Abstract
Rodent models show decreased neuronal responses to estradiol (E2) during aging (E2-desensitization) in association with reduced neuronal estrogen receptor (ER)-α, but little is known about age changes of E2-dependent astrocytic neurotrophic support. Because elevated expression of astrocyte glial fibrillary acidic protein (GFAP) is associated with impaired neurotrophic activity and because the GFAP promoter responds to ERα, we investigated the role of astrocytic ERα and ERβ in impaired astrocyte neurotrophic activity during aging. In vivo and in vitro, ERα was increased greater than 50% with age in astrocytes from the cerebral cortex of male rats (24 vs 3 months), whereas ERβ did not change. In astrocytes from 3-month-old males, experimentally increasing the ERα to ERβ ratio induced the aging phenotype of elevated GFAP and impaired E2-dependent neurite outgrowth. In 24-month-old male astrocytes, lowering ERα reversed the age elevation of GFAP and partially restored E2-dependent neurite outgrowth. Mixed glia (astrocytes to microglia, 3:1) of both sexes also showed these age changes. In a model of perimenopause, mixed glia from 9- to 15-month rats showed E2 desensitization: 9-month regular cyclers retained young-like ERα to ERβ ratios and neurotrophic activity, whereas 9-month noncyclers had elevated ERα and GFAP but low E2-dependent neurotrophic activity. In vivo, ERα levels in cortical astrocytes were also elevated. The persisting effects of ovarian acyclicity in vitro are hypothesized to arise from steroidal perturbations during ovarian senescence. These findings suggest that increased astrocyte ERα expression during aging contributes to the E2 desensitization of the neuronal responses in both sexes.
The two classical estradiol (E2) receptors, estrogen receptor (ER)-α and ERβ, undergo changes with age in brain systems of both sexes in humans (1, 2) and rodents (1, 3–6). Thus, aging female rats have decreased levels of both ERs in the cerebral cortex (4, 6). The age-impairments of responses to E2 by ERα in hippocampal axospinous synapses (7) and of neurite outgrowth after axotomy of cortical afferents (8), may be described as E2 desensitization. Although both ERs bind to the same promoter estrogen response elements, deficits of either ER influence transcription, eg, in the hippocampus (9). Moreover, the ERα to ERβ ratio can influence the sensitivity of transcriptional (9, 10) and behavioral responses to E2 (9–11).
Whether ER expression changes with age in astrocytes has not been reported. The coexpression of ERα and ERβ in astrocytes is critical to analysis of neuronal functions because of astrocyte-neuron interdependency throughout life. Although neonatal cultured astrocytes expressed both (12), the evidence is mixed on coexpression in vivo: in the unlesioned brain, only ERβ was detected (13), whereas after injury only ERα expression was definitive (14). During middle age and later adulthood, astrocytes show an increased expression of the intermediate filament protein glial fibrillary acidic protein (GFAP) and other genes responsive to inflammation and oxidative stress (15–18), concurrently with synapse attrition in many regions (15, 17, 19). These mild glial and neuronal changes are not associated with neuron death in aged rodents, which normally lack cerebrovascular or Alzheimer-like lesions (15). Thus, glial activation during normal aging differs from the reactive gliosis induced by injury or neurodegenerative disease.
To analyze glial roles in brain aging, we developed a heterochronic glial-neuron coculture model of enriched primary astrocytes from cerebral cortex of different aged rats seeded with embryonic day (E) 18 neurons as test cells (20). E2-dependent neurotrophic activities of astrocytes are mediated in part by laminin secretion, which varies inversely with GFAP expression, as shown by GFAP-cDNA manipulations (20–22). Cultured mixed glia containing astrocytes and microglia (3:1) were also examined because the standard shaking procedure to remove microglia might impact gene expression through hydrodynamic forces, as documented for astrocytes and other cells (23, 24). The age loss of E2-dependent neurotrophic activity was reversed by the small interfering RNA (siRNA) reduction of GFAP (20). Conversely, increasing GFAP with cDNA in young astrocytes impaired E2-dependent neurotrophic activity.
Because E2 regulates GFAP transcription via estrogen response elements that bind ERα (25), we characterized ERα and ERβ in enriched astrocytes from aged rats of both sexes. The male ages examined (3 vs 24 months) represent young adulthood and early senescence in the lab rat life span of about 30 months. Females aged 9–15 months are models of premenopausal stages during transitions from irregular cycles to acyclicity. We tested the role of ERα and ERβ in E2 responsivity of astrocytes with siRNA and cDNA for effects on GFAP and neurotrophic activity. With the heterochronic in vitro astrocyte model (20), Lewis et al (26) showed impaired E2-dependent neurotrophic support of astrocytes from acyclic 10-month vs 3-month cycling rats. The importance of resolving the effects of acyclicity from age is indicated by impairments of spatial learning in irregular cyclers relative to regular cyclers in rats of the same age (27). We found that astrocytic ERα increased with age and with ovarian senescence, which we specifically link to increased GFAP and to impaired E2-dependent neurotrophic activity.
