Abstract
Background
Delirium is characterized by acute cognitive impairment. We examined the effect of delirium on long-term cognitive trajectory in older adults with Alzheimer's disease (AD).
Methods
Prospectively collected longitudinal data from a nested cohort of hospitalized patients with AD (n=263) in the Massachusetts Alzheimer's Disease Research Center Patient Registry during 1991–2006 (median follow-up: 3.2 years). Cognitive function was measured using the Information-Memory-Concentration (IMC) section of the Blessed Dementia Rating Scale. Delirium was identified using a validated chart review method. The pace of cognitive deterioration was contrasted using random effect regression models.
Results
Over half of the sample of patients with AD developed delirium during hospitalization (56%). The pace of cognitive deterioration prior to hospitalization did not differ between patients who developed delirium (1.4 IMC points/year, 95% confidence interval, CI,0.7,2.1) and those who did not (0.8 IMC points/year, 95% CI: 0.3,1.3) (P=0.24). In the year following hospitalization, patients who had developed delirium experienced greater cognitive deterioration (3.1 IMC points/year, 95% CI: 2.1,4.1) relative to patients who did not develop delirium (1.4 IMC points/year, 95% CI: 0.2,2.6) after adjusting for confounders. The ratio of these changes suggests that following delirium, cognitive deterioration proceeds at 2.2 times the rate in patients without delirium in the year after hospitalization. The delirium group maintained a more rapid pace of cognitive deterioration throughout the 5-year period following hospitalization. Sensitivity analyses excluding rehospitalized patients and matching on baseline cognitive function and baseline pace of cognitive deterioration produced essentially identical results. The acceleration due to delirium was independent of dementia severity, comorbidity, and demographic characteristics.
Conclusions
Delirium is highly prevalent among persons with AD who are hospitalized and associated with an increased pace of cognitive deterioration which is maintained for up to 5 years. Strategies to prevent delirium may offer a promising avenue to explore for ameliorating cognitive deterioration in AD.
Introduction
Alzheimer’s disease (AD) is a relentlessly progressive and devastating disorder characterized by impaired memory and loss of ability to function independently (1). An estimated 4.5 million older adults in the United States currently have AD (2). Without advances in prevention or treatment, that number is expected to triple to 13.2 million by 2050 (3). Identification of modifiable risk factors for progressive cognitive deterioration in AD has been identified as a top national priority to develop preventive strategies for slowing progression of AD severity and reducing morbidity (4,5).
Delirium is a preventable medical syndrome, which is common among older hospitalized patients (6–8), and characterized by acute change in cognitive status, particularly attention and executive function (9). A recent meta-analysis demonstrated that delirium is a risk factor for death and institutionalization (14). The odds of institutionalization among hospitalized older adults who develop delirium are 2.4 times higher than among those who do not develop delirium (14). The one-year mortality rate for hospitalized seniors who develop delirium is 35–40% (15), comparable to the mortality associated with sepsis and acute myocardial infarction (11). Notably, the adverse impact of delirium on cognition and function among older adults without dementia has been demonstrated extensively in previous research (10,11,16–22).
In persons with AD, the adverse impact of delirium is further magnified. Although delirium complicates care for more than 20% of all hospitalized adults over 65, its prevalence rises to 60–89% of patients with AD. Moreover, older adults with AD are nearly three times more likely to experience delirium than those without dementia (6–8,10–13). Despite its frequency and adverse impact, relatively little attention has been paid to consequences of delirium on cognitive deterioration among patients with AD (23). The few studies completed to date have focused on relatively short-term cognitive outcomes. Fong and colleagues (24) reported significant change in global cognitive function among patients with AD up to six months following delirium. However, that study was unable to address whether this change resulted in an enduring alteration in the trajectory of cognitive function, for which a longer view is necessary (25–27).
The purpose of the present study was to examine the pace of cognitive deterioration for up to five years before and five years after the occurrence of delirium among hospitalized persons with AD. We hypothesized that development of delirium would accelerate the long-term pace of cognitive deterioration in patients with AD, defined by change observed on a test of global cognitive function.
Methods
Study Sample
Patients were drawn from a nested longitudinal cohort of participants enrolled in the Massachusetts Alzheimer’s Disease Research Center (MADRC) patient registry. The MADRC began in 1984 at the Massachusetts General Hospital (MGH) in Boston, MA as a specialized research center devoted to the study of memory impairment. Examining MADRC neurologists diagnosed AD using National Institute of Neurological and Communicative Diseases and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) guidelines (28).
