Abstract
Objective
Eating in the absence of hunger (EAH) typically is assessed by measuring snack intake after consumption of a meal. There are no validated self-report measures of EAH. We sought to examine the relationship of adolescent self-report and parent-reported EAH to adolescents’ measured intake in the absence of hunger.
Design and Methods
Ninety adolescents completed the Eating in the Absence of Hunger Questionnaire for Children and Adolescents (EAH-C) to describe eating when not hungry. Parents described children’s EAH on a parallel version designed for parents (EAH-P). In a randomized crossover study, adolescent EAH in response to external cues was measured as snack intake after a lunch meal standardized to provide 50% of daily energy requirements and after a large array (>10,000 kcal).
Results
Parents’ reports of children’s EAH in response to external cues were associated with greater EAH after both meals, adjusting for body composition, sex, age, race, puberty, and meal intake. Adolescent-reported EAH was unrelated or showed an inverse association with observed EAH.
Conclusions
Parent-reported EAH showed a positive association with adolescents’ observed EAH and may be a useful research and clinical tool for assessing EAH in response to external cues in conditions when laboratory assessments are not feasible.
Keywords: Eating Behaviors, Adolescents, Overweight, Obesity, Questionnaire Design
Introduction
Eating in the absence of hunger (EAH), which is generally defined as eating palatable foods in the absence of perceived physiological hunger (1), is believed to be a form of disinhibited eating that could predispose youth to develop obesity. EAH typically is measured objectively by observing snack food intake after a meal designed to reduce hunger (2, 3). Children and adolescents who are overweight or obese (body mass index [BMI, kg/m2] ?≥ 85th percentile for age and sex) consume more energy in the absence of hunger in the laboratory (3, 4, 5, 6), at home (7, 8), and at school (7) than their non-overweight peers. EAH appears to be moderately heritable (4). EAH, on average, increases as children age, but youth with overweight or obese parents consume more energy during the EAH paradigm and show the greatest increases in EAH over time compared to youth without overweight parents (9, 10).
The widespread exposure to large portions of palatable, inexpensive, readily-available, and energy-dense foods in today’s “obesogenic” environment has made it increasingly likely that youth will, at least occasionally, eat in the absence of physiological hunger. Yet, a propensity to frequently eat in response to external or environmental cues such as the availability of palatable foods or their taste or smell, rather than eating in response to physiological hunger, would seem to be an eating style likely to promote positive energy balance (11). The degree to which EAH prospectively contributes to excessive weight gain is yet to be established (12). In part, the study of EAH in well-powered longitudinal studies of children’s and adolescents’ growth is hampered by the time-intensive and costly nature of observational paradigms for assessing EAH. Questionnaire measures of eating behavior have the marked advantage of being inexpensive and easy to administer; however, children and adolescents’ self-reports of eating behavior are significantly limited by the tendency to underestimate and underreport food intake, especially among heavier youth (13, 14). Parents, on the other hand, may be more valid reporters of their children’s eating patterns. Among 4–5-year-olds, parental reports of their children’s satiety responsiveness, an eating behavioral trait posited to underlie EAH (15), explained 11% of the variance in children’s observed EAH, before accounting for children’s body size or other relevant covariates (16). The relationship of adolescents’ or their parents’ reports of EAH to observed EAH in the laboratory is unknown. Likewise, the degree to which questionnaire measures of EAH capture observed EAH beyond what can be explained by adolescents’ current body weight is not known. Adolescence is a crucial developmental period for understanding eating behaviors that may promote excessive weight gain and obesity (17). Eating patterns developed during adolescence likely set the stage for the development and/or persistence of obesity in adulthood.
In the current study, we aimed to examine the relationship of adolescents’ self-reports of EAH in response to external cues with their observed EAH in the laboratory. We also studied the relationship of parents’ reports of their children’s EAH in response to external cues with adolescents’ observed EAH. Although adolescents spend less time with parents relative to childhood and consequently exercise greater control over their food selection and intake (18), parents continue to monitor and exert an influence on the eating behavior of their adolescent children (19). Therefore, based upon prior data showing poor correspondence between questionnaire and observational measures of eating behavior (13, 14), we hypothesized that parents would be more accurate reporters of their children’s EAH than adolescents’ own self-reports. We posited that the relationships of reported to observed EAH would exist even after accounting for adolescents’ current body composition.
In addition, we expected that adolescents’ and parents’ reports of EAH in response to external cues would show better correspondence with observed EAH in response to external cues than self- and parent-reports of EAH in response to other precipitants such as emotional triggers. This hypothesis was based upon the notion that eating when not hungry in response to internal cues such as negative feelings is a discriminant construct from eating because palatable foods are available (20). Although the two constructs likely have some overlap, the former (EAH in response to external triggers) is thought to be more prevalent (8), whereas the latter (EAH in response to emotional triggers) is thought to overlap more with disordered eating behaviors that begin to emerge during adolescence (20).
Methods and Procedures
Participants
Adolescents were recruited for a study on eating behavior (ClinicalTrials.gov ID: NCT00631644) through flyers and notices to school parent e-mail listservs in Washington, DC and the greater metropolitan area. The present study is a secondary analysis of a randomized crossover feeding study designed to validate a modified laboratory paradigm for assessing EAH in adolescents (5). Results examining the association between body weight and EAH from 78 subjects included in the current report have been previously published (5). Individuals were eligible to participate if they were between 13 and 17 years of age and were in good general health. Exclusion criteria included chronic illnesses, use of medications likely to affect body weight or appetite, pregnancy, ongoing weight-loss treatment, or a psychiatric condition that would impede adherence to study procedures. Adolescents provided written assent and parents/guardians gave written consent for participation. Families were told that the purpose of the study was to better understand eating behaviors in teenagers and to determine how genes or DNA are related to how people eat. Youth were financially compensated for their participation. The study was approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board.
