Abstract
AIM: To increase the understanding, diagnosis and treatment of pneumatosis cystoides intestinalis (PCI) and to find the characteristics and potential cause of the disease in China.
METHODS: We report here one case of PCI in a 70-year-old male patient who received a variety of treatment methods. Then, we systematically searched the PCI eligible literature published from an available Chinese database from May 2002 to May 2012, including CBM, CBMDisc, CMCC, VIP, Wanfang, and CNKI. The key words were pneumatosis cystoides intestinalis, pneumatosis, pneumatosis intestinalis, pneumatosis coli and mucosal gas. The patients’ information, histories, therapies, courses, and outcomes were reviewed.
RESULTS: The study group consisted of 239 PCI cases (male:female = 2.4:1) from 77 reported incidents. The mean age was 45.3 ± 15.6 years, and the median illness course was 6 mo. One hundred and sixty patients (66.9%) were in high altitude areas. In addition, 43.5% (104/239) of the patients had potential PCI-related disease, and 16.3% had complications with intestinal obstruction and perforation. The most common symptom was abdominal pain (53.9%), followed by diarrhea (53.0%), distention (42.4%), nausea and vomiting (14.3%), bloody stool (12.9%), mucous stool (12.0%) and constipation (7.8%). Most multiple pneumocysts developed in the submucosa of the colon (69.9%). The efficacy of the treatments by combined modalities, surgery, endoscopic treatment, conservative approach, oxygen, and antibiotics were 100%, 100%, 100%, 93.3%, 68.3% and 26.3%, respectively.
CONCLUSION: PCI can be safely managed by conservative treatments, presents more frequently in males, in the large bowel and submucosa, than in females, in the small intestine and subserosa. High altitude residence maybe associated with the PCI etiology.
Keywords: Pneumatosis cystoides intestinalis, Pneumatosis, Cyst, Intestinal, Colon
Core tip: Pneumatosis cystoids intestinalis (PCI) is a rare disease characterized by the presence of multiple gas-filled cysts in the submucosa and/or subserosa of the intestinal wall. PCI is still a poorly understood entity, and nearly all of the studies for PCI are case reports. In this work, we systematically evaluated and demonstrated for the first time the characteristics of PCI patients in China.
INTRODUCTION
Pneumatosis cystoids intestinalis (PCI) is an uncommon disease with an unknown etiology, characterized by the presence of gas within the submucosa or subserosa of the intestine[1,2]. Since it was first described by Du Vernoy[1] in autopsy specimens in 1730 and subsequently named by Mayer as PCI in 1825, it has been reported in various publications. However, after reviewing the literature, we found no epidemiologic studies, no randomized clinical trials, very few case series, and a large number of case reports. Many of the patients underwent misdiagnosis, mistreatment or even surgical exploration[3-5].
Our case report and systematic analysis are based on the Chinese publications to increase the understanding, diagnosis and treatment of PCI and to find the potential cause of the disease in China.
MATERIALS AND METHODS
Materials
A 70-year-old male was admitted for intermittent diarrhea accompanied by abdominal pain and bloody purulent stool for almost 2 years. The abdomen showed no relevant physical findings. Routine biochemical tests, inflammation indices and tumor markers were within normal values. He was diagnosed with hypertension and diabetes 10 years ago. Barium enema (Figure 1A) and colonoscopy (Figure 1B) disclosed multiple submucosal cysts protruding into the lumen of the whole colon. When a cyst was biopsied, it disappeared immediately. Endoscopic ultrasonography revealed gas in the cysts (Figure 1C). PCI was diagnosed. The patient received antibiotics and became asymptomatic with normal bowel movements. However, the diarrhea recurred after 4 mo. The patient then started hyperbaric oxygenation therapy. Unfortunately, he suffered from a hearing disorder and could not tolerate the hyperbaric therapy. Thus, the conservative approach was employed (observation only). Regular follow-up visits half a year later revealed improved clinical and radiological signs of PCI (Figure 1D).
Figure 1.