Eligible participants for the present study were those diagnosed with definite, possible, or probable AD who visited the study clinic at least three times between January 1, 1991 and June 30, 2006; who were age 65 and older at the first MADRC visit; and who consented to participation in research (n=895). Data from Medicare and the National Death Index were obtained from January 1, 1991 through December 31, 2007. Chart reviews were conducted for this study from 2007–2009. Of the initial 895 patients, 379 (42%) were hospitalized, a further inclusion criterion for this study. We excluded those enrolled in a Medicare health maintenance organization (n=68) because hospitalizations for this group are not consistently identifiable in Medicare data. Additionally, because hospital records were needed to classify delirium, we excluded patients if their hospital record was unavailable (n=48). These exclusions resulted in a sample of 263 patients with AD.
The informed consent policy of the MADRC includes obtaining joint consent of patients and their next of kin, health care proxy, or legal guardian. The current study, conducted using data from the MADRC and medical record review, merged with Medicare and National Death Index data, was approved by the institutional review boards of MGH, Hebrew Rehabilitation Center, and 46 Massachusetts hospitals where medical records were reviewed.
Cognitive Function
Cognitive function was measured during MADRC clinic visits with the Information-Memory-Concentration (IMC) section of the Blessed Dementia Rating Scale (29). The Blessed Dementia Rating Scale is a brief test of global cognitive function. It has been validated as a sensitive tool for dementia using neuropathological features of AD and consensus diagnosis by psychiatrists (30). The IMC assesses orientation, memory, knowledge of personal information and public events, and concentration. It is scored from 0–37 points based on number of errors. A score of 0–2 is considered typical and a score above 14 indicates major cognitive impairment (31). The Blessed IMC score is highly correlated with other widely used clinical measures of global cognitive function, including the Mini-Mental State Examination (r=0.81–0.85) and the Alzheimer’s Disease Assessment Scale-Cognitive subscale (r=0.82)(32).
Hospitalization
Hospitalizations were retrospectively identified using the Medicare Provider Analysis and Review (MEDPAR) database and confirmed by review of hospital records. The index hospitalization was defined as the first hospitalization occurring during a patient’s follow-up period at the MADRC (time between first and last clinical visit). Because of the study inclusion criteria, all patients had an IMC score before and after their index hospitalization. We defined the baseline MADRC visit as the most proximal MADRC visit preceding the index hospitalization.
Delirium
The primary exposure of interest was delirium. To classify delirium, hospital records from the index hospitalization were examined using a validated chart review method (33). Compared to ratings using direct patient interview with cognitive testing and the Confusion Assessment Method (CAM) (34), the chart review approach has a sensitivity of 74%, specificity of 83%, overall agreement of 82%, and chance-corrected agreement statistic (kappa) of 0.41 (33), which is considered moderate agreement (35). The main reason for missed diagnoses was lack of adequate documentation, which is more likely to occur with mild delirium cases. Sensitivity approached 90% when restricted to more severe cases of delirium (33). Our chart review method has been successfully applied in previous studies (24,36), and retrospective methods like it are increasingly being utilized (e.g., 37).
Covariates
Demographic variables included age, sex, years of education, race (white vs. other races), and marital status. Date of birth was corroborated with Medicare and National Death Index records. Health-related variables included history of smoking (yes vs. no), history of depression (yes vs. no), and the number of comorbidities at the index hospitalization. Comorbidity burden was measured by the Charlson comorbidity index, which was calculated using diagnoses from MADRC, Medicare, and hospital records (38). Dementia-related variables included informant-reported duration of symptoms before diagnosis, speed of initial onset (rapid vs. slow), course (fluctuating/stepwise vs. stable/improving), family history of dementia, and physician-rated dementia severity (range: 0–5, 5 profoundly impaired). This severity scale is highly correlated with Clinical Dementia Rating (Spearman r=0.87, P<0.001)(23,39). Missing data did not exceed 4% for any covariate. To account for the small amount of missing data, we used Bayesian imputation methods with 25 random draws for each observation with missing data (40).
Handling of Time
We defined the time-scale as the number of years from a patient’s index hospitalization. Patients contributed time between their first and last MADRC visits, between which the index hospitalization occurred. We excluded data from MADRC visits that took place beyond five years of the index hospitalization because of sparse data.
Statistical Analyses
Baseline descriptive statistics were used to characterize patients. Distributions of age, sex, race, and education in the sample were compared with those in the National Alzheimer’s Disease Coordinating Center (NACC) to assess generalizability of the present sample to persons with AD living in the United States.
For the main analysis, we used linear regression models with random effects to characterize change in IMC score over time. This approach minimizes bias in parameter estimates and standard errors given the longitudinal repeated measures design. Random effects were estimated for intercept and slope parameters to accommodate individually varying IMC scores, individually varying paces of change, and clustering of repeated observations over time within a patient. Models were stratified by delirium status. To address the possibility that the association of delirium with the pace of cognitive deterioration was limited to follow-up shortly after the index hospitalization, we included discrete breaks in time at the index hospitalization and at one and two years after the index hospitalization. To help interpret the differences in paces of deterioration, we calculated the ratio of pace of cognitive deterioration comparing the group with delirium to the group without delirium. This ratio represents the acceleration in pace of cognitive deterioration attributable to delirium beyond the typical rate of aging in hospitalized patients with AD.