Procedures and Measures
Participants were screened for eligibility at an initial visit that included a medical history and a physical examination performed by an endocrinologist or nurse practitioner. Testicular volume (mL) was measured using a set of orchidometer beads as standards according to Prader (21), and breast development was assigned according to the five stages of Tanner (22, 23). Testicular volume for males and Tanner breast staging for females were used to categorize youth as those in pre-puberty (for boys testes < 4 mL; for girls, Tanner stage I), early/mid-puberty (for boys: testes 4–15 mL; for girls: Tanner stages 2–3), or late puberty (for boys: testes ≥ 15 mL; for girls: Tanner stages 4–5). Height was measured three times to the nearest millimeter by a stadiometer (Holtain, Crymmych, Wales) calibrated before each participant’s measurement. Fasting weight was measured to the nearest 0.1 kg with a calibrated digital scale (Scale-Tronix, Wheaton, IL). Height and weight were used to compute BMI, calculated as weight (kg) divided by the square of height (m). BMI standard deviation (BMI z) scores for sex and age were calculated according to the Centers for Disease Control and Prevention 2000 standards (24). Overweight status was dichotomized into non-overweight (BMI < 85th percentile) and overweight or obese (BMI ≥ 85th percentile). Fat-free mass (kg) and percentage body fat were assessed with air-displacement plethysmography (Life Measurement Inc, Concord, CA). Body composition measurements were obtained as recommended, with participants fasted, wearing only underclothes or a form-fitting bathing suit (25).
Adolescent Self-Report of EAH
Also at the initial screening appointment, adolescents reported on their perceived eating in the absence of hunger or eating past satiation in response to external cues on the Eating in the Absence of Hunger Questionnaire for Children and Adolescents (EAH-C) (26). The EAH-C is designed to assess the frequency of precipitants for eating when not physically hungry among 6–19-year-old youth. The form instructs participants: “imagine that you are eating a meal or snack at home, school, or in a restaurant; imagine that you eat enough of your meal so that you are no longer hungry.” For the external cues subscale (4 items), youth are queried about how often in this situation they keep eating in response to external precipitants such as sensory cues (e.g., “how often do you keep eating because the food looks, tastes, or smells so good?”) or social cues (e.g., “how often do you keep eating because others are still eating”). The EAH-C also contains scales assessing EAH in response to negative affect (6 items; e.g., “how often do you keep eating because you are feeling sad or depressed?”) and fatigue/boredom (4 items; e.g., “how often do you keep eating because you are feeling bored?”). Although EAH in response to emotional triggers was not the focus of this study, we examined these scales in relationship to observed EAH in response to external cues to assess discriminant validity. Items were rated on a 5-point Likert scale ranging from 1 = “never” to 5 = “always.” Scales were calculated by taking the average of the items. The measure has demonstrated good internal consistency and temporal stability for all scales and convergent validity with obesity status for the EAH-C negative affect subscale (26). All scales have shown low to moderate correspondence with measures of emotional eating, depressive symptoms, and anxiety symptoms (26).
Parent Report of Adolescent EAH
At the screening appointment, parents reported their perceptions of their children’s eating in the absence of hunger or eating past satiation in response to external cues on a parallel version of the EAH-C designed for parents (EAH-P). The EAH-P items were scored, and parallel subscales were calculated in the same manner as the EAH-C. In a separate sample of 140 children 8–17 years of age (M ± SD age 12.5 ± 2.8 years) with BMI z 0.86 ± 1.11 (Range = −1.50–3.20) and their parents, all three EAH-P scales demonstrated good internal reliability (αs = 0.85–0.94) and temporal stability (rs = 0.63–0.70, Ps < 0.001) over 10.5 ± 7.9 weeks (range 17.4–65.4 weeks). Also, the EAH-P’s convergent validity is supported by significant associations between EAH-P scales and children’s interview-assessed overeating and disordered eating behaviors (27).
Observational Assessment of EAH
Following the initial screening visit, observed EAH was assessed on two separate days (3.2 ± 4.0 days apart) using laboratory paradigms that differed only in the type of meal served prior to EAH measurement – either a standardized lunch meal or a large array of lunch-type foods, presented in random order. Both types of test meals have been demonstrated to capture the construct of EAH (4, 5). A comparison and validation of these paradigms for assessing EAH in 78 adolescents from the current sample has been previously reported: EAH measured after a large array produces a more conservative assessment of EAH by ensuring that snack intake occurs in the absence of hunger (5). In the current study, we examined the correspondence of EAH-C and EAH-P questionnaires with both observational assessments of EAH in order to provide two tests of convergent validity. On both days, the participant was instructed not to eat anything or drink anything other than water after 10:00 p.m. on the evening prior to the visits, in order to minimize any acute influences of food consumed outside of the laboratory on eating behavior in the laboratory. Adherence to overnight fasting was encouraged by reminder calls from research staff the night before, and any adolescent who did not adhere to fasting was rescheduled for a different day. On both days, an ad libitum lunch meal was served at 11:00 a.m. in a private room, and the participant was given the following tape-recorded instruction: “Please eat until you are no longer hungry. Take as much time as you need and open the door when you’re done.” In the standardized lunch meal condition, chicken nuggets, grapes, baby carrots, tortilla chips, sandwich cookies, ketchup, 2% milk, and lemonade were served in amounts adjusted for each participant such that the meal provided 50% of each adolescent’s total daily estimated energy needs based on age, sex, BMI, and a “low active” physical activity coefficient (28), consistent with previous laboratory studies of EAH in adolescents (4) (standardized meal total energy provided = 1367.0 ± 260.7 kcal; 55.4 ± 2.3% carbohydrate, 11.6 ± 0.2% protein, 35.2 ± 0.4% fat). In the large array lunch meal condition designed to ensure that adolescents were completely sated prior to EAH measurement (5), the meal consisted of a 10,934 kcal multi-item buffet with individual items that varied in macronutrient composition (overall: 54% carbohydrate, 12% protein, 33% fat). Items were a wide assortment of foods: 6 slices white bread, 6 slices whole wheat bread, 3 Kaiser rolls, ham (180 g), turkey (180 g), American cheese (240 g), peanut butter (120 g), grape jelly (120 g), tomatoes (200 g), lettuce (50 g), chocolate candy (120 g), 18 chicken nuggets, tortilla chips (120 g), pretzel rods (150 g), grapes (250 g), 3 bananas, 3 oranges, baby carrots (200 g), 12 sandwich cookies, 12 vanilla wafers, jelly beans (120 g), mayonnaise (90 g), mustard (90 g), BBQ sauce (90 g), mild salsa (250 g), ranch dressing (90 g), 2% milk (850 g), lemonade (850 g), apple juice (850 g), and water (850 g).
