (17 of 38, 44.74% vs 30 of 135, 22.22%) (χ2 = 7.257, P < 0.05).
CONCLUSION: MRCP and MRI can depict the features of pancreatitis associated with divisum. Recurrent acute pancreatitis and isolated dorsal involvement are more common in patients with divisum.
Keywords: Pancreas divisum, Pancreatitis, Diagnosis, Magnetic resonance imaging, Magnetic resonance cholangiopancreatography
Core tip: We reviewed 1439 cases of abdominal magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP). There were 122 cases of pancreas divisum (PD) and 38 of them were diagnosed as pancreatitis. The pancreatitis associated with PD was usually distributed in dorsal pancreas and presented as recurrent acute type. MRCP in combination with MRI can accurately detect ductal and parenchymal abnormalities of pancreas. Therefore, MRCP and MRI should be referred to as primary diagnostic tools for pancreatitis with PD whereas endoscopic retrograde cholangiopancreatography can be reserved for those who require therapeutic interventions.
INTRODUCTION
Pancreas divisum (PD) is the most common developmental anatomic variant of pancreatic duct with a reported incidence of 4%-14% in the population at autopsy series, 3%-8% at endoscopic retrograde cholangiopancreatography (ERCP), and 9% at magnetic resonance cholangiopancreatography (MRCP)[1-5]. This abnormality occurs when the dorsal and ventral pancreas anlage fails to fuse during the 6th-8th week of gestation. PD is characterized not only by the anatomical morphology but also by the physiology in which the majority of pancreatic juice drains through the duct of Santorini into the duodenum at orifice of minor papilla while the minority (about 10%) drains through the (ventral) duct of Wirsung into the duodenum at major papilla[1]. Although the clinical significance still remains controversial, there seems to be an association between PD and chronic abdominal pain and recurrent acute pancreatitis. Moreover, the timely and appropriate therapeutic interventions such as minor papillotomy or stent placement in the dorsal pancreatic duct or surgical procedures can benefit the patients with symptomatic PD remarkably from reducing the pressure in the main pancreatic duct[6].
The manifestations of acute and chronic pancreatitis at magnetic resonance imaging (MRI) and magnetic resonance (MR) cholangiopancreatography (MRCP) have been well described in previous studies[7-10], however, to our knowledge, there is no published literature on imaging features of pancreatitis in patients with PD using MRI together with MRCP without secretin injection. Although ERCP is considered as a gold standard of diagnosis, prior studies have shown that there is a great correlation between MRCP and ERCP in detecting PD[11,12]. Currently, the multidetector computed tomography (MDCT) has been reported to be valuable in the detection of PD[13]. As a noninvasive approach, MRCP can be used much more extensively than ERCP when radiation is in consideration and can always be performed together with MRI, which can depict the morphologic changes in detail[9,14]. Therefore, since MRI and MRCP can be employed to establish a diagnosis non-invasively, including for patients who are unable to undergo diagnostic ERCP, the ERCP can be reserved for those who require therapeutic intervention.
Therefore, the purpose of this study was to retrospectively evaluate the imaging features of pancreatitis in patients with PD at MRI and MRCP without injection of secretin and to describe the differences of MR imaging between pancreatitis with and without divisum.
MATERIALS AND METHODS
Patient population and proof of diagnosis
During one year period, a total of 1439 consecutive patients (age range, 16-95 years; 698 men and 741 women) consecutively underwent successful abdominal MRI and MRCP without injection of secretin in our institution for a variety of clinical indications. Among them, 173 cases were finally diagnosed as pancreatitis based on clinical presentations, laboratory values, and imaging findings. Of the 173 cases, 38 cases associated with PD constituted the study group in this study. A total of 42 times of ERCP examination and interventional therapy were performed in 21 cases in the study group. Among them, minor papillotomy and temporary transpapillary stent placement in main pancreatic duct (n = 15) through minor papilla, stent placement in common bile duct (CBD) (n = 3), and surgery of Puestow procedure (n = 1) were performed. Eighteen patients were male and 20 were female, with a mean age of 43.6 years (range, 20-79 years). The remaining 135 cases of pancreatitis without PD (66 male and 69 female) served as control group, aged from 19 to 85 years with a mean age of 53.4 years. We obtained the institutional review board approval and waiver of informed consent for this retrospective HIPPA-compliant study.
The recurrent acute pancreatitis was defined in this study as a clinical setting in which the clinical or/and serologic features were characteristic of acute pancreatitis with a history of recurrence at least 2 times. The clinical data, which included symptoms at presentation, history of previous episodes of pancreatitis, associated symptoms involving other systems, and laboratory findings, were reviewed for all of the 173 cases of pancreatitis in this study.
Imaging techniques
All the MR studies including coronal MRCP and axial MRI were performed with a 1.5-T MR imager (Magneton Vision; Siemens, Erlangen, Germany) using a phase array body coil. The MRCP images were initially obtained and axial MR imaging followed. The pancreaticobiliary tract was localized with a thick-slab (40 mm) half-Fourier RARE image in coronal-oblique (25 degrees) and axial planes, which necessitated an acquisition time of 7 s. The thin-slab MRCP acquisitions were obtained at various angles that allowed optimal visualization of the bile and pancreatic ducts; the number of thin-slab acquisitions per patient ranged from 3 to 15 (mean, 7 acquisitions). Both the thick-slab and thin slab images were obtained during breath hold. The half-Fourier RARE parameters included repetition time ms/echo time ms (effective) of ∞/95.0; echo train length, 128; flip angle, 150 degrees; section thickness, 3.0 mm with no gap; field of view, 270 mm × 270 mm; number of signals acquired, 1; matrix, 240 × 256 and acquisition time, 20 s. Fat saturation and shim adjustments were used in all cases.