To control for potential confounding, models were adjusted for age, sex, years of education, comorbidity, duration of AD symptoms, family history of dementia, and dementia severity. To address differences in pre-index trajectory and baseline IMC scores by delirium status, we performed a matched analysis by individually matching, with replacement, patients who developed delirium to patients who did not by quintiles of estimated cognitive function at index hospitalization and pace of cognitive deterioration prior to hospitalization. In another sensitivity analysis, we excluded rehospitalized patients to assess whether differences in rehospitalization rates by delirium status affected results.
Analyses were conducted with Mplus statistical software (version 6.12) (41) using robust maximum likelihood estimation that assumes observations for the outcome variable are missing at random, conditional on variables in the model (42). Model fit was evaluated by correlating model-predicted and observed IMC scores, the square of which is the proportion of variability in observed IMC scores explained by the model (the empirical R2) (43). Diagnostic plots of model residuals were checked for normality and random scatter over time. In a sensitivity analysis, we excluded outlying observations to verify that inferences remained unchanged.
Results
Patients were mostly female, most had at least a high school education, and the average age was 78.3 years. Characteristics in this cohort were compared with those available in NACC, a national sample of 74,169 patients with AD. There were no clinically relevant differences with respect to age, sex, education, baseline cognitive function or dementia severity (Table 1).
Table 1.
Comparison of Baseline Characteristics in the Massachusetts Alzheimer’s Disease Research Center (MADRC) Hospitalized Cohort and the National Alzheimer's Disease Coordinating Center (NACC) Cohort
| MADRC Hospitalized Cohort (n=263) |
NACC (n=74,169) |
|
|---|---|---|
| Demographic | ||
| Age, mean ± SD | 78.3 ± 6.0 | 77.9 ± 6.8 |
| Sex, female, n (%) | 150 (57.0) | 44,414 (59.9) |
| Race, White, n (%) | 14 (5.3) | 12,298 (16.7) |
| Years of education, mean ± SD | 13.7 ± 3.6 | 13.2 ± 4.0 |
| Married, n (%) | 168 (63.9) | 40,070 (56.9) |
| Dementia-related | ||
| IMC score at baseline MADRC visit*, mean ± SD | 10.7 (6.3) | -- |
| Mini-Mental State Examination at baseline clinic visit† | -- | 21.5 (7.7) |
| Significant cognitive impairment at baseline‡, n (%) | 68 (27.0) | 18,667 (30.2) |
Note: comparisons of demographic characteristics in MADRC and NACC revealed trivial to small effect size differences (REF 52).
Sample statistics were calculated at the baseline MADRC visit, which was the MADRC visit immediately prior to the index hospitalization.
The baseline NACC visit was the first visit to an AD clinic.
Significant cognitive impairment was considered to be above 14 on the Blessed IMC and below 18 on the Mini-Mental State Examination.
SD: standard deviation. IMC: Blessed Information-Memory-Concentration test. Higher Blessed IMC scores indicate poorer cognitive function.
Patients were evaluated at the MADRC approximately every 6 months over a median duration of 3.2 years of follow-up (range: 0.7–14.5 years). On average, patients had two MADRC visits before their index hospitalization and three visits afterwards. The median time between the baseline MADRC visit and the index hospitalization was 10.5 months (range: 0.1–75.2 months). Missing IMC scores were more likely at MADRC visits after a patient’s index hospitalization (P<0.001), but missingness did not vary by delirium status (P=0.42).
Delirium Incidence
The overall incidence of delirium by chart review in the hospitalized sample was 56.3% (95% confidence interval, CI, 50.2%, 62.3%) (Table 2). Patients who developed delirium during hospitalization were more likely to be male, have less education, be married, smoke, and have greater cognitive impairment at the baseline MADRC visit than patients in the non-delirium group.
Table 2.