On both days, after completing the meal, participants were escorted to a separate room where they completed rating forms assessing hunger and fullness on visual analog scales ranging from 0 = “not at all” to 100 = “extremely.” Present emotional state was assessed with adolescents’ reports on the well-validated Brunel Mood Scale (29), which generates six subscales pertaining to anger, confusion, depression, fatigue, tension, and vigor. They were invited to view magazines screened to be devoid of content related to food, eating, body shape, or weight. One half-hour after meal termination, adolescents were told that they would be completing a snacks taste test. Participants were escorted back to the test meal room where they were provided with a 4,055-kcal array of highly palatable snack food items in generous portions: popcorn (65 g), potato chips (70 g), pretzel twists (70 g), 8 fig bars, 8 chocolate chip cookies, 30 fruit chew candies, chocolate malt balls (120 g), 2 half cup containers of three ice cream flavors (chocolate, strawberry and vanilla), cherry and lemon Italian ice (2 half cup containers each). Adolescents were played a tape-recorded instruction that stated, “Please taste each of the foods. Rate your preferences for how much you like or dislike the foods on this rating form. Try to take at least two bites of each food. When you’re done, feel free to use any of the activities in the room, and eat as much of the foods as you like. The investigator will return in 15 minutes.” Participants were provided with a rating form to describe their preferences on a 10-point Likert scale from 1 = “I hate the food” to 10 = “I love the food.” On a separate side table there were non-food activities including a hand-held computer game, playing cards, magazines, word and drawing games, paper and crayons/markers. The adolescent was then left alone for a 15-minute period. Since participants were expected to be in a neutral emotional state and were offered plenty of activities, the snack array intake served as a measure of EAH in response to external precipitants as opposed to emotional triggers such as negative affect or boredom/fatigue.
The amounts of each food and beverage consumed from the meals (standardized lunch meal and array lunch meal) and from the snack array (EAH after the standardized lunch meal and EAH after the array lunch meal) were measured by using the difference in weight (g) of each item before and after the meal. Energy intakes (kcal) were calculated with data from the US Department of Agriculture (USDA) National Nutrient Database for Standard Reference (USDA, Agricultural Research Service, Beltsville, MD) and food manufacturer nutrient information obtained from food labels.
Data Analysis
Analyses were conducted with SPSS 18.0. Data were screened for problems of outliers, skew, and kurtosis. To prepare data for analyses, outliers (< 3% of all data points) were adjusted to fall 1.5 times the interquartile range below the 25th percentile or above the 75th percentile (e.g., to the whiskers in Tukey’s boxplot) (30). We routinely utilize this strategy because it minimizes outliers’ influence on the characteristics of the distribution, minimally changes the distribution overall, and avoids potential bias associated with eliminating outliers altogether. Skew and kurtosis were satisfactory on all variables. Descriptive information was generated on study variables. Paired samples t-tests were used to describe and compare subjective hunger/fullness and mood ratings and energy intake in the standardized lunch meal and array lunch meal conditions. Bivariate correlations were conducted to describe the univariate relationships among body composition, adolescent- and parent-reported EAH, observed standardized lunch meal and array lunch meal intake, and observed EAH after the standardized lunch meal and after the array lunch meal. A series of multiple regressions were utilized to investigate whether adolescent- and parent-reported EAH related to adolescents’ observed EAH after accounting for adolescent age (years), sex, race (Non-Hispanic White vs. Other), puberty (pre/early/mid vs. late), percent fat mass, fat-free mass (kg), height (cm), and prior meal energy intake (standardized lunch meal or array lunch meal). Consistent with previous laboratory eating studies, we included anthropometric and demographic covariates shown to be related to energy intake (4, 5, 31, 32). We used this analytic strategy so that any observed links between reported and observed EAH would be beyond what would be accounted for by these third factors. Meal order (i.e., standardized or array condition first) was not significant, and its inclusion did not alter the significance or direction of any result. For all analyses, associations and differences were considered significant when P < 0.05. All tests were two-tailed.