Materials and Methods
Animal care and cycling status
Animal procedures conformed to National Institutes of Health guidelines, as approved by the University of Southern California Institutional Animal Care and Use Committee. Male F344 rats, aged 3–4 and 24 months (National Institutes of Health), and female Sprague Dawley retired breeders, aged 5 months, 9–10 months, 13 months, and 15 months, (Harlan Laboratories, Indianapolis, Indiana) were housed on 12-hour light, 12-hour dark cycles with ad libitum water and lab chow. Ovarian cycles were assessed in vaginal lavages for proportions of leukocytes, nucleated epithelia, and cornified epithelia (28). Regular cyclers (RCs) were selected by 2 consecutive 4- or 5-day cycles; acyclic (AC) had more than 10-day constant estrus (CEs) identified by keratinized (cornified) vaginal epithelial cells; alternate terminology for AC is CE or persistent vaginal cornification (28, 29). In 50%:75% of individuals, irregular lengthening cycles are followed by CE, characterized by elevated blood E2-progesterone (P4) due to multiple growing follicles secreting low levels of E2 and minimal P4 (28, 30).
Because stages of reproductive aging in rodents are driven by ovarian senescence as in women, rodents are a model for a subset of perimenopausal changes according to the updated Stages of Reproductive Aging Workshop (STRAW) criteria (31, 32). The CE rodent is a model for STRAW premenopause stage −2 with hyperestrogenic cycles and elevated plasma E2-P4 (32–34). In late ovarian senescence, rodents develop major hypothalamus-pituitary dysfunctions with persistently impaired E2-induced LH surges (29, 30, 35), whereas premenopausal women show milder, sporadic impairments of the E2-induced LH surge (36), implying transient hypothalamic dysfunction. Ovarian senescence is complete from follicular exhaustion after 18 months, about 10 months before the rodent life span (29, 37, 38), with ensuing ovariectomy-equivalent low levels of E2 and elevations of LH (39). The rodent end stage of ovarian senescence, although functionally equivalent to human menopause, is not described as such because rodents lack menses (32, 35, 40).
Brain collection
Animals were anesthetized with isoflurane and euthanized by decapitation (approved by the University of Southern California Institutional Animal Care and Use Committee). For primary glia culture, both cerebral hemispheres were used from the male, whereas only the right cerebral hemisphere was used from the female. For immunohistochemistry (IHC) only the left cerebral cortex was analyzed for both males and females. Brains were prepared for cryosection by fixation in 4% paraformaldehyde for 2 days before cryoprotection in 30% sucrose/PBS.
IHC and image analysis
Astrocyte ERs were analyzed from cerebral cortex layers 1 and 2/3, primary visual and retrosplenial regions, on the same sagittal sections: 18 μm; 3 sections per slide per condition (age or cycling status); ERs were analyzed from the left hemisphere of both sexes by IHC. Sections were postfixed in 4% paraformaldehyde and permeabilized in 1% Nonidet P-40, followed by blocking in 5% BSA, as developed for Western blots to minimize nonspecific binding. Sections were incubated overnight in primary antibodies: GFAP (primary; Millipore AB5541, 1:400; secondary, Alexa Fluor 488 Conjugate IgG; Millipore, Billerica, Massachusetts); ERβ (primary; Santa Cruz H-150; 1:100; secondary, Alexa Fluor 594 Conjugate IgG; Santa Cruz Biotechnology, Santa Cruz, California); ERα (primary, Millipore C1355, 1:100; secondary, Alexa Fluor 594 Conjugate IgG; Millipore). Triple colocalization used antimouse ERα (primary, Abcam ab2746; 1:100; secondary, Cy3 conjugated IgG; Abcam, Cambridge, Massachusetts) for compatibility with antirabbit ERβ (primary, Santa Cruz H-150, 1:100; secondary, Alexa Fluor 488 Conjugate IgG; Santa Cruz Biotechnology) and antichicken GFAP (primary, Millipore AB5541, 1:400; secondary, Alexa Fluor 647 Conjugate IgG; Millipore) and secondary antibodies (Invitrogen, Grand Island, New York). For ERα colocalization (Abcam ab2746), tyramide signal amplification was necessary (PerkinElmer, Downers Grove, Illinois) but was not used for ER quantification. Controls that omitted the primary antibody lacked immunostaining. Nonspecific binding was minimized by inclusion of 5% albumin (BSA), as developed for Western blots (see below). Images were analyzed by a Nikon TE2000 scanning confocal microscope and EZ-C1 software (both from Nikon, Melville, New York). Z-stack images of 1-μm steps were analyzed with ImageJ (National Institutes of Health, Bethesda, Maryland). The threshold was optimized to reduce background and maximize signal; the same settings were used throughout. Cells uniformly showed colocalization of ERs with GFAP in cells throughout the image stack. Immunostaining represents total integrated density of the cell body.
