Abstract
Adipose tissue plays an important role in skeletal homeostasis, and there is interest in identifying adipokines that influence bone mass. One such adipokine may be apelin, a ligand for the Gi-G protein-coupled receptor APJ, which has been reported to enhance mitogenesis and suppress apoptosis in MC3T3-E1 cells and primary human osteoblasts (OBs). However, it is unclear whether apelin plays a physiological role in regulating skeletal homeostasis in vivo. In this study, we compared the skeletal phenotypes of apelin knockout (APKO) and wild-type mice and investigated the direct effects of apelin on bone cells in vitro. The increased fractional cancellous bone volume at the distal femur was observed in APKO mice of both genders at 12 weeks of age and persisted until the age of 20. Cortical bone perimeter at the femoral midshaft was significantly increased in males and females at both time points. Dynamic histomorphometry revealed that APKO mice had increased rates of bone formation and mineral apposition, with evidences of accelerated OB proliferation and differentiation, without significant alteration in osteoclast activity. An in vitro study showed that apelin increased proliferation of primary mouse OBs as well as suppressed apoptosis in a dose-dependent manner with the maximum effect at 5nM. However, it had no effect on the formation of mineralized nodules. We did not observed significantly altered in osteoclast parameters in vitro. Taken together, the increased bone mass in mice lacking apelin suggested complex direct and paracrine/endocrine effects of apelin on bone, possibly via modulating insulin sensitivity. These results indicate that apelin functions as a physiologically significant antianabolic factor in bone in vivo.
Adipose tissue plays key roles in the regulation of skeletal homeostasis. Several potential mechanisms have been proposed to explain the complex relationship between fat and bone mass. In addition to the effect of soft tissue mass on skeletal loading, the influence of fat on bone is likely to be both paracrine and endocrine, such as the secretion of bone active hormones from pancreatic β-cells (eg, insulin, amylin, and preptin) and from adipocytes (eg, estrogens, adiponectin, and leptin) (1).
Apelin was identified in 1998 as an endogenous ligand of the Gi-G protein-coupled receptor (GPCR), APJ (2). Apelin is initially synthesized as a 77-amino acid prepropeptide that can be cleaved into fragments of different size. To date, the main active forms of apelin are apelin-13, apelin-17, and apelin-36 and the pyroglutamated isoform of apelin-13 ([Pyr1]-Apelin-13) characterized by a higher resistance to degradation (3). Apelin and its receptor are distributed in a wide variety of tissues, including brain, heart, lung, kidney, gastrointestinal tract, retina, and mammary gland (3–7). Apelin exerts a broad range of physiological roles, including effects on heart contractility, blood pressure, blood vessel growth, appetite and drink behavior, and pituitary hormone secretion (8–11). Recent evidences suggested that apelin/APJ may hold promise as a target for the development of novel therapeutics for patients with metabolic and cardiovascular disease (12–18). Apelin is also produced and secreted by adipocytes (19, 20), and its expression in adipose tissue is regulated by factors such as fasting and refeeding, insulin, GH, TNFα, and peroxisome proliferator-activated receptor γ coactivator 1α (3). Apelin, as an adipokine, influences glucose and lipid metabolism. Increases in plasma apelin in obesity have been associated with insulin resistance and hyperinsulinemia, and a decline in apelin levels after diet-induced weight loss or barietric surgery in obese individuals has also been described (19, 21–23). Apelin-deficient mice have reduced insulin sensitivity and increased abdominal and epididymal fat mass (24, 25). Moreover, long-term apelin treatment of obese and insulin-resistant mice has also been shown to improve insulin sensitivity (25, 26).
Human and murine osteoblasts (OBs) have been reported to express APJ (27, 28), suggesting that apelin/APJ might be another metabolic factor that impacts bone. There is limited data about the effect of apelin on human bone, with one study showing no significant correlation between the level of serum apelin and bone mineral density and bone turnover markers in postmenopausal Chinese woman (29). Apelin was shown to enhance mitogenesis, as well as suppress apoptosis, in MC3T3-E1 cells and primary human OBs in vitro, and these actions were mediated via c-Jun N-terminal kinase and phosphatidylinositol 3-kinase/Akt signaling pathways (27, 28, 30). Until now, there has been no published data regarding the effect of apelin on bone in vivo. In the present study, we have addressed the question of whether apelin influences skeletal homeostasis in vivo by determining the bone phenotype of apelin knockout (APKO) mice. We also investigated the direct effects of apelin on primary cultures of OBs and osteoclasts in vitro.
Materials and Methods
Animals
Generalized APKO mice used in this study were developed by Charo et al (31) at Stanford University School of Medicine. Briefly, the embryonic stem cells were originally from 129Sv/J line, which was injected into the C57Bl6 blastocysts, and the 80%–90% chimeric mice were back bred with 129Sv/J mice to get the germ line transmission into 129Sv/J background. The mice were then backcrossed with 129Sv/J mice later on for 6 generations. APKO mice are born in the expected Mendelian ratios, appear healthy, and have normal fertility. Real-time reverse transcription polymerase chain reaction (RT-qPCR) demonstrated no apelin mRNA expression in various tissues, including brain, heart, lung, liver, and skeletal muscle (25). Lack of apelin mRNA expression in 12-week-old tibias was also confirmed by RT-PCR (Supplemental Figure 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Animals were maintained on a normal chow diet (Prolab RMH 3000; Purina-Mills, St Louis, Missouri) containing 26% protein, 14% fat, and 60% carbohydrate by calorie count; given free access to tap water; and housed in a room with a 12-hour light, 12-hour dark cycle and an ambient temperature of 22°C. Male and female mice at 12 and 20 weeks were characterized. Age- and sex-matched 129Sv/J wild-type (WT) mice were used as controls. All protocols were approved by the Administrative Panel on Laboratory Animal Care at Stanford University School of Medicine, where the animals were raised, and by the Animal Use Committee of the San Francisco Veterans Affairs Medical Center, where the animals were studied.
Microcomputed tomography (μCT)
Left femurs from 12- and 20-week-old mice were isolated and cleaned of adherent tissue. Before μCT analysis, bones were fixed for 1–2 days in 10% neutral-buffered formalin (NBF) (Fisher Scientific, Pittsburgh, Pennsylvania) and stored in 70% ethanol for μCT scanning. The femurs were imaged using a vivaCT-40 μCT system (ScancoMedical AG, Bruttisellen, Switzerland). Imaging of cancellous bone was carried out at the distal metaphysis, and imaging of diaphyseal cortical bone was carried out at the midpoint of the femur. All images were obtained using an x-ray energy of 55 kV with a voxel size of 10.5 μm on each side and integration time of 1000 msec. The cancellous region of interest was at a distance of 0.30–1.35 mm from the primary spongiosa. The cancellous region of interest was assessed in femurs of 20-week-old mice using a global thresholding protocol with segmentation values of 0.4/1/270. Quantitative assessment of diaphyseal cortex was conducted using data from 40 slices (0.42 mm) at the midfemur. Cortical bone from 20-week-old mice was assessed using a global thresholding protocol with segmentation values of 0.8/1/365.
Static histomorphometry
Femurs from 12- and 20-week-old mice were isolated at the time of euthanasia and fixed in 10% NBF for 1–2 days and stored in 70% ethanol. After μCT analysis, the undecalcified bone samples were embedded in methyl methacrylate and sectioned with Jung 2065 and 2165 microtomes (Leica, Bannockburn, Illinois). Sectioned bones were processed for Von Kossa (VK) staining and tartrate-resistant acid phosphatase (TRAP) staining as previously described (32).