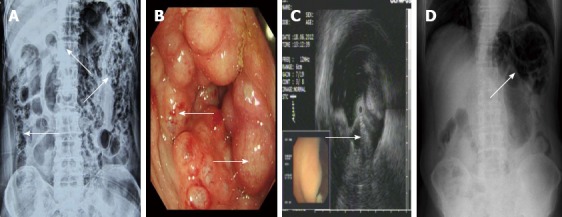
Imaging features of pneumatosis cystoides intestinalis. A: Barium enema study revealing multiple polypoid lesions with air shadows (arrow) and grape-like intramural gas in the whole colon; B: Colonoscopy revealing multiple round and smooth-surfaced elevated lesions (arrow) similar to submucosal tumors in the colon; C: Endoscopic ultrasonography revealing hyperechoic lesions and acoustic shadows in the submucosal layer (arrow); D: Plain radiography of the left upper quadrant abdomen revealing dilatation of the intestine and small linear, round radiolucent areas (arrow) on the clusters in the wall of the colon.
Search strategy
The literature search used the available Chinese databases from May 2002 to May 2012, including CBM, CBMDisc, CMCC, VIP, Wanfang, and CNKI. The key words were pneumatosis cystoides intestinalis, pneumatosis, pneumatosis intestinalis, pneumatosis coli and mucosal gas. The patients’ information, histories, therapies, courses, and outcomes were reviewed. Moreover, extended information was collected with regard to the nature and pathophysiology of PCI, and the incomplete reports were removed.
Statistical analysis
All data are presented as the mean ± SE. The demographic characteristics are presented as number (%).
RESULTS
The study group included 77 reports that contained an adequate amount of clinical information on 239 PCI cases (168 male:71 female = 2.4:1). The number of case reports was 62 (80.5%). The mean age was 45.3 ± 15.6 years (range: 2-81 years). The group was nationwide and particularly included high altitude areas and poor areas, including Qinghai, Sinkiang and Gansu (Table 1).
Table 1.
Geographical distribution of pneumatosis cystoides intestinalis in China n (%)
| Province | Mean altitude (m) | Cases |
| Qinghai | 4000 | 92 (38.5) |
| Sinkiang | 2000 | 28 (11.7) |
| Beijing | 50 | 21 (8.8) |
| Gansu | 3000 | 17 (7.1) |
| Shanghai | 4 | 10 (4.2) |
| Yunnan | 1500 | 8 (3.3) |
| Shanxi | 1000 | 8 (3.3) |
| Henan | 1500 | 7 (2.9) |
| Tianjin | 5 | 7 (2.9) |
| Sichuan | 500 | 7 (2.9) |
| Tibet | 4000 | 7 (2.9) |
| Shandong | 1500 | 4 (1.7) |
| Shaanxi | 1000 | 4 (1.7) |
| Zhejiang | 50 | 3 (1.3) |
| Jiangxi | 50 | 2 (0.8) |
| Guangdong | 100 | 2 (0.8) |
| Kiangsu | 50 | 2 (0.8) |
| Hainan | 200 | 2 (0.8) |
| Heilongjiang | 200 | 2 (0.8) |
| Jilin | 800 | 2 (0.8) |
| Liaoning | 500 | 2 (0.8) |
| Inner mongolia | 1000 | 1 (0.4) |
| Hebei | 400 | 1 (0.4) |
One hundred and four cases (43.5%) had comorbidities that may be related to PCI, with peptic ulcer being the most common concomitant disorder. In addition, 16.3% of the patients had complications including intestinal obstruction and perforation (Table 2).
Table 2.
Pneumatosis cystoids intestinalis concomitant diseases and pneumatosis cystoids intestinalis complications n (%)
| Total | n = 239 |
| PCI concomitant diseases | 104 (43.5) |
| Pyloric obstruction | 31 (29.8) |
| Duodenal ulcer | 18 (17.3) |
| Gastric ulcer | 17 (16.3) |
| Pulmonary diseases | 15 (14.4) |
| Intestinal diseases | 17 (16.3) |
| Abdominal external injury or surgery | 10 (9.6) |
| Malnutrition | 27 (26.0) |
| Connective tissue disease | 3 (2.9) |
| Diabetes mellitus | 5 (4.8) |
| Hypertension | 3 (2.9) |
| PCI complications | 39 (16.3) |
| Intestinal obstruction | 20 (51.3) |
| Intestinal perforation | 14 (35.9) |
| Atypical hyperplasia and canceration | 4 (10.2) |
| Intussusception and intestinal necrosis | 1 (2.6) |
PCI: Pneumatosis cystoids intestinalis.