Baseline Characteristics of the Hospitalized Alzheimer's Disease Cohort (N=263)
| Delirium (n=148) |
No delirium (n=115) |
P-values for group differences |
|
|---|---|---|---|
| Demographic | |||
| Age, mean ± SD | 78.3 ± 6.1 | 78.3 ± 5.8 | P = 0.92 |
| Sex, female, n (%) | 75 (50.7) | 75 (65.2) | P = 0.02 |
| Race, White, n (%) | 139 (93.9) | 110 (95.7) | P = 0.54 |
| Years of education, mean ± SD | 13.2 ± 3.7 | 14.3 ± 3.5 | P = 0.02 |
| Married, n (%) | 102 (68.9) | 66 (57.4) | P = 0.05 |
| Health-related | |||
| Deyo-Charlson comorbidity index, n (%) | P = 0.09 | ||
| None | 64 (43.2) | 59 (51.3) | |
| One | 41 (27.7) | 36 (31.3) | |
| Two or more | 43 (29.1) | 20 (17.4) | |
| History of smoking, n (%) | 45 (31.5) | 20 (17.5) | P = 0.01 |
| History of depression, n (%) | 32 (26.2) | 25 (28.1) | P = 0.77 |
| Rehospitalization, n (%) | 79 (53.4) | 44 (38.3) | P < 0.01 |
| Dementia-related | |||
| Duration of symptoms, years, mean ± SD | 2.8 ± 1.8 | 3.2 ± 2.6 | P = 0.16 |
| Speed of initial onset*, n (%) | 11 (7.4) | 6 (5.2) | P = 0.47 |
| Course†, n (%) | 4 (2.7) | 2 (1.7) | P = 0.60 |
| Family history of AD, n (%) | 12 (8.1) | 9 (7.8) | P = 0.93 |
| Dementia severity‡, mean ± SD | 2.2 ± 0.8 | 2.0 ± 0.8 | P = 0.06 |
| Length of follow-up, years, mean ± SD | 3.6 ± 2.2 | 4.1 ± 2.7 | P = 0.06 |
SD: standard deviation. AD: Alzheimer’s disease.
Missing data were as follows: education (n=5); history of smoking (n=6); depression (n=52); duration of AD symptoms prior to diagnosis (n=4); dementia severity (n=5).
Rapid versus slow, informant-report
Fluctuating/stepwise versus stable/improving, informant-report
Physician rating of dementia severity (range 0–5, 5 profound)
Cognitive Deterioration
Comparisons of observed and model-implied changes in cognitive trajectory over time by delirium group are summarized in Figure 1. The model was adjusted for age, sex, education, medical comorbidities, duration of AD symptoms, family history of AD, and dementia severity, and fit the data well (empirical R2: 0.89). At baseline, the observed mean IMC score was higher in the delirium group (difference: 1.5 IMC points, 95% CI: 0.2, 2.9), but this difference was not significant after adjustment for potential confounders (Table 3). In addition, the observed and model-implied paces of cognitive deterioration were similar preceding the index hospitalization between the two groups (observed difference: 0.8 IMC points/year, 95% CI: −0.1, 1.7; model-implied difference: 0.6 IMC points/year, 95% CI: −0.1, 1.3) (Table 4).
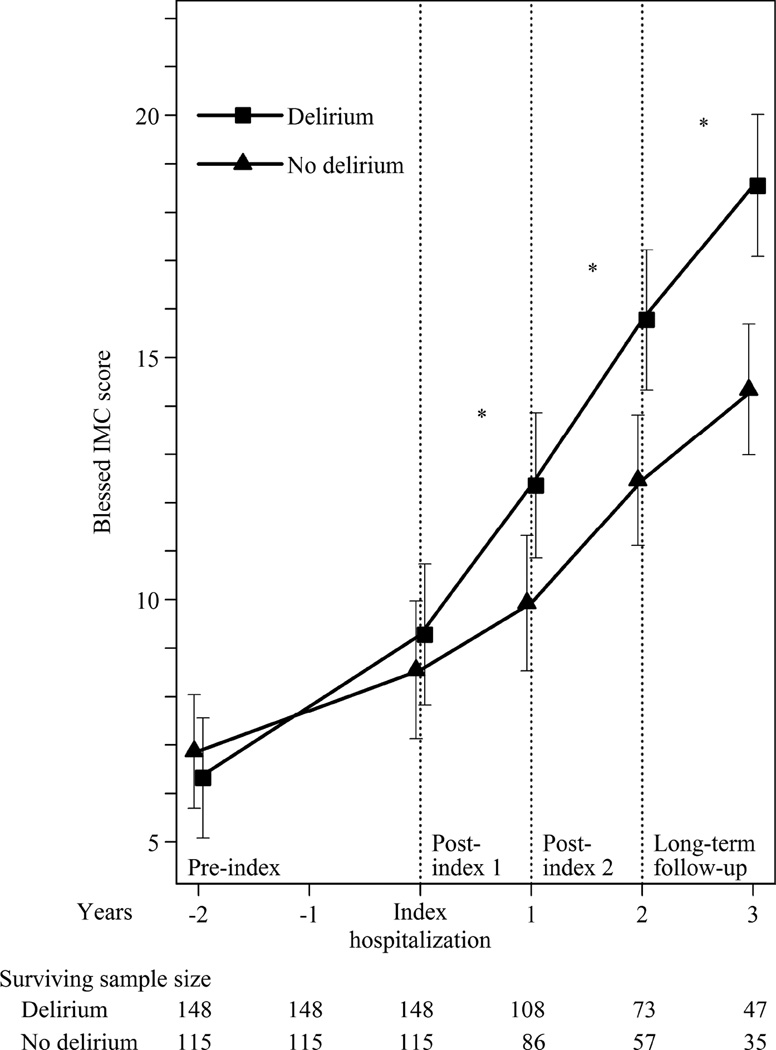
Figure 1. Estimated Trajectory of Cognitive Function with and without Delirium (N=263).