Dynamic histomorphometry
Mice were injected with 20 mg/kg of calcein (Sigma-Aldrich, St Louis, Missouri) 21 and 7 days before euthanasia and with 15 mg/kg of demeclocycline (Sigma-Aldrich) 2 days before euthanasia. Mice were euthanized at 12 and 20 weeks, and femurs were isolated, fixed in 10% NBF, and stored in 70% ethanol. After μCT analysis, the undecalcified bone samples were embedded in methyl methacrylate. Assessment of cancellous bone was performed on 10-μm longitudinal sections from the left femur. Assessment of cortical bone was performed on 10-μm transverse sections at the midpoint of the left femur. Mosaic-tiled images were acquired at ×20 magnification with a Zeiss Axioplan Imager M1 microscope (Carl Zeiss MicroImaging, Thornwood, New York) fitted with a motorized stage. The tiled images were stitched and converted to a single image using the Axiovision software (Carl Zeiss MicroImaging) before blinded analyses being performed using image-analysis software (Bioquant Image Analysis Corp, Nashville, Tennessee). Cancellous bone was assessed in the region 100 μm from the lowest point on the growth plate, extending 1 mm down the metaphysis. The dynamic indices of bone formation that were measured include mineralizing surface per bone surface (MS/BS), mineral apposition rate (MAR), and surface-based bone-formation rate (BFR).
RNA extraction and RT-qPCR
Tissue samples were isolated and kept frozen in liquid nitrogen until processing. Before freezing, epiphyses were removed, and bone marrow was flushed from tibial bone samples. Frozen tissues were pulverized using a biopulverizer (Biospec Products, Inc, Bartlesville, Oklahoma), followed by RNA extraction using RNA STAT60 (Tel-Test, Inc, Friendswood, Texas) and subsequent purification using Microto-Midi Total RNA Purification kit (Invitrogen, Carlsbad, California). cDNA was synthesized using TaqMan Reverse Transcription reagents (Applied Biosystems, Inc, Foster City, California) and random hexamer primers according to the recommendations of the manufacturer. Gene amplification was measured with SYBR Green using the ABI Prism 7300 real-time thermocycler (Applied Biosystems, Inc). Analysis was carried out using the SDS software supplied with the thermocycler. The sequences of the primer sets are listed in Supplemental Table 1. All reactions were performed in triplicate, and target gene expression was displayed normalized to glyceraldehyde-3-phosphate dehydrogenase.
Apelin null mice were generated using a construct that replaced the start codon and signal sequence of the apelin gene with a bacterial lacZ reporter gene, whose expression can be assessed by RT-PCR. For analyzing the expression of apelin and lacZ mRNA in tibias, total RNA and cDNA synthesis was performed as described above. The sequence of the apelin primer is 5′-CTGCTGCTCTGGCTCTCCT-3′ and 5′- AGGAAGCAAAGCCTACATGG-3′, and the lacZ primer is 5′-ATACGCCGAACGATCGCCAGTTCT-3′ and 5′- CACTACGCGTACTGTGAGCCAGAG-3′. PCR was done as follows: 94°C for 3 minutes, 94°C for 1 minute, 58°C for 45 seconds, 72°C for 45 seconds, and 58°C for 45 seconds for 39 cycles, followed by a 5-minute incubation at 72°C. The PCR products (200 base-pairs for apelin and 350 base-pairs for lacZ) were visualized in a 2% agarose gel stained with SYBR Green.
Serum chemistry
Blood was collected from mice at the time of euthanasia and processed in MicroTainer serum separator tubes (BD Biosciences, San Jose, California). Serum procollagen type I amino-terminal propeptide (PINP) and serum pyridinoline (PYD) measurements were carried out using the rat/mouse PINP enzyme immunoassay kit AC-33F1 from Immunodiagnostic Systems (Scottsdale, Arizona) and the MetraBiosystems SerumPYD kit 8019 (MetaBiosystems, Santa Clara, California) according to manufacturers' directions. Insulin (Mouse Ultrasensitive ELISA kit; Alpco Diagnostics, Salem, New Hampshire) and adiponectin (mouse ELISA kit; Alpco Diagnostics) were also analyzed. Plasma glucose levels were assessed using a glucometer to measure tail-nick blood samples.
Primary mouse OB cultures
Calvarial OBs (COBs) were harvested using techniques previously described (33). Briefly, calvariae were dissected from 7- to 9-day-old 129Sv/J WT mice. After removal of sutures, pooled calvariae (6–8 animals) were subjected to 5 sequential, 15-minute digestions in an enzyme mixture containing 0.05% trypsin (25300; GIBCO, Rockville, Maryland) and 1.5-U/mL collagenase P (Boehringer Mannheim, Mannheim, Germany) at 37°C on a rocking platform. Cell fractions 2–5 were collected, pooled, and chilled by the addition of an equal volume of cold primary culture media (PCM) consisting of DMEM (Mediatech, Inc, Manassas, Virginia), 10% fetal bovine serum (FBS) (Thermo Scientific, Lagan, Utah), 100-U/mL penicillin, 100-μg/mL streptomycin (Mediatech, Inc), and 0.25-μg/mL fungizone (Mediatech, Inc). Cells were centrifuged, resuspended, and filtered through a 70-μm cell strainer. An aliquot of cells was diluted 1:1 with 0.4% trypan blue in PBS, and viable cells were counted.
Cell proliferation
Effect of apelin on COB proliferation was determined by measuring bromodeoxyuridine (BrdU) incorporation using a Cell Proliferation ELISA kit (Roche Applied Science, Indianapolis, Indiana). Briefly, COBs were plated onto 96-well plates at a seeding density of 5 × 103 cells/well in PCM. On day 4, 0.5nM to 25nM [Pyr1]-Apelin-13 (ANASPEC, Fremont, California) was added to the media for 48 hours, and BrdU was added 24 hours before the assay. The incorporated BrdU in each culture was quantified according to the manufacturer's instruction. The results of this assay were confirmed by repeating the experiment 3 times.
Cell apoptosis
Effect of apelin on OB apoptosis was determined by using a Cell Death Detection ELISA kit (Roche Applied Science) according to the manufacturer's instruction. Briefly, bone marrow stromal cells (BMSCs) were plated onto 96-well plates at a seeding density of 5 × 103 cells/well in PCM. At day 5, 50-μg/mL ascorbic acid (Sigma-Aldrich) and 5mM β-glycerol phosphate (Sigma-Aldrich) were added to the PCM to induce OB differentiation for the next 7 days. To assess cell apoptosis, cells were then cultured in serum-free media for 48 hours in the absence or presence of 0.5nM to 25nM [Pyr1]-Apelin-13 before the assay. One percent FBS treatment was used as a negative control. The results of this assay were confirmed by repeating the experiment 3 times.
Mineralization
COBs were plated onto 6-well plates at a density of 2.4 × 106 cells/well. Cells were incubated in a humidified atmosphere of 5% CO2 at 37°C. On day 7, 50-μg/mL ascorbic acid (Sigma-Aldrich) and 5mM β-glycerol phosphate (Sigma-Aldrich) were added to the PCM to induce OB differentiation. Media were changed every 2–3 days. To assess mineralization, 2% silver nitrate solution (Sigma-Aldrich) was added to cell culture dishes on days 28–35 for VK staining and UV-cross-linked for 10 minutes. Stained cultures were scanned and quantified using Improvision Openlab software version 5.0.2. The results of this assay were confirmed by repeating the experiment 5 times.