Cell culture, immunocytochemistry (ICC), and image analysis
Primary glia from adult rat cerebral cortex were cultured by standard methods, which maintain in vivo phenotypes of aging (20, 41). Note that male-derived cultures used both hemispheres, whereas those from females used only the right hemisphere. Cerebral hemispheres were dissociated by mechanical and enzymatic treatment and filtration. Cells were resuspended in fresh complete medium (DMEM/F12 media; Invitrogen), supplemented with 20% fetal bovine serum (HyClone, Logan, Utah), 100 U/mL penicillin, 50 U/mL streptomycin, and 2.5 mM L-glutamine (Sigma-Aldrich, St Louis, Missouri), plated on T-75 flasks, and grown 48 hours before medium replacement. After 12 days, fetal bovine serum was lowered to 10%. At confluence (3–4 weeks), cultures were trypsinized and replated on 4-well slides (200 000 cells/well). After 24 hours in secondary culture, neurons from E18 rat whole cerebral cortex (both sexes) were plated upon the glial monolayer (1:3, neuron-astrocyte) (22). Media were replaced with DMEM with 4.5 mg/mL glucose (Invitrogen), 100 U/mL penicillin, and 50 U/mL streptomycin with neuronal supplement (B-27) but without P4 (Invitrogen). For monotypic astrocytes (>95%), microglia were removed by shaking 4 hours (41, 42). One hour after plating neurons, loose cells were aspirated, and media were refreshed with 100 pM E2 (Steraloids, Inc, Newport, Rhode Island) or vehicle (0.08% ethanol). After 48 hours, cultures were fixed in cold methanol, washed with saline, and incubated with antirabbit GFAP (Dako Z0334; Dako Corp, Carpinteria, California) 1:400 and antimouse microtubule-associated protein 5 (MAP5) (Invitrogen M4528) 1:200, followed by fluorescent secondary antibodies, Alexa Fluor 488 goat antimouse and Alexa Fluor 594 goat antirabbit. MAP5+ neurons were visualized on an inverted fluorescent microscopy. Neurite outgrowth was measured by MAP-5 immunopositivity, with a Sobel edge detection algorithm (IPLab, Scanalytics, Fairfax, Virginia) (20) to skeletonize the image exclusive of cell bodies as a single pixel line, converted to neurite length. GFAP was measured as total immunoreactive area of the cell body.
Western blots
Cells were lysed in 10 mM Tris, 2% sodium dodecyl sulfate, 10% β-mercaptoethanol, and 0.5 mM EDTA, followed by boiling 5 minutes. For ERα and ERβ, proteins were electrophoresed on 10% acrylamide gels, followed by immunoblotting with primary anti-ERα (Millipore C1355, 1:100; Santa Cruz Biotechnology MC-20, 1:200; or Abcam ab2746, 1:100); anti-ERβ (Santa Cruz Biotechnology H150, 1:200) and anti β-tubulin (Santa Cruz Biotechnology H-235, 1:500), or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Millipore MAB374, 1:1000), and then processed with peroxidase-conjugated secondary antibodies and SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, Illinois). For ERs, to minimize nonspecific binding, we introduced a blocking step with 5% BSA prior to primary antibody incubation, which yielded a single protein band of each ER. Integrated density was measured by ImageJ software (National Institutes of Health); ER signals were normalized to GAPDH. The 3 ERα antibodies (from Abcam, Millipore, and Santa Cruz Biotechnology) detected similar changes with age and responses to siRNA or cDNA. Signals were normalized to β-actin or GAPDH. Data are presented as a percentage control because we cannot compute the actual prevalence of the transcriptionally relevant ERs per cell, which include minor splice variants.
Transfection of siRNA and cDNA
After plating onto poly-D-lysine-coated 4-chamber slides (see above), astrocytes were transfected with siRNA (rat, 30 nM) or ERα/β cDNA (human, 0.1 μg): ERα cDNA (2.0 kb; PCMV5 from C. J. Pike (University of Southern California) and ERβ cDNA (1.6 kb; pSG5 vector from C. Gaudon, Institut Génétique de Biologie Moléculaire et Cellulaire, Illkirch-Strasbourg, France), as follows: ERα siRNA, wt, AA/GTCTCTGGAAGAGAAGGAC/CA, mut, AA/GTCTCGTGAAGAGAAGGAC/CA; and ERβ siRNA, wt, AA/AGCTGCCAGGCCTGCCGAC/TT, mut, AA/AGCTGCACGGCCTGCCGAC/TT.
Transfection of astrocytes was done in serum-reduced OPTI-MEMI (Gibco, Grand Island, New York) for 6 hours and then incubated overnight in serum containing media and changed 24 hours after transfection. Two days later, E18 neurons were plated at 1:3 neuron-astrocyte. After 1 hour, 100 pM E2 was added with fresh media; 48 hours later, cultures were fixed in cold methanol.
Quantitative real-time PCR
cDNA was prepared from total cellular RNA (Super Script III; Invitrogen) to target the DNA binding domain shared by both ERs: ERα exons 2-3, 189 nt, G+C, 57%; ERβ exons 6–7, 493 nt, G+C, 55%. Both ERs had similar cycle threshold (CT) values: ERα, 31 ± 0.39; ERβ, 32 ± 0.39 (n = 12, control young male astrocytes). CT values were converted copy numbers with standard curves (r2 > 0.95) of plasmid controls, normalized to actin. The similar CT values suggest close to a 1:1 prevalence of ERα and ERβ. However, actual levels of functional ER mRNAs per cell cannot be determined because multiple isoforms are associated with these amplicons that could include nuclear and membrane isoforms. Because of these uncertainties, data are presented as percentage of control.
Primers were as follows: ERα forward, AATTCTGACAATCGACGCCAG, ERα reverse, GTGCTTCAACATTCTCCCTCCTC; ERβ forward, ATCTGTCCAGCCACGAATCA, ERβ reverse, ATTAGCACCTCCATCCAGCA; and actin forward, CTGGCACCACACCTTCTACAATG, actin reverse, GAAATCGTGCGTGACATCAAAGAG.
Statistical analysis
ERs were quantified in 4 brains from each condition, 3 images per brain, and 40–50 cells per image. Statistics represent average per brain ANOVA with Tukey post hoc text (α = .05). Male-derived glia were from 3–5 culture preparations from 2–3 rats per preparation. Female-derived glia were from 4 culture preparations from 4 rats per cycling stage. In vitro experiments consisted of 3–4 wells per condition. Statistics represent the average of 5 images per well (ANOVA and Tukey, as above).