Results
Descriptive Information
Ninety-six adolescents attended a screening appointment for eligibility. Six were excluded from participation: two were vegetarian and consequently did not like enough of the foods offered; one reported active suicidal ideation and was referred for a psychiatric consult; one reported self-induced vomiting and was referred for treatment; and two were not compliant with study procedures. Eligible participants were 43 adolescent girls (47.8%) and 47 boys ages 13–17 years (M ± SD 15.27 ± 1.39; Table 1). The sample was 46.7% Non-Hispanic White (n = 42) and 53.3% Other race/ethnicity, including 40.0% non-Hispanic Black (n = 36), 6.7% Asian (n = 6), 3.3% Hispanic White (n = 3), and 3.3% multiple races (n = 3). The vast majority of adolescents (83.3%) were in late puberty, with the remainder in early/mid- (15.5%) or pre-puberty (1.1%). For analyses taking pubertal development into account, pre-pubertal children were therefore combined with the early/mid group. Forty percent of youth were overweight or obese (BMI ≥ 85th percentile for age and sex), which is slightly higher than current estimates of overweight or obese observed among U.S. adolescents (34%). Descriptive information about body composition (fat mass and fat-free mass), adolescent self- and parent-reported EAH, and observed eating behavior is presented in Table 1.
Table 1.
Descriptive characteristics of study sample
| M ± SD or % | Range | |
|---|---|---|
| Age (years) | 15.27 ± 1.39 | 13 – 17 |
| Sex (% female) | 47.8 | -- |
| Race (% non-Hispanic white) | 46.7 | -- |
| BMI z | 0.77 ± 1.07 | −1.53 – 2.57 |
| Fat Mass (%) | 24.54 ± 12.62 | 6.20 – 57.85 |
| Fat-Free Mass (kg) | 50.21 ± 9.57 | 30.90 – 71.25 |
| Teen EAH External | 2.46 ± 0.72 | 1.00 – 4.25 |
| Teen EAH Fatigue | 1.68 ± 0.66 | 1.00 – 4.25 |
| Teen EAH Negative | 1.24 ± 0.36 | 1.00 – 3.33 |
| Parent EAH External | 2.59 ± 0.80 | 1.00 – 5.00 |
| Parent EAH Fatigue | 1.75 ± 0.68 | 1.00 – 4.00 |
| Parent EAH Negative | 1.38 ± 0.52 | 1.00 – 4.00 |
| Standard Lunch (kcal) | 853.99 ± 249.90 | 345.30 – 1458.20 |
| Array Lunch (kcal) | 1295.43 ± 482.65 | 351.70 – 2620.16 |
| EAH Standard Lunch (kcal) | 359.73 ± 151.54 | 56.56 – 607.01 |
| EAH Array Lunch (kcal) | 300.86 ± 160.95 | 8.21 – 686.02 |
EAH = Eating in the absence of hunger;
Teen EAH and Parent EAH scales possible range = 1.00 – 5.00.
Adolescents reported little to no hunger after both lunch meals, but they reported feeling less hungry after the array lunch meal (8.37 ± 10.74 on a 0 – 100 visual analog scale) compared to after the standardized lunch meal (14.24 ±16.05, t(89) = 3.75, P < 0.001). Similarly, participants reported greater fullness after the array lunch meal (75.27 ±19.90) than after the standardized lunch meal (65.54 ± 21.01, t(89) = 3.51, P = 0.001). Mood state ratings after lunch and prior to the snacks did not vary between conditions. On a scale with possible scores ranging from 0 to 16, adolescents reported little to no anger (0.23 ± 0.89 vs. 0.16 ± 0.56, after standardized and array lunch meals, respectively), confusion (0.47 ± 0.91 vs. 0.37 ± 0.85), depression (0.17 ± 0.52 vs. 0.14 ± 0.44), fatigue (3.02 ± 2.78 vs. 3.39 ± 3.17), or tension (0.40 ± 0.95 vs. 0.44 ± 1.04) and small to moderate amounts of vigor (6.02 ± 3.44 vs. 5.74 ± 3.34, all ts(89) < 1.13, all Ps > 0.26). Adolescents ate more at the array lunch meal than at the standardized lunch meal (d = 441.44 ± 369.20 kcal, t(89) = 11.34, P < 0.001), and they consumed less snacks after the array lunch meal than after the standardized lunch meal (d = 58.87 ± 145.04 kcal, t(89) = 3.85, P < 0.001).
Correspondence within and between Adolescent- and Parent-Reported EAH Questionnaires
The internal reliability for each scale of the EAH-C ranged from adequate to good, αs = 0.78 – 0.90. Likewise, the three subscales of the EAH-P were internally reliable: external eating, α = 0.86, negative affect, α = 0.92, and fatigue/boredom, α = 0.79.
Adolescents’ reports of EAH in response to external cues, fatigue/boredom, and negative affect were interrelated (Ps < 0.05; Table 2). Likewise, parents’ reports of their children’s EAH in response to external cues, fatigue/boredom, and negative affect were interrelated (Ps < 0.001). As shown in Table 2, the EAH-C fatigue/boredom scale was related to all three EAH-P scales (rs = 0.22 – 0.30, Ps < 0.05); the other EAH-C external eating and negative affect scales were not significantly related to the EAH-P (Ps > .05).
Table 2.
Correlations among adolescent- and parent-reported eating in the absence of hunger (EAH), adolescent observed intake during a standardized lunch meal and an array lunch meal, and EAH after a standardized lunch meal and after an array lunch meal
| Teen EAH External | Teen EAH Fatigue | Teen EAH Negative | Parent EAH External | Parent EAH Fatigue | Parent EAH Negative | Standard Lunch | Array Lunch | EAH- Standard Lunch | |
|---|---|---|---|---|---|---|---|---|---|
| Teen EAH External | 1.00 | ||||||||
| Teen EAH Fatigue | 0.50*** | 1.00 | |||||||
| Teen EAH Negative | 0.26*** | 0.46*** | 1.00 | ||||||
| Parent EAH External | 0.17 | 0.22* | 0.18 | 1.00 | |||||
| Parent EAH Fatigue | 0.16 | 0.30** | 0.08 | 0.55*** | 1.00 | ||||
| Parent EAH Negative | 0.01 | 0.24* | 0.18 | 0.39*** | 0.69*** | 1.00 | |||
| Standard Lunch (kcal) | −0.14 | −0.01 | −0.08 | 0.03 | 0.06 | 0.11 | 1.00 | ||
| Array Lunch (kcal) | −0.13 | −0.01 | −0.01 | 0.15 | 0.09 | 0.04 | 0.66*** | 1.00 | |
| EAH-Standard Lunch (kcal) | −0.28** | −0.08 | −0.04 | 0.32** | 0.15 | 0.12 | 0.25* | 0.31** | 1.00 |
| EAH-Array Lunch (kcal) | −0.14 | −0.01 | 0.01 | 0.33** | 0.19 | 0.26** | 0.11 | 0.08 | 0.57*** |
P < 0.05.