The illness course from onset to identification ranged from 0 to 20 years, with a median of 6 mo. Overall, 217 cases (90.8%) had symptoms, and the most common symptom was abdominal pain (n = 117, 53.9%), followed by diarrhea (n = 115, 53.0%), distention (n = 92, 42.4%), nausea and vomiting (n = 31, 14.3%), bloody stool (n = 28, 12.9%), mucous stool (n = 26, 12.0%) and constipation (n = 17, 7.8%) (Figure 2A). PCI was most frequently diagnosed by colonoscopy (51.9%, 124/239), followed by surgery (40.6%, 97/239) and X-ray (10.9%, 26/239) (Figure 2B). The primary involved site was the colon, followed by the small intestine, especially the descending colon (n = 79, 33.1%), sigmoid (n = 77, 32.2%) and ileum (n = 72, 30.1%) (Figure 2C). The majority of the cysts were found in the submucosa (69.9%, 167/239). Only 11 (4.6%) patients had both submucosa and subserosa involvement (Figure 2D).
Figure 2.
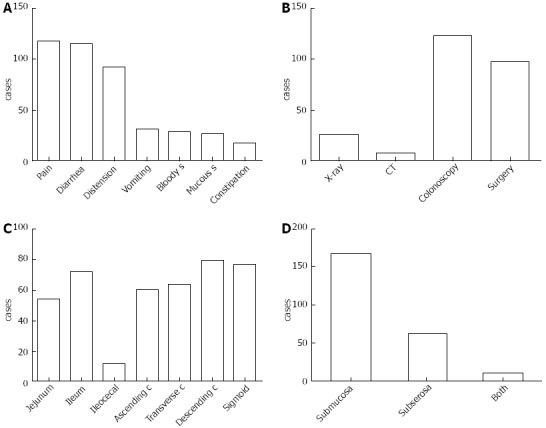
Clinical information of all 217 cases. A: The chief complaints; B: The methods of diagnosis; C: The primary involved site; D: The localization of gas in the intestinal wall. S: Stool; C: Colon; CT: Computed tomography.
The management of PCI included antibiotics, oxygen therapy, endoscopic therapy, surgery and the conservative approach. The efficiency of the conservative treatment reached up to 93.3% (Table 3). During the follow-up, which ranged from 1 mo to 20 years (median, 1 year), no symptoms recurred.
Table 3.
Pneumatosis cystoids intestinalis therapies and their efficiency n (%)
| Methods | n | Efficiency |
| Antibiotics | 19 | 5 (26.3) |
| Oxygen | 41 | 28 (68.3) |
| Endoscopy | 12 | 12 (100) |
| Surgery | 97 | 97 (100) |
| Conservative | 15 | 14 (93.3) |
| Antibiotics + Oxygen | 1 | 1 (100) |
| Oxygen + Endoscopic | 53 | 53 (100) |
| Oxygen + Surgery | 6 | 6 (100) |
DISCUSSION
PCI is a rare disease and is still poorly understood. In a retrospective review of PCI, Koss[6] found a 3.5:1 male-to-female ratio of the occurrence of PCI in an age group of 30-50 years, and Jamart[7] found a 3:1 male-to-female ratio (aged from 41-50 years old) of the occurrence of PCI. However, both of these old reports contained few patients. A prospective study by Knechtle et al[8] showed equal incidence among males and females. PCI was previously thought to occur most frequently in the small intestine, but in recent barium enemas and colonoscopies studies, PCI has been reported to more commonly affect the colon. Most older studies showed PCI to occur more commonly in the small intestine. However, Horiuchi et al[9] showed that PCI appeared more commonly in the colon (61.8%) of females (mean age 55.4 years), followed by the small intestine (15.4%). Recently, Morris et al[10] showed the incidence of PCI was 46% in the colon; 27% in the small intestine, only 7% in the colon and small intestine combined. In contrast to previous reports using different ethnic cohorts, PCI in the patients in this study (Chinese cohort) showed a male-to-female ratio of 2.4:1 and a mean age of 45.3 ± 15.6 years. The most frequent location of PCI was the colon instead of the small bowel (rate of 1.3:1), with only 2.9% (7/239) of the cases being combined colon and small intestine. The most common localization of gas was in the submucosa (69.9%) (Figure 1).