Results
Age increase of male rat astrocytic ERα in vivo and in vitro
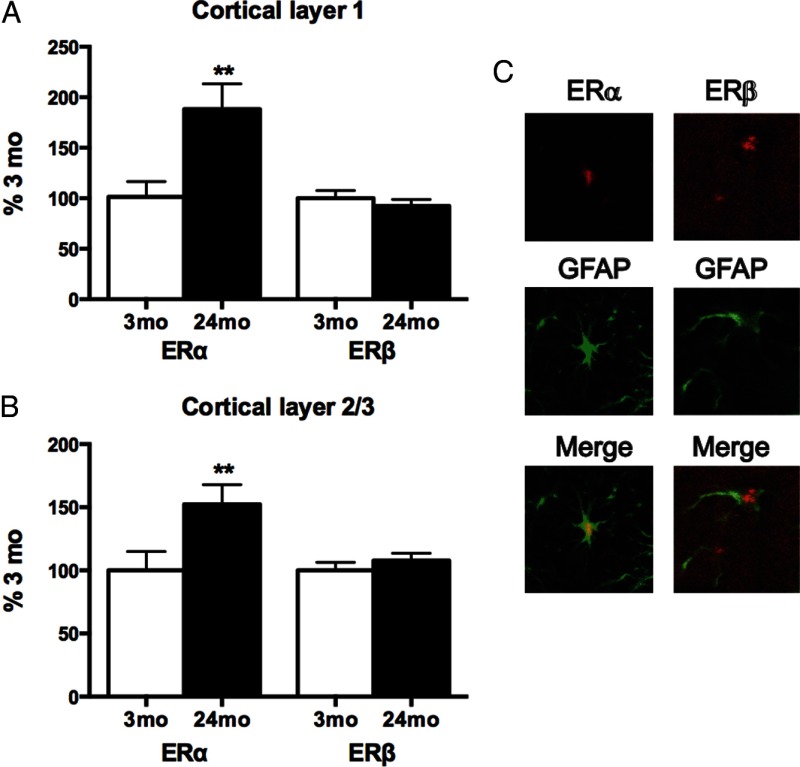
Age effects on expression of astrocyte ERα and ERβ were evaluated by IHC in the cerebral cortex. Astrocytes were identified by their characteristic intermediate filament GFAP. In the primary visual cortex, ERα immunoreactivity per astrocyte increased with age by 50% or greater in layer 1 and layer 2/3, whereas ERβ was unchanged (Figure 1, A and B). ERα and ERβ colocalized with GFAP (Figure 1C). Retrosplenial cortex astrocytes showed similar increases of ERα with age (Supplemental Figure 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Age did not alter the continued coexpression of both ERs in GFAP-immunopositive astrocytes (triple IHC, Supplemental Figure 2).
Figure 1.
Astrocyte ERs per cell of male rat cerebral cortex (primary visual region): 3 months (young adult) and 24 months (senescent) (4 brains per age group). Total integrated density of immunoreactive ERα or ERβ per GFAP-positive astrocyte, represented as percentage at 3 months (mean ± SEM). **P < .01. A, ERα per astrocyte in cortical layer 1 increased 1.9-fold with age, with no change in ERβ per astrocyte. B, ERα per astrocyte in cortical layers 2/3 increased 1.5-fold with age, with no change in ERβ per astrocyte. C, Confocal ICC images of GFAP-immunopositive astrocytes, costained for ERα or ERβ (pseudocolor with merged images).
In vitro, ERα mRNA and protein increased with age 3-fold in 24- vs 3-month primary astrocyte cultures, whereas ERβ was unchanged (Figure 2, A–C). Western blots showed a single band corresponding to the major isoforms, as expressed in neonatal astrocyte cultures (12, 43). Note the strong correlation (r2 = 0.72) of ER mRNA encoding the DNA binding site with the levels of a single band on Western blots in the same samples.
Figure 2.
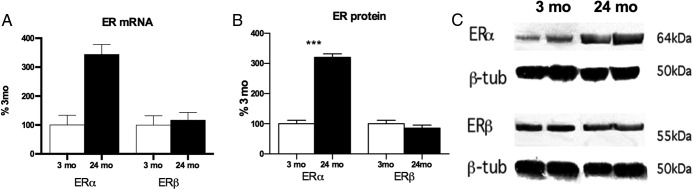
ERα and ERβ mRNA and protein levels in primary astrocyte cultures from 3 months and 24 months male rat cerebral cortex (n = 4 cultures, 4 brains per age group). Data are represented as percentage at 3 months (mean ± SEM). ***P < .001. A, ERα mRNA (RT-PCR) increased 3-fold by age; ERβ mRNA was unchanged. B, ERα protein (Western) increased 3-fold, with no change in ERβ. C, Representative Western blot showing single bands of ERα and ERβ. β-tub, β-tubulin loading control.
Age loss of E2-dependent neurotrophic activity
Prior studies of astrocyte neurotrophic activity during aging were based on the wounding-in-a-dish model with cortical astrocytes from 24- vs 3-month-old male rats cocultured with E18 neurons in 100 pM E2 (20). Because astrocyte ERs are induced by injury (14, 44), we first determined whether 24-month-old astrocyte cultures without lesioning also showed impaired E2-dependent neurotrophic activity. Astrocyte cultures from 24-month cerebral cortex supported 35% less neurite outgrowth than astrocytes from 3-month cerebral cortex. Moreover, in 3-month astrocytes, 100 pm E2 induced a further 40% increase of neurites, whereas 24-month astrocytes did not respond to E2 (Figure 3, A–C). Despite astrocyte age impairments on E18 neurite outgrowth, the number of E18 neurons in the cocultures was not altered by age or E2 treatment (Supplemental Fig. 3).