P < 0.01.
P < 0.001.
On average, parents reported greater adolescent EAH in response to negative affect than adolescents reported about themselves (1.38 ± 0.52 vs. 1.24 ± 0.36, t(89) = 2.25, P = 0.03). Adolescents and their parents reported similar perceived levels of EAH in response to external cues (2.46 ± 0.72 vs. 2.59 ± 0.80, t(89) = 1.21, P = 0.23) and fatigue/boredom (1.68 ± 0.66 vs. 1.75 ± 0.68, t(89) = 0.75, P = 0.46). Adolescent-reported EAH was not significantly associated with overweight status, BMI z, or body composition (Ps > 0.07). In contrast, parents of overweight adolescents reported significantly greater adolescent EAH to all three triggers than parents of non-overweight youth (ts(89) > 2.86, Ps < 0.01). Likewise, EAH-P subscales were positively correlated with BMI z and percent fat mass (rs > .23, Ps < 0.05), but not adolescent fat-free mass (Ps > 0.30).
Relationships of Reported and Observed EAH
In bivariate correlations (Table 2), adolescent-reported EAH-C fatigue/boredom and negative affect subscales were not significantly related to observed EAH. The EAH-C external subscale showed an inverse association with EAH measured after the standardized lunch meal only (r = −0.28, P = 0.01). In contrast, parent-reported EAH to external cues was positively associated with adolescent EAH measured after the standardized lunch meal (r = 0.32, P < 0.01) and after the array lunch meal (r = 0.33, P < 0.01). The EAH-P negative affect scale also was positively related to adolescent EAH after the array lunch meal (r = 0.26, P = 0.01).
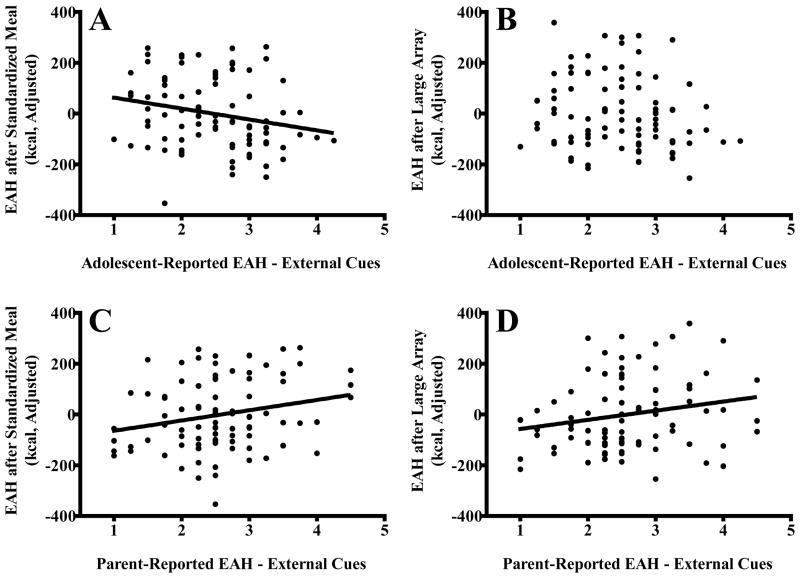
In regression models, the covariates of age, sex, race, percent fat mass, fat-free mass, height, puberty, and standardized or array lunch meal intake accounted for 22% of the variance in EAH after the array lunch meal and 13% of the variance in EAH after the standardized lunch meal. After accounting for covariates, adolescent-reported EAH to external cues remained inversely associated with observed EAH after the standardized lunch meal (β = −0.23, P = 0.03; Figure 1A). The EAH-C external scale explained 4.7% of the variance in observed EAH after the standardized lunch meal, after accounting for covariates (P = 0.04). Adolescent-reported EAH to external cues was not significantly related to observed EAH after the array lunch meal (β = −0.17, P = 0.11; Figure 1B). The EAH-C fatigue/boredom and negative affect subscales were unrelated to observed EAH in both conditions (Ps > 0.20).
Figure 1.
Associations of adolescent-reported eating in the absence of hunger (EAH) in response to external cues and adolescent observed EAH after a standardized lunch meal (A; β = −0.23, P = 0.03) and EAH after an array lunch meal (B; β = −0.17, P = 0.11), and associations of parent-reported EAH in response to external cues and adolescent observed EAH after a standardized lunch meal (C; β = −0.30, P = 0.01) and EAH after an array lunch meal (D; β = −0.26, P = 0.03). All analyses adjusted for adolescent age (years), sex, race (Non-Hispanic White vs. Other), puberty (pre/early/mid vs. late), percent fat mass, fat-free mass (kg), height (cm), and prior meal energy intake (standardized lunch meal or array lunch meal).
Accounting for age, sex, race, percent fat mass, fat-free mass, height, puberty, and lunch meal energy intake, parent-reported EAH to external cues remained significantly positively associated with adolescents’ observed EAH after the standardized lunch meal (β = 0.30, P = 0.01; Figure 1C) and after the array lunch meal (β = 0.26, P = 0.03; Figure 1D). After accounting for all covariates, the EAH-P external scale explained an additional 4.6 – 6.5% of the variability in observed EAH (Ps < 0.05). Parent-reported EAH in response to fatigue/boredom or negative affect were not related to observed adolescent EAH in the multivariate models (Ps > 0.27).