After MRCP, conventional axial MR imaging was conducted to examine the abdomen. MR imaging sequences included unenhanced T1-weighted breath-hold spoiled gradient echo (148/5 ms; flip angle, 70 degrees; section thickness, 10 mm; gap, 30%), unenhanced T2-weighted breath-hold fast SE (3500/138 ms; section thickness, 8 mm; gap, 25%), unenhnaced in phase and out-of-phase T1-weighted gradient recalled echo, unenhanced and double-phased dynamic contrast-enhanced T1-weighted fat suppression (200/4.4 ms; flip angle, 70 degrees; section thickness, 8 mm; gap, 20%) 30 and 60 s after beginning of intravenous administration of the contrast materials. Gadopentetate dimeglumine (Magnevist; Berlex Laboratories, Wayne, NJ, United States) was administered intravenously using an automatic injector at a dose of 0.1 mmol per kilogram of body weight as a bolus followed by a normal-saline flush.
Imaging analysis
All the images in this study were reviewed retrospectively using interactive picture archiving and communicating system (PACS) workstations by two experienced (10-15 years of practice) abdominal radiologists (Wang D and Yu J) in consensus. The disputes were resolved via consultation with a third experienced abdominal radiologist (Fulcher AS). During the reading, the following items were taken into account: (1) classification of PD, complete PD or incomplete PD; (2) distribution of pancreatitis in the pancreas (ventral, dorsal or ventral plus dorsal pancreas); (3) morphologic changes including pancreatic parenchyma (enlargement or atrophy of pancreas), and pancreatic duct changes (side branch ectasia, pancreatic ductal dilatation and strictures, and intraductal calculi); (4) signal intensity abnormalities on unenhanced or enhanced images, including necrosis or cystic changes in pancreas; (5) changes outside of the pancreas, i.e., peripancreatic stranding, fluid collections, and involvement of the adjacent organs and vessels, etc; and (6) abscess inside pancreas and outside of pancreas. The classifications and distributions of pancreatitis were compared between the study group and control group. After careful analysis of the abovementioned findings, the imaging features of pancreatitis associated with PD were established.
Statistical analysis
Pearson χ2 and Fisher exact probability test were introduced for classification and distribution of pancreatitis in patients with PD in the study group compared with those in the control group. A P value less than 0.05 was considered to indicate a statistically significant difference.
RESULTS
Clinical features of the pancreatitis superimposed on PD
The classifications of pancreatitis in the 38 cases with PD in the study group included: recurrent acute pancreatitis in 21 cases (all cases with abdominal pain, 5 with gallstones, 1 with jaundice, and 16 with hyperlipasemia and hyperamylasemia), chronic pancreatitis in 8 cases (all cases with abdominal pain, 3 with mild serologic enzymes elevation, and 1 with gallstones) and the other 8 cases revealed with chronic pancreatitis incidentally at MRI and MRCP primarily for detecting hepatic lesions or biliary abnormalities, and acute pancreatitis in 1 case with worsening abdominal pain and serum lipase elevation of more than 1000 U/LH (normal, 23-300 U/LH). The gallstone pancreatitis was the dominant type in the control group (75.6%, 102/135). The other etiologies included intrapancreatic calculi (11.1%, 15/135), pancreatic ductal strictures (5.9%, 8/135), and autoimmune pancreatitis (4.4%, 6/135); and no distinct etiologic factors were found in 4 of the 135 cases (3%). The recurrent acute pancreatitis accounted for 55.26% (21 of 38) in pancreatitis with PD, which was higher in percentage than 6.67% (9 of 135) in the control group, whereas the chronic pancreatitis was a dominant type in the control group (85.19%, 115 of 135) when compared to the study group (42.11%, 16 of 38) (χ2 = 40.494, P < 0.0001) (Table 1). The pancreatitis in patients with PD accounted for 21.96% (38 of 173) in the total population of pancreatitis in this study.
Table 1.
Comparison of classification of pancreatitis with and without pancreas divisum n (%)
| Acute pancreatitis | Chronic pancreatitis | Recurrent acute pancreatitis | Total | |
| Pancreatitis with PD | 1 (2.63) | 16 (42.11) | 21(55.26) | 38 |
| Pancreatitis without PD | 11 (8.14) | 115 (85.19) | 9 (6.67) | 135 |
| Total | 12 (6.93) | 131 (75.72) | 30(17.34) | 173 |
PD: Pancreas divisum.
Imaging features of the pancreatitis superimposed on PD
Ductal and parenchymal changes of pancreas: Pancreatic duct in patients with PD and without PD both showed dilatation (6 vs 65), irregularity (16 vs 86), dilatation with irregularity (8 vs 80), focal stricture (6 vs 31), intrapancreatic duct calculi ( 2 vs 15), and side branch ectasia (36 vs 116) (Figure 1). In the study group, 7 cases of the isolated dorsal pancreatitis showed dilatation of main pancreatic duct all the way proximally to minor papilla. Two of recurrent cases each had a santorinicele of 5 mm (Figure 2) and 15 mm, respectively. Totally, 38 segments of duct of Santorini, 32 of duct of Wirsung, and 36 of duct in the body and in the tail as well were visualized clearly enough to be measured. The mean duct diameter was 3.00 ± 1.17 mm (SD) for the duct of Santorini, 2.22 ± 0.906 mm for the duct of Wirsung, 4.11 ± 2.72 mm for the body, and 3.27 ± 2.14 mm in the tail segments (Table 2). There were 3 severe chronic cases and 3 recurrent cases of pancreatitis showing the maximum of dorsal pancreatic ductal dilatation measured from 5 mm to 13.5 mm.
