Abstract
The T cell receptor (TCR) recognizes self or foreign antigens presented by major histocompatibility complex (MHC) molecules. Engagement of the TCR triggers the formation of multi-molecular signalosomes that lead to the generation of second messengers and subsequent activation of multiple distal signaling cascades, such as the Ca+2-calcineurin-NFAT, RasGRP1-Ras-Erk1/2, PKCθ-IKK-NFκB, and TSC1/2-mTOR pathways. These signaling cascades control many aspects of T cell biology. Mechanisms have been evolved to fine-tune TCR signaling to maintain T cell homeostasis and self-tolerance, and to properly mount effective responses to microbial infection. Defects or deregulation of TCR signaling has been implicated in the pathogenesis of multiple human diseases.
Keywords: T cell receptor, T cell activation, T cell development, T cell tolerance, T cell anergy, Mammalian target of rapamycin, Diacylglycerol kinase
Introduction
T cells play a major role in mounting an effective adaptive immune response. Evidence demonstrates that signals from T cell receptor (TCR) are critical for T cell development, homeostasis, activation, and tolerance. T cell development in the thymus requires the expression of a functional TCR, following proper VDJ recombination [1,2]. Moreover, signal strength from the TCR on CD4+CD8+ double-positive (DP) thymocytes dictate the fate of these immature T cells during thymic selection [3]. DP thymocytes that receive weak TCR signals are positively selected and undergo maturation to either CD4 or CD8 single-positive (SP) mature T cells [4], whereas those that receive strong TCR stimuli proceed for apoptosis by negative selection [5]. Negative selection deletes highly self-reactive T cells to establish central T cell tolerance. Thymic selection ensures generation of mature T cells that are mostly self–tolerant, are capable of mounting effective immune responses against foreign antigens, and can maintain homeostasis [6].
In the peripheral lymphoid organs and tissues, naïve T cells are activated upon TCR engagement with foreign peptides presented by dendritic cells and other antigen-presenting cells in response to pathogenic infection [7]. Intracellular signal cascades generated from the TCR leads to plethoric effects, such as increased cell size, transcription, translation, and subsequent production of various cytokines, chemokines, and effector molecules [8]. TCR signaling in mature T cells is also tightly regulated via multiple mechanisms, and dysregulaton of TCR signaling can cause T cell hyperactivation that can lead to autoimmune diseases [9].
Proximal TCR Signal Transduction
TCR complex
Intense investigations during the mid-1980s, using biochemical and immunochemical methods, unveiled the structural components of TCR complex [9]. These efforts, followed by powerful genetic manipulation studies, led to the current knowledge regarding the composition of the TCR complex. The TCR complex is composed of TCRα and β, and the CD3 proteins [10,11]. TCRα and β are generated by somatic VDJ recombination, and TCRαβ dimer is responsible for recognition of peptide-MHC complexes [12,13]. The invariant CD3 proteins, consisting of δ, ε, γ and ζ chains, associate with TCR via hydrophobic interaction [14]. Unlike TCRα and β chains, CD3 proteins are not directly involved in antigen recognition, but their cytosolic domains contain the immunoreceptor tyrosine-based activation motifs (ITAMs) that are responsible for transmitting the TCR signal [15,16]. ITAMs consist of two tyrosine residues flanked by leucine/isoleucine and spaced by bulky aromatic aminoacid. Engagement of TCR triggers ITAM phosphorylation at the tyrosine residues by Src-family protein tyrosine kinases (PTKs), leading to the formation of a proximal signaling complex and activation of downstream signaling events [17].
Proximal protein tyrosine kinases and substrates
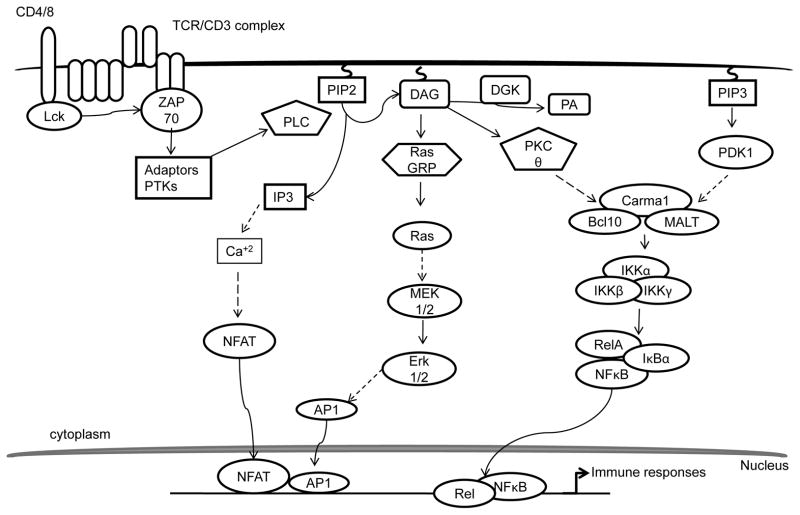
Studies using PTK inhibitors revealed the roles of PTKs in TCR signaling. Cytosolic PTKs, such as the Src-family PTKs, Lck and Fyn, and the Syk-family kinase ζ-associated protein of 70 kDa (ZAP-70), are activated by TCR ligation (Figure 1) [18–20]. Lck activation triggers the phosphorylation of ITAMs of CD3 proteins [21]. Evidence garnered from genetically modified mouse models has demonstrated that Lck is required for T cell development [22]. Dephosphorylation of the inhibitory Y505 residue of Lck by CD45 tyrosine phosphatase facilitates its auto-phosphorylation at Y394, which is a key event for Lck activation [23,24]. In contrast, phosphorylation of Y505 by C-terminal Src kinase (Csk) inhibits Lck activation, which is important for maintaining T cell homeostasis in the absence of TCR engagement [25]. Several lines of evidence have shown that Fyn is also involved in phosphorylation of ITAMs [26]. However, Fyn is not essential for T cell development. Phosphorylated ITAMs form docking sites for several PTKs and other proteins. The phosphorylation of ITAMs at ζ chain recruits ZAP-70 via its SH2 domains, which specifically bind to phospho-tyrosine residues on ITAMs [27,28]. This binding triggers phosphorylation of ZAP-70 by Lck, leading to its activation. Direct association of ZAP-70 with the activated TCR/CD3 complex results in phosphorylation/activation of other proteins and recruitment of adaptors, leading to the formation of a multi-nucleated signaling complex [29].
Figure 1.
Schematic illustration of TCR signaling.
Formation of signalosomes nucleated by adaptor molecules and activation of PLCγ
Several studies have identified the primary role of ZAP-70 phosphorylation in the regulation of phospho lipase C γ1 (PLCγ1) activation, cytosolic Ca+2 influx, and activation of distal signaling pathways, such as NFAT, AP-1 and NF-κB [30]. However, the manner in which ZAP-70 mediate these events was unknown until the characterization of the components of the proximal signaling complex. Rigorous investigations have identified adaptor proteins, protein kinases, and key regulatory molecules that assemble the proximal signalosome for TCR signal transduction. ZAP-70 phosphorylates two key adaptor proteins: Linker for the activation of T cells (LAT), a transmembrane adaptor protein, and the cytosolically localized SH2, containing leukocyte phosphoprotein of 76 kDa (SLP-76) [31,32]. These two adaptors together form a proximal signaling complex and orchestrate the recruitment of various effector proteins [33,34]. Phosphorylated LATs recruit SH2 domain-bearing proteins, including PLCγ1, the p85 regulatory subunit of PI3K, Grb2, and Gads, to form a sub cellular assembly [35,36]. SLP-76 joins the complex by binding to Gads and PLCγ1 via a proline-rich region. Diminished activation of Ras was manifested in LAT- and SLP-76-deficient mouse models and Jurkat T cell line models due to the impairment in the formation of the proximal signaling complex [37,38]. Grb2 is constitutively bound with Son of Sevenless (Sos), which is a dual-specific GTP exchange factor (GEF) for both Ras and Rho GTPases. Grb-Sos complex binds to phosphorylated tyrosines on LAT, leading to activation of Ras. However, LAT mutants, defective of PLCγ1 binding, fail to induce complete activation of Ras, suggesting that Grb2 is not sufficient for Ras activation, at least in T cells [9,39].
Concurrently, SLP-76 also undergoes ZAP-70 mediated phosphorylation, leading to the recruitment of Vav1, a GEF, IL-2-induced tyrosine kinase (Itk, a Tec family of PTK), and other adaptor proteins, such as non-catalytic tyrosine kinase (Nck) and ADAP [40]. Coordinated and precise loading of these effector molecules into the complex is not only important for maintaining the stability of the complex but also for its optimal activation. Ligation of TCR induces cellular polarization by regulating cytoskeleton and integrin affinity. Recruitment of Vav1 to SLP-76 via its SH2 domain activates Rho family of GTPases, such as Rac1, to promote actin reorganization [41]. ADAP also binds to SLP-76 at C-terminally located phosphorylated tyrosines to activate integrin signaling [42].