Adipocyte differentiation
Primary BMSCs were isolated from the femurs and tibias of 12-week-old 129Sv/J WT male mice. The cells were collected in PCM and plated onto 12-well plates at a density of 5 × 106 cells/well. Cells were treated with 0.5nM to 25nM [Pyr1]-Apelin-13 for the first 5 days of culture and remained in PCM for 10 days. At day 10, the medium was replaced with secondary adipogenic differentiation medium (addition of 1μM rosiglitazone, 1μM dexamethasone, 5-μg/mL insulin, and 500μ 3-isobutyl-1-methylxanthine), and cells were maintained in it for 2 days. After 2 days, this medium was replaced with PCM containing 5-μg/mL insulin, and cells were incubated in it for another 2 days. Cells were then maintained in PCM until day 19. At day 19, cells were fixed in PBS containing 10% formaldehyde. After fixation, cells were stained in Oil Red O for 15 minutes. Cells were rinsed several times with distilled water to remove excess stain. Stained cultures were scanned and quantified using Improvision Openlab software version 5.0.2. for identifying the lipid accumulation in the differentiated cells.
BMSC cultures
A previously described procedure (34) was used to harvest BMSC from 20-week-old APKO and WT mice. The marrow from each individual mouse was cultured in α-modification of Eagle's medium (MEM) (Thermo Scientific), supplemented with 10% fetal calf serum, 100-U/mL penicillin, 100-μg/mL streptomycin, and 20-ng/mL macrophage-colony stimulating factor (M-CSF) (R&D Systems, Minneapolis, Minnesota). After 2 days of M-CSF stimulation, nonadherent osteoclast precursors were transferred onto 48-well plates at a density of 0.75 × 106 cells/well and cultured in α-MEM with 60-ng/mL receptor activator of nuclear factor-κB ligand (RANKL) (R&D Systems) and 10-ng/mL M-CSF for an additional 8 days. Fresh media were added every 3 days. At the end of culture, TRAP staining was performed using a Leukocyte Acid Phosphatase kit (Sigma-Aldrich) to identify osteoclasts as TRAP+ cells containing 3 or more nuclei. TRAP+ osteoclast numbers were counted by light microscopy. Resorption activity was evaluated using a 96-well Corning Osteo Assay Surface polystyrene Stripwell Microplate (Corning, Inc, Tewksbury, Massachusetts). Cells (1 × 106) were plated and treated with 60-ng/mL RANKL and 10-ng/mL M-CSF for 10 days. Individual pits or multiple pit clusters were visualized by light microscopy, and digital images were recorded. The resorption area was determined by using a Bioquant Image Analysis system.
The osteoclast cultures were also performed in the absence or presence of [Pyr1]-Apelin-13. BMSCs were isolated from WT mice and cultured in 10% FBS/MEM + 1%penicillin/streptomycin (Mediatech, Inc). At day 2, nonadherent cells (mainly osteoclast precursor) were collected and replating onto 48-well plates with an addition of M-CSF and RANKL. [Pyr1]-Apelin-13 was added in different concentration from 0.5nM to 25nM. TRAP staining was performed on day 9 to assess TRAP+ osteoclast numbers. Resorption activity was evaluated using a 96-well Corning Osteo Assay Surface polystyrene Stripwell Microplate (Corning, Inc).
Statistical analysis
All data are presented as mean ± SD. Statistical significance was ascertained by comparison between APKO mice vs age- and sex-matched controls using a 2-tailed Student's t tests assuming equal variance. Statistical significance was taken as P < .05.
Results
General phenotype of APKO mice
There were no significant differences observed in the body weights and femoral lengths of 12- and 20-week-old APKO and age-matched WT mice of both genders (Table 1). APKO mice exhibited hyperinsulinemia, albeit only significantly changed in males, without alteration in fasting plasma glucose levels. A decrease in adiponectin level was also observed in APKO males. These results were consistent with Yue et al (24, 25), which found that 12-week-old APKO males displayed hyperinsulinemia and hypoadiponectimenia with increased free fatty acid and abdominal adiposity. However, a significantly altered adiponectin levels were not observed in our female APKO.
Table 1.
Phenotypic Characteristics of APKO and WT Mice Aged 12 and 20 Weeks
| Male |
Female |
|||||||
|---|---|---|---|---|---|---|---|---|
| 12 weeks |
20 weeks |
12 weeks |
20 weeks |
|||||
| WT | APKO | WT | APKO | WT | APKO | WT | APKO | |
| Body weight (g) | 26.2 ± 2.3 | 28.6 ± 2.7 | 27.2 ± 2.6 | 29.6 ± 3.5 | 20.7 ± 0.7 | 21.8 ± 1.0 | 21.4 ± 1.0 | 22.9 ± 1.8 |
| Femoral length (mm) | 15.9 ± 0.2 | 15.4 ± 0.3 | 15.6 ± 0.3 | 15.3 ± 0.3 | 15.8 ± 0.4 | 15.6 ± 0.3 | 16.0 ± 0.2 | 15.9 ± 0.3 |
| Glucose (mg/dL) | 66.1 ± 14.8 | 61.7 ± 11.1 | 71.8 ± 16.7 | 63.8 ± 14.2 | 67.2 ± 15.3 | 62.8 ± 14.2 | 67.0 ± 12.4 | 58.5 ± 13.9 |
| Insulin (ng/mL) | 0.92 ± 0.15 | 1.31 ± 0.31a | 0.77 ± 0.43 | 1.23 ± 0.14a | 0.44 ± 0.22 | 0.75 ± 0.51 | 0.50 ± 0.26 | 0.93 ± 0.27 |
| Adiponectin (μg/mL) | 21.4 ± 2.7 | 17.4 ± 1.9a | 17.2 ± 2.7 | 13.8 ± 2.2a | 30.7 ± 5.3 | 28.9 ± 3.5 | 24.7 ± 2.6 | 26.1 ± 2.6 |
The values are the mean ± SD (n = 6–10 per group).
Statistically significant changes relative to age- and sex-matched controls, P < .05.
Skeletal phenotype of APKO mice
Cortical bone structure
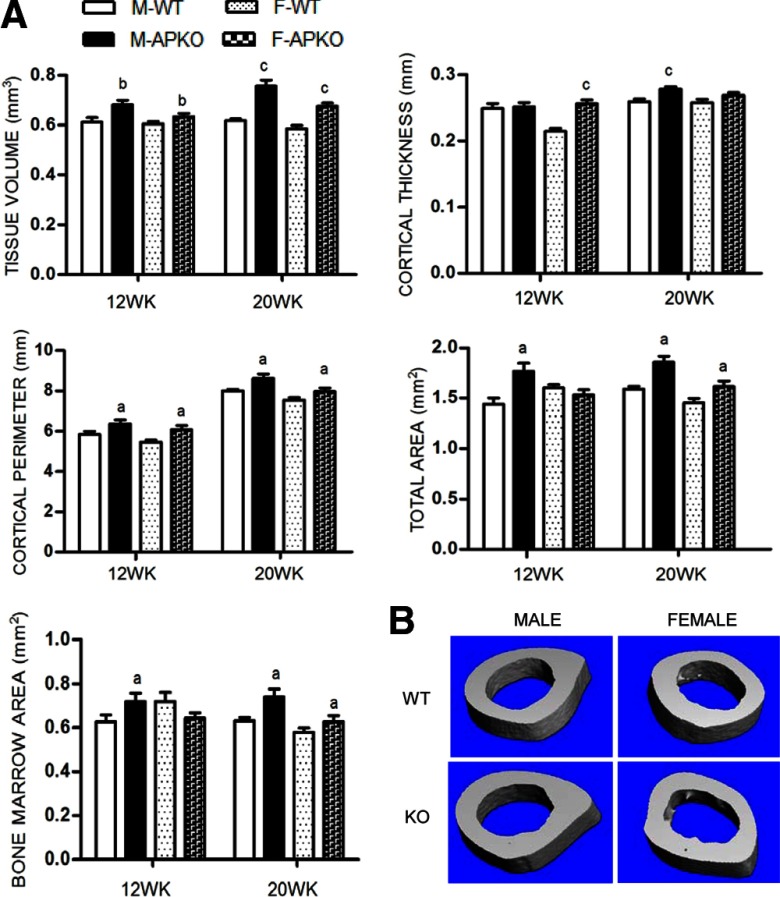
Cortical bone of APKO mice was assessed by μCT at the femoral midshaft of 12- and 20-week-old mice (Figure 1). Cortical tissue volume was increased in APKO mice of both genders. We observed an increase in the cortical thickness in younger APKO females (12 wk old) and adult APKO males (20 wk old). As assessed by 2-dimension illustration, total area and cortical perimeter were significantly greater in male and female APKO mice, suggesting a mechanically stronger cortical architecture in these mice. This phenomenon was further examined by dynamic histomorphometry. An increase in the periosteal BFR, resulting from increased mineralizing surface (MS/BS), and MAR, was observed in male APKO mice. Female APKO mice displayed the same trend, but the periosteal parameters did not reach statistical significance. No change in endosteal BFR was observed in both genders (Table 2).