Discussion
Consistent data indicate that youth are inaccurate reporters of how much they consume (13, 14). In the present study, we found that adolescents’ reports on the EAH-C questionnaire are also an imprecise measure of how much they eat in the absence of hunger. By contrast, parents appear to be better informants.
Parental reports of their children’s eating in the absence of hunger (EAH) in response to external cues were significantly, positively related to adolescents’ observed EAH in laboratory paradigms designed to assess EAH in response to environmental cues. This pattern was visible when EAH was measured after a standardized lunch meal and when EAH was measured after a very large array of lunch foods. These results are consistent with prior data illustrating a relationship between parent-reported satiety responsiveness and young children’s observed EAH (16). Among adolescents, the consistent, significant relationship between parent-reported EAH and adolescents’ observed EAH remained even when accounting for youths’ body composition, a significant correlate of both parent-reported and observed EAH.
Parental report of their children’s EAH in response to negative affect was positively correlated with adolescents’ observed EAH after an array lunch meal. Yet, after accounting for body composition and other covariates, parental reports of EAH in response to affective triggers – negative affect and fatigue/boredom – were unrelated to intake. The weaker correspondence with scales measuring perceived EAH in response to affective triggers provides some evidence for discriminant validity. The laboratory paradigms in the current study observed EAH in response to the availability of palatable foods, not in response to emotional cues per se. Furthermore, the EAH paradigm offered participants games, puzzles, and reading material that were intended to prevent boredom, and the lunchtime paradigm should have minimized fatigue in participants. Mood ratings just prior to the EAH paradigm confirmed that participants were in a neutral mood state. Thus, it is logical that parental reports of EAH specifically in response to external cues were more consistently related to measured EAH than parental reports of EAH in response to negative affect or fatigue/boredom. Preliminary evidence suggests that EAH in response to emotional triggers may represent a distinct construct from EAH in response to external precipitants (33). EAH in response to negative emotions may be more closely aligned with adolescent disordered eating behaviors (e.g., loss of control or binge eating), which often involve eating to cope with negative affect (34). Given the emergence of disordered eating among adolescents, an important goal for future research is to examine the correspondence of parental and adolescent reports of EAH in response to affective triggers, boredom, or fatigue with adolescents’ EAH in paradigms specifically designed to capture EAH in response to such emotional triggers. It is possible, for example, that EAH in response to external cues may be more observable – and hence, parents may be better reporters of this behavior – than EAH in response to internal, emotional triggers.
Consistent with previous studies examining cross-informant reports of eating patterns such as external eating, emotional eating, loss of control or binge eating (35, 36, 37), adolescents’ reports of EAH showed low correspondence with their parents’ reports of EAH. Furthermore, adolescents’ reports of EAH were unrelated to body composition and to measured EAH after a standardized lunch meal and after an array lunch meal. The one unexpected exception was that adolescent report of EAH in response to external cues was inversely related to observed EAH after a standardized meal. Said differently, lower self-reports of EAH were related to greater observed EAH in the laboratory, even when accounting for body composition and demographic factors. This pattern was observed only when EAH was assessed after a standardized meal, but did not differ by sex or overweight status. Thus, these data suggest that adolescents’ EAH-C reports are generally inaccurate measures of eating when not hungry in the laboratory setting. Indeed, the observed inverse relationship between self-reported and observed EAH after the standardized meal may be reflective of the notion that EAH often results from poor awareness of appetitive cues in the presence of highly palatable food environmental stimuli (20).
Study limitations include the relatively small sample size and the cross-sectional nature of the data. Future studies are required to examine the EAH-C’s and EAH-P’s predictive validity of adolescent EAH over time and/or increases in children’s weight or adiposity. Also, in the current study, we focused on 13–17-year-old youth, and the percentage of overweight youth was somewhat greater than U.S. national norms (40% vs. 34%). Thus, the utility of the EAH-C and EAH-P may differ in younger or older groups or samples less enriched for overweight youth. It is also possible that demand characteristics of the experimental paradigm might have altered either reported or actual EAH. Similarly, ecological validity may have been limited by serving a lunch-type meal at 11:00 a.m., which – although a majority (~60%) of adolescents skip breakfast several times per week (38) – may have represented a departure from some youths’ usual eating patterns. The snacks foods offered during the EAH assessment period shared sensory properties to snacks foods offered at the array lunch meal, which might have lead to sensory-specific satiety and diminished the degree of EAH in this condition. We did not obtain objective measurements of parental height and weight, and parents’ own body weight may have an influence on their perceptions of their children’s EAH. Finally, questionnaire measures of EAH explained a significant but relatively small percentage (~5–7%) of the variance in observed eating behavior, reflecting that other phenotypic factors (e.g., youths’ current body composition (5)) and genotype (4) very likely contribute to EAH.
Strengths of the current study include the use of several measures of observed eating behavior in a sample of adolescents diverse in race/ethnicity, sex, and body weight. Since EAH has been observed following both a standardized meal (4, 5) and a large array (5), our findings suggest that the EAH-P may be a useful screening tool following either type of meal. Data suggest that parents can provide valid information on their children’s eating behavior in the absence of hunger in response to external cues. When observational measures of EAH are not feasible, the EAH-P may be a reasonable measure of adolescents’ EAH. Likewise, the EAH-P may prove useful to clinicians working with youths who have weight- or eating-related problems. There is evidence to suggest that interventions that explicitly target reducing EAH by cue exposure and responsiveness to satiety signals have the potential to reduce children’s EAH (39, 40). Parental assessment of EAH by questionnaire may be useful in identifying youth prone to frequent eating when not hungry.