A key event in connecting proximal signaling to distal signaling branches of the TCR signaling pathway is the activation of PLCγ1 [43]. Mutational analyses of PLCγ1 have demonstrated that association of PLCγ1 with LAT, Gads, and SLP-76 is required for its optimal activation [39]. PLCγ1 activation also requires Itk, which is activated by both SLP-76 and Lck [44,45]. Association of Itk with SLP-76 maintains its close proximity to its substrate PLCγ1, whereas Lck directly phosphorylates Itk at Y511 and promotes its activation [40,46]. Activated Itk phosphorylates PLCγ1, resulting in its activation. Additionally, Rlk, another member of Tec family PTKs, is also known to participate in the activation of PLCγ1, as Itk and Rlk double-deficient mouse models exhibited complete loss of PLCγ1 activity [47,48].
Activated PLCγ1 hydrolyzes membrane-bound phosphatidylinositol 4, 5-bisphosphate (PIP2) into two essential second messengers—inositol-3-phosphate (IP3) and diacylglycerol (DAG) in equal stoichiometry [49]. IP3 and DAG initiate a variety of distal signaling pathways. IP3 triggers the activation of a Ca+2-dependent calcineurin-NFAT signaling pathway. The membrane-bound DAG can activate at least couple of major signaling pathways that include RasGRP1, PKCθ, and PDK1-mediated pathways [49,50].
Distal Signaling Pathway
The Ras-Erk1/2-AP1 pathway
Phosphorylation of extracellular signal-regulated kinase 1/2 (Erk1/2) has been a designated biochemical marker to assess the quality of TCR signaling for decades. However, the exact pathway that regulates activation of Erk1/2 was unclear until the identification of Ras [51,52]. Ras is a small G protein whose activated state is dependent on the presence of GTP. The GTP-bound form is active, while the GDP-bound form is inactive. Ras-GTP activates Raf-1, which, itself, is a serine/threonine kinase. Raf-1 phosphorylates and activates mitogen-activated protein kinase kinase (Mek1/2), which subsequently phosphorylates and activates Erk1/2. Erks trigger the phosphorylation and activation of transcription factor, Elk [53]. Elk induces the expression of c-fos transcription factor, leading to the formation of AP-1, a dimeric complex of Jun/Fos that plays a critical role in immune response.
Two guanine nucleotide exchange factors, RasGRP1 and Sos, are known to be responsible for Ras activation in T cells. RasGRP1 binds to DAG, which induces its membrane translocation and activation [54]. Deficiency of RasGRP1 causes defective activation of the Ras- Erk1/2 pathway, mTOR, and PI3K/Akt [55,56]. RasGRP1 is crucial for conventional αβ T cell and iNKT but not γδ T cell development [55,57,58]. RasGRP1 also mediates the activation of conventional αβ T cells and is critical for γδ T cell activation and expression IL-17 [58]. Different from RasGRP1, Sos is recruited to LAT via Grb, and its activation is independent of DAG. Recent evidence indicates that So spromotes Ras activation and is dependent on RasGRP1; it may also play a selective role for Ras activation downstream of the pre- TCR and is important for β-selection [51,59–61]. Although RasGRP1 is not essential for β-selection, it appears to be important for efficient β selection [58]. Moreover, abnormal expression of RasGRP1 and Ras were noted in systemic lupus erythematous (SLE) patients and in SLE mouse models, indicating that the RasGRP1 and Ras pathway may be implicated in the generation of SLE [62], furthermore suggesting the therapeutic potential in targeting this pathway.
IP3-Ca+2-NFAT pathway
Calcium is an essential second messenger and plays key roles in the activation of T cells. IP3, generated by PLCγ1, binds to its receptor on the endoplasmic reticulum, leading to a release of intracellular calcium stores. It has been noticed for years that calcium depletion in the ERs can trigger an influx of extracellular Ca+2 into T cells through a calcium release-activated calcium channel (CRAC) [63]. Only until recently, it was found that ERs constitutively express a transmembrane protein called stromal interaction molecule (STIM), which senses the intracellular Ca+2 store levels and facilitates the Ca+2 influx from Orai1 type CRAC channels [64].
Ca+2 influx triggers the activation of downstream events that subsequently activate transcriptional targets [50]. Ca+2 activates calcineurin, a protein phosphatase, leading to dephosphorylation and subsequent nuclear translocation of NFAT. Nuclear NFATs, together with AP-1 transcription factors (Jun/Fos) derived from DAG signaling, bind to cognate DNA response elements and induce the expression of genes related to T cell activation such as IL-2 and other effector molecules. However, in the absence of DAG-mediated signaling, selective activation of the Ca+2-calcineurin-NFAT pathway induces T cell anergy by promoting the expression of anergy-relative molecules, such as several E3 ubiquitin ligases and DGKα [65–68]. In addition to NFAT, Ca+2 also activates calmodulin-dependent kinase (CaMK), which in turn activates transcription factor cyclic-AMP-responsive- element-binding protein (CREB) and myocyte enhancer factor 2 (MEF2) to mediate T cell activation [69]. Besides regulating T cell activation and anergy, Ca+2-mediated signaling is also important for positive selection of thymocytes and for iNKT cell development [70–73]. Impaired Ca+2 signaling causes SCID in humans, due to the missense mutation in Orai1, which leads to aberrant calcineurin- NFAT activation [74,75].
The PKCθ-IKK-NFκB pathway
In addition to activating RasGRP1, DAG also triggers PKCθ signaling. PKCθ belongs to a class of novel-type PKCs that are primarily activated by DAG, through the C1 lipid-binding domain [76]. Although T cells express other forms/classes of PKCs, PKCθ plays major and non-redundant roles in T cell activation, particularly in the peripheral immune compartment [77,78]. Intense investigations have deciphered the underlying mechanisms by which IKKs are regulated. Activated PKCθ phosphorylates adaptor proteins, CARMA1, Bcl10, and MALT1, which subsequently triggers the formation of a tri-molecular complex called the CARMA1/Bcl10/MALT1 (CBM) complex [79–81]. Association of Bcl10 with MALT1 may recruit E3 ubiquitin ligase, called tumor necrosis factor receptor-associated factor 6 (TRAF6), to degrade IKKγ, or NF-κB essential modifier (NEMO), a regulatory protein of the IKK complex [82,83]. This relieves the inhibition on catalytic IKKs α and β, leading to phosphorylation of IκB. Phosphorylation of IκB by IκB kinases (IKK) induces ubiquitination and degradation of IκB. As result, NF-κB is released and translocated into the nucleus to regulate gene expression [84,85]. Genetic evidence has clearly demonstrated the critical roles of the PKCθ-IKK-NFκB pathway for T cell activation, survival, homeostasis, and effector function along with iNKT and regulatory T cell (Treg) development [86,87]. Deregulation of this pathway can cause several consequences, such as severe combined immunodeficiency (SCID), autoimmunity, lymphoma, and defective T cell activation and survival [88–90].
TSC1/2-mTOR signaling
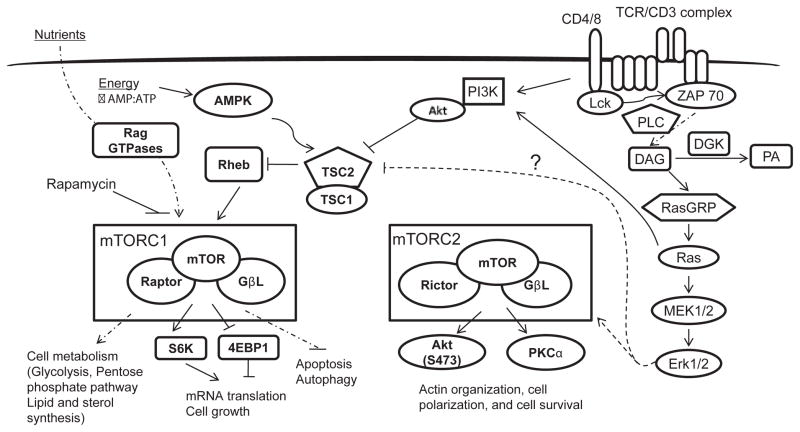
Mammalian target of rapamycin (mTOR) has evolved as a master regulator of cell growth, proliferation, and metabolism by integrating extracellular signals/stimuli, such as nutrients, energy, stress, and growth factors/cytokines. mTOR, a serine/threonine kinase, exists in two functionally distinct complexes— namely, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2)— to phosphorylate and activate a unique choice of substrates [91]. mTORC1 complexes with raptor, an adaptor protein, and other accessory proteins to phosphorylate substrates p70 ribosomal S6 kinase1 (S6K1), 4 elongation factor-binding protein 1 (4E-BP1), and Unc-51 like kinase (ULK1) in a rapamycin-sensitive and dependent manner (Figure 2) [92–94]. However, mTORC2 is insensitive to acute rapamycin treatment, and it associates with rictor to phosphorylate PKCα and Akt on serine 473 residue [95,96]. Phospho-S6K1 phosphorylates S6, a key component of ribosome, whose phosphorylation is critical for the reconstitution of ribosome and for protein synthesis. Hyper-phosphorylation of 4E-BP1 releases the inhibition on eIF4E, a key eukaryotic translational initiation factor, leading to initiation of cap-dependent translation [97]. In contrast, the mTORC2 substrates PKCα and Akt, upon phosphorylation, directly act as signaling proteins to modulate various cellular processes, which include cytoskeletal reorganization and cell survival [98]. mTORC1 is activated by a Ras-like, small GTPase, called Rheb. The TSC1/2 heterodimeric complex is a key negative regulator of mTORC1 [99]. TSC2 is a GTPase-activating protein, by which TSC2 inhibits the activation of Rheb [100]. Phosphorylation of TSC2 by Akt and Erk induces inactivation of TSC, releasing the inhibition on Rheb, and leading to the activation of mTOR [101,102]. TSC1 binds to and stabilizes TSC2.