Model-fitted trajectory of cognitive performance with 95% confidence intervals at discrete time points from a random effects regression model of Blessed IMC score during MADRC follow-up. The model is adjusted for age, sex, education, number of comorbidities, duration of AD symptoms, family history of AD, and dementia severity. Missing covariate data were multiply imputed using Bayesian imputation methods with 25 datasets. The timescale depicted includes the middle 80% of study visits nearest the index hospitalization (although statistical models used data up to 5 years before and 5 years after the index hospitalization). The surviving sample size is the number of patients who survived up to each year.
IMC: Blessed Information-Memory-Concentration test; AD: Alzheimer’s disease
Table 3.
Baseline Cognitive Scores by Delirium Group in the Hospitalized Alzheimer’s Disease Cohort (N=263)
| Baseline IMC Score ‡ |
|||
|---|---|---|---|
| Value. | (95% CI) | ||
| Delirium (n = 148) | |||
| Observed | 9.8 | (8.9, 10.7) | |
| Adjusted model† | 9.3 | (7.9, 10.7) | |
| No delirium (n = 115) | |||
| Observed | 8.3 | (7.3, 9.3) | |
| Adjusted model† | 8.5 | (7.4, 9.6) | |
| Group differences (n = 263) | |||
| Observed | 1.5 ‡ | (0.2, 2.9) | |
| Adjusted model† | 0.8 | (−0.8, 2.2) | |
Baseline observed IMC scores taken from the MADRC visit prior to index hospitalization. Adjusted values are predicted (model-implied) values at the index hospitalization.
Adjusted for age, sex, education, medical comorbidities, duration of AD symptoms, family history of AD, and dementia severity.
Test of group differences in Blessed IMC (Information-Memory-Concentration) score between delirium and no delirium groups, P<0.05.
Table 4.
Comparison of Change in Cognitive Score During Follow-up Periods (N=263)
| Annual change in interval from time of index hospitalization |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-index (Years before index hospitalization) |
Post-index 1 (Years 0 to 1) |
Post-index 2 (Years 1 to 2) |
Long term follow-up (Years 2 to 5) |
||||||
| IMC points/ year |
95% CI | IMC points/ year |
95% CI | IMC points/ year |
95% CI | IMC points/ year |
95% CI | ||
| Delirium (n = 148) | |||||||||
| Observed | 1.7 | (1.2, 2.2) | 2.1 | (1.6, 2.6) | 1.7 | (1.3, 2.1) | 2.2 | (1.7, 2.7) | |
| Adjusted model* | 1.4 | (0.7, 2.1) | 3.1 | (2.1, 4.1) | 3.2 | (2.6, 3.8) | 3.1 | (2.5, 3.7) | |
| No delirium (n = 115) | |||||||||
| Observed | 0.9 | (0.5, 1.3) | 1.3 | (0.8, 1.8) | 0.9 | (0.4, 1.4) | 1.7 | (1.2, 2.2) | |
| Adjusted model* | 0.8 | (0.3, 1.3) | 1.4 | (0.2, 2.6) | 2.0 | (1.3, 2.7) | 1.9 | (1.4, 2.4) | |
| Group differences (n = 263) | |||||||||
| Observed | 0.8 | (−0.1, 1.7) | 0.8 † | ( 0.1, 1.6) | 0.8 † | (0.0, 1.7) | 0.5 | (−0.7, 1.7) | |
| Adjusted model* | 0.6 | (−0.0, 1.3) | 1.7 † | (0.3, 3.1) | 1.2 † | (0.5, 2.1) | 1.2 † | (0.5, 1.8) | |
Adjusted for age, sex, esducation, medical comorbidities, duration of AD symptoms, family history of AD, and dementia severity.
Test of group differences in observed, annualized change in Blessed IMC (Information-Memory-Concentration) score between delirium and no delirium groups, P<0.05.
The observed and model-implied pace of cognitive deterioration accelerated during the year following the index hospitalization in both groups (Table 4). This acceleration was greater for the delirium group. The model-implied pace of cognitive deterioration in the delirium group was significantly worse than in the non-delirium group during the year following the index hospitalization (difference: 1.7 IMC points/year, 95% CI: 0.3, 3.1), and remained so through the end of the study period (P=0.003). The ratio of these group differences suggests that delirium accelerates the rate of cognitive deterioration by 2.2-fold in the year following the index hospitalization and by an average of 1.7-fold over the 5 year period following the index hospitalization.
Sensitivity Analyses
To control for baseline cognitive status, a sensitivity analysis using individual matching on baseline IMC score and pre-index trajectory was performed. Findings were consistent with the main analysis. Although an exact match was not achieved, results are likely conservative because the raw mean IMC score in the delirium group was nearly 1 point lower (less impaired) at baseline than in the non-delirium group (9.5 IMC points vs. 10.4 IMC points, respectively). However, both raw and model-implied mean IMC scores were higher (more impaired) in the delirium group by the end of the study.