PCI has been associated with a wide variety of underlining etiologies to explain the abnormal accumulation of gas[11-23]. There are five major theories: (1) The mechanical theory: Intestinal obstruction, inflammatory bowel disease, ischemic bowel disease, gastroenteric tumor, anorectal surgery, bowel preparation or colonoscopy resulting in intestinal wall injury or increased intraluminal pressure serve as the driving force in PCI that causes the intramural gas[14,15]. However, this theory cannot explain how the cysts are maintained once they have formed; (2) The pulmonary theory: Pulmonary diseases, such as chronic obstructive pulmonary disease, asthma, and interstitial pneumonia, may result in pulmonary alveolar rupture and then produce a pneumomediastinum that dissects along the aorta and then along the mesenteric vessels to the bowel wall. However, this theory alone also fails to account for the finding that hydrogen, a gas never produced by mammalian cells, may comprise up to 50% of the gas content of the cysts[16]; (3) The bacterial theory: The gas is produced by gas-forming bacteria that enter the mucosal barrier through mucosal rents or increased mucosal permeability and produce the gas within the bowel wall. Indirect support for this theory was obtained by the successful treatment of PCI with antibiotics. However, the presence of aerogenic bacteria in the cysts has not yet been proven. Although Yale et al[17] reported that pneumatosis has been produced in germ-free rats by the injection of Clostridium species into the wall of the intestine, the isolation and cultivation of these organisms is rarely possible. Conversely, many of the patients who have pneumoperitoneum resulting from the rupture of cysts show no signs of peritonitis, prompting the theory that in this population, the gas is not caused by bacteria; (4) The chemical theory or nutritional deficiency theory: Malnutrition can prevent the digestion of carbohydrates and increased bacterial fermentation in the intestine, producing large volumes of gas leading to distention and ischemia and subsequently the submucosal dissection of gas. Recently, the development of PCI during the treatment with α-glucosidase inhibitors (α-GI) has been reported. The cessation of α-GI therapy is the key to the successful treatment of PCI[18,19], which supports the fourth theory; and (5) There have been some recent reports on PCI associated with chemotherapy, hormonal therapy and connective tissue disease that are not generally accepted[20-23].
Although many theories exist to explain the etiology and pathogenesis of PCI, no theory can explain the entire pathologic processes. Our experiments showed that many patients accompanied with pyloric obstruction, peptic ulcer, malnutrition and pulmonary diseases may support the theories of mechanical, pulmonary and nutritional deficiency (Table 2). Moreover, we also found that many of the patients came from highland areas, such as Qinghai, Sinkiang and Gansu (Table 1). The passage of intraluminal gas into the submucosa requires damage to the mucosa, which might be possible in these geographic areas. Further studies should be performed in the highland areas.
Although there are many symptoms of PCI, including abdominal pain, abdominal distention, diarrhea, mucous stool and bloody stool, none is disease specific (Figure 2A). The cysts may cause obstruction by internal or external compression of the bowel lumen when the cysts become larger. Complications associated with PCI occur in approximately 16.3% of cases and include intestinal obstruction or intestinal perforation (Table 2).
Approximately 85% of cases fall under secondary PCI[6], which results from other diseases. In these cases, the main symptoms and also the main treatments are related to the primary disease. Thus, some scholars do not think PCI is a disease by itself but rather a secondary manifestation of the reaction of the body to a variety of conditions; therefore, they believe that it does not have a single etiology[24].