Figure 1.
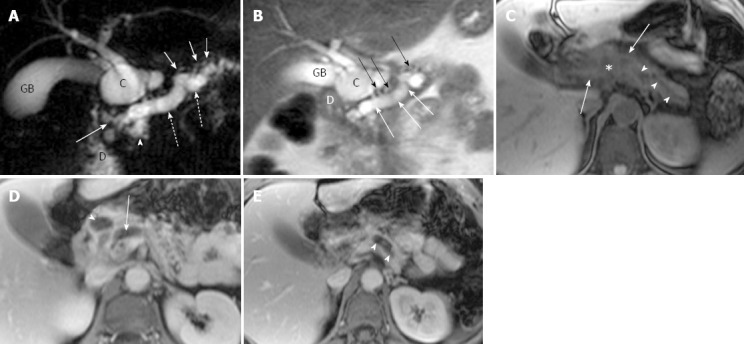
Magnetic resonance cholangiopancreatography and magnetic resonance imaging of recurrent acute pancreatitis involving the entire pancreas in a 44-year-old woman with several episodes of abdominal pain. A: Coronal oblique thick-section rapid acquisition with relaxation enhancement magnetic resonance (RARE-MR) cholangiogram [infinite/1100 (effective), 40-mm section thickness] shows severe dilatation of pancreatic duct (dotted arrows) with stricture (solid arrow) just before entering the duodenum (D) and side branch ectasia (short arrows) as well. There is a pseudocyst (C) formation in pancreatic parenchyma. The gallbladder (GB) is distended and the common bile duct is dilated (arrowhead) as well; B: Thin-section half-Fourier RARE-MR cholangiogram [infinite/95 (effective), 3-mm section thickness] shows remarkable dilatation of dorsal pancreatic duct (white arrows) with severe side branch ectasia (black arrows). The pseudocyst (C) is formed in the pancreatic head region immediately adjacent to the duodenum (D) and GB; C: Axial precontrast T1WI SPGR shows the dilatation of pancreatic duct (arrowheads) and the pseudocyst (star) formation in the pancreatic neck were not easily appreciated. The signal intensity of the dorsal pancreas (arrows) is dramatically decreased; D: Axial postcontrast T1WI SPGR shows delayed enhancement of the dorsal pancreas and the wall of the pseudocysts (arrowhead). Dilatation of the pancreatic duct (arrow) was noted; E: Axial postcontrast T1WI SPGR shows delayed enhancement of the dorsal pancreas and the dilatation of the pancreatic duct (arrowheads).
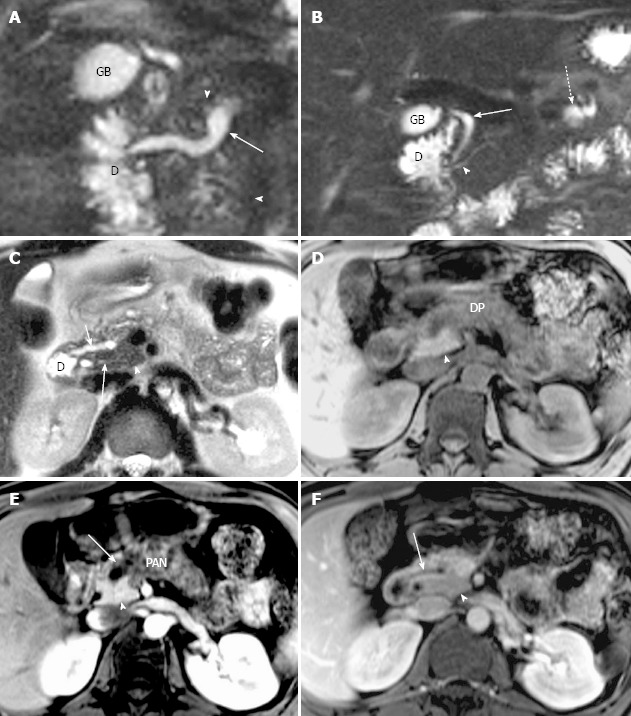
Figure 2.
Magnetic resonance cholangiopancreatography and magnetic resonance imaging of recurrent acute pancreatitis with pancreas divisum only involving the pancreatic tail with a small santorinicele in a 44-year-old man with several episodes of abdominal pain. A: Thin-section half-Fourier rapid acquisition with relaxation enhancement magnetic resonance (RARE- MR) cholangiogram [infinite/95 (effective), 3-mm section thickness] shows mild dilatation of the duct of Santorini (white arrows) with a focal enlargement consistent with santorinicele (arrowhead) at the entrance into the duodenum (D) via minor papilla. The side branch ectasia (black arrows) are noted in the pancreatic tail consistent with pancreatitis. Distended gallbladder (GB) and the CBD (dotted black arrow) are noted. B: Axial precontrast T1WI SPGR shows swelling and decrease of signal intensity in the pancreatic tail (black arrowhead), and thickening of the left anterior renal fascia (white arrowheads). C: Axial postcontrast T1WI SPGR shows mild delayed enhancement of pancreatic tail (arrowheads) with focal cystic changes (arrow).
Table 2.