Figure 3.
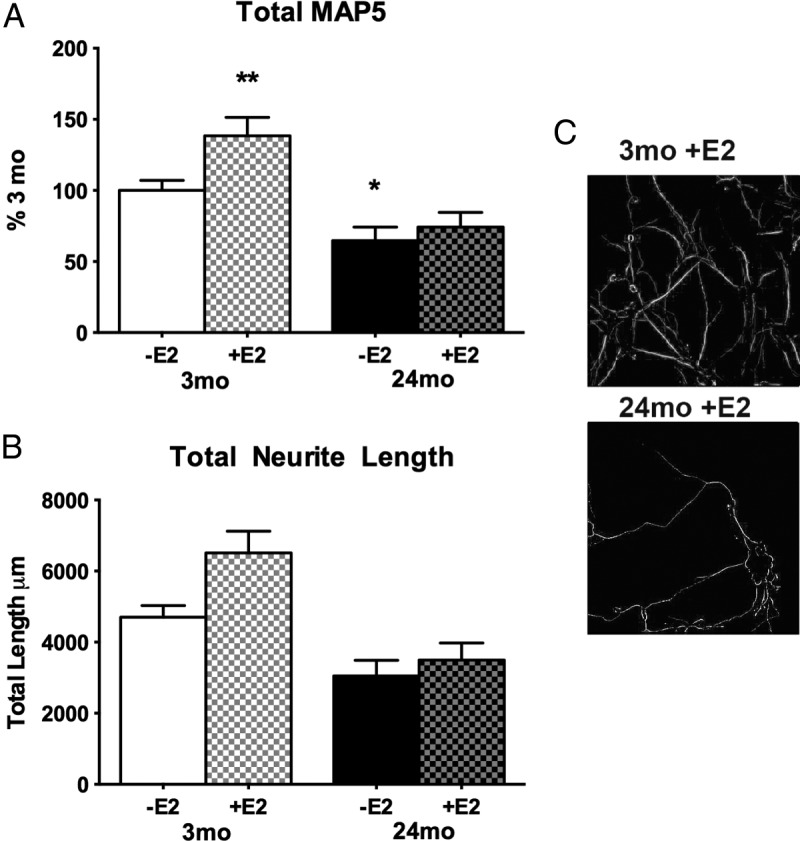
Neurite outgrowth (MAP5 immunostaining) in cocultures of astrocytes from male cerebral cortex with E18 cortical neurons: effects of age (3 vs 24 months) on response to 100 pm E2 (4 cultures, 4 brains per age group; mean ± SEM). **P < .01; *P < .05. A, Total MAP5 immunoreactivity from skeletonized images (panel C). Data are expressed as percentage at 3 months. Astrocytes from 3-month-old rats responded to 100 pM E2 by supporting 40% more neurite outgrowth, whereas 24-month-old astrocytes were unresponsive to E2. Absent E2, 24-month-old astrocytes supported 35% less neurite outgrowth than 3-month-old controls. B, Length of MAP5 immunopositive neurites from skeletonized images (panel C). C, Skeletonized images of MAP5 immunopositive neurites (see Materials and Methods).
Manipulations of ERα and ERβ in astrocytes influence GFAP expression and neurotrophic activity
To study their role in neurotrophic activities, astrocyte ERs were manipulated by transfecting with siRNA (decrease) or cDNA (increase) prior to adding neurons and 100 pM E2. There was no receptor cross talk in the absence of E2, ie, manipulating either ERα or ERβ with siRNA or cDNA did not alter mRNA or protein of the other ER within 24 hours (Supplemental Figure 4).
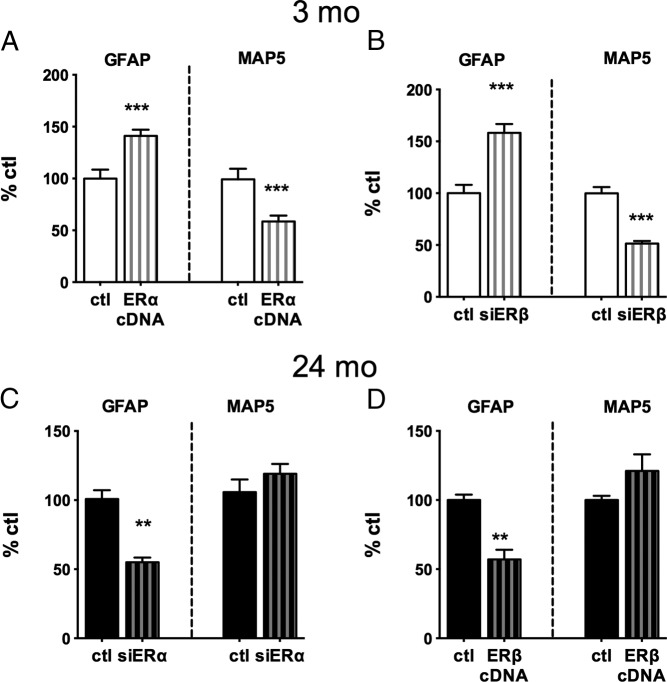
Young adult (3 months) astrocytes showed reciprocal responses of GFAP and neurite outgrowth from ER manipulation. Transfection with ERα cDNA caused 35% increase of astrocytic GFAP and 40% decrease of neurite outgrowth, assayed by neuronal MAP5 (Figure 4A). Similarly, ERβ-siRNA treatment caused increased GFAP and decreased MAP5 (Figure 4B). Thus in 3-month astrocytes, E2-dependent GFAP expression and its link to neurite outgrowth are both sensitive to the ERα to ERβ ratio.