In general, the diagnosis of PCI is based on endoscopy or plain radiography of the abdomen and is usually not difficult because the typical radiolucency appears as grape-like clusters or honeycomb-shaped shadows along the wall of the intestine. After the identification of PCI, a prompt further evaluation, including concomitant radiographic findings, of the patient should be conducted. Although only a few patients were diagnosed through an abdominal CT scan in our study, CT is a useful method for diagnosing PCI and is important because it provides data on other abdominal pathologies[25-27]. Radiographic signs of bowel perforation or peritonitis and endoscopic signs of a tumor often indicate the need for emergency surgery[28]. Using two different preoperative imaging modalities to make a precise diagnosis is necessary.
The appropriate therapy is related to the underlying cause of PCI. The majority of patients (93.3%, Table 3) without pronounced symptoms were cured without any treatments. If the symptoms are pronounced, a conservative approach to treatment is allowed, such as gastrointestinal decompression, intestinal “rest”, parenteral nutrition, and fluid and electrolyte supplementation. However, in contrast to the case reports, we found the efficiency of treatment by antibiotics was only 26.3%. The most efficient treatment was therapeutic alliance. Although oxygen was first used by Forgacs et al[16] in 1973, the optimal concentration, duration and effect of oxygen have not been established. The application of 200-300 mmHg PO2 pressure for 1.5-2.5 h/d for 2-14 d or 55%-75% oxygen inhalation for 1-3 h/d for 2-5 d was suggested to lead to gas absorption within the cysts. Surgery is reserved either for cases of suspected inconvertible intestinal obstruction or perforation or cases with precancerous conditions[29]. The extremely high rate of surgical resection in China (40.6%) is associated with the lack of realization and the misdiagnosis of PCI as many cases can recover with non-operative management. Therefore, diagnosing PCI as early as possible and providing fast and adequate therapy to treat PCI are extremely important[30,31].
In conclusion, after recognizing the disease for almost three centuries, PCI is still a poorly understood phenomenon. Several theories explaining PCI and the variety of treatments reflect the lack of knowledge regarding the underlying pathophysiology. A long-term follow-up study is suggested to evaluate the long-term outcome of these therapies.
COMMENTS
Background
Pneumatosis cystoides intestinalis (PCI) is a rare disease characterized by the presence of multiple gas-filled cysts in the submucosa and/or subserosa of the intestinal wall. PCI is still a poorly understood entity, and nearly all of the studies for PCI are case reports.
Research frontiers
PCI is a rare disease that has not been unequivocally addressed. In this study, the authors systematically evaluated and demonstrated for the first time the characteristics of PCI patients in China.
Innovations and breakthroughs
For the first time, the authors determined the prevalence and the characteristics of PCI in Chinese people.
Applications
The study results suggest that PCI in Chinese patients presents in the large bowel more often than in the small intestine and more frequently in middle-aged males than in females. The majority of the cysts are found in the submucosa rather than the subserosa. High altitude residence may be associated with the PCI etiology. The majority of PCI can be safely managed by conservative treatment.
Peer review
The authors review PCI in the published literature from China and report on 239 cases. Stats on geography and altitude is hare to interpret - as population density may be more important - would incidence per 100000 population at high or low altitude be more helpful.