Figure 1.
A, The cortical bone parameters of the midshaft of femurs isolated from male and female mice at 12 and 20 weeks, assessed by μCT (tissue volume and cortical thickness) and cross-sectional area analysis (total area, bone marrow area, and cortical perimeter) (n ≥ 10 per group). B, Three-dimensional reconstruction μCT renderings of the midshaft of femurs from 20-week-old APKO mice compared with age- and sex-matched controls. Representative images were selected from animals whose μCT parameters were closest to the mean of their representative group. All data are mean ± SD. Statistical significance ascertained compared with age- and sex-matched controls. aP < .05; bP < .01; cP < .001. M, male; F, female; KO, apelin knockout.
Table 2.
Periosteal and Endocortical Dynamic Histomorphometric Measurements Performed on 12- and 20-Week-Old APKO Mice and Sex- and Age-Matched WT Mice at the Femoral Midshaft
| Mice | Periosteum |
Endosteum |
||||
|---|---|---|---|---|---|---|
| MS/BS (%) | MAR (μm/d) | BFR/BS (μm3/μm2·d) | MS/BS (%) | MAR (μm/d) | BFR/BS (μm3/μm2·d) | |
| Male | ||||||
| 12 weeks, WT | 32.5 ± 10.8 | 1.57 ± 0.14 | 0.50 ± 0.14 | 43.4 ± 17.3 | 1.37 ± 0.29 | 0.56 ± 0.08 |
| 12 weeks, APKO | 50.9 ± 19.7 | 2.48 ± 0.78a | 1.21 ± 0.49a | 30.9 ± 13.2 | 0.97 ± 0.69 | 0.35 ± 0.31 |
| 20 weeks, WT | 13.5 ± 9.8 | 1.03 ± 0.11 | 0.14 ± 0.10 | 42.3 ± 5.0 | 0.69 ± 0.27 | 0.30 ± 0.15 |
| 20 weeks, APKO | 32.7 ± 13.4a | 1.97 ± 0.29a | 0.67 ± 0.37a | 36.5 ± 13.3 | 0.65 ± 0.43 | 0.27 ± 0.19 |
| Female | ||||||
| 12 weeks, WT | 33.0 ± 15.8 | 2.12 ± 0.77 | 0.70 ± 0.37 | 16.9 ± 19.6 | 0.96 ± 1.25 | 0.32 ± 0.53 |
| 12 weeks, APKO | 43.7 ± 9.3 | 1.53 ± 0.44 | 0.68 ± 0.30 | 25.0 ± 17.3 | 1.86 ± 1.23 | 0.36 ± 0.32 |
| 20 weeks, WT | 21.6 ± 7.1 | 1.29 ± 0.44 | 0.30 ± 0.15 | 34.8 ± 9.5 | 0.60 ± 0.55 | 0.36 ± 0.15 |
| 20 weeks, APKO | 44.2 ± 17.4 | 1.26 ± 0.34 | 0.56 ± 0.23 | 40.9 ± 11.6 | 1.06 ± 0.49 | 0.41 ± 0.32 |
The values are the mean ± SD (n = 3–4 per group).
Statistically significant changes relative to age- and sex-matched controls, P < 0.05.
Cancellous bone structure
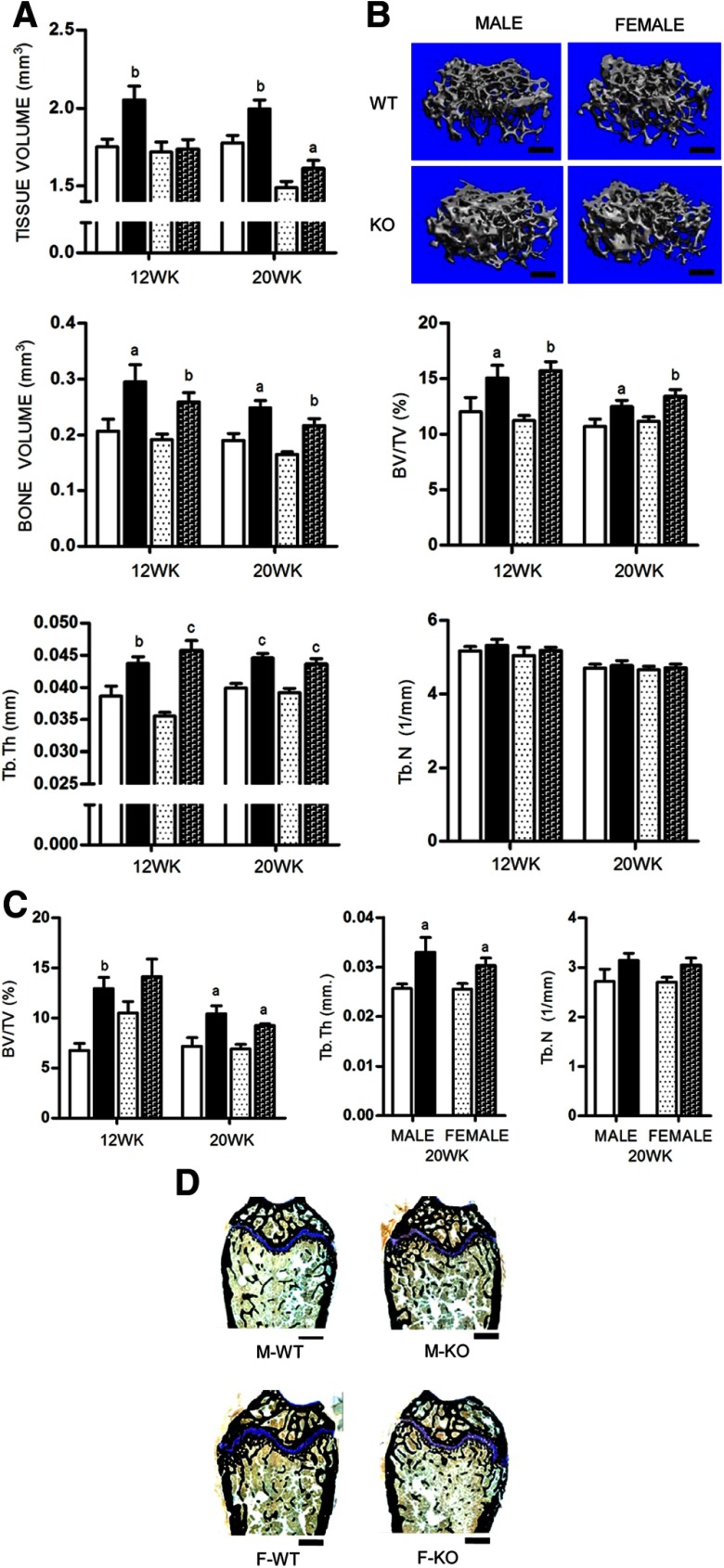
To assess cancellous bone mass in APKO mice, femurs from male and female mice at 12 and 20 weeks were examined using both μCT and histomorphometry. The cancellous fractional bone volume (bone volume/tissue volume [BV/TV]), assessed by μCT, was significantly increased in both male and female APKO mice at the age of 12 weeks and persisted until 20 weeks (10.43 ± 1.4% in male KO vs 7.17 ± 1.5% in WT; and 9.24 ± 0.3% in female KO vs 6.91 ± 0.8% in WT, P < .05). The increase in BV/TV was associated with an increase in trabecular thickness (Figure 2A). These differences can be clearly observed from comparison of representative 3-dimensional reconstruction images of distal femurs (Figure 2B). Histomorphometric analysis concurred with the μCT data, indicating that the increase in BV/TV resulted from increased trabecular thickness with no change in trabecular number (Figure 2, C and D).