Patients in the delirium group were more likely to be rehospitalized than patients in the non-delirium group. In the delirium group, 79 (53%) patients were rehospitalized, and in the non-delirium group, 44 (38%) patients were rehospitalized (P<0.01) (Table 2). Most patients were rehospitalized within two years of their index hospitalization, during which time the proportion differed significantly between delirium (n=70, 47%) and non-delirium (n=37, 32%) groups (P=0.01). The proportion of patients rehospitalized after two years did not differ significantly between delirium (n=23, 16%) and non-delirium (n=15, 13%) groups (P=0.60). When we excluded rehospitalized patients from the analysis, the pace of cognitive deterioration during the year following the index hospitalization remained worse in the delirium group (3.5 IMC points/year) than the non-delirium group (1.5 IMC points/year) (P=0.07); these estimates are slightly larger than those including rehospitalized patients (Table 4). This trend continued 1–2 years after index hospitalization (P=0.04) and after 2 years (P=0.02). Thus, the overall findings were similar when rehospitalized patients were excluded.
Discussion
In this prospective study of hospitalized older adults with AD, we investigated the effect of delirium on the long-term pace of cognitive deterioration. Delirium developed in 56% of the patients. The development of delirium during hospitalization was associated with accelerated cognitive deterioration. This effect was independent of dementia severity, demographic characteristics, and the level and pace of cognitive deterioration prior to hospitalization. This acceleration persisted throughout the five-year duration of follow-up. Thus, delirium significantly accelerates the pace of cognitive deterioration in patients with AD from baseline levels, and this acceleration is sustained long-term.
Rehospitalization after the index hospitalization was common during MADRC follow-up (36), and we do not have information about the delirium status during these subsequent hospitalizations. However, rehospitalization is a highly prevalent consequence of delirium, and may serve--at least in part--as a marker or mediator through which delirium affects cognitive trajectory. Patients with delirium tend to be discharged sicker, have worse prognosis for rehabilitation, and have an elevated risk of rehospitalization (11,44). To adjust for rehospitalizations in the analysis may potentially remove part of the total effect of delirium (45). Despite this conjecture, our sensitivity analysis excluding rehospitalizations found little evidence to suggest that rehospitalizations account for the findings in this study.
Several caveats deserve comment. First, the semi-annual time intervals between MADRC visits did not allow us to capture the maximal acute effect of delirium on the pace of cognitive deterioration, which would be most pronounced shortly after delirium onset. However, this limitation is likely to introduce a conservative bias in the present study, thus supporting the robustness of our findings. We included breaks in the cognitive trajectory at one and two years after the index hospitalization to accommodate the potential for only short term deterioration, but we observed deterioration over 5 years. This finding suggests that delirium may fundamentally alter the cognitive trajectory in a sustained fashion. Second, the use of the chart review method is an imperfect strategy for classifying delirium, and likely leads to an underestimate of the true delirium prevalence. However, the method has been shown to have good overall criterion validity. Further, using the published sensitivity and specificity for chart review delirium compared with a standardized full CAM diagnostic interview, we would expect that 41% of the non-delirium group would include false negative cases but only 9% of the delirium group would include false positives (33). While we cannot determine with certainty, observed group differences in the pace of cognitive deterioration are likely conservative estimates.
We used a well-characterized clinical sample of community-dwelling patients with AD and high-quality longitudinal information about cognition, hospitalization, and control variables. Our results challenge highly ingrained attitudes about the transient, reversible nature of delirium in AD. Delirium is recognized by physicians and nurses in fewer than 30% of hospital patients (46–48). This lack of recognition may be attributed at least in part to the widely-held notion that delirium is an unimportant, transient condition that is inevitable during hospitalization and has no long-term significance in persons with AD (9). If delirium worsens the long-term course of cognitive function among persons with AD, then it should be handled as a genuine medical emergency in all cases, and would merit changes to incorporate routine delirium prevention in the standard practice for dementia patients to ensure timely intervention to prevent long-term cognitive deterioration and subsequent outcomes.
Previous research suggests that proven, targeted delirium prevention programs, such as the Hospital Elder Life Program (9), might help to reduce delirium for at least 27% of AD patients, which represents 1.2 million persons in the U.S. and 4 million worldwide (10). Given that the national costs of delirium are estimated to be $40 to $150 billion annually (49), strategies that impact even a fraction of these costs have the potential for far-reaching cost savings for our health care system (50–51), far exceeding those of current pharmacologic treatments for AD. Most importantly, delirium prevention holds the potential to improve quality of life for AD patients and their families.