Footnotes
P- Reviewers Li XA, Misra SP S- Editor Zhai HH L- Editor A E- Editor Ma S
References
- 1.Du Vernoy JG. Aer intestinorum tam sub extima quam intima tunica inclusus: observationes anatomicae: comment. Acad Acient Imp Petropol. 1730;5:213–225. [Google Scholar]
- 2.Ivanović A, Kovač J, Mašulović D, Stefanović A, Jakšić E, Saranović D. Education and imaging. Gastrointestinal: the role of multidetector computer tomography in diagnosis of pneumatosis cystoides intestinalis. J Gastroenterol Hepatol. 2012;27:182. doi: 10.1111/j.1440-1746.2011.06952.x. [DOI] [PubMed] [Google Scholar]
- 3.Slesser AA, Patel PH, Das SC, Leahy A, Livingstone J, Riaz AA. A rare case of segmental small bowel pneumatosis intestinalis: A case report. Int J Surg Case Rep. 2011;2:185–187. doi: 10.1016/j.ijscr.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rath T, Roeb E, Doppl WE. Pneumatosis coli as a rare complication of bowel preparation. Endoscopy. 2010;42 Suppl 2:E344–E345. doi: 10.1055/s-0030-1255977. [DOI] [PubMed] [Google Scholar]
- 5.Buckle C, Holdridge C, Xu T, Akhwais F, Sinha A, Doddi S, Sinha P. Acute abdominal pain and radiological pneumoperitoneum - always an indication for laparotomy. J Clin Med Res. 2013;5:132–134. doi: 10.4021/jocmr929w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.KOSS LG. Abdominal gas cysts (pneumatosis cystoides intestinorum hominis); an analysis with a report of a case and a critical review of the literature. AMA Arch Pathol. 1952;53:523–549. [PubMed] [Google Scholar]
- 7.Jamart J. Pneumatosis cystoides intestinalis. A statistical study of 919 cases. Acta Hepatogastroenterol (Stuttg) 1979;26:419–422. [PubMed] [Google Scholar]
- 8.Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg. 1990;212:160–165. doi: 10.1097/00000658-199008000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horiuchi A, Akamatsu T, Mukawa K, Ochi Y, Arakura N, Kiyosawa K. Case report: Pneumatosis cystoides intestinalis associated with post-surgical bowel anastomosis: a report of three cases and review of the Japanese literature. J Gastroenterol Hepatol. 1998;13:534–537. doi: 10.1111/j.1440-1746.1998.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 10.Morris MS, Gee AC, Cho SD, Limbaugh K, Underwood S, Ham B, Schreiber MA. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195:679–682; discussion 682-683. doi: 10.1016/j.amjsurg.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Rahim H, Khan M, Hudgins J, Lee K, Du L, Amorosa L. Gastrointestinal sarcoidosis associated with pneumatosis cystoides intestinalis. World J Gastroenterol. 2013;19:1135–1139. doi: 10.3748/wjg.v19.i7.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JY, Han HS, Lim SN, Shim YK, Choi YH, Lee OJ, Lee KH, Kim ST. Pneumatosis intestinalis and portal venous gas secondary to Gefitinib therapy for lung adenocarcinoma. BMC Cancer. 2012;12:87. doi: 10.1186/1471-2407-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rittenhouse DW, Chojnacki KA. Massive portal venous air and pneumatosis intestinalis associated with cocaine-induced mesenteric ischemia. J Gastrointest Surg. 2012;16:223–225. doi: 10.1007/s11605-011-1579-6. [DOI] [PubMed] [Google Scholar]
- 14.Nancy Fu YT, Kim E, Bressler B. Pneumatosis intestinalis after colonoscopy in a Crohn’s disease patient with mucosal healing. Inflamm Bowel Dis. 2013;19:E7–E8. doi: 10.1002/ibd.22840. [DOI] [PubMed] [Google Scholar]
- 15.Wertkin MG, Wetchler BB, Waye JD, Brown LK. Pneumatosis coli associated with sigmoid volvulus and colonoscopy. Am J Gastroenterol. 1976;65:209–214. [PubMed] [Google Scholar]
- 16.Forgacs P, Wright PH, Wyatt AP. Treatment of intestinal gas cysts by oxygen breathing. Lancet. 1973;1:579–582. [PubMed] [Google Scholar]