Caliber measurement in four portions of pancreatic duct at magnetic resonance-cholangiopancreatography
| Santorini | Wirsung | Body | Tail | |
| Acute pancreatitis (mm) | 4.5 | 2.4 | 3.5 | 2.2 |
| Recurrent pancreatitis (mm) | 3.81 ± 1.02 | 2.40 ± 1.360 | 4.62 ± 3.49 | 3.37 ± 1.99 |
| Chronic pancreatitis (mm) | 4.06 ± 1.26 | 2.11 ± 0.597 | 3.74 ± 2.01 | 3.24 ± 2.35 |
| Mean (mm) | 4.00 ± 1.17 | 2.22 ± 0.906 | 4.11 ± 2.72 | 3.27 ± 2.14 |
Data are expressed as absolute numbers or mean ± SD.
In the study group, pancreatic edematous enlargement (n = 3), peripancreatic stranding (n = 13), atrophy with T1 signal intensity decrease (n = 12), atrophy with normal signal (n = 12), only T1 signal decrease (n = 3), and small intrapancreatic necrosis (n = 4) were detected in pancreatitis patients with PD. Six intrapancreatic pseudocysts were detected in 6 cases with a size ranging from 5 to 20 mm while 4 extrapancreatic fluid collections and pseudocysts were formed in the other 3 cases sized 35 to 76 mm. Thirty-two cases (recurrent acute, n = 21; chronic pancreatitis, n = 8) demonstrated delayed enhancement of pancreas (Figure 3). There were no adjacent organ and vessel involvement, and no hemorrhage or abscess formation due to pancreatitis in patients with PD.
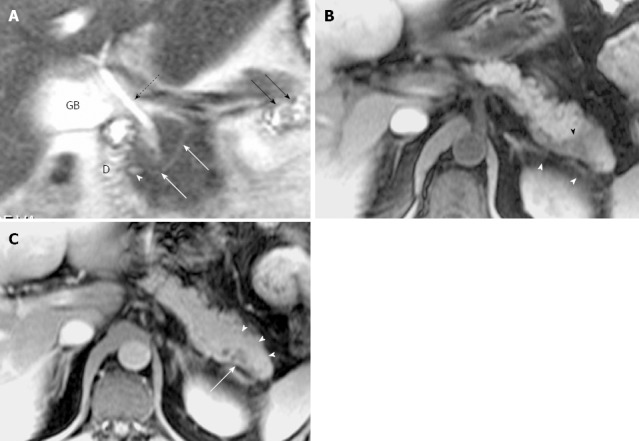
Figure 3.
Magnetic resonance cholangiopancreatography and magnetic resonance imaging of incidental chronic pancreatitis in a 47-year-old woman with a history of liver cirrhosis. A: Coronal oblique thick-section rapid acquisition with relaxation enhancement magnetic resonance (RARE-MR) cholangiogram [infinite/1100 (effective), 40-mm section thickness] of the pancreaticobiliary ducts shows slight dilatation with irregularity and strictures in the main pancreatic duct (dotted arrows) and in the duct of Santorini (arrowhead) just before entering the duodenum (D) via the minor papilla. Several side branch ectasia (long arrows) arising from the ventral duct and a normal common bile duct (short arrows) are demonstrated; B: Axial T1WI with fat saturation shows severe atrophy of the entire pancreas parenchyma (arrowheads). The liver (L) cirrhosis and splenomegaly (S) are noted; C: Axial T2WI shows main pancreatic duct (solid arrows) continued by duct of Santorini entering the duodenum (D) anterior to the common bile duct (arrowhead).
Classification of pancreas divisum and distribution of pancreatitis: In the study group, 35 cases were classified as complete PD and 3 cases as incomplete PD. Totally, 16 cases with complete divisum and 1 with incomplete divisum comprised dorsal pancreatitis in patients with PD. In cases of pancreatitis with PD, the dorsal pancreatitis accounted for a much higher percentage than in pancreatitis without PD (17 of 38, 44.74% vs 30 of 135, 22.22%) (χ2 =7.257, P < 0.05) (Table 3, Figures 4 and 5). Fifteen cases of dorsal pancreatitis were classified as recurrent acute pancreatitis. According to the anatomical involvement of lesion, the 17 cases of dorsal pancreatitis could be classified as complete dorsal involvement (n = 7), suproanterior portion of head and neck involvement (n = 3), dominant body involvement (n = 2), and dominant tail involvement (n = 5) with ductal stricture at body-tail junction resulting in upstream ductal dilatation in the tail.
Table 3.
Comparison of distribution of pancreatitis with and without pancreas divisum n (%)
| Dorsal | Ventral | Entire | Total | |
| Pancreatitis with PD | 17 (44.74) | 0 (0) | 21 (55.26) | 38 |
| Pancreatitis without PD | 30 (22.22) | 4 (2.96) | 101 (74.81) | 135 |
| Total | 47 (27.17) | 4 (2.31) | 122 (70.52) | 173 |
PD: Pancreas divisum.
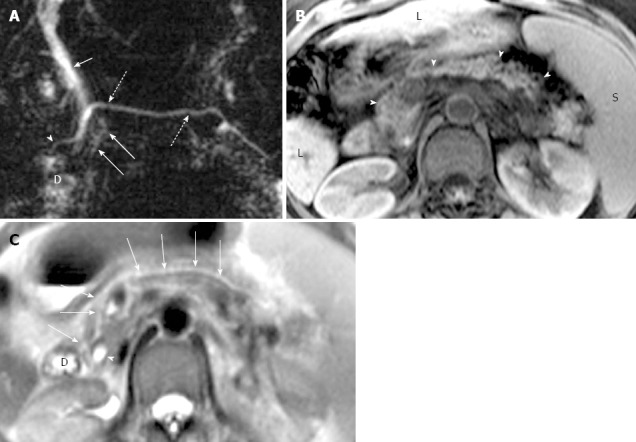
Figure 4.