Figure 2.
mTOR signaling and its regulation in T cells.
TCR engagement induces the activation of both mTORC1 and mTORC2 through the PI3K-Akt and DAG-RasGRP1-Ras-Mek1/2- Erk1/2 pathways [56,103]. DGKα and ζ inhibit mTORC1 and mTORC2 activation via negative control of the DAG-RasGRP1-Ras- Erk1/2 pathway [56]. Studies from cell line models have shown that Akt or Erk1/2 can phosphorylate TSC2 and promote the activation of mTORC1 [101]. The underlying mechanisms by which mTORC2 is regulated are not known.
Using rapamycin, shRNA, and genetically engineered mouse models, multiple studies have demonstrated that mTOR regulates the key processes in adaptive immunity. Inhibition of mTORC1 with rapamycin induces T cell anergy [104]. In contrast, elevated mTORC1 signaling, due to TSC1 deficiency, prevents T cell anergy [105].
Signals from both TCR and specific cytokine milieus play a crucial role in the generation of appropriate CD4+ helper effector cells (Th) [106]. mTORC1 and mTORC2 differentially regulate the generation of effector T cell types [107]. Naïve CD4 T cells derived from rictor-deficient mice are impaired in differentiation into IL-4-producing Th2 and IFNγ-producing Th1 effector cells, whereas T cells from Rheb or raptor-deficient mice are defective in Th17 differentiation [108,109]. mTORC2 induces the phosphorylation of PKCθ at S660/676 and Akt at S473 to promote Th2 and Th1 differentiation, respectively [109]. mTORC1 promotes Th17 differentiation by down-regulating Socs5 to promote STAT3 activity [108]. In addition to controlling Th differentiation, mTORC1 positively controls primary but inhibits memory CD8 T cell-mediated, anti-viral immune response and regulates the T cell trafficking by modulating L-selectin (CD62L) and CCR7 [103,110,111]. Given the importance of mTOR, its activity also needs to be tightly controlled. Indeed, several recent studies have demonstrated that dysregulation of mTOR signaling, due to deficiency of TSC1, results in increased T cell death, resistance to T cell anergy, loss of T cell quiescence, and the abnormal survival and function of macrophages and mast cells [105,112–119].
TCR Signaling in Tregs
Tregs mediated immune suppression is crucial for immune tolerance, dysregulation of which results autoimmunity [120–122]. Natural Tregs (nTreg) are a subset of T cells whose lineage differentiation is primarily governed by Forkhead box transcription factor FOXP3 in the thymus [123]. In addition, FOXP3 can be induced in peripheral conventional T cells to generate inducible Tregs (iTreg) especially in mucosal tissues. Tregs constitutively express the high affinity IL-2 receptor α-chain (CD25), GITR, and CTLA-4 [124–126]. They also produce suppressive cytokines such as TGF-β and IL-10 [127].
Emerging investigations have shed light on some of key aspects of TCR signaling in Treg development and function. Like conventional T cells, both avidity and duration of TCR signals play important roles in determining Treg differentiation. The signals received from TCR, IL-2R, TGF-βR and retinoic acid play pivotal roles in induction of AP-1, NFAT, and NF-κB that modulate OXP3 transcription and subsequently Treg differentiation [128]. The proximal TCR signaling components are absolutely required for Treg development and function. Mice deficient of Lck, ZAP-70, and LAT are devoid of both conventional T cells and Tregs, suggesting that these two subsets of T cells at least share some common promoximal components for TCR signal transduction. ZAP-70 mutation in its ITAM binidng SH2 domain leads to decreased nTreg, resulting in autoimmunity [129,130]. Impaired PLCγ1 activation also impacts the Treg development and function. A mutation in PLCγ1 binding site of LAT (Y136F) causes severe impairment in Treg development due to attenuation of downstream signals such as Ca+2 influx [131].
Multiple distal signaling cascades play important roles in Treg development and function. Ca+2 influx and its downstream signaling play essential roles for generating and maintaining the sustainable calcineurin-NFAT signaling pathway that is important for Treg development and function [132]. Combined deficiency of endoplasmic reticulum Ca+2 sensors STIM1 and STIM2 markedly reduces Treg cellularity and impairs immune suppression [133]. However, the ablation of Orai Ca+2 channels appears not affecting Treg development and function suggesting that these channels may not be crucial for Ca+2 entry in Treg [134]. The NF-κB signaling pathway is another major contributor of nTreg development. Specific ablation of PKCθ, CARMA1, Bcl 10 or TAK in mice inhibits the generation of nTregs in the thymus [135–138]. In contrast to its role for nTreg development, PKCθ inhibits Treg function as blockade of PKCθ activity enhances Treg-mediated suppression [139]. Furthermore, PKCθ signaling negatively regulates iTreg function via Akt-Foxo1/3A axis [140]. Tregs display decreased Erk1/2 activation [141]. Although the RasGRP1-Ras-Erk1/2 pathway plays indispensable roles for postive selection of conventional T cells in the thymus, deficiency of RasGRP1 does not cause a drastic decrease of Tregs or γδ T cells [58,142]. The independence of Treg development on RasGRP1 is consistent with the notion that Tregs are selected with relatively strong TCR signaling during positive selection and RasGRP1 is preferentially required for positive selection of developing T cells with low affinity TCRs to self-peptide/MHC complexes [143]. Compared with conventional T cells, Tregs have decreased PI3K and mTOR activities [56,144]. Although mTOR signaling plays pivotal roles during T cell activation, the PI3K-Akt pathway and mTOR signaling appear to inhibit Treg differentiation and function [145]. Mouse model with inactive form of PI3K-p110 catalytic subunit exhibited increased thymic Treg cellularity [146]. Inhibition of mTOR by rapamycin or genitic deficiency of mTOR induces Treg differentiation in vitro [147–151]. Overall, both common signaling pathways and distinct regulations of TCR signaling exist between conventional T cells and Tregs. However, the limited availability of Tregs prevents in depth analyses of TCR in this subset of T cells.
Negative Control of TCR Signaling
TCR signaling play key roles in T cell development and function, deregulation of TCR signal has been proven detrimental to the host. Various negative regulatory molecules including phosphatases, ubiquitin ligases, and lipid kinases play key roles in T cells by fine-tuning TCR signaling.
Phosphatases
Formation of proximal signaling complex is initiated through phosphorylation and activation of Lck. The SH2-domain, containing protein tyrosine phosphatase (SHP-1), inactivates Lck by dephosphorylating its active site [152]. SHP-1 dampens TCR signaling events, both at early and late time points by mediating Lck dephosphorylation. TCR ligation of antagonists or weak antigens induce Lck-mediated phosphorylation as well as activation of SHP-1. As a result, SHP-1 dephosphorylates Lck, leading to its rapid inactivation. At later time points, TCR stimulation induces the expression of adhesion molecules called carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1). Phosphorylated immunoreceptor tyrosine-based inhibitory motifs (ITIM) of CEACAM1 recruit SHP-1 and dephosphorylate Lck to terminate the TCR signaling pathway [153,154]. Under optimal conditions, Erk1/2 indirectly regulates the SHP-1 activity. Erk1/2 phosphorylates Lck at S59 to prevent the binding of SHP-1, leading to sustained TCR signaling [155].
Suppressor of T cell receptor signaling (Sts), another novel class of proteins, is hypothesized to possess protein phosphatase activity. Sts-1 targets Syk, whereas Sts-2 dephosphorylates ZAP-70 [156,157]. Genetic ablation of these two isoforms resulted in T cell hyperproliferation and autoimmunity. The underlying mechanisms are not known but may be uncovered in future studies. In addition to dephosphorylating Lck, phosphatases act on many other downstream signaling events, such as dephosphorylating PIP3 by Pten and MAPKs by dual specificity phosphatases, to negatively control TCR signaling [158,159].