Acknowledgements
This work was supported in part by grants from the National Institute on Aging (P01AG031720, SKI), the Massachusetts Alzheimer’s Disease Research Center (P50AG005134, SKI, LY), and the Alzheimer’s Association (IIRG-08-88738, SKI). Dr. Gross was supported by a National Institutes of Health Translational Research in Aging fellowship (T32AG023480-07). Dr. Inouye holds the Milton and Shirley F. Levy Family Chair in Alzheimer’s Disease. The contents do not necessarily represent the views of the funding entities. This work is dedicated to the memories of Mary Margaret Brady, Mitsuo Inouye, and Jordan Helfand.
Dr. Gross had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Author contributions are as follows. Study concept and design: Gross, Jones, Inouye. Acquisition of data: Inouye, Jones, Yap. Analysis and interpretation of data: Gross, Jones, Tommet, Quach, Habtemariam, Inouye. Drafting the manuscript: Gross, Jones, Inouye. Critical revision of the manuscript for important intellectual content: Gross, Jones, Inouye, Habtemariam, Fong, Tommet, Quach, Schmitt, Yap. Statistical analysis: Gross. Obtained funding: Inouye. Administrative, technical, and material support: Habtemariam, Tommet, Schmitt, Inouye, Yap. Study supervision: Jones, Inouye.
Footnotes
Previous presentation: This work was presented at the Massachusetts Alzheimer's Disease Research Center & Boston University Alzheimer's Disease Center's 25th Annual Poster Session; February 8, 2012; Boston, MA, USA.
Financial Disclosure: No authors claim financial conflicts of interest. The contents do not necessarily represent views of the funding entities. Funders had no deciding roles in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- 1.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 2.Administration on Aging. A profile of older Americans. Washington, D.C.: Department of Health and Human Services; 2007. [Google Scholar]
- 3.Cummings JL. Alzheimer’s disease. N Eng J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 4.Fratiglioni L. Epidemiology of Alzheimer's disease and current possibilities for prevention. Acta Neurol Scand Suppl. 1996;165:33–40. doi: 10.1111/j.1600-0404.1996.tb05870.x. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services. [Accessed March 1, 2012];Draft national plan to address Alzheimer’s disease. 2012 via http://aspe.hhs.gov/daltcp/napa/NatlPlan.pdf.
- 6.Edlund A, Lundström M, Brännström B, Bucht G, Gustafson Y. Delirium before and after operation for femoral neck fracture. J Am Geriatr Soc. 2001;49:1335–1340. doi: 10.1046/j.1532-5415.2001.49261.x. [DOI] [PubMed] [Google Scholar]
- 7.Fick DM, Foreman M. Consequences of not recognizing delirium superimposed on dementia in hospitalized elderly individuals. J Gerontol Nurs. 2000;26:30–40. doi: 10.3928/0098-9134-20000101-09. [DOI] [PubMed] [Google Scholar]
- 8.Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50:1723–1732. doi: 10.1046/j.1532-5415.2002.50468.x. [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 10.Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 11.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 12.Marcantonio ER, Ta T, Duthie E, Resnick NM. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50(5):850–857. doi: 10.1046/j.1532-5415.2002.50210.x. [DOI] [PubMed] [Google Scholar]
- 13.Weiler PG, Lubben JE, Chi I. Cognitive impairment and hospital use. Am J Public Health. 1991;81:1153–1157. doi: 10.2105/ajph.81.9.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: A meta-analysis. JAMA. 2010;304(4):443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 15.Moran JA, Dorevitch MI. Delirium in the hospitalized elderly. Aust J Hosp Pharm. 2001;31:35–40. [Google Scholar]
- 16.Cole MG. Delirium in elderly patients. Am J Geriatr Psychiatry. 2004;12:7–21. [PubMed] [Google Scholar]
- 17.Francis J, Kapoor WN. Prognosis after hospital discharge of older medical patients with delirium. J Am Geriatr Soc. 1992;40:601–606. doi: 10.1111/j.1532-5415.1992.tb02111.x. [DOI] [PubMed] [Google Scholar]
- 18.Inouye SK, Charpentier P. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852–857. [PubMed] [Google Scholar]
- 19.Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14:87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 20.Katz IR, Curyto KJ, TenHave T, Mossey J, Sands L, Kallan MJ. Validating the diagnosis of delirium and evaluating its association with deterioration over a one-year period. Am J Geriatr Psychiatry. 2001;9:148–159. [PubMed] [Google Scholar]
- 21.McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ. 2001;165:575–583. [PMC free article] [PubMed] [Google Scholar]
- 22.Rahkonen T, Eloniemi-Sulkava U, Paanila S, Halonen P, Sivenius J, Sulkava R. Systematic intervention for supporting community care of elderly people after a delirium episode. Int Psychogeriatr. 2001;13:37–49. doi: 10.1017/s104161020100744x. [DOI] [PubMed] [Google Scholar]