Figure 2.
A, Cancellous bone was assessed by μCT at the distal femurs isolated from male and female mice at 12 and 20 weeks (n ≥ 10 per group). B, Three-dimensional reconstruction μCT renderings of distal femurs from 20-week-old APKO mice compared with age- and sex-matched controls. Representative images were selected from animals whose μCT parameters were closest to the mean of their representative group. C, Histomorphometric analysis of 12- and 20-week-old femurs (n = 5 per group). D, Histomorphometric sections of distal femurs of 20-week-old mice stained for VK and Tetrachrome. All data are mean ± SD. Statistical significance ascertained compared with age- and sex-matched controls. aP < .05; bP < .01; cP < .001. Tb.N, trabecular number; Tb.Th, trabecular thickness. KO, apelin knockout.
Osteoblasts and bone formation
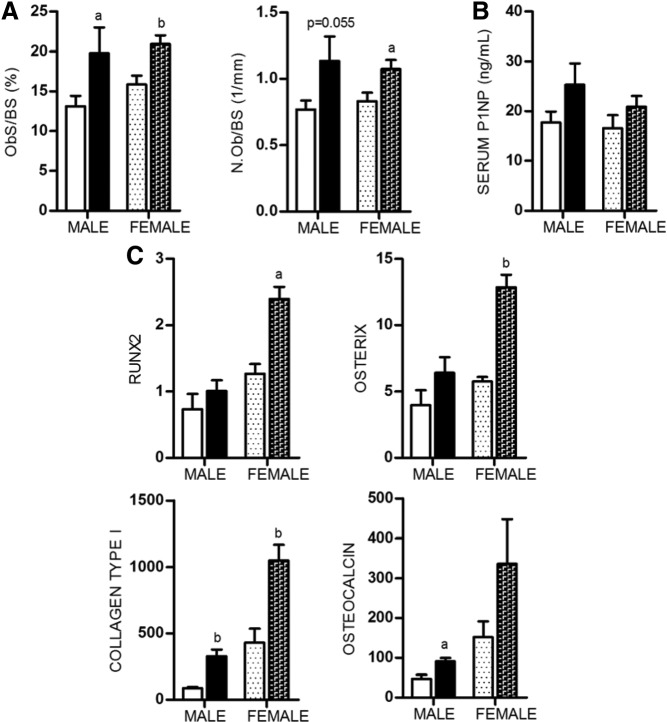
Static histomorphometric measurements were taken at the metaphysis of the distal femurs and revealed that male APKO mice had increased OB surface and OB number per bone surface (Figure 3A). These changes correlated with alteration in the expression of OB marker genes, collagen type I and osteocalcin, in RNA isolated from 20-week-old femurs (Figure 3C). The level of serum PINP, a marker of bone formation, was unaltered in APKO mice compared with WT mice (Figure 3B). These findings were also observed in female APKO mice but with some differences, such as an increase in early OB marker genes, Runx2 and osterix. To assess whether the increase in cancellous bone mass observed in APKO mice could be due to increased OB activity, dynamic histomorphometry was performed in 12- and 20-week-old femurs (Table 3). Both male and female APKO mice displayed an increase in BFR compared with age- and sex-matched controls. These findings support the idea that the increased bone mass in AKPO mice was caused by increased numbers of active OBs.
Figure 3.
A, Osteoblast surface per bone surface (Ob.S/BS) and OB number per bone surface (N.Ob/BS) assessed by histomorphometry in sections of 20-week-old femurs (n = 4 per group). B, Serum PINP, a marker of OB activity, measured in serum from 20-week-old animals (n = 6–12 per group). C, Expression level of OB marker genes: Runx2, osterix, collagen type 1, and osteocalcin, in bone. All expression data were obtained by RT-qPCR analysis of RNA isolated from 20-week-old femurs (n = 3 per group). All data are shown as mean ± SD. Statistical significance ascertained compared with age- and sex-matched controls. aP < .05; bP < .01.
Table 3.
Cancellous Dynamic Histomorphometric Measurements Performed on 12- and 20-Week-Old APKO Mice and Age- and Sex-Matched Controls at the Distal Femur
| Mice | MS/BS (%) | MAR (μm/d) | BFR/BS (μm3/μm2·d) |
|---|---|---|---|
| Male | |||
| 12 weeks, WT | 38.9 ± 6.4 | 1.37 ± 0.07 | 0.53 ± 0.11 |
| 12 weeks, APKO | 51.2 ± 15.8 | 2.08 ± 0.19a | 1.05 ± 0.40a |
| 20 weeks, WT | 33.7 ± 5.7 | 1.49 ± 0.34 | 0.49 ± 0.14 |
| 20 weeks, APKO | 41.9 ± 4.9 | 1.90 ± 0.01 | 0.79 ± 0.09a |
| Female | |||
| 12 weeks, WT | 46.2 ± 7.0 | 2.16 ± 0.36 | 0.90 ± 0.08 |
| 12 weeks, APKO | 46.5 ± 15.8 | 2.60 ± 0.19 | 1.19 ± 0.40 |
| 20 weeks, WT | 32.1 ± 2.3 | 2.47 ± 0.28 | 0.79 ± 0.09 |
| 20 weeks, APKO | 44.8 ± 4.2a | 2.14 ± 0.36 | 0.95 ± 0.13a |
The values are the mean ± SD (n = 3–5 per group).
Statistically significant changes relative to age- and sex-matched controls, P < 0.05.
Osteoclasts and bone resorption
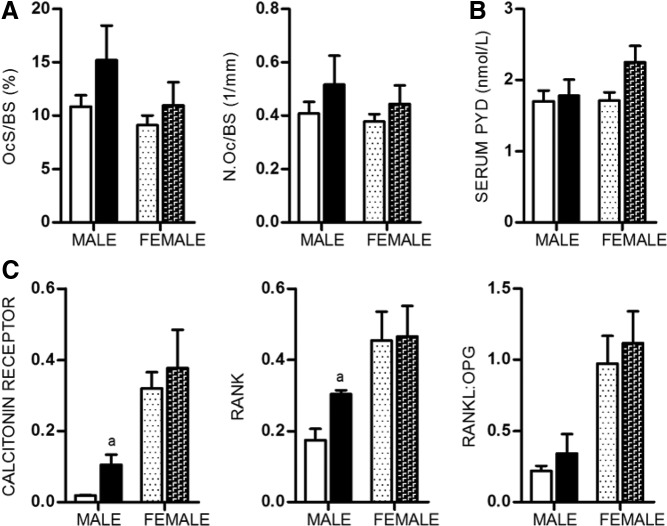
We saw little evidence suggesting that apelin has downstream effects on osteoclasts. Histomorphometry revealed a slight increase in osteoclast surface and osteoclast number per bone surface in both male and female APKO mice (Figure 4A). Similarly, levels of serum PYD cross-links, a marker of bone resorption, were unaltered in these mice (Figure 4B). RANKL, mainly produced by osteocytes, and osteoprotegerin (OPG) are important factors that regulate the development of osteoclasts (35–37). Expression of RANKL, OPG, and the ratio of RANKL to OPG remain unchanged in 20-week-old femurs. However, expression of calcitonin receptor and RANK, a gene highly expressed in osteoclasts, were increased in male APKO mice (Figure 4C). These findings suggest that apelin has a minor role in osteoclastogenesis.