Magnetic resonance cholangiopancreatography and magnetic resonance imaging of recurrent acute pancreatitis only involving the dorsal pancreas in a 43-year-old man. A: Coronal-oblique, thin-section half-Fourier rapid acquisition with relaxation enhancement magnetic resonance (RARE-MR) cholangiogram [infinite/95 (effective), 3-mm section thickness] shows marked dilatation (1 cm in diameter) of duct of Santorini (arrow) with apparent side branch ectasia (arrowheads). The gallbladder (GB) and the duodenum (D) are demonstrated clearly; B: Coronal-oblique, thin-section half-Fourier RARE MR cholangiogram [infinite/95 (effective), 3-mm section thickness] shows normal common bile duct (arrow) and ventral duct (arrowhead) of pancreas. The cystic changes secondary to pancreatitis in the pancreatic tail (dotted arrow) is shown while the GB and duodenum (D) appear normal; C: Axial T2WI shows dilatation and irregularity of duct of Santorini (short arrow) which enters the duodenum (D) via minor papilla. The ventral duct (solid arrow) and the pancreatic uncinate (arrowhead) are normal in size and signal intensity while the anterior portion of pancreatic head is abnormal with elevation of signal intensity; D: Axial precontrast T1WI SPGR shows that the pancreatic uncinate (arrowhead) is normal in size and signal intensity and the dorsal pancreas (DP) is abnormal with decreased T1 signal intensity and the swelling of the parenchyma; E: Axial T1WI SPGR after administration of Gd-DTPA at arterial phase shows normal enhancement of the pancreatic uncinate (arrowhead) and the enhancement of dorsal pancreas (PAN) is remarkably compromised with duct dilatation (arrow) and cystic changes; F: Axial T1WI SPGR at portal venous phase after administration of Gd-DTPA shows normal wash-out of contrast material in pancreatic uncinate (arrowhead) and delayed enhancement of the anterior portion of pancreatic head with dilatation of duct of Santorini (arrow).
Figure 5.
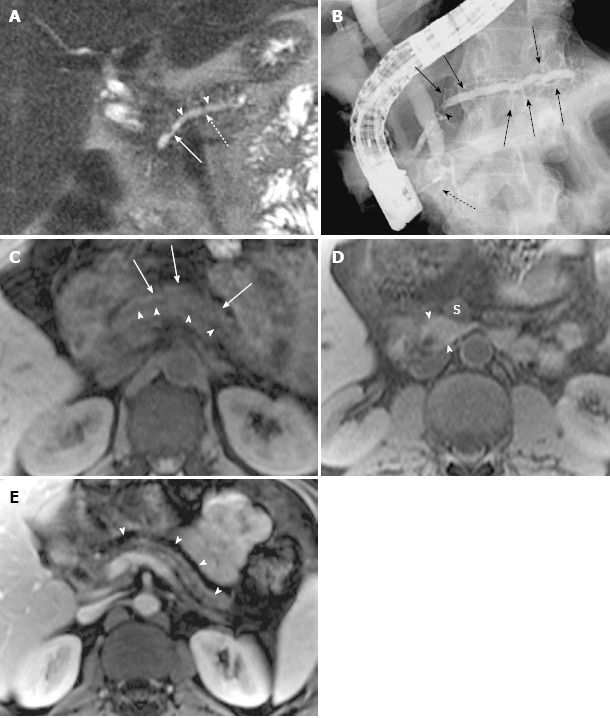
Magnetic resonance cholangiopancreatography and magnetic resonance imaging of recurrent acute pancreatitis only involving the dorsal pancreas, with endoscopic retrograde cholangiopancreatography correlation, in a 42-year-old man with several episodes of abdominal pain. A: Coronal-oblique, thin-section half-Fourier rapid acquisition with relaxation enhancement magnetic resonance (RARE-MR) cholangiogram [infinite/95 (effective), 3-mm section thickness] shows remarkable dilatation of the dorsal pancreatic duct (dotted arrow) with a conspicuous stricture (solid arrow) and severe side branch ectasia (arrowheads); B: Endoscopic retrograde cholangiopancreatography shows the dilatation of dorsal pancreatic duct and duct of Santorini with a well-seen stricture (arrowhead) and remarkable side branch ectasia (arrows) continual and proximal to the minor papilla. The intrapancreatic segment of common bile duct (C) is very narrow while the other part of the CBD is dilated. The ventral duct is normal in size (dotted arrow); C: Axial precontrast T1WI SPGR shows a marked decrease of signal intensity of dorsal pancreas (arrows) with ductal dilatation (arrowheads); D: Axial precontrast T1WI SPGR shows that the uncinate of pancreas is normal in size and signal intensity (arrowheads) is much higher than the superior mesenteric vein (S) and similar to the liver; E: Axial postcontrast T1WI SPGR shows the atrophy of dorsal pancreas with delayed enhancement (arrowheads) and dilatation of the allied pancreatic duct.
DISCUSSION
Classic PD (type 1) is defined as complete failure of fusion of the ducts of Santorini and Wirsung; other fusion anomalies with dominant dorsal drainage include absence of duct of Wirsung (type 2) and the presence of a filamentous or tiny caliber communication between the dominant dorsal duct of Santorini and the duct of Wirsung (type 3, incomplete PD)[15]. In patient with complete PD, a larger amount secretion from the dorsal pancreas could exert a significant burden on the relatively smaller orifice of minor duodenal papilla causing elevation of endoluminal pressure in pancreatic duct, resulting in subsequent pancreatitis. It has been reported that the clinical implications of incomplete PD may be similar to those of complete PD though the precise physiology may differ from each other[16,17].