Ubiquitination and degradation: E3 ubiquitin ligases
Interest has been growing in the ability to understand the roles of protein degradation mechanisms that regulate TCR signaling. Protein degradation occurs mainly by ubiquitin-mediated proteosomal and/ or lysosomal mediated processes. Ubiquitination or conjugation of ubiquitin (Ub) to protein involves a cascade of enzymatic reactions [160]. First, Ub activating enzyme, E1 initiates the formation of activated Ub from an inactivated state. Second, activated Ubis further transferred to a Ub-conjugating enzyme called E2. Finally, E3 ubiquitin ligases couple the transfer of the activated Ub from E2 to the target proteins. Thus, E3 ligases facilitate the actual attachment of Ub to substrates by defining the substrate specificity [161]. Although the list of E3 ligases is increasing, the manners which specify the binding to their substrates are still not well defined [162].
Several studies have demonstrated that certain E3 ligases function as regulators of T cell tolerance and that their mutation or deletion can lead to the generation and activation of autoreactive T cells, subsequently resulting in the onset of autoimmunity [163]. E3 ligases primarily regulate the T cell activation by regulating TCR signaling to induce both central and peripheral T cell tolerance [164,165]. Casitas B cell lymphoma (Cbl-b) is a well-studied E3 ligase belonging to the RING finger family, which marks numerous target proteins by ubiquitination to initiate protein degradation. Cbl-b, along with other members of the Cbl family such as the protein c-Cbl, promotes negative regulation in the TCR signaling pathway [166]. Cbl proteins ubiquitinate TCRζ and several proteins in a proximal signaling complex and target them for degradation to attenuate the TCR signaling [167]. The intracellular domain of the TCR/CD3ζ chain is rich in multiple lysine residues, acting as a substrate for ubiquitination. Cbl-b triggers the conjugation of Ub to the TCRζ chain in activated T cells to sequester or degrade surface TCRs, thus resulting in the attenuation of TCR signaling. ZAP-70 facilitates Cbl-mediated TCRζ ubiquitination by acting as an adaptor [168]. Downstream of TCR, Src- and Syk-family PTKs undergo Cbl-mediated ubiquitination, followed by degradation, to dampen the TCR signaling. Furthermore, Cbl-b interacts with Vav1, specifically in naïve T cells, and this interaction is important for maintaining negative regulation of TCR signaling in the absence of antigen engagement [169]. In the absence of a DAG-AP1 signaling pathway, Ca+2 flux activates NFAT, which positively regulates the expression of Cbl-b via Egr transcription factors [170].
TRAF6 is a member of the RING finger family E3 ligase whose functions were well documented in APCs but poorly understood in T cells [171]. Similar to Cbl-b, the genetic ablation of TRAF6 had also been shown to induce T cell hyperproliferation [172]. The gene related to anergy in lymphocytes (GRAIL) is a transmembrane protein, the expression of which regulates T cell activation. Anergic T cells express high levels of GRAIL, accompanied by a characteristic decrease in IL-2 production [173]. Itch, a HECT family ubiquitin ligase, is known to regulate T cell tolerance, particularly T cell anergy, by modulating TCR signaling [174]. Itch, by virtue of its ubiquitin ligase activity, targets PLC-γ1 and PKCθ [175,176]. Further, Itch also marks Jun, causing diminished activation of AP-1 [177]. This is at least one of the mechanisms that accounts for autoimmune and proinflammatory-prone phenotypes in Itch-deficient mouse models. Roquin was identified as a novel RING finger E3 ligase and has been shown to play a role in T cell tolerance [178]; however, underlying mechanisms are yet to be elucidated. Because Cbl-b, GRAIL and Itch possess overlapping functions, it has been postulated that these E3 ligases translocate to immune synaptic regions where they target TCR signaling proteins [179]. This results in compromised immunological synaptic function and subsequent diminished TCR signaling.
Diacyglycerol kinases
Given the crucial roles of DAG in TCR signaling, it is important to understand the mechanisms through which DAG is regulated. DGKs are lipid kinases that phosphorylate DAG to produce phosphatidic acid (PA), thereby regulating the subcellular DAG levels [180,181]. As a result, TCR induced Ras-Mek-Erk signaling is attenuated when DGK activity is increased [182,183]. Ten isoforms of DGKs are expressed in mammals, consisting of the kinase domain and at least two cysteine-rich C1 domains that share the DAG/phorbol ester-binding region of PKCs [118,181]. The genetic ablation of DGKα or ζ, DGK isoforms, expressed at high levels in T cells, resulted in increased activation of the Ras-Mek-Erk-AP1 pathway, the PKCθ-NFκB pathway, and mTOR signaling, which in turn led to T cell hyperactivation, loss of T cell anergy, and enhanced primary but impaired anti-viral responses by CD8 T cells [49,67,68,184–186]. Moreover, deficiency of both DGKα and ζ resulted in a severe T cell developmental blockade at the DP stage, indicating that these two isoforms perform redundant roles in T cells. Interestingly, treatment of DGKα and ζ double-deficient thymi with phosphatidic acid partially restored T cell development, suggesting that DGKα and ζ may function as a signal switch by terminating DAG mediated signaling and at the same time initiating PA-mediated signaling [187,188]. A particularly important question to be addressed is the identity of the downstream effector molecules that bind to and mediate PA signaling.
Dysregulation of TCR Signaling in Diseases
Given the importance of the TCR signal in T cell development, activation, and tolerance, it is not surprising that dysregulation in TCR signaling causes or contributes to various diseases. Defects in TCR signaling may lead to failure in generating optimal immune responses, which can lead to immune deficiency. For example, mutations on genes that encode for CD3δ, ε, and ζ chain results in SCID, which is characterized by the absence or defective function of T cells [189]. Defective expression of Lck is associated with suboptimal activity that in turn leads to immunodeficiency in both humans and mice [190,191]. Deficiency or mutation of ZAP-70 leads to a rare form of SCID in humans, with specific absence of peripheral CD8+ cells and functionally impaired CD4+ cells [192,193]. However, a mouse deficient in ZAP-70 is devoid of both CD4+ and CD8+, as its development was blocked at the CD4+ CD8+ DP stage.
Altered TCR signaling can cause abnormal thymic selection or uncontrolled T cell activation, which can cause autoimmune diseases. Reduced expression of CD3ζ is associated with SLE and rheumatoid arthritis (RA) [194–196]. Altered signal transduction from ZAP-70 causes aberrant changes in tyrosine phosphorylation and Ca+2 mobilization and, subsequently, thymic selection [197]. Mutations observed in ZAP-70 were transitions and deletions leading to transcriptional loss, destabilized protein, and loss of kinase function. Spontaneous mutations in the SH3 domain of ZAP-70 in mice produce autoimmune arthritis, which resembles human RA [129].
A deficiency of CD45 expression results in SCID, similar to its phenotype in humans [198,199]. Patients carrying certain CD45 polymorphisms were predisposed to multiple sclerosis (MS). A similar protein tyrosine phosphatase PTPN22 (LYP/PEP) acts on several of its substrates, including Lck, ZAP-70, and Syk, to inactivate a TCR signaling pathway by direct dephosphorylation [200]. A genetic variant of human PTPN2 is involved in a range of autoimmune diseases, such as type1 diabetes mellitus, SLE, RA, Graves’ disease, and Hashimoto thyroiditis [201–205].
Conclusions
Initiation of T cell immune responses begins with the generation of signals from the TCR, which is further conveyed to various downstream effectors to shape T cell development, activation, homeostasis, and tolerance. Advances in biochemical, molecular biological, and genetic tools/reagents have significantly contributed to the appreciation of today’s TCR signaling. While we have focused on discussing proximal TCR signaling events, downstream signaling cascades, and negative control of TCR signaling, but the transduction and regulation of TCR signal is much more complex. Each of the signaling events may be controlled by counter-mechanisms to ensure proper TCR signaling and T cell homeostasis and activation. Cross talks among distal TCR signaling pathways and feedback mechanisms add to the complexity of regulation. Current knowledge of TCR signaling is brought to the stage mainly based on naïve conventional αβ T cells and T cell line models. TCR signaling in other rare but important populations of T cells, such as memory T cells, Tregs, and NKT cells, remains poorly understood. Identification of distinct regulation of TCR signaling among the different T cell subsets allows selective modulation of T cell responses. Although traditional immunoblotting and genetic approaches have greatly advanced our understanding of TCR signaling, new imaging technologies should illustrate the dynamic signal transduction and its regulation in different subcellular compartments. Comprehensive and in-depth knowledge of TCR signaling may improve the understanding of the pathogenesis and aid in the design of effective therapies for immunological diseases including autoimmunity, immunodeficiencies, and cancers.
Acknowledgments
We regret that we could not cite all important literatures due to space limitation. This work was supported by funding from National Institutes of Health (R01AI076357, R01AI079088, and R01AI101206) and the American Cancer Society (RSG-08-186-01-LIB) to X-P.Z.