- 17.Yale CE, Balish E. The natural course of Clostridium perfringens--induced pneumatosis cystoides intestinalis. J Med. 1992;23:279–288. [PubMed] [Google Scholar]
- 18.Wu SS, Yen HH. Images in clinical medicine. Pneumatosis cystoides intestinalis. N Engl J Med. 2011;365:e16. doi: 10.1056/NEJMicm1013439. [DOI] [PubMed] [Google Scholar]
- 19.Tsujimoto T, Shioyama E, Moriya K, Kawaratani H, Shirai Y, Toyohara M, Mitoro A, Yamao J, Fujii H, Fukui H. Pneumatosis cystoides intestinalis following alpha-glucosidase inhibitor treatment: a case report and review of the literature. World J Gastroenterol. 2008;14:6087–6092. doi: 10.3748/wjg.14.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groninger E, Hulscher JB, Timmer B, Tamminga RY, Broens PM. Free air intraperitoneally during chemotherapy for acute lymphoblastic leukemia: consider pneumatosis cystoides intestinalis. J Pediatr Hematol Oncol. 2010;32:141–143. doi: 10.1097/MPH.0b013e3181ced397. [DOI] [PubMed] [Google Scholar]
- 21.Vendryes C, Hunter CJ, Harlan SR, Ford HR, Stein J, Pierce JR. Pneumatosis intestinalis after laparoscopic appendectomy: case report and review of the literature. J Pediatr Surg. 2011;46:e21–e24. doi: 10.1016/j.jpedsurg.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 22.Balbir-Gurman A, Brook OR, Chermesh I, Braun-Moscovici Y. Pneumatosis cystoides intestinalis in scleroderma-related conditions. Intern Med J. 2012;42:323–329. doi: 10.1111/j.1445-5994.2011.02557.x. [DOI] [PubMed] [Google Scholar]
- 23.Korhonen K, Lovvorn HN, Koyama T, Koehler E, Calder C, Manes B, Evans M, Bruce K, Ho RH, Domm J, et al. Incidence, risk factors, and outcome of pneumatosis intestinalis in pediatric stem cell transplant recipients. Pediatr Blood Cancer. 2012;58:616–620. doi: 10.1002/pbc.23242. [DOI] [PubMed] [Google Scholar]
- 24.Arikanoglu Z, Aygen E, Camci C, Akbulut S, Basbug M, Dogru O, Cetinkaya Z, Kirkil C. Pneumatosis cystoides intestinalis: a single center experience. World J Gastroenterol. 2012;18:453–457. doi: 10.3748/wjg.v18.i5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee KS, Hwang S, Rúa SM, Janjigian YY, Gollub MJ. Distinguishing benign and life-threatening pneumatosis intestinalis in patients with cancer by CT imaging features. AJR Am J Roentgenol. 2013;200:1042–1047. doi: 10.2214/AJR.12.8942. [DOI] [PubMed] [Google Scholar]
- 26.Adar T, Paz K. Images in clinical medicine. Pneumatosis intestinalis. N Engl J Med. 2013;368:e19. doi: 10.1056/NEJMicm1205591. [DOI] [PubMed] [Google Scholar]
- 27.Kim D, Lee J, Yeh J. Pneumatosis intestinalis on plain film. J Emerg Med. 2013;44:175–176. doi: 10.1016/j.jemermed.2011.05.056. [DOI] [PubMed] [Google Scholar]
- 28.Kim YG, Kim KJ, Noh SH, Yang DH, Jung KW, Ye BD, Byeon JS, Myung SJ, Yang SK. Clear water filling and puncture: sufficient for endoscopic diagnosis of pneumatosis cystoides intestinalis (with video) Gastrointest Endosc. 2011;74:1170–1171. doi: 10.1016/j.gie.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Jun SL, Brennan TV. Pneumatosis intestinalis: not always a surgical indication. Case Rep Surg. 2012;2012:719713. doi: 10.1155/2012/719713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinagare AB, Howard SA, Krajewski KM, Zukotynski KA, Jagannathan JP, Ramaiya NH. Pneumatosis intestinalis and bowel perforation associated with molecular targeted therapy: an emerging problem and the role of radiologists in its management. AJR Am J Roentgenol. 2012;199:1259–1265. doi: 10.2214/AJR.12.8782. [DOI] [PubMed] [Google Scholar]
- 31.Khalil PN, Huber-Wagner S, Ladurner R, Kleespies A, Siebeck M, Mutschler W, Hallfeldt K, Kanz KG. Natural history, clinical pattern, and surgical considerations of pneumatosis intestinalis. Eur J Med Res. 2009;14:231–239. doi: 10.1186/2047-783X-14-6-231. [DOI] [PMC free article] [PubMed] [Google Scholar]