Eating in the absence of hunger has emerged as a robust endophenotype for child and adolescent obesity. Yet, whether this behavior represents a specific eating pattern that promotes excessive weight gain beyond other risk factors remains to be conclusively established. In the current study, we provide evidence that a parent questionnaire to assess eating when not hungry is significantly associated with adolescents’ observed eating when not hungry, even when accounting for adolescents’ current body weight. Parental reports of EAH in response to external cues may prove useful as the field moves forward in trying to elucidate the role of EAH in risk for obesity.
Acknowledgments
JAY is a Commissioned Officer in the U.S. Public Health Service, Department of Health and Human Services. The authors’ responsibilities were as follows: MTK, LBS, ABC, SZY, and JAY designed the study. LBS, MTK, and JAY analyzed the data, interpreted the results, and drafted the manuscript. All authors (LBS, MTK, MM, SAR, ABC, SEF, BEM, SMB, SZY, and JAY) contributed to the collection and assembly of data, provided critical revision of the article for content, andapproved the final version of the manuscript. We thank the volunteers who participated for their help in completing these studies. The funding organization played no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; nor preparation or review of the manuscript.
Research support: NICHD National Research Service Award 1F32HD056762 and Career Development Award K99HD069516 (to L. Shomaker), NIDDK grant 1R01DK080906 and USUHS grant R072IC (to M. Tanofsky-Kraff), and NICHD Intramural Research Program grant ZIAHD000641 with supplemental funding from NIMHD and OBSSR (to J. Yanovski).
Footnotes
ClinicalTrials.gov ID: NCT00631644
Disclosure Statement
None of the authors had any conflict of interest. Disclaimer: The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the U.S. Public Health Service, the Uniformed Services University of the Health Sciences, or the U.S. Department of Defense.
References
- 1.Kral TV, Faith MS. Child eating patterns and weight regulation: a developmental behaviour genetics framework. Acta Paediatr Suppl. 2007;96:29–34. doi: 10.1111/j.1651-2227.2007.00167.x. [DOI] [PubMed] [Google Scholar]
- 2.Birch LL, Fisher JO, Davison KK. Learning to overeat: Maternal use of restrictive feeding practices promotes girls’ eating in the absence of hunger. Am J Clin Nutr. 2003;78:215–220. doi: 10.1093/ajcn/78.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher JO, Birch L. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. Am J Clin Nutr. 2002;76:226–231. doi: 10.1093/ajcn/76.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher JO, Cai G, Jaramillo SJ, Cole SA, Comuzzie AG, Butte NF. Heritability of hyperphagic eating behavior and appetite-related hormones among Hispanic children. Obesity. 2007;15:1484–1495. doi: 10.1038/oby.2007.177. [DOI] [PubMed] [Google Scholar]
- 5.Shomaker LB, Tanofsky-Kraff M, Zocca JM, Courville A, Kozlosky M, Columbo KM, et al. Eating in the absence of hunger in adolescents: intake after a large-array meal compared with that after a standardized meal. Am J Clin Nutr. 2010;92:697–703. doi: 10.3945/ajcn.2010.29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cutting TM, Fisher JO, Grimm-Thomas K, Birch LL. Like mother, like daughter: familial patterns of overweight are mediated by mothers’ dietary disinhibition. Am J Clin Nutr. 1999;69:608–613. doi: 10.1093/ajcn/69.4.608. [DOI] [PubMed] [Google Scholar]
- 7.Hill C, Llewellyn CH, Saxton J, Webber L, Semmler C, Carnell S, et al. Adiposity and ‘eating in the absence of hunger’ in children. Int Journal Obes. 2008;32:1499–1505. doi: 10.1038/ijo.2008.113. [DOI] [PubMed] [Google Scholar]
- 8.Moens E, Braet C. Predictors of disinhibited eating in children with and without overweight. Behav Res Ther. 2007;45:1357–1368. doi: 10.1016/j.brat.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Francis LA, Ventura AK, Marini M, Birch LL. Parent overweight predicts daughters’ increase in BMI and disinhibited overeating from 5 to 13 years. Obesity. 2007;15:1544–1553. doi: 10.1038/oby.2007.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faith MS, Berkowitz RI, Stallings VA, Kerns J, Storey M, Stunkard AJ. Eating in the absence of hunger: a genetic marker for childhood obesity in prepubertal boys? Obesity. 2006;14:131–138. doi: 10.1038/oby.2006.16. [DOI] [PubMed] [Google Scholar]
- 11.Carnell S, Wardle J. Appetitive traits and child obesity: measurement, origins and implications for intervention. Proc Nutr Soc. 2008;67:343–355. doi: 10.1017/S0029665108008641. [DOI] [PubMed] [Google Scholar]
- 12.Butte NF, Cai G, Cole SA, Wilson TA, Fisher JO, Zakeri IF, et al. Metabolic and behavioral predictors of weight gain in Hispanic children: the Viva la Familia Study. Am J Clin Nutr. 2007;85:1478–1485. doi: 10.1093/ajcn/85.6.1478. [DOI] [PubMed] [Google Scholar]
- 13.Wolkoff LE, Tanofsky-Kraff M, Shomaker LB, Kozlosky M, Columbo KM, Elliott CA, et al. Self-reported vs. actual energy intake in youth with and without loss of control eating. Eat Behav. 2011;12:15–20. doi: 10.1016/j.eatbeh.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher JO, Johnson RK, Lindquist C, Birch LL, Goran MI. Influence of body composition on the accuracy of reported energy intake in children. Obes Res. 2000;8:597–603. doi: 10.1038/oby.2000.77. [DOI] [PubMed] [Google Scholar]