Figure 4.
A, Osteoclast surface per bone surface (Oc.S/BS) and osteoclast number per bone surface (N.Oc/BS) assessed by histomorphometry in sections of 20-week-old femurs (n = 4 per group). B, Serum PYD cross-links, a marker of osteoclast activity, measured in serum from 20-week-old animals (n = 6–12 per group). C, Expression level of the osteoclast marker genes: Calcitonin receptor and RANK, as well as the RNA expression ratio of the osteoclastogenic factor, RANKL, and its decoy receptor, OPG, in bone. All expression data were obtained by RT-qPCR analysis of RNA isolated from 20-week-old femurs (n = 3 per group). All data are shown as mean ± SD. Statistical significance ascertained compared with age- and sex-matched controls. aP < .05.
Effects of apelin on OB proliferation in vitro
We confirmed the APJ expression in primary COBs by RT-qPCR (Supplemental Figure 2). The effect of apelin on OB proliferation was assessed using a BrdU incorporation assay. Apelin significantly increased proliferation in primary COBs (+41%, P < .01) in a dose-dependent manner with the maximum effect at 5nM (Figure 5A). This concentration was about 2-fold higher than normal circulating levels (25). The effect of apelin on OB differentiation was assessed by VK staining. Continuous apelin treatment (0.5nM, 5nM, and 50nM) over 28–35 days had no effect on the formation of mineralized nodules in COB cultures (Figure 5B).
Figure 5.

A, Effect of apelin (0.5nM to 50nM) on COB proliferation was assessed by using a BrdU incorporation assay. B, Effect of apelin on COB differentiation and mineralization was assessed by VK staining at days 28–35. The total colony area for VK staining was expressed as fold over basal. C, Effect of apelin on OB apoptosis was assessed by Cell Death Detection ELISA kit. D, Expression level of the adipocyte marker genes: eroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer-binding protein α (C/EBPα). All expression data were obtained by RT-qPCR analysis of RNA isolated from 20-week-old femurs (n = 3 per group). E, Effect of apelin on adipogenesis. Oil Red O staining of lipid contents of the cell indicated the degree of adipogenesis. Total colony area stained positive for Oil Red O staining and total Oil Red O-positive colony number (CFU) were quantified as percent of basal level of control. The results of these assays were confirmed by repeating the experiment 3 times. All data are shown as mean ± SD. aP < .05; bP < .01.
Effect of apelin on OB apoptosis in vitro
Effect of apelin on OB apoptosis was determined by Cell Death Detection ELISA photometric enzyme immunoassay kit. Apelin at the concentration of 5nM significantly reduced OB apoptosis, and the effect was observed until the concentration of 25nM (Figure 5C).
Effects of apelin on adipogenesis
We have assessed the marrow fat in APKO mice by measuring mRNA expression of adipocyte marker genes, CCAAT/enhancer-binding protein α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ), by RT-qPCR from femurs and tibias of 20-week-old mice. We found no difference in gene expression levels between KO and WT mice, suggesting that the APKO mice have unaltered adipose differentiation (Figure 5D). We have also examined the effect of exposing BMSCs to apelin on subsequent adipogenesis in vitro. We found no difference in the Oil Red O positive colonies after 19 days of culture, suggesting that apelin treatment on BMSCs did not alter the subsequent adipogenesis (Figure 5E).
Effects of apelin on osteoclastogenesis and osteoclast activity
Osteoclasts were derived in vitro from bone marrow macrophages by 8 days of treatment with M-CSF and RANKL. RNA isolated from these osteoclasts was examined for expression of APJ and osteoclast marker genes by RT-qPCR analysis and compared with the expression of these transcripts in bone marrow-derived macrophages treated with M-CSF alone for 2 days. Macrophages in M-CSF alone expressed low levels of APJ and RANK but not calcitonin receptor and cathepsin K. The presence of mature osteoclasts derived in vitro from 129Sv/J mice was supported by high expression levels of cathepsin K. We demonstrated that osteoclasts from bone marrow precursor cells express APJ, and the expression level was comparable with calcitonin receptor (Supplemental Figure 2). However, in BMSC cultures from APKO and WT mice, TRAP+ multinucleated cell numbers and area of pit formation were unaltered in the presence of M-CSF and RANKL. Also, the effect of exogenous apelin on osteoclastogenesis in vitro was not demonstrated at the presence of 0.5nM–25nM of [Pyr1]-Apelin-13.
Discussion
This is the first study to date investigating the physiological role of apelin on skeletal homeostasis in vivo, using APKO mice. We demonstrated that mice lacking apelin increased both trabecular and cortical bone mass. These findings suggest that apelin is an adipokine that plays a physiological role in regulating skeletal homeostasis. The phenotypic changes were discovered from 12 weeks in the similar patterns of the older mice, suggesting that the observed increased bone mass is a consequence of increased mineral accumulation during skeletal development. Dynamic histomorphometric analysis of APKO mice revealed a significant increase in parameters of trabecular bone formation, indicating that the increase in their trabecular bone volume can be attributed to an increase in bone formation. The increase in bone formation in APKO mice was associated with an increase in OB surfaces and OB numbers, together with and an increase in the expression of OB marker genes. We saw little evidence for a role of altered bone resorption in APKO mice. Although the increased bone marrow area was consistent with the possibility that endocortical resorption was elevated in the knockout mice at some point, the osteoclast marker genes, the osteoclast surfaces, and serum PYD cross-link were unaltered in these mice. These have indicated that the primary cause of an increase in bone mass was increased bone formation. Interestingly, the effect of apelin deletion on cortical bone differed in males and females. Cortical tissue volume and cortical perimeter were increased in both genders. However, an increase in cortical thickness was observed earlier in females APKO, and it disappeared when the mice became mature adulthood. In contrast, the cortical thickness was increased in adult APKO males. We found that the bone mineral content, assessed by μCT, was significantly increased in APKO mice compared with controls, suggesting that there was more mineralizing matrix in these mice. Together with the increased bone formation parameters, we believed that APKO mice should have better biomechanical properties. However, the effect of the changes on the biomechanical properties of bone remains to be investigated.
It is well known that adipokines, such as leptin or adiponectin, may impact the skeleton in several ways. They could affect on bone cells directly, via autocrine/paracrine actions, and/or indirectly via an endocrine mechanism. The integration of these effects may contribute to the overall skeletal changes in vivo (38–41). Thus, to assess a possible direct effect of apelin on bone cells, we have confirmed that APJ is expressed in OBs and osteoclasts. In vitro studies showed that apelin enhanced primary mouse OB proliferation as well as suppressed apoptosis, without significantly altered mineralization. Our results are similar to those of Xia et al (27, 28, 30), who found that apelin promoted proliferation and suppressed apoptosis of MC3T3-E1 OB-like cells and primary human OBs, with unchanged alkaline phosphatase mRNA expression. APKO mice have unaltered adipocyte differentiation in bone marrow environment. Moreover, apelin had no effect on subsequent adipogenesis of BMSCs in vitro.