Abbreviations
- PIP3
Phosphatidylinositol-tris-phosphate
- MHC
Major Histocompatibility Complex
- Lck
Lymphocyte-specific protein tyrosine kinase
- Syk
Spleen tyrosine kinase
- SH2
Src Homology 2
- SH3
Src Homology 3
- PI3K
Phosphatidylinositol 3-kinases
- Grb2
Growth factor receptor-bound protein 2
- Gads
Grb2-like adaptor protein
- NFAT
Nuclear Factor of Activated T-cells
- AP-1
Activator Protein 1
- NF-κB
Nuclear Factor-Kappa B
- ADAP
Adhesion and Degranulation-promoting Adapter Protein
- Rlk
Resting lymphocyte kinase
- RasGRP1
RAS Guanyl-Releasing Protein 1
- PKCθ
Protein Kinase C θ
- PDK1
3-phosphoinositide dependent protein kinase-1
- Elk
Ets-related protein
- mTOR
Mammalian Target of Rapamycin
- iNKT
invariant Natural Killer T cell
- CCR7
Chemokine receptor 7
- Bcl10
B-cell lymphoma/leukemia 10
- MALT1
Mucosa-Associated Lymphoid Tissue lymphoma translocation protein 1
- IκB
Inhibitor of Kappa B
- Treg
regulatory T cells
- SLE
Systemic Lupus Erythematous
- RA
Rheumatoid Arthritis
- SCID
Severe Combined Immunodeficiency
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Krangel MS. Mechanics of T cell receptor gene rearrangement. Curr Opin Immunol. 2009;21:133–139. doi: 10.1016/j.coi.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Möröy T, Karsunky H. Regulation of pre-T-cell development. Cell Mol Life Sci. 2000;57:957–975. doi: 10.1007/PL00000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayes SM, Love PE. Strength of signal: a fundamental mechanism for cell fate specification. Immunol Rev. 2006;209:170–175. doi: 10.1111/j.0105-2896.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 4.Takahama Y, Nitta T, Mat Ripen A, Nitta S, Murata S, et al. Role of thymic cortex-specific self-peptides in positive selection of T cells. Semin Immunol. 2010;22:287–293. doi: 10.1016/j.smim.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 6.Zúñiga-Pflücker JC. T-cell development made simple. Nat Rev Immunol. 2004;4:67–72. doi: 10.1038/nri1257. [DOI] [PubMed] [Google Scholar]
- 7.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 8.Pearce EL. Metabolism in T cell activation and differentiation. Curr Opin Immunol. 2010;22:314–320. doi: 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedrick SM, Cohen DI, Nielsen EA, Davis MM. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984;308:149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- 11.Malissen M, Minard K, Mjolsness S, Kronenberg M, Goverman J, et al. Mouse T cell antigen receptor: structure and organization of constant and joining gene segments encoding the beta polypeptide. Cell. 1984;37:1101–1110. doi: 10.1016/0092-8674(84)90444-6. [DOI] [PubMed] [Google Scholar]
- 12.Saito T, Germain RN. Predictable acquisition of a new MHC recognition specificity following expression of a transfected T-cell receptor beta-chain gene. Nature. 1987;329:256–259. doi: 10.1038/329256a0. [DOI] [PubMed] [Google Scholar]
- 13.Dembić Z, Haas W, Weiss S, McCubrey J, Kiefer H, et al. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature. 1986;320:232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- 14.Borst J, Coligan JE, Oettgen H, Pessano S, Malin R, et al. The delta-and epsilon-chains of the human T3/T-cell receptor complex are distinct polypeptides. Nature. 1984;312:455–458. doi: 10.1038/312455a0. [DOI] [PubMed] [Google Scholar]
- 15.Samelson LE, Patel MD, Weissman AM, Harford JB, Klausner RD. Antigen activation of murine T cells induces tyrosine phosphorylation of a polypeptide associated with the T cell antigen receptor. Cell. 1986;46:1083–1090. doi: 10.1016/0092-8674(86)90708-7. [DOI] [PubMed] [Google Scholar]
- 16.Letourneur F, Klausner RD. Activation of T cells by a tyrosine kinase activation domain in the cytoplasmic tail of CD3 epsilon. Science. 1992;255:79–82. doi: 10.1126/science.1532456. [DOI] [PubMed] [Google Scholar]
- 17.Letourneur F, Klausner RD. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- 18.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 19.Siegel JN, Egerton M, Phillips AF, Samelson LE. Multiple signal transduction pathways activated through the T cell receptor for antigen. Semin Immunol. 1991;3:325–334. [PubMed] [Google Scholar]
- 20.Chan AC, Desai DM, Weiss A. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu Rev Immunol. 1994;12:555–592. doi: 10.1146/annurev.iy.12.040194.003011. [DOI] [PubMed] [Google Scholar]
- 21.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 22.Molina TJ, Kishihara K, Siderovski DP, van Ewijk W, Narendran A, et al. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 23.Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol Rev. 2009;228:9–22. doi: 10.1111/j.1600-065X.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- 24.McNeill L, Salmond RJ, Cooper JC, Carret CK, Cassady-Cain RL, et al. The differential regulation of Lck kinase phosphorylation sites by CD45 is critical for T cell receptor signaling responses. Immunity. 2007;27:425–437. doi: 10.1016/j.immuni.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 26.Samelson LE, Phillips AF, Luong ET, Klausner RD. Association of the fyn protein-tyrosine kinase with the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990;87:4358–4362. doi: 10.1073/pnas.87.11.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 28.Irving BA, Chan AC, Weiss A. Functional characterization of a signal transducing motif present in the T cell antigen receptor zeta chain. J Exp Med. 1993;177:1093–1103. doi: 10.1084/jem.177.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Kadlecek TA, Au-Yeung BB, Goodfellow HE, Hsu LY, et al. ZAP-70: an essential kinase in T-cell signaling. Cold Spring Harb Perspect Biol. 2010;2:a002279. doi: 10.1101/cshperspect.a002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macián F, López-Rodríguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–2489. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 32.Bubeck Wardenburg J, Fu C, Jackman JK, Flotow H, Wilkinson SE, et al. Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. J Biol Chem. 1996;271:19641–19644. doi: 10.1074/jbc.271.33.19641. [DOI] [PubMed] [Google Scholar]
- 33.Sommers CL, Samelson LE, Love PE. LAT: a T lymphocyte adapter protein that couples the antigen receptor to downstream signaling pathways. Bioessays. 2004;26:61–67. doi: 10.1002/bies.10384. [DOI] [PubMed] [Google Scholar]
- 34.Koretzky GA, Abtahian F, Silverman MA. SLP76 and SLP65: complex regulation of signalling in lymphocytes and beyond. Nat Rev Immunol. 2006;6:67–78. doi: 10.1038/nri1750. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Trible RP, Samelson LE. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 36.Liu SK, Fang N, Koretzky GA, McGlade CJ. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr Biol. 1999;9:67–75. doi: 10.1016/s0960-9822(99)80017-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, et al. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 38.Pivniouk V, Tsitsikov E, Swinton P, Rathbun G, Alt FW, et al. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell. 1998;94:229–238. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]
- 39.Sommers CL, Lee J, Steiner KL, Gurson JM, Depersis CL, et al. Mutation of the phospholipase C-gamma1-binding site of LAT affects both positive and negative thymocyte selection. J Exp Med. 2005;201:1125–1134. doi: 10.1084/jem.20041869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bunnell SC, Diehn M, Yaffe MB, Findell PR, Cantley LC, et al. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J Biol Chem. 2000;275:2219–2230. doi: 10.1074/jbc.275.3.2219. [DOI] [PubMed] [Google Scholar]
- 41.Dombroski D, Houghtling RA, Labno CM, Precht P, Takesono A, et al. Kinase-independent functions for Itk in TCR-induced regulation of Vav and the actin cytoskeleton. J Immunol. 2005;174:1385–1392. doi: 10.4049/jimmunol.174.3.1385. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, McCann FE, Gordan JD, Wu X, Raab M, et al. ADAP-SLP-76 binding differentially regulates supramolecular activation cluster (SMAC) formation relative to T cell-APC conjugation. J Exp Med. 2004;200:1063–1074. doi: 10.1084/jem.20040780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beach D, Gonen R, Bogin Y, Reischl IG, Yablonski D. Dual role of SLP-76 in mediating T cell receptor-induced activation of phospholipase C-gamma1. J Biol Chem. 2007;282:2937–2946. doi: 10.1074/jbc.M606697200. [DOI] [PubMed] [Google Scholar]
- 44.Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PL. Tec family kinases in T lymphocyte development and function. Annu Rev Immunol. 2005;23:549–600. doi: 10.1146/annurev.immunol.22.012703.104743. [DOI] [PubMed] [Google Scholar]