Figure 4.
Manipulations of ERs by cDNA and siRNA in astrocytes from adult male rats before coculture with E18 neurons, assayed as responses of astrocyte GFAP and E18 neurite outgrowth (MAP5) (4 cultures, 4 brains per age group). Data are expressed as percentage of respective age control (ctl) (mean ± SEM). ***P < .001; **P < .01; *P < .05. A, ERα-cDNA transfection of 3-month-old astrocytes increased GFAP 40% and decreased neurite outgrowth 50%. B, ERβ knockdown by siRNA in 3-month-old astrocytes increased GFAP 50% and decreased neurite outgrowth 50%. C, ERα knockdown by siRNA in 24-month-old astrocytes decreased GFAP 50%. D, ERα-cDNA transfection of 24-month-old astrocytes decreased GFAP 50%.
Because increasing the ERα to ERβ ratio inhibited E2-dependent neurite outgrowth in young astrocytes in association with decreased GFAP, we hypothesized that age-related changes in astrocyte responsivity to E2 would be reversed by experimentally decreasing ERα. Figure 4C shows that ERα knockdown in 24-month astrocytes decreased GFAP by 50%. ERβ-cDNA transfection, which also results in decreased ERα levels relative to ERβ, similarly decreased GFAP by 50% (Figure 4D).
A mixed glia culture model for studies of neurotrophic support
Cultured mixed glia (3:1, astrocytes-microglia) from 3-month-old males showed E2-dependent reduction of GFAP and increased neurite outgrowth (Figure 5, A and B) equivalent to neurotrophic responses of enriched astrocytes (Figure 3). Moreover, aging changes were similar. Mixed glia from 24-month-old rats had elevated GFAP and deficient neurotrophic activity that did not respond to E2, as in primary astrocytes (20). Neuron numbers did not differ by age or E2 treatment of mixed glia (Supplemental Figure 3).
Figure 5.
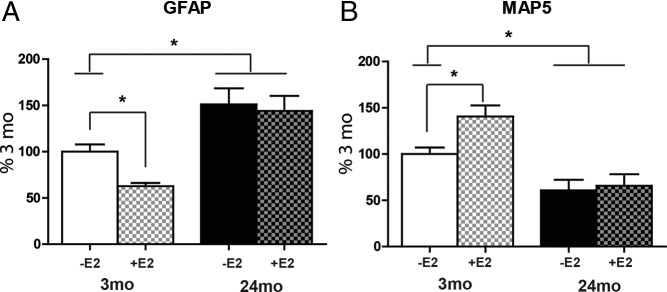
Effects of age and E2 (100 pM) on GFAP and MAP5 (neurite outgrowth) in mixed glia (3:1, astrocytes-microglia) from adult male rat cerebral cortex cocultured with E18 neurons (4 cultures, 4 brains per age group). Data are expressed as percentage at 3 months (mean ± SEM). *P < .05. A, Mixed glia from 3-month-old rats showed an E2-dependent decrease of GFAP, whereas 24-month-old mixed glia did not respond to E2. B, MAP5 increased in response to E2 in 3-month mixed glia but not in 24-month mixed glia. Absent E2, 24-months mixed glia supported 50% less neurite outgrowth than that at 3 months.
Glial neurotrophic support in females: age vs stage of reproductive senescence
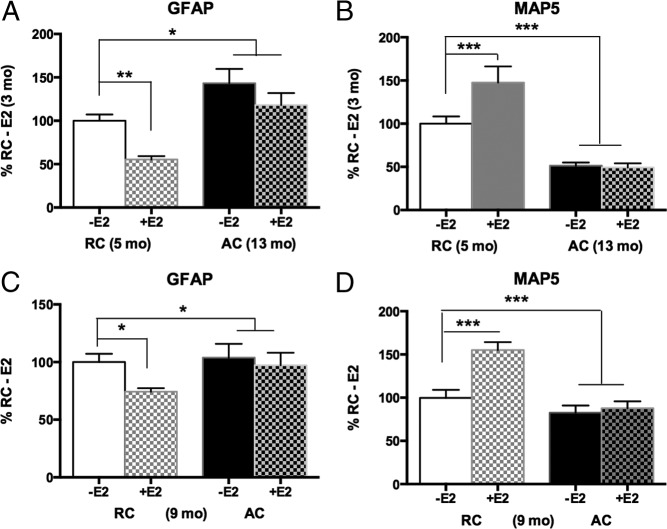
Mixed glia were evaluated at female rodent aging stages corresponding to the STRAW stages of human premenopause (see Materials and Methods) for E2-dependent neurotrophic support in 2 experiments: 5-month RCs vs 13-month ACs (constant estrus) (Figure 6, A and B) and 9-month-old RC vs 9-month AC subgroups (Figure 6, C and D). In both experiments, mixed glia from aged 5- to 9-month RCs supported equivalent E2-induced neurite outgrowth with decreased GFAP in response to E2. In contrast, mixed glia from 9- and 13-month AC did not respond to E2. The number of E18 neurons did not differ by age, cycling status, or E2 treatment (Supplemental Figure 3).