Recently, we have demonstrated that blockade of GPCR induces Gi signaling in mature OBs in vivo-enhanced bone formation (42). APJ is one of the identified OB Gi-GPCRs that might be responsible for negatively regulating bone formation. It is interesting to address that the increase in bone mass in APKO mice may resulted from an inhibition of apelin/APJ Gi-GPCR signaling in mature OBs. Further studies assessing the bone phenotypes in APJ OB-specific knockout mice are needed to clarify this issue.
We further demonstrated that apelin and its receptor were also expressed in bone marrow macrophages and mature osteoclasts at the similar level of calcitonin receptor. However, no significant changes of osteoclast differentiation and bone resorption activity were observed in the culture of mouse bone marrow macrophages from APKO mice compared with controls. Therefore, the direct effect of apelin on bone cells did not clearly explain overall bone phenotypes in APKO mice, suggesting that apelin affects the skeleton in a complex manner, involving both direct and indirect pathways.
Apelin and APJ are widely expressed throughout the body and exert a broad range of physiological and pathological roles (8–11). Apelin plays a protective role in cardiovascular and whole-body metabolism. In clinical studies, serum apelin levels were positively correlated with body mass index, and the levels were increased in patients with impaired glucose intolerance or with type 2 diabetes. Reduced plasma apelin levels were found in patients after diet-induced weight loss or bariatric surgery (22, 23, 43). Both short- and long-term apelin treatments of obese and insulin-resistant mice have been shown to improve insulin sensitivities by enhancing insulin-stimulated glucose uptake in muscles, decreased insulin production, and reduced fat mass (24, 26). In addition, insulin can stimulate adipose apelin expression, and apelin, in turn, can inhibit insulin secretion, suggesting a close interaction between the 2 systems (3, 19, 44, 45). In our study, we found that 20-week-old APKO mice had significantly increased in insulin levels compared with controls. The result was consistent with a report by Yue et al (25), in which 12-week-old male APKO mice were shown to be hyperinsulinemic, and the phenotypic features of insulin resistance were restored after reintroduction of apelin. However, the adiponectin level remained decreased only in 20-week-old males. Insulin and adiponectin have been shown to influence bone metabolism (38, 39, 46–49), and the alteration in serum insulin and adiponectin levels in our mouse model might be one explanation for the increased bone mass.
Apelin might be associated with inflammation. Its expression was induced by inflammatory mediators, such as TNFα (50), IL-6, and interferon-γ (51); and plasma apelin was correlated with markers of inflammation. Moreover, it had been shown that apelin can act as proinflammation adipokine that participates in vascular wall inflammation (52). It remains to establish the role of apelin as the mediating inflammation in bone environment.
Apelin/APJ are expressed widely in different areas in the central nervous system and has also been shown to be a neuropeptide, acting through autocrine, involved in the modulation of the hypothalamus-hypophysis axis activity as well as in body fluid balance. A recent study had demonstrated that apelin was typically localized in corticotropic cells and promoted autocrine/paracrine ACTH release (53). Apelin might affect the other regions of hypothalamus or nervous system, and it is interesting to consider the possibility that apelin effects on bone could have a neurogenic basis.
In conclusion, the present study indicates that the adipokine apelin has overall negative effects on bone formation in vivo. Further studies are needed to determine the detailed cellular and molecular bases for this effect and the extent to which apelin contributes to the negative effects of fat on bone.
Supplementary Material
Acknowledgments
L.W. thanks Richard Kao, Zhiqiang cheng, and Yongmei Wang for valuable technical assistance and discussion. R.A.N. is a Senior Research Career Scientist of the Department of Veterans Affairs.
This work was supported by National Institutes of Health Grant DK072071 and the Department of Veterans Affairs Merit Review Grant 1I01BX001496 (to R.A.N.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- APKO
- apelin knockout
- BFR
- bone-formation rate
- BMSC
- bone marrow stromal cell
- BrdU
- bromodeoxyuridine
- BV/TV
- bone volume/tissue volume
- COB
- calvarial OB
- μCT
- microcomputed tomography
- FBS
- fetal bovine serum
- GPCR
- G protein-coupled receptor
- MAR
- mineral apposition rate
- M-CSF
- macrophage-colony stimulating factor
- MEM
- modification of Eagle's medium
- MS/BS
- mineralizing surface per bone surface
- NBF
- neutral-buffered formalin
- OB
- osteoblast
- OPG
- osteoprotegerin
- PCM
- primary culture media
- PINP
- procollagen type I amino-terminal propeptide
- PYD
- pyridinoline
- [Pyr1]-Apelin-13
- pyroglutamated isoform of apelin-13
- RANKL
- receptor activator of nuclear factor-κB ligand
- RT-qPCR
- real-time reverse transcription polymerase chain reaction
- TRAP
- tartrate-resistant acid phosphatase
- VK
- Von Kossa
- WT
- wild type.
References
- 1. Reid IR. Relationships between fat and bone. Osteoporos Int. 2008;19:595–606 [DOI] [PubMed] [Google Scholar]
- 2. Tatemoto K, Hosoya M, Habata Y, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–476 [DOI] [PubMed] [Google Scholar]
- 3. Pitkin SL, Maguire JJ, Bonner TI, Davenport AP. International union of basic and clinical pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and function. Pharmacol Rev. 2010;62:331–342 [DOI] [PubMed] [Google Scholar]
- 4. Carpéné C, Dray C, Attané C, et al. Expanding role for the apelin/APJ system in physiopathology. J Physiol Biochem. 2007;63:359–373 [PubMed] [Google Scholar]
- 5. Kleinz MJ, Skepper JN, Davenport AP. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul Pept. 2005;126:233–240 [DOI] [PubMed] [Google Scholar]
- 6. Medhurst AD, Jennings CA, Robbins MJ, et al. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J Neurochem. 2003;84:1162–1172 [DOI] [PubMed] [Google Scholar]
- 7. O'Carroll AM, Selby TL, Palkovits M, Lolait SJ. Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochim Biophys Acta. 2000;1492:72–80 [DOI] [PubMed] [Google Scholar]
- 8. Ashley E, Chun HJ, Quertermous T. Opposing cardiovascular roles for the angiotensin and apelin signaling pathways. J Mol Cell Cardiol. 2006;41:778–781 [DOI] [PubMed] [Google Scholar]
- 9. Cox CM, D'Agostino SL, Miller MK, Heimark RL, Krieg PA. Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev Biol. 2006;296:177–189 [DOI] [PubMed] [Google Scholar]
- 10. Reaux A, De Mota N, Skultetyova I, et al. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J Neurochem. 2001;77:1085–1096 [DOI] [PubMed] [Google Scholar]
- 11. Taheri S, Murphy K, Cohen M, et al. The effects of centrally administered apelin-13 on food intake, water intake and pituitary hormone release in rats. Biochem Biophys Res Commun. 2002;291:1208–1212 [DOI] [PubMed] [Google Scholar]
- 12. Atluri P, Morine KJ, Liao GP, et al. Ischemic heart failure enhances endogenous myocardial apelin and APJ receptor expression. Cell Mol Biol Lett. 2007;12:127–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dai T, Ramirez-Correa G, Gao WD. Apelin increases contractility in failing cardiac muscle. Eur J Pharmacol. 2006;553:222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foussal C, Lairez O, Calise D, et al. Activation of catalase by apelin prevents oxidative stress-linked cardiac hypertrophy. FEBS Lett. 2010;584:2363–2370 [DOI] [PubMed] [Google Scholar]