- 45.Qi Q, August A. Keeping the (kinase) party going: SLP-76 and ITK dance to the beat. Sci STKE. 2007;2007:pe39. doi: 10.1126/stke.3962007pe39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bogin Y, Ainey C, Beach D, Yablonski D. SLP-76 mediates and maintains activation of the Tec family kinase ITK via the T cell antigen receptor-induced association between SLP-76 and ITK. Proc Natl Acad Sci U S A. 2007;104:6638–6643. doi: 10.1073/pnas.0609771104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, et al. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25:79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Readinger JA, Mueller KL, Venegas AM, Horai R, Schwartzberg PL. Tec kinases regulate T-lymphocyte development and function: new insights into the roles of Itk and Rlk/Txk. Immunol Rev. 2009;228:93–114. doi: 10.1111/j.1600-065X.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong XP, Guo R, Zhou H, Liu C, Wan CK. Diacylglycerol kinases in immune cell function and self-tolerance. Immunol Rev. 2008;224:249–264. doi: 10.1111/j.1600-065X.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oh-hora M, Rao A. Calcium signaling in lymphocytes. Curr Opin Immunol. 2008;20:250–258. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ebinu JO, Stang SL, Teixeira C, Bottorff DA, Hooton J, et al. RasGRP links T-cell receptor signaling to Ras. Blood. 2000;95:3199–3203. [PubMed] [Google Scholar]
- 52.Genot E, Cantrell DA. Ras regulation and function in lymphocytes. Curr Opin Immunol. 2000;12:289–294. doi: 10.1016/s0952-7915(00)00089-3. [DOI] [PubMed] [Google Scholar]
- 53.Janknecht R, Ernst WH, Pingoud V, Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 1993;12:5097–5104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ebinu JO, Bottorff DA, Chan EY, Stang SL, Dunn RJ, et al. RasGRP, a Ras guanyl nucleotide- releasing protein with calcium- and diacylglycerol-binding motifs. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 55.Dower NA, Stang SL, Bottorff DA, Ebinu JO, Dickie P, et al. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 56.Gorentla BK, Wan CK, Zhong XP. Negative regulation of mTOR activation by diacylglycerol kinases. Blood. 2011;117:4022–4031. doi: 10.1182/blood-2010-08-300731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen S, Chen Y, Gorentla BK, Lu J, Stone JC, et al. Critical roles of RasGRP1 for invariant NKT cell development. J Immunol. 2011;187:4467–4473. doi: 10.4049/jimmunol.1003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Y, Ci X, Gorentla B, Sullivan SA, Stone JC, et al. Differential requirement of RasGRP1 for γδ T cell development and activation. J Immunol. 2012;189:61–71. doi: 10.4049/jimmunol.1103272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roose JP, Mollenauer M, Ho M, Kurosaki T, Weiss A. Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Mol Cell Biol. 2007;27:2732–2745. doi: 10.1128/MCB.01882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kortum RL, Sommers CL, Pinski JM, Alexander CP, Merrill RK, et al. Deconstructing Ras signaling in the thymus. Mol Cell Biol. 2012;32:2748–2759. doi: 10.1128/MCB.00317-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kortum RL, Sommers CL, Alexander CP, Pinski JM, Li W, et al. Targeted Sos1 deletion reveals its critical role in early T-cell development. Proc Natl Acad Sci U S A. 2011;108:12407–12412. doi: 10.1073/pnas.1104295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mor A, Philips MR, Pillinger MH. The role of Ras signaling in lupus T lymphocytes: biology and pathogenesis. Clin Immunol. 2007;125:215–223. doi: 10.1016/j.clim.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 63.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca+2 entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Penna A, Demuro A, Yeromin AV, Zhang SL, Safrina O, et al. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature. 2008;456:116–120. doi: 10.1038/nature07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 66.Macián F, García-Cózar F, Im SH, Horton HF, Byrne MC, et al. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 67.Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, et al. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat Immunol. 2006;7:1166–1173. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- 68.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, et al. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 69.Savignac M, Mellström B, Naranjo JR. Calcium-dependent transcription of cytokine genes in T lymphocytes. Pflugers Arch. 2007;454:523–533. doi: 10.1007/s00424-007-0238-y. [DOI] [PubMed] [Google Scholar]
- 70.Neilson JR, Winslow MM, Hur EM, Crabtree GR. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity. 2004;20:255–266. doi: 10.1016/s1074-7613(04)00052-4. [DOI] [PubMed] [Google Scholar]
- 71.Li S, Symonds AL, Zhu B, Liu M, Raymond MV, et al. Early growth response gene-2 (Egr-2) regulates the development of B and T cells. PLoS One. 2011;6:e18498. doi: 10.1371/journal.pone.0018498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seiler MP, Mathew R, Liszewski MK, Spooner C, Barr K, et al. Elevated and sustained expression of the transcription factors Egr1 and Egr2 controls NKT lineage differentiation in response to TCR signaling. Nat Immunol. 2012;13:264–271. doi: 10.1038/ni.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lazarevic V, Zullo AJ, Schweitzer MN, Staton TL, Gallo EM, et al. The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat Immunol. 2009;10:306–313. doi: 10.1038/ni.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Le Deist F, Hivroz C, Partiseti M, Thomas C, Buc HA, et al. A primary T-cell immunodeficiency associated with defective transmembrane calcium influx. Blood. 1995;85:1053–1062. [PubMed] [Google Scholar]
- 75.Feske S, Müller JM, Graf D, Kroczek RA, Dräger R, et al. Severe combined immunodeficiency due to defective binding of the nuclear factor of activated T cells in T lymphocytes of two male siblings. Eur J Immunol. 1996;26:2119–2126. doi: 10.1002/eji.1830260924. [DOI] [PubMed] [Google Scholar]
- 76.Melowic HR, Stahelin RV, Blatner NR, Tian W, Hayashi K, et al. Mechanism of diacylglycerol-induced membrane targeting and activation of protein kinase Ctheta. J Biol Chem. 2007;282:21467–21476. doi: 10.1074/jbc.M700119200. [DOI] [PubMed] [Google Scholar]
- 77.Szamel M, Resch K. T-cell antigen receptor-induced signal-transduction pathways--activation and function of protein kinases C in T lymphocytes. Eur J Biochem. 1995;228:1–15. doi: 10.1111/j.1432-1033.1995.tb20221.x. [DOI] [PubMed] [Google Scholar]
- 78.Sun Z. Intervention of PKC-theta as an immunosuppressive regimen. Front Immunol. 2012;3:225. doi: 10.3389/fimmu.2012.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matsumoto R, Wang D, Blonska M, Li H, Kobayashi M, et al. Phosphorylation of CARMA1 plays a critical role in T Cell receptor-mediated NF-kappaB activation. Immunity. 2005;23:575–585. doi: 10.1016/j.immuni.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 80.Sommer K, Guo B, Pomerantz JL, Bandaranayake AD, Moreno-García ME, et al. Phosphorylation of the CARMA1 linker controls NF-kappaB activation. Immunity. 2005;23:561–574. doi: 10.1016/j.immuni.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 81.Narayan P, Holt B, Tosti R, Kane LP. CARMA1 is required for Akt-mediated NF-kappaB activation in T cells. Mol Cell Biol. 2006;26:2327–2336. doi: 10.1128/MCB.26.6.2327-2336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol Cell. 2004;14:289–301. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- 83.Zhou H, Wertz I, O’Rourke K, Ultsch M, Seshagiri S, et al. Bcl10 activates the NF-kappaB pathway through ubiquitination of NEMO. Nature. 2004;427:167–171. doi: 10.1038/nature02273. [DOI] [PubMed] [Google Scholar]
- 84.Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 85.Hoffmann A, Natoli G, Ghosh G. Transcriptional regulation via the NF-kappaB signaling module. Oncogene. 2006;25:6706–6716. doi: 10.1038/sj.onc.1209933. [DOI] [PubMed] [Google Scholar]
- 86.Schulze-Luehrmann J, Ghosh S. Antigen-receptor signaling to nuclear factor kappa B. Immunity. 2006;25:701–715. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 87.Shen S, Wu J, Srivatsan S, Gorentla BK, Shin J, et al. Tight regulation of diacylglycerol-mediated signaling is critical for proper invariant NKT cell development. J Immunol. 2011;187:2122–2129. doi: 10.4049/jimmunol.1100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krappmann D, Emmerich F, Kordes U, Scharschmidt E, Dörken B, et al. Molecular mechanisms of constitutive NF-kappaB/Rel activation in Hodgkin/ Reed-Sternberg cells. Oncogene. 1999;18:943–953. doi: 10.1038/sj.onc.1202351. [DOI] [PubMed] [Google Scholar]