A prior study with ERCP showed that the highest incidence of PD associated with idiopathic acute pancreatitis reached 50% in a total of 58 cases, which is significantly higher than in both controls and the whole population[18]. The recurrent acute pancreatitis in our study accounted for 55.26% (21 of 38) of pancreatitis in patients with PD, which is higher than in control group (6.67%, 9 of 135) (P < 0.0001, Table 1). The recurrent acute pancreatitis is a clinical entity that is characterized by repeated episodes of pancreatitis, which evolves over time with recurrent attacks of acute pancreatitis in otherwise normal pancreas until interventional procedures were performed in this clinical setting[19]. The pancreatitis in patients with PD accounted for 21.97% (38 of 173) in total population of pancreatitis in this study, which is higher than in the results based on ERCP by Bernard et al[18] and Kamisava et al[20].
In Western countries, the incomplete PD is uncommon with a reported incidence of 0.13%-0.9%. However, there was a much higher prevalence of incomplete PD in the recent reports from Japan and Korea, indicating 48% and 52% of PD[16,17]. In the present study, the incomplete PD occurred in 7.9% (3 of 38) among the pancreatitis patients with PD. Partially, the fluctuation of the frequency of incomplete PD could result from the different techniques employed for the detection of PD, i.e., the ERCP or MRCP, even for the same imaging modality, the techniques may be different with time due to intrinsic advances resulting in improved resolution[16,21-24].
In our study, 94.1% (16 of 17) of dorsal pancreatitis were detected in cases with complete PD. According to the pathophysiology of PD, the majority of pancreatic juice drained through minor papilla can result in endoluminal pressure elevation or obstruction with subsequent pancreatitis likely involving the dorsal pancreas and sparing the ventral pancreas instead.
Although the incidence of dorsal pancreatitis (44.74%, 17 of 38) in patients with PD was significantly higher than in patients without PD (30 of 135, 22.22%) (P < 0.05, Table 3) in this study and in the study with ERCP conducted by Kamisava et al[20], it is lower than the prior study performed with ERCP by Morgan et al[4]. The presence of pancreas divisum may reduce the severity of acute gallstone pancreatitis, as stone impaction at the major papilla only affects the ventral pancreas, a smaller portion (about 10%) of pancreas compared to the dorsal pancreas[25].
Among the isolated dorsal pancreatitis cases, severe strictures at the duct junction of body and tail were responsible for upstream ductal dilatation in tail with atrophy of the affected pancreas, which presented with recurrent acute pancreatitis clinically. The pancreatic parenchyma in pancreatitis associated with PD demonstrated a spectrum of abnormalities including low-signal-intensity of pancreas on T1-weighted fat-suppressed images due to edema or fibrosis, decreased and delayed enhancement after intravenous contrast administration, parenchymal atrophy or enlargement, and pseudocysts. Inflammation and fibrosis can diminish the proteinaceous fluid content in the pancreas, leading to the loss of the usual high signal intensity on T1-weighted fat-suppressed images; therefore, the pancreatitis in patients with PD can have some findings similar to the pancreatitis without PD as indicated in literature[8].
Although the ERCP is still considered as a gold standard for diagnosing PD, it is an invasive technique and expensive, particularly it has several drawbacks including failure to cannulate minor papilla[12,26], a high rate of complications such as ERCP-induced pancreatitis[26], radiation, and use of iodinated contrast medium. It was reported that as high as 35% of patients with pancreatitis had no abnormalities on ERCP[27]. A recent article reported that the MDCT could detect the PD via visualization of the Santorini duct[13]. However, MRCP together with MRI is a non-invasive technique without radiation; MRCP can always be done together with MRI in a single study, which can delineate the parenchymal morphology in detail[28]. Comparing to ERCP and MDCT, MRCP and MRI can be repeated more safely in the follow-up of the patients of pancreatitis with PD since patients in this subgroup are likely younger and more sensitive to radiation. MRCP with secretin stimulation can provide better visualization of pancreatic duct, resulting in higher sensitivity and specificity for diagnosis of the pancreatic abnormalities[29,30]. MRI can have the same accuracy as CT for pancreatitis at present. Additionally, MRCP is thought to depict the pancreatic duct in more physiologic states than under exogenous pressure such as ERCP. Therefore, MRCP and MRI can serve as a comprehensive diagnostic tool without radiation for the pancreatitis associated with PD whereas the ERCP can be reserved for those who require interventional procedures for therapeutic purpose.
This study had several limitations. One limitation was that not all cases (21 of 38) underwent ERCP procedure for reference or interventional management. Owing to the resolution of MRCP without secretin stimulation at present, there could be some compromises resulting in possible false-negative consequences in detection and characterization of PD and pancreatitis associated with PD in some cases. Finally, the subjects enrolled into the study was based on the referring criteria for MRCP and MRI studies; the severity of pancreatitis or the classifications of pancreatitis might not exactly reflect the real profile of pancreatitis in patients with PD since most of severe and acute patients prefer CT because it is quicker than MRI in examination.
In conclusion, recurrent acute pancreatitis is more common in patients with divisum than in patients without divisum (21 of 38, 55.26% vs 9 of 135, 6.67%). In divisum patients, the dorsal pancreatitis accounts for a much higher percentage than in patients without PD (17 of 38, 44.74% vs 30 of 135, 22.22%). Therefore, MRCP and MRI could be a comprehensive diagnostic tool without radiation for the pancreatitis associated with PD whereas the ERCP can be reserved for those who require therapeutic interventions.