Figure 6.
Female perimenopausal rats: influences of age (A and B) and ovarian cycle status (C and D) on E2 responses of GFAP and MAP5 (neurite outgrowth); mixed glia from cerebral cortex of young (5 months) and perimenopausal (9 or 13 months) rats, RCs vs AC, cocultured with E18 neurons (4 cultures, 4 brains per age/cycling stage group). Data are expressed as a percentage at RC control (mean ± SEM). ***P < .001;**P < .01; *P < .05. A, Mixed glia from young 5-month-old RCs showed E2-dependent decrease of GFAP. The 13-month mixed glia from AC had elevated GFAP, which did not respond to E2. B, Neurite outgrowth (MAP5) was increased in response to 100 pM E2 in 5-month mixed glia but unresponsive to E2 in 13-month mixed glia. With or without added E2, 13-month mixed glia supported 50% less neurite outgrowth than 5-month-old controls. C, GFAP responses in mixed glia from 9-month-old rats: the RCs responded to 100 pM E2 with decreased GFAP, but age-matched (9 months) ACs were unresponsive. D, Neurite outgrowth (MAP5) on mixed glia from 9-month-old rats was increased by 100 pM E2 in RCs but not in the age-matched AC rats.
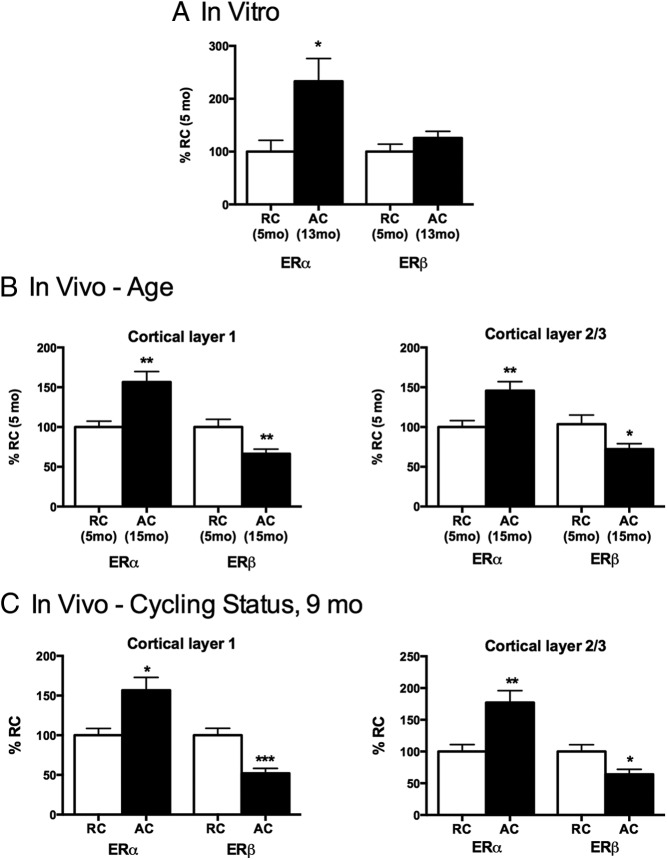
The ERs were examined for effects of age and for cycling status because hormonal changes alter astrocyte GFAP transcription in vivo (25). In mixed glia grown without neurons, ERα on Western blots was higher in 13-month AC vs 5-month RCs, without change in ERβ (Figure 7A). Individual astrocyte ER levels in mixed glia could not be resolved by ICC because of overlapping cell processes. Cell limitations precluded an ER assay in 9-month glia. In vivo, cerebral cortex astrocytes (primary visual cortex, layers 1 and 2/3) also had increased ERα: 15 month AC vs 5-month RCs (Figure 7B) and 9-month AC vs RCs (Figure 7C). ERβ was 40% lower in the AC groups of both experiments. Retrosplenial cortex astrocytes showed similar trends (Supplemental Figure 1).
Figure 7.
ERα and ERβ expression in cerebral cortex astrocytes of perimenopausal female rats: influences of age and ovarian cycle status. Data are expressed as percentage of RC control (mean ± SEM). ***P < .001; **P < .01; *P < .05. A, In vitro age comparison: mixed glia from cerebral cortex without neurons (4 cultures, 4 brains per age/cycling stage group). ERα levels were higher in mixed glia from 13-month-old AC rats than from 5-month-old RCs; ERβ did not differ by age. B, In vivo age comparison of astrocyte ERs in primary visual cortex layers 1 and 2/3: ER expression in GFAP-identified astrocytes, 5-month-old RC vs 15-month-old ACs (4 brains per age group). ERα immunostaining per astrocyte was higher in 15-month-old ACs than 5-month-old RCs. ERβ was lower in 15-month-old ACs vs 5-month-old RCs. C, In vivo comparison of astrocyte ERs in primary visual cortex in layers 1 and 2/3 of 9-month-old rats, RCs vs ACs (4 brains per cycle status). ERα immunostaining per astrocyte (GFAP immunopositive) was higher ACs than RCs, whereas ERβ was lower in ACs.