- 89.Staudt LM. Oncogenic activation of NF-kappaB. Cold Spring Harb Perspect Biol. 2010;2:a000109. doi: 10.1101/cshperspect.a000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krishna S, Xie D, Gorentla B, Shin J, Gao J, et al. Chronic activation of the kinase IKKβ impairs T cell function and survival. J Immunol. 2012;189:1209–1219. doi: 10.4049/jimmunol.1102429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Laplante M, Sabatini DM. mTOR Signaling. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 93.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 96.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 97.Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- 98.Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 99.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28:4104–4115. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 102.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 103.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, et al. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 105.Xie DL, Wu J, Lou YL, Zhong XP. Tumor suppressor TSC1 is critical for T-cell anergy. Proc Natl Acad Sci U S A. 2012;109:14152–14157. doi: 10.1073/pnas.1119744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Delgoffe GM, Powell JD. mTOR: taking cues from the immune microenvironment. Immunology. 2009;127:459–465. doi: 10.1111/j.1365-2567.2009.03125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Finlay D, Cantrell D. Phosphoinositide 3-kinase and the mammalian target of rapamycin pathways control T cell migration. Ann N Y Acad Sci. 2010;1183:149–157. doi: 10.1111/j.1749-6632.2009.05134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.O’Brien TF, Zhong XP. The role and regulation of mTOR in T-lymphocyte function. Arch Immunol Ther Exp (Warsz) 2012;60:173–181. doi: 10.1007/s00005-012-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O’Brien TF, Gorentla BK, Xie D, Srivatsan S, McLeod IX, et al. Regulation of T-cell survival and mitochondrial homeostasis by TSC1. Eur J Immunol. 2011;41:3361–3370. doi: 10.1002/eji.201141411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shin J, Pan H, Zhong XP. Regulation of mast cell survival and function by tuberous sclerosis complex 1. Blood. 2012;119:3306–3314. doi: 10.1182/blood-2011-05-353342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pan H, O’Brien TF, Zhang P, Zhong XP. The role of tuberous sclerosis complex 1 in regulating innate immunity. J Immunol. 2012;188:3658–3666. doi: 10.4049/jimmunol.1102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011;12:888–897. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu Q, Liu Y, Chen C, Ikenoue T, Qiao Y, et al. The tuberous sclerosis complex-mammalian target of rapamycin pathway maintains the quiescence and survival of naive T cells. J Immunol. 2011;187:1106–1112. doi: 10.4049/jimmunol.1003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhong XP, Shin J, Gorentla BK, O’Brien T, Srivatsan S, et al. Receptor signaling in immune cell development and function. Immunol Res. 2011;49:109–123. doi: 10.1007/s12026-010-8175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhong XP. An expanded role of the tumor suppressor TSC1 in T cell tolerance. Cell Cycle. 2012:11. doi: 10.4161/cc.22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 121.Esensten JH, Wofsy D, Bluestone JA. Regulatory T cells as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:560–565. doi: 10.1038/nrrheum.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 123.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 124.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 125.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 128.Yuan X, Malek TR. Cellular and molecular determinants for the development of natural and induced regulatory T cells. Hum Immunol. 2012;73:773–782. doi: 10.1016/j.humimm.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426:454–460. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 130.Tanaka S, Maeda S, Hashimoto M, Fujimori C, Ito Y, et al. Graded attenuation of TCR signaling elicits distinct autoimmune diseases by altering thymic T cell selection and regulatory T cell function. J Immunol. 2010;185:2295–2305. doi: 10.4049/jimmunol.1000848. [DOI] [PubMed] [Google Scholar]
- 131.Koonpaew S, Shen S, Flowers L, Zhang W. LAT-mediated signaling in CD4+CD25+ regulatory T cell development. J Exp Med. 2006;203:119–129. doi: 10.1084/jem.20050903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oh-hora M, Rao A. The calcium/NFAT pathway: role in development and function of regulatory T cells. Microbes Infect. 2009;11:612–619. doi: 10.1016/j.micinf.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gwack Y, Srikanth S, Oh-Hora M, Hogan PG, Lamperti ED, et al. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol Cell Biol. 2008;28:5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Medoff BD, Sandall BP, Landry A, Nagahama K, Mizoguchi A, et al. Differential requirement for CARMA1 in agonist-selected T-cell development. Eur J Immunol. 2009;39:78–84. doi: 10.1002/eji.200838734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gupta S, Manicassamy S, Vasu C, Kumar A, Shang W, et al. Differential requirement of PKC-theta in the development and function of natural regulatory T cells. Mol Immunol. 2008;46:213–224. doi: 10.1016/j.molimm.2008.08.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schmidt-Supprian M, Tian J, Grant EP, Pasparakis M, Maehr R, et al. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation. Proc Natl Acad Sci U S A. 2004;101:4566–4571. doi: 10.1073/pnas.0400885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wan YY, Chi H, Xie M, Schneider MD, Flavell RA. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat Immunol. 2006;7:851–858. doi: 10.1038/ni1355. [DOI] [PubMed] [Google Scholar]
- 139.Zanin-Zhorov A, Ding Y, Kumari S, Attur M, Hippen KL, et al. Protein kinase C-theta mediates negative feedback on regulatory T cell function. Science. 2010;328:372–376. doi: 10.1126/science.1186068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ma J, Ding Y, Fang X, Wang R, Sun Z. Protein kinase C-θ inhibits inducible regulatory T cell differentiation via an AKT-Foxo1/3a-dependent pathway. J Immunol. 2012;188:5337–5347. doi: 10.4049/jimmunol.1102979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hickman SP, Yang J, Thomas RM, Wells AD, Turka LA. Defective activation of protein kinase C and Ras-ERK pathways limits IL-2 production and proliferation by CD4+CD25+ regulatory T cells. J Immunol. 2006;177:2186–2194. doi: 10.4049/jimmunol.177.4.2186. [DOI] [PubMed] [Google Scholar]
- 142.Chen X, Priatel JJ, Chow MT, Teh HS. Preferential development of CD4 and CD8 T regulatory cells in RasGRP1-deficient mice. J Immunol. 2008;180:5973–5982. doi: 10.4049/jimmunol.180.9.5973. [DOI] [PubMed] [Google Scholar]
- 143.Priatel JJ, Teh SJ, Dower NA, Stone JC, Teh HS. RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity. 2002;17:617–627. doi: 10.1016/s1074-7613(02)00451-x. [DOI] [PubMed] [Google Scholar]
- 144.Chen Y, Shen S, Gorentla BK, Gao J, Zhong XP. Murine regulatory T cells contain hyperproliferative and death-prone subsets with differential ICOS expression. J Immunol. 2012;188:1698–1707. doi: 10.4049/jimmunol.1102448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Patton DT, Garden OA, Pearce WP, Clough LE, Monk CR, et al. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6598–6602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 147.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 148.Strauss L, Czystowska M, Szajnik M, Mandapathil M, Whiteside TL. Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin. PLoS One. 2009;4:e5994. doi: 10.1371/journal.pone.0005994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, et al. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 150.Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111:453–462. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev. 2009;228:342–359. doi: 10.1111/j.1600-065X.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nagaishi T, Pao L, Lin SH, Iijima H, Kaser A, et al. SHP1 phosphatase-dependent T cell inhibition by CEACAM1 adhesion molecule isoforms. Immunity. 2006;25:769–781. doi: 10.1016/j.immuni.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 154.Beauchemin N, Kunath T, Robitaille J, Chow B, Turbide C, et al. Association of biliary glycoprotein with protein tyrosine phosphatase SHP-1 in malignant colon epithelial cells. Oncogene. 1997;14:783–790. doi: 10.1038/sj.onc.1200888. [DOI] [PubMed] [Google Scholar]
- 155.Stefanová I, Hemmer B, Vergelli M, Martin R, Biddison WE, et al. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 156.Carpino N, Turner S, Mekala D, Takahashi Y, Zang H, et al. Regulation of ZAP-70 activation and TCR signaling by two related proteins, Sts-1 and Sts-2. Immunity. 2004;20:37–46. doi: 10.1016/s1074-7613(03)00351-0. [DOI] [PubMed] [Google Scholar]
- 157.Mikhailik A, Ford B, Keller J, Chen Y, Nassar N, et al. A phosphatase activity of Sts-1 contributes to the suppression of TCR signaling. Mol Cell. 2007;27:486–497. doi: 10.1016/j.molcel.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Caunt CJ, Armstrong SP, Rivers CA, Norman MR, McArdle CA. Spatiotemporal regulation of ERK2 by dual specificity phosphatases. J Biol Chem. 2008;283:26612–26623. doi: 10.1074/jbc.M801500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 160.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 161.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 162.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 163.Wang J, Maldonado MA. The ubiquitin-proteasome system and its role in inflammatory and autoimmune diseases. Cell Mol Immunol. 2006;3:255–261. [PubMed] [Google Scholar]