COMMENTS
Background
Pancreas divisum (PD) is the most common developmental anatomic variant of pancreatic duct. Elevation of the intraluminal pressure of the pancreatic duct could result in pancreatitis. Magnetic resonance-cholangiopancreatography (MRCP) can always be performed together with magnetic resonance imaging (MRI), which can accurately detect both the ductal and the parenchymal abnormalities of the pancreas in detail.
Research frontiers
The pancreatitis in patients with PD could be different from the cases without PD both in clinical presentations and distribution of the abnormalities in pancreas since the congenital anomaly, however, the clinical significance remains controversial. To their knowledge, there is no published literature on imaging features of pancreatitis in patients with PD using MRI together with MRCP without secretin injection.
Innovations and breakthroughs
The pancreatitis associated with PD was usually distributed in dorsal pancreas and presented as recurrent acute type. MRCP in combination with MRI can accurately detect ductal and parenchymal abnormalities of pancreatitis in patients with PD.
Applications
The results of the present study indicated that repeated attacks of acute pancreatitis or isolated dorsal involvement of pancreas could imply a congenital PD in pancreas. MRCP and MRI should be referred to as a primary diagnostic tool for pancreatitis patients associated with PD whereas ERCP can be reserved for those who require therapeutic interventions.
Terminology
Recurrent acute pancreatitis was defined in this study as a condition in which the clinical or/and serologic features were characteristic of acute pancreatitis with a history of recurrence at least 2 times.
Peer review
Pancreas divisum is a common congenital anomaly of the pancreatic duct and possible cause of recurrent pancreatitis and chronic pancreatitis. But, there are still controversies in clinical significance as a cause of pancreatitis. This study revealed that recurrent acute pancreatitis is more common in divisum patients compared with those without divisum. The sample size was large to reach a conclusion and those results can help clinicians understand clinical significance of pancreas divisum. The imaging quality of presented figures is excellent and representative.
Footnotes
Supported by National Natural Science Foundation of China, No. 81171389; Key Basic Research Project of Shanghai Municipal Science and Technology Commission, No. 12JC1406500
P- Reviewers Lee KT, Motoo Y S- Editor Wen LL L- Editor Ma JY E- Editor Ma S
References
- 1.Kozu T, Suda K, Toki F. Pancreatic development and anatomical variation. Gastrointest Endosc Clin N Am. 1995;5:1–30. [PubMed] [Google Scholar]
- 2.Lehman GA, Sherman S. Diagnosis and therapy of pancreas divisum. Gastrointest Endosc Clin N Am. 1998;8:55–77. [PubMed] [Google Scholar]
- 3.Bret PM, Reinhold C, Taourel P, Guibaud L, Atri M, Barkun AN. Pancreas divisum: evaluation with MR cholangiopancreatography. Radiology. 1996;199:99–103. doi: 10.1148/radiology.199.1.8633179. [DOI] [PubMed] [Google Scholar]
- 4.Morgan DE, Logan K, Baron TH, Koehler RE, Smith JK. Pancreas divisum: implications for diagnostic and therapeutic pancreatography. AJR Am J Roentgenol. 1999;173:193–198. doi: 10.2214/ajr.173.1.10397125. [DOI] [PubMed] [Google Scholar]
- 5.Agha FP, Williams KD. Pancreas divisum: incidence, detection, and clinical significance. Am J Gastroenterol. 1987;82:315–320. [PubMed] [Google Scholar]
- 6.Gerke H, Byrne MF, Stiffler HL, Obando JV, Mitchell RM, Jowell PS, Branch MS, Baillie J. Outcome of endoscopic minor papillotomy in patients with symptomatic pancreas divisum. JOP. 2004;5:122–131. [PubMed] [Google Scholar]
- 7.Lecesne R, Taourel P, Bret PM, Atri M, Reinhold C. Acute pancreatitis: interobserver agreement and correlation of CT and MR cholangiopancreatography with outcome. Radiology. 1999;211:727–735. doi: 10.1148/radiology.211.3.r99jn08727. [DOI] [PubMed] [Google Scholar]
- 8.Miller FH, Keppke AL, Wadhwa A, Ly JN, Dalal K, Kamler VA. MRI of pancreatitis and its complications: part 2, chronic pancreatitis. AJR Am J Roentgenol. 2004;183:1645–1652. doi: 10.2214/ajr.183.6.01831645. [DOI] [PubMed] [Google Scholar]
- 9.Sica GT, Miller FH, Rodriguez G, McTavish J, Banks PA. Magnetic resonance imaging in patients with pancreatitis: evaluation of signal intensity and enhancement changes. J Magn Reson Imaging. 2002;15:275–284. doi: 10.1002/jmri.10066. [DOI] [PubMed] [Google Scholar]
- 10.Varghese JC, Masterson A, Lee MJ. Value of MR pancreatography in the evaluation of patients with chronic pancreatitis. Clin Radiol. 2002;57:393–401. doi: 10.1053/crad.2001.0888. [DOI] [PubMed] [Google Scholar]