- 23.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s Disease in the United States and the Public Health Impact of Delaying Disease Onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72(18):1570–1575. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole MG, McCusker J, Dendukuri N, Han L. The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003;51:754–760. doi: 10.1046/j.1365-2389.2003.51255.x. [DOI] [PubMed] [Google Scholar]
- 26.Levkoff SE, Evans DA, Litpzin B, et al. Delirium: the occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med. 1992;152:334–340. doi: 10.1001/archinte.152.2.334. [DOI] [PubMed] [Google Scholar]
- 27.McCusker J, Cole M, Dendukuri N, Han L, Belzile E. The course of delirium in older medical inpatients: A prospective study. J Gen Intern Med. 2003;18:696–704. doi: 10.1046/j.1525-1497.2003.20602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA group under the auspices of Department of HHS Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 29.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 30.Heun R, Papassotiropoulos A, Jennssen F. The validity of psychometric instruments for detection of dementia in the elderly general population. Int J Geriatr Psychiatry. 1998;13:368–380. doi: 10.1002/(sici)1099-1166(199806)13:6<368::aid-gps775>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 31.Stern Y, Mayeux R, Sano M, Hauser WA, Bush T. Predictors of disease course in patients with probable Alzheimer's disease. Neurology. 1987;37:1649–1653. doi: 10.1212/wnl.37.10.1649. [DOI] [PubMed] [Google Scholar]
- 32.Thal L, Grundman M, Golden R. Alzheimer’s disease: A correlational analysis of the Blessed-Information-Memory-Concentration Test and the Mini-Mental State Exam. Neurology. 1986;36:262–264. doi: 10.1212/wnl.36.2.262. [DOI] [PubMed] [Google Scholar]
- 33.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST, Jr, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53(2):312–318. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- 34.Inouye SK, Van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 35.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 36.Rudolph JL, Zanin NM, Jones RN, et al. Hospitalization in community-dwelling persons with Alzheimer's disease: frequency and causes. J Am Geriatr Soc. 2010;58(8):1542–1548. doi: 10.1111/j.1532-5415.2010.02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerbier-Colomban S, Bourjault M, Cêtre JC, Baulieux J, Metzger MH. Evaluation Study of Different Strategies for Detecting Surgical Site Infections Using the Hospital Information System at Lyon University Hospital, France. Annals of Surgery. 2012 doi: 10.1097/SLA.0b013e31824e6f4f. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 39.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 40.Rubin DB. Multiple imputation for nonresponse in surveys. New York: J. Wiley & Sons; 1987. [Google Scholar]
- 41.Muthén LK, Muthén BO. Mplus user's guide: Sixth Edition. Los Angeles, CA: Muthén & Muthén; 1998–2010. [Google Scholar]
- 42.Little RJ, Rubin D. Statistical analysis with missing data. New York: Wiley Inc.; 1987. [Google Scholar]
- 43.Singer JD, Willet J. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 44.Dosa D, Intrator O, McNicoll L, Cang Y, Teno J. Preliminary derivation of a Nursing Home Confusion Assessment Method based on data from the Minimum Data Set. J Am Geriatr Soc. 2007;55(7):1099–1105. doi: 10.1111/j.1532-5415.2007.01239.x. [DOI] [PubMed] [Google Scholar]
- 45.Cole SR, Hernán MA. Fallibility in estimating direct effects. International Journal of Epidemiology. 2002;31:163–165. doi: 10.1093/ije/31.1.163. [DOI] [PubMed] [Google Scholar]
- 46.Fick DM, Hodo DM, Lawrence F, Inouye SK. Recognizing delirium superimposed on dementia: assessing nurses' knowledge using case vignettes. J Gerontol Nurs. 2007;33(2):40–47. doi: 10.3928/00989134-20070201-09. quiz 48–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM., Jr Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161(20):2467–2473. doi: 10.1001/archinte.161.20.2467. [DOI] [PubMed] [Google Scholar]
- 48.Laurila JV, Pitkala KH, Strandberg TE, Tilvis RS. Detection and documentation of dementia and delirium in acute geriatric wards. Gen Hosp Psych. 2004;26:31–35. doi: 10.1016/j.genhosppsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27–32. doi: 10.1001/archinternmed.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubin FH, Neal K, Fenlon K, Hassan S, Inouye SK. Sustainability and Scalability of the Hospital Elder Life Program at a Community Hospital. J Am Geriatr Soc. 2011;59(2):359–365. doi: 10.1111/j.1532-5415.2010.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubin FH, Williams JT, Lescisin DA, Mook WJ, Hassan S, Inouye SK. Replicating the Hospital Elder Life Program in a community hospital and demonstrating effectiveness using quality improvement methodology. J Am Geriatr Soc. 2006;54(6):969–974. doi: 10.1111/j.1532-5415.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 52.Cohen J. Statistical Power Analysis for the Behavioral Sciences. second ed. Lawrence Erlbaum Associates; 1988. [Google Scholar]