- 15.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children’s Eating Behaviour Questionnaire. J Child Psychol Psychiatry. 2001;42:963–970. doi: 10.1111/1469-7610.00792. [DOI] [PubMed] [Google Scholar]
- 16.Carnell S, Wardle J. Measuring behavioural susceptibility to obesity: validation of the child eating behaviour questionnaire. Appetite. 2007;48:104–113. doi: 10.1016/j.appet.2006.07.075. [DOI] [PubMed] [Google Scholar]
- 17.Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101:539–549. [PubMed] [Google Scholar]
- 18.Larson R, Richards MH. Daily companionship in late childhood and early adolescence: Changing developmental contexts. Child Dev. 1991;62:284–300. doi: 10.1111/j.1467-8624.1991.tb01531.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaur H, Li C, Nazir N, Choi WS, Resnicow K, Birch LL, et al. Confirmatory factor analysis of the child-feeding questionnaire among parents of adolescents. Appetite. 2006;47:36–45. doi: 10.1016/j.appet.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Shomaker LB, Tanofsky-Kraff M, Yanovski JA. Disinhibted eating and body weight in youth. In: Preedy VR, Watson RR, Watson CR, editors. Handbook of Behavior, Food, and Nutrition. Springer; 2011. [Google Scholar]
- 21.Tanner JM. Growth and maturation during adolescence. Nutr Rev. 1981;39:43–55. doi: 10.1111/j.1753-4887.1981.tb06734.x. [DOI] [PubMed] [Google Scholar]
- 22.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 25.Nicholson JC, McDuffie JR, Bonat SH, Russell DL, Boyce KA, McCann S, et al. Estimation of body fatness by air displacement plethysmography in African American and white children. Pediatr Res. 2001;50:467–473. doi: 10.1203/00006450-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Tanofsky-Kraff M, Ranzenhofer LM, Yanovski SZ, Schvey NA, Faith M, Gustafson J, et al. Psychometric properties of a new questionnaire to assess eating in the absence of hunger in children and adolescents. Appetite. 2008;51:148–155. doi: 10.1016/j.appet.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shomaker LB, Tanofsky-Kraff M, Elliott C, Wolkoff LE, Columbo KM, Ranzenhofer LM, et al. Salience of loss of control for pediatric binge episodes: does size really matter? Int J Eat Disord. 2010;43:707–716. doi: 10.1002/eat.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Institute of Medicine of the National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: 2005. [DOI] [PubMed] [Google Scholar]
- 29.Terry PC, Lane AM, Lane HJ, Keohane L. Development and validation of a mood measure for adolescents. J Sports Sci. 1999;17:861–872. doi: 10.1080/026404199365425. [DOI] [PubMed] [Google Scholar]
- 30.Tukey JW. Exploratory data analysis. Addison-Wesley; Reading, MA: 1977. [Google Scholar]
- 31.Tanofsky-Kraff M, McDuffie JR, Yanovski SZ, Kozlosky M, Schvey NA, Shomaker LB, et al. Laboratory assessment of the food intake of children and adolescents with loss of control eating. Am J Clin Nutr. 2009;89:738–745. doi: 10.3945/ajcn.2008.26886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shomaker LB, Tanofsky-Kraff M, Savastano DM, Kozlosky M, Columbo KM, Wolkoff LE, et al. Puberty and observed energy intake: boy, can they eat! Am J Clin Nutr. 2010;92:123–129. doi: 10.3945/ajcn.2010.29383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zocca JM, Shomaker LB, Tanofsky-Kraff M, Columbo KM, Raciti GR, Brady SM, et al. Links between mothers’ and children’s disinhibited eating and children’s adiposity. Appetite. 2011;56:324–331. doi: 10.1016/j.appet.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanofsky-Kraff M, Goossens L, Eddy KT, Ringham R, Goldschmidt A, Yanovski SZ, et al. A multisite investigation of binge eating behaviors in children and adolescents. J Consult Clin Psychol. 2007;75:901–913. doi: 10.1037/0022-006X.75.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanofsky-Kraff M, Yanovski SZ, Yanovski JA. Comparison of child interview and parent reports of children’s eating disordered behaviors. Eat Behav. 2005;6:95–99. doi: 10.1016/j.eatbeh.2004.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinberg E, Tanofsky-Kraff M, Cohen ML, Elberg J, Freedman RJ, Semega-Janneh M, et al. Comparison of the child and parent forms of the Questionnaire on Eating and Weight Patterns in the assessment of children’s eating-disordered behaviors. Int J Eat Disord. 2004;36:183–194. doi: 10.1002/eat.20022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braet C, Soetens B, Moens E, Mels S, Goossens L, Van Vlierberghe L. Are two informants better than one? Parent-child agreement on the eating styles of children who are overweight. Eur Eat Disord Rev. 2007;15:410–417. doi: 10.1002/erv.798. [DOI] [PubMed] [Google Scholar]
- 38.Pastore DR, Fisher M, Friedman SB. Abnormalities in weight status, eating attitudes, and eating behaviors among urban high school students: correlations with self-esteem and anxiety. J Adolesc Health. 1996;18:312–319. doi: 10.1016/1054-139X(95)00321-I. [DOI] [PubMed] [Google Scholar]
- 39.Boutelle KN, Zucker NL, Peterson CB, Rydell SA, Cafri G, Harnack L. Two novel treatments to reduce overeating in overweight children: A randomized controlled trial. J Consult Clin Psychol. 2011;79:759–771. doi: 10.1037/a0025713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson SL. Improving Preschoolers’ self-regulation of energy intake. Pediatrics. 2000;106:1429–1435. doi: 10.1542/peds.106.6.1429. [DOI] [PubMed] [Google Scholar]