- 11.Mortelé KJ, Rocha TC, Streeter JL, Taylor AJ. Multimodality imaging of pancreatic and biliary congenital anomalies. Radiographics. 2006;26:715–731. doi: 10.1148/rg.263055164. [DOI] [PubMed] [Google Scholar]
- 12.Sica GT, Braver J, Cooney MJ, Miller FH, Chai JL, Adams DF. Comparison of endoscopic retrograde cholangiopancreatography with MR cholangiopancreatography in patients with pancreatitis. Radiology. 1999;210:605–610. doi: 10.1148/radiology.210.3.r99fe55605. [DOI] [PubMed] [Google Scholar]
- 13.Asayama Y, Fang W, Stolpen A, Kuehn D. Detectability of pancreas divisum in patients with acute pancreatitis on multi-detector row computed tomography. Emerg Radiol. 2012;19:121–125. doi: 10.1007/s10140-011-1008-x. [DOI] [PubMed] [Google Scholar]
- 14.Barish MA, Yucel EK, Ferrucci JT. Magnetic resonance cholangiopancreatography. N Engl J Med. 1999;341:258–264. doi: 10.1056/NEJM199907223410407. [DOI] [PubMed] [Google Scholar]
- 15.Warshaw AL, Simeone JF, Schapiro RH, Flavin-Warshaw B. Evaluation and treatment of the dominant dorsal duct syndrome (pancreas divisum redefined) Am J Surg. 1990;159:59–64; discussion 64-66. doi: 10.1016/s0002-9610(05)80607-5. [DOI] [PubMed] [Google Scholar]
- 16.Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A. Clinical implications of incomplete pancreas divisum. JOP. 2006;7:625–630. [PubMed] [Google Scholar]
- 17.Kim MH, Lee SS, Kim CD, Lee SK, Kim HJ, Park HJ, Joo YH, Kim DI, Yoo KS, Seo DW, et al. Incomplete pancreas divisum: is it merely a normal anatomic variant without clinical implications. Endoscopy. 2001;33:778–785. doi: 10.1055/s-2001-16521. [DOI] [PubMed] [Google Scholar]
- 18.Bernard JP, Sahel J, Giovannini M, Sarles H. Pancreas divisum is a probable cause of acute pancreatitis: a report of 137 cases. Pancreas. 1990;5:248–254. doi: 10.1097/00006676-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Testoni PA. Recurrent acute pancreatitis. Introduction. JOP. 2001;2:355–356. [PubMed] [Google Scholar]
- 20.Kamisawa T, Egawa N, Tsuruta K, Okamoto A, Mtsukawa M. Pancreatitis associated with congenital abnormalities of the pancreaticobiliary system. Hepatogastroenterology. 2005;52:223–229. [PubMed] [Google Scholar]
- 21.Fulcher AS, Turner MA, Capps GW, Zfass AM, Baker KM. Half-Fourier RARE MR cholangiopancreatography: experience in 300 subjects. Radiology. 1998;207:21–32. doi: 10.1148/radiology.207.1.9530295. [DOI] [PubMed] [Google Scholar]
- 22.Leyendecker JR, Elsayes KM, Gratz BI, Brown JJ. MR cholangiopancreatography: spectrum of pancreatic duct abnormalities. AJR Am J Roentgenol. 2002;179:1465–1471. doi: 10.2214/ajr.179.6.1791465. [DOI] [PubMed] [Google Scholar]
- 23.Fulcher AS, Turner MA, Capps GW. MR cholangiography: technical advances and clinical applications. Radiographics. 1999;19:25–41; discussion 41-44. doi: 10.1148/radiographics.19.1.g99ja0525. [DOI] [PubMed] [Google Scholar]
- 24.Soto JA, Yucel EK, Barish MA, Chuttani R, Ferrucci JT. MR cholangiopancreatography after unsuccessful or incomplete ERCP. Radiology. 1996;199:91–98. doi: 10.1148/radiology.199.1.8633178. [DOI] [PubMed] [Google Scholar]
- 25.Boon N, Delhaye M, Le Moine O, De Maertelaer V, Devière J. Severity of acute gallstone pancreatitis in patients with pancreas divisum. Endoscopy. 2003;35:407–410. doi: 10.1055/s-2003-38771. [DOI] [PubMed] [Google Scholar]
- 26.Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, Overby CS, Aas J, Ryan ME, Bochna GS, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425–434. doi: 10.1067/mge.2001.117550. [DOI] [PubMed] [Google Scholar]
- 27.Gold RP, Berman H, Fakhry J, Heier S, Rosenthal W, DelGuercio L. Pancreas divisum with pancreatitis and pseudocyst. AJR Am J Roentgenol. 1984;143:1343–1344. doi: 10.2214/ajr.143.6.1343. [DOI] [PubMed] [Google Scholar]
- 28.Yu J, Turner MA, Fulcher AS, Halvorsen RA. Congenital anomalies and normal variants of the pancreaticobiliary tract and the pancreas in adults: part 2, Pancreatic duct and pancreas. AJR Am J Roentgenol. 2006;187:1544–1553. doi: 10.2214/AJR.05.0774. [DOI] [PubMed] [Google Scholar]
- 29.Matos C, Metens T, Devière J, Delhaye M, Le Moine O, Cremer M. Pancreas divisum: evaluation with secretin-enhanced magnetic resonance cholangiopancreatography. Gastrointest Endosc. 2001;53:728–733. doi: 10.1067/mge.2001.114784. [DOI] [PubMed] [Google Scholar]
- 30.Manfredi R, Costamagna G, Brizi MG, Spina S, Maresca G, Vecchioli A, Mutignani M, Marano P. Pancreas divisum and “santorinicele”: diagnosis with dynamic MR cholangiopancreatography with secretin stimulation. Radiology. 2000;217:403–408. doi: 10.1148/radiology.217.2.r00nv29403. [DOI] [PubMed] [Google Scholar]