- 15. Francia P, Salvati A, Balla C, et al. Cardiac resynchronization therapy increases plasma levels of the endogenous inotrope apelin. Eur J Heart Fail. 2007;9:306–309 [DOI] [PubMed] [Google Scholar]
- 16. Pchejetski D, Foussal C, Alfarano C, et al. Apelin prevents cardiac fibroblast activation and collagen production through inhibition of sphingosine kinase 1. Eur Heart J. 2012;33(18):2360–2369 [DOI] [PubMed] [Google Scholar]
- 17. Tao J, Zhu W, Li Y, et al. Apelin-13 protects the heart against ischemia-reperfusion injury through inhibition of ER-dependent apoptotic pathways in a time-dependent fashion. Am J Physiol Heart Circ Physiol. 2011;301:H1471–H1486 [DOI] [PubMed] [Google Scholar]
- 18. Goetze JP, Rehfeld JF, Carlsen J, et al. Apelin: a new plasma marker of cardiopulmonary disease. Regul Pept. 2006;133:134–138 [DOI] [PubMed] [Google Scholar]
- 19. Boucher J, Masri B, Daviaud D, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–1771 [DOI] [PubMed] [Google Scholar]
- 20. Kawamata Y, Habata Y, Fukusumi S, et al. Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta. 2001;1538:162–171 [DOI] [PubMed] [Google Scholar]
- 21. Castan-Laurell I, Dray C, Knauf C, Kunduzova O, Valet P. Apelin, a promising target for type 2 diabetes treatment? Trends Endocrinol Metab. 2012;23:234–241 [DOI] [PubMed] [Google Scholar]
- 22. Castan-Laurell I, Vítkova M, Daviaud D, et al. Effect of hypocaloric diet-induced weight loss in obese women on plasma apelin and adipose tissue expression of apelin and APJ. Eur J Endocrinol. 2008;158:905–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soriguer F, Garrido-Sanchez L, Garcia-Serrano S, et al. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Obes Surg. 2009;19:1574–1580 [DOI] [PubMed] [Google Scholar]
- 24. Yue P, Jin H, Xu S, et al. Apelin decreases lipolysis via G(q), G(i), and AMPK-dependent mechanisms. Endocrinology. 2011;152:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yue P, Jin H, Aillaud M, et al. Apelin is necessary for the maintenance of insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;298:E59–E67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Attané C, Foussal C, Le Gonidec S, et al. Apelin treatment increases complete fatty acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin-resistant mice. Diabetes. 2012;61:310–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tang SY, Xie H, Yuan LQ, et al. Apelin stimulates proliferation and suppresses apoptosis of mouse osteoblastic cell line MC3T3-E1 via JNK and PI3-K/Akt signaling pathways. Peptides. 2007;28:708–718 [DOI] [PubMed] [Google Scholar]
- 28. Xie H, Tang SY, Cui RR, et al. Apelin and its receptor are expressed in human osteoblasts. Regul Pept. 2006;134:118–125 [DOI] [PubMed] [Google Scholar]
- 29. Zhang H, Xie H, Zhao Q, et al. Relationships between serum adiponectin, apelin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in post-menopausal Chinese women. J Endocrinol Invest. 2010;33:707–711 [DOI] [PubMed] [Google Scholar]
- 30. Xie H, Yuan LQ, Luo XH, et al. Apelin suppresses apoptosis of human osteoblasts. Apoptosis. 2007;12:247–254 [DOI] [PubMed] [Google Scholar]
- 31. Charo DN, Ho M, Fajardo G, et al. Endogenous regulation of cardiovascular function by apelin-APJ. Am J Physiol Heart Circ Physiol. 2009;297:H1904–H1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iwaniec UT, Yuan D, Power RA, Wronski TJ. Strain-dependent variations in the response of cancellous bone to ovariectomy in mice. J Bone Miner Res. 2006;21:1068–1074 [DOI] [PubMed] [Google Scholar]
- 33. Kalajzic I, Kalajzic Z, Kaliterna M, et al. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J Bone Miner Res. 2002;17:15–25 [DOI] [PubMed] [Google Scholar]
- 34. Huang JC, Sakata T, Pfleger LL, et al. PTH differentially regulates expression of RANKL and OPG. J Bone Miner Res. 2004;19:235–244 [DOI] [PubMed] [Google Scholar]
- 35. Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakashima T, Hayashi M, Fukunaga T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234 [DOI] [PubMed] [Google Scholar]
- 37. Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Williams GA, Wang Y, Callon KE, et al. In vitro and in vivo effects of adiponectin on bone. Endocrinology. 2009;150:3603–3610 [DOI] [PubMed] [Google Scholar]
- 39. Oshima K, Nampei A, Matsuda M, et al. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun. 2005;331:520–526 [DOI] [PubMed] [Google Scholar]
- 40. Cirmanova V, Bayer M, Starka L, Zajickova K. The effect of leptin on bone: an evolving concept of action. Physiol Res. 2008;57(suppl 1):S143–S151 [DOI] [PubMed] [Google Scholar]
- 41. Williams GA, Callon KE, Watson M, et al. Skeletal phenotype of the leptin receptor-deficient db/db mouse. J Bone Miner Res. 2011;26:1698–1709 [DOI] [PubMed] [Google Scholar]
- 42. Millard SM, Louie AM, Wattanachanya L, Wronski TJ, Conklin BR, Nissenson RA. Blockade of receptor-activated G(i) signaling in osteoblasts in vivo leads to site-specific increases in cortical and cancellous bone formation. J Bone Miner Res. 2011;26:822–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li L, Yang G, Li Q, et al. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp Clin Endocrinol Diabetes. 2006;114:544–548 [DOI] [PubMed] [Google Scholar]
- 44. Sörhede Winzell M, Magnusson C, Ahrén B. The apj receptor is expressed in pancreatic islets and its ligand, apelin, inhibits insulin secretion in mice. Regul Pept. 2005;131:12–17 [DOI] [PubMed] [Google Scholar]
- 45. Wei L, Hou X, Tatemoto K. Regulation of apelin mRNA expression by insulin and glucocorticoids in mouse 3T3-L1 adipocytes. Regul Pept. 2005;132:27–32 [DOI] [PubMed] [Google Scholar]
- 46. Cornish J, Callon KE, Reid IR. Insulin increases histomorphometric indices of bone formation In vivo. Calcif Tissue Int. 1996;59:492–495 [DOI] [PubMed] [Google Scholar]
- 47. Yang J, Zhang X, Wang W, Liu J. Insulin stimulates osteoblast proliferation and differentiation through ERK and PI3K in MG-63 cells. Cell Biochem Funct. 2010;28:334–341 [DOI] [PubMed] [Google Scholar]
- 48. Fujiwara N, Tabata MJ, Endoh M, Ishizeki K, Nawa T. Insulin-like growth factor-I stimulates cell proliferation in the outer layer of Hertwig's epithelial root sheath and elongation of the tooth root in mouse molars in vitro. Cell Tissue Res. 2005;320:69–75 [DOI] [PubMed] [Google Scholar]
- 49. Pun KK, Lau P, Ho PW. The characterization, regulation, and function of insulin receptors on osteoblast-like clonal osteosarcoma cell line. J Bone Miner Res. 1989;4:853–862 [DOI] [PubMed] [Google Scholar]
- 50. Daviaud D, Boucher J, Gesta S, et al. TNFα up-regulates apelin expression in human and mouse adipose tissue. FASEB J. 2006;20:1528–1530 [DOI] [PubMed] [Google Scholar]
- 51. Han S, Wang G, Qi X, Englander EW, Greeley GH., Jr Involvement of a Stat3 binding site in inflammation-induced enteric apelin expression. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1068–G1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Leeper NJ, Tedesco MM, Kojima Y, et al. Apelin prevents aortic aneurysm formation by inhibiting macrophage inflammation. Am J Physiol Heart Circ Physiol. 2009;296:H1329–H1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reaux-Le Goazigo A, Alvear-Perez R, et al. Cellular localization of apelin and its receptor in the anterior pituitary: evidence for a direct stimulatory action of apelin on ACTH release. Am J Physiol Endocrinol Metab. 2007;292:E7–E15 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.