- 164.Fang N, Fang D, Wang HY, Altman A, Liu YC. Regulation of immune responses by E3 ubiquitin-protein ligases. Curr Dir Autoimmun. 2002;5:161–175. doi: 10.1159/000060552. [DOI] [PubMed] [Google Scholar]
- 165.Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435:590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 166.Naramura M, Jang IK, Kole H, Huang F, Haines D, et al. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat Immunol. 2002;3:1192–1199. doi: 10.1038/ni855. [DOI] [PubMed] [Google Scholar]
- 167.Wang HY, Altman Y, Fang D, Elly C, Dai Y, et al. Cbl promotes ubiquitination of the T cell receptor zeta through an adaptor function of Zap-70. J Biol Chem. 2001;276:26004–26011. doi: 10.1074/jbc.M010738200. [DOI] [PubMed] [Google Scholar]
- 168.Murphy MA, Schnall RG, Venter DJ, Barnett L, Bertoncello I, et al. Tissue hyperplasia and enhanced T-cell signalling via ZAP-70 in c-Cbl-deficient mice. Mol Cell Biol. 1998;18:4872–4882. doi: 10.1128/mcb.18.8.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 170.Safford M, Collins S, Lutz MA, Allen A, Huang CT, et al. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 171.Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kobayashi T, Walsh MC, Choi Y. The role of TRAF6 in signal transduction and the immune response. Microbes Infect. 2004;6:1333–1338. doi: 10.1016/j.micinf.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 173.Anandasabapathy N, Ford GS, Bloom D, Holness C, Paragas V, et al. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–547. doi: 10.1016/s1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 174.Liu YC. The E3 ubiquitin ligase Itch in T cell activation, differentiation, and tolerance. Semin Immunol. 2007;19:197–205. doi: 10.1016/j.smim.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Heissmeyer V, Macián F, Im SH, Varma R, Feske S, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 176.Scharschmidt E, Wegener E, Heissmeyer V, Rao A, Krappmann D. Degradation of Bcl10 induced by T-cell activation negatively regulates NF-kappa B signaling. Mol Cell Biol. 2004;24:3860–3873. doi: 10.1128/MCB.24.9.3860-3873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gao M, Labuda T, Xia Y, Gallagher E, Fang D, et al. Jun turnover is controlled through JNK-dependent phosphorylation of the E3 ligase Itch. Science. 2004;306:271–275. doi: 10.1126/science.1099414. [DOI] [PubMed] [Google Scholar]
- 178.Glasmacher E, Hoefig KP, Vogel KU, Rath N, Du L, et al. Roquin binds inducible costimulator mRNA and effectors of mRNA decay to induce microRNA-independent post-transcriptional repression. Nat Immunol. 2010;11:725–733. doi: 10.1038/ni.1902. [DOI] [PubMed] [Google Scholar]
- 179.Lin AE, Mak TW. The role of E3 ligases in autoimmunity and the regulation of autoreactive T cells. Curr Opin Immunol. 2007;19:665–673. doi: 10.1016/j.coi.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 180.Shulga YV, Topham MK, Epand RM. Regulation and functions of diacylglycerol kinases. Chem Rev. 2011;111:6186–6208. doi: 10.1021/cr1004106. [DOI] [PubMed] [Google Scholar]
- 181.Mérida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008;409:1–18. doi: 10.1042/BJ20071040. [DOI] [PubMed] [Google Scholar]
- 182.Zhong XP, Hainey EA, Olenchock BA, Zhao H, Topham MK, et al. Regulation of T cell receptor-induced activation of the Ras-ERK pathway by diacylglycerol kinase zeta. J Biol Chem. 2002;277:31089–31098. doi: 10.1074/jbc.M203818200. [DOI] [PubMed] [Google Scholar]
- 183.Carrasco S, Merida I. Diacylglycerol-dependent binding recruits PKCtheta and RasGRP1 C1 domains to specific subcellular localizations in living T lymphocytes. Mol Biol Cell. 2004;15:2932–2942. doi: 10.1091/mbc.E03-11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, et al. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat Immunol. 2003;4:882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- 185.Zhong XP, Olenchock BA, Koretzky GA. The role of diacylglycerol kinases in T cell anergy. Ernst Schering Found Symp Proc. 2007:139–149. [PubMed] [Google Scholar]
- 186.Shin J, O’Brien TF, Grayson JM, Zhong XP. Differential regulation of primary and memory CD8 T cell immune responses by diacylglycerol kinases. J Immunol. 2012;188:2111–2117. doi: 10.4049/jimmunol.1102265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Guo R, Wan CK, Carpenter JH, Mousallem T, Boustany RM, et al. Synergistic control of T cell development and tumor suppression by diacylglycerol kinase alpha and zeta. Proc Natl Acad Sci U S A. 2008;105:11909–11914. doi: 10.1073/pnas.0711856105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu CH, Machado FS, Guo R, Nichols KE, Burks AW, et al. Diacylglycerol kinase zeta regulates microbial recognition and host resistance to Toxoplasma gondii. J Exp Med. 2007;204:781–792. doi: 10.1084/jem.20061856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fischer A. Severe combined immunodeficiencies (SCID) Clin Exp Immunol. 2000;122:143–149. doi: 10.1046/j.1365-2249.2000.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sawabe T, Horiuchi T, Nakamura M, Tsukamoto H, Nakahara K, et al. Defect of lck in a patient with common variable immunodeficiency. Int J Mol Med. 2001;7:609–614. doi: 10.3892/ijmm.7.6.609. [DOI] [PubMed] [Google Scholar]
- 191.Goldman FD, Ballas ZK, Schutte BC, Kemp J, Hollenback C, et al. Defective expression of p56lck in an infant with severe combined immunodeficiency. J Clin Invest. 1998;102:421–429. doi: 10.1172/JCI3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Elder ME, Lin D, Clever J, Chan AC, Hope TJ, et al. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 1994;264:1596–1599. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- 193.Arpaia E, Shahar M, Dadi H, Cohen A, Roifman CM. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking zap-70 kinase. Cell. 1994;76:947–958. doi: 10.1016/0092-8674(94)90368-9. [DOI] [PubMed] [Google Scholar]
- 194.Roberts JL, Lauritsen JP, Cooney M, Parrott RE, Sajaroff EO, et al. T-B+NK+ severe combined immunodeficiency caused by complete deficiency of the CD3zeta subunit of the T-cell antigen receptor complex. Blood. 2007;109:3198–3206. doi: 10.1182/blood-2006-08-043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rieux-Laucat F, Hivroz C, Lim A, Mateo V, Pellier I, et al. Inherited and somatic CD3zeta mutations in a patient with T-cell deficiency. N Engl J Med. 2006;354:1913–1921. doi: 10.1056/NEJMoa053750. [DOI] [PubMed] [Google Scholar]
- 196.Wu J, Edberg JC, Gibson AW, Tsao B, Kimberly RP. Single-nucleotide polymorphisms of T cell receptor zeta chain in patients with systemic lupus erythematosus. Arthritis Rheum. 1999;42:2601–2605. doi: 10.1002/1529-0131(199912)42:12<2601::AID-ANR13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 197.Chu DH, Morita CT, Weiss A. The Syk family of protein tyrosine kinases in T-cell activation and development. Immunol Rev. 1998;165:167–180. doi: 10.1111/j.1600-065x.1998.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 198.Kung C, Pingel JT, Heikinheimo M, Klemola T, Varkila K, et al. Mutations in the tyrosine phosphatase CD45 gene in a child with severe combined immunodeficiency disease. Nat Med. 2000;6:343–345. doi: 10.1038/73208. [DOI] [PubMed] [Google Scholar]
- 199.Tchilian EZ, Wallace DL, Wells RS, Flower DR, Morgan G, et al. A deletion in the gene encoding the CD45 antigen in a patient with SCID. J Immunol. 2001;166:1308–1313. doi: 10.4049/jimmunol.166.2.1308. [DOI] [PubMed] [Google Scholar]
- 200.Wilkinson B, Downey JS, Rudd CE. T-cell signalling and immune system disorders. Expert Rev Mol Med. 2005;7:1–29. doi: 10.1017/S1462399405010264. [DOI] [PubMed] [Google Scholar]
- 201.Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75:330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Smyth D, Cooper JD, Collins JE, Heward JM, Franklyn JA, et al. Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus. Diabetes. 2004;53:3020–3023. doi: 10.2337/diabetes.53.11.3020. [DOI] [PubMed] [Google Scholar]
- 203.Kyogoku C, Langefeld CD, Ortmann WA, Lee A, Selby S, et al. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am J Hum Genet. 2004;75:504–507. doi: 10.1086/423790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Velaga MR, Wilson V, Jennings CE, Owen CJ, Herington S, et al. The codon 620 tryptophan allele of the lymphoid tyrosine phosphatase (LYP) gene is a major determinant of Graves’ disease. J Clin Endocrinol Metab. 2004;89:5862–5865. doi: 10.1210/jc.2004-1108. [DOI] [PubMed] [Google Scholar]
- 205.Criswell LA, Pfeiffer KA, Lum RF, Gonzales B, Novitzke J, et al. Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet. 2005;76:561–571. doi: 10.1086/429096. [DOI] [PMC free article] [PubMed] [Google Scholar]