Discussion
We report 3 findings on ERs of rat cerebral cortex astrocytes relevant to age-related impairments of neuronal responsiveness to E2 (E2 desensitization). First, in astrocytes from aged rats of both sexes, ERα was increased per cell in vivo and in primary glial cultures by 50% or more, whereas ERβ changed little or decreased. Thus, the ERα to ERβ ratio in astrocytes consistently increased with age. Second, in primary astrocytes from 24-month-old male rats, the elevation of ERα was associated with the desensitization of astrocyte GFAP responses to E2 and decreased neurotrophic support. Experimental reduction of the ERα to ERβ ratio in 24-month-old astrocytes reversed the age loss of E2 sensitivity, whereas an aging phenotype was induced in 3-month astrocytes by increasing the ERα to ERβ ratio. These findings extend the inverse relationship between GFAP expression and neurotrophic support (20–22). And third, mixed glia from acyclic middle-aged female rats also had elevated ERα and GFAP, with impaired E2-dependent neurotrophic support, whereas regular cyclers of the same age had a young phenotype. We propose that these changes in cultured glia arise from steroidal perturbations during ovarian senescence, which expose the brain to persistently unopposed estrogens.
Two glial models were used: mixed glia, containing astrocytes and microglia (3:1), and monotypic astrocytes (>95%); in both models, E18 neurons were seeded at 1:3, neurons-glia. We examined mixed glia because of concern that the standard shaking protocol to remove microglia (42) would introduce artifacts from hydrodynamic perturbations that can alter gene expression in astrocytes (23) and other cells (24). Fortunately, the age changes of astrocyte ER expression and support of E2-dependent neurite outgrowth were indistinguishable in both monotypic astrocytes and mixed glia. Mixed glia also allow interactions between astrocytes and microglia that may more closely model in vivo processes, eg, apolipoprotein E (apoE), a neurotrophic factor, is induced by E2 in both glial types in vivo and in vitro but not in cultured astrocytes alone (45). Mixed glia also show E2-P4 interactions in lesion-induced sprouting that depend on the presence of microglia (46, 47).
Astrocyte ERα and ERβ
Contrary to in vivo studies of astrocytes, which detected only ERβ in the unlesioned brain (13), we observed coexpression of both ERα and ERβ in astrocytes of rat cerebral cortex of both sexes, aged 3 and 24 months. These findings extend to adult astrocytes the reports on neonatal-derived astrocyte cultures, which documented expression of both ERs (12, 43). In vivo, at least 50% of astrocytes of 2 cortical regions showed triple colocalization of GFAP, ERα and ERβ; however, this may underestimate the coexpression because of the weaker ERα ICC signal. Prior studies that detected ERβ but not ERα in cerebral cortical astrocytes (13) were confirmed with the antibody used (not shown). Primary astrocytes from adult rat cerebral cortex of both sexes also coexpressed both ERs by ICC, Westerns blots, and PCR. The coexpression of ERα and ERβ in astrocytes could allow formation of ERα/β heterodimers with special properties, as observed in breast cancer cells in which the induction of ERα/β heterodimers by a novel phytoestrogen inhibited cell growth (48). However, we cannot assess nuclear colocalization of ERs because we assayed immunoreactivity across the cell area that colocalized with cytoplasmic GFAP. Another caveat is that ERs were evaluated from only the left hemisphere. Although there may be hemispheric asymmetry in astrocyte ER expression, there were similar ERα changes with age or ovarian status in glial cultures from both sides (male) or the right side (female). Lastly, there could be regional differences in ER changes within functional domains of the cerebral cortex that were not surveyed in this initial analysis of 2 cortical regions.
Age increase of astrocyte ERα
During aging, cortical astrocyte ERα was increased 2-fold in both sexes, in vivo and in vitro, whereas ERβ showed little change or a decrease. This specificity is relevant because both ERs can regulate E2-dependent neurotrophic activities of astrocytes. In young astrocytes, bidirectional manipulations of ERs by cDNA and siRNA showed that higher ERα diminished E2-dependent neurite outgrowth, whether due to increased ERα or decreased ERβ. Treatment with ERα-siRNA or ERß-cDNA restored the responsiveness of GFAP expression to E2 in astrocytes from senescent males (24 months old). The present studies and another on breast and prostate cell lines (48) did not find cross talk between ERs; however, ER cross talk was reported for the EtC.1 neuronal line (49) and other breast cell lines (50), which may be due to cell type differences or to the absence of E2 during ER transfection in our studies. These findings extend hypotheses about the role of the ERα to ERβ ratio in the sensitivity of neuronal responses to E2 (1, 9–11) to the regulation of GFAP- and of E2-dependent neurotrophic activity of astrocytes.
The ovarian cycle status is also critical: ERα at 9 months was increased only in the acyclic subgroup in vivo. Correspondingly, in vitro, only regular cyclers retained normal E2-dependent glial neurotrophic activity. These findings extend those of Lewis et al (26), which showed that astrocytes from 10-month-old acyclic rats had impaired neurotrophic activity with embryonic neural progenitor cells; however, this study did not distinguish effects of acyclicity from age. Thus, astrocyte response to E2 is sensitive to ovarian changes that are not a strict function of chronological age. The close relationship of astrocyte neurotrophic support to synaptic functions could be a mechanism in the impaired learning of irregularly cycling middle-aged rats relative to regular cyclers of the same age (27). We argue below for a role of elevated E2-P4 in astrocyte E2 desensitization. Future studies may identify age effects that are separate from ovarian interactions, as observed in males.
These findings on the glial side of ERs in brain aging are relevant to discussions of neuronal ERs, which mediate brain functions during development and throughout the life span in both sexes (1, 9–11, 51, 53). Some studies associated neuronal ERβ more than ERα in regulation of synaptic proteins (1, 53). Nonetheless, in an astrocyte-neuron culture model, ERα was specifically associated with E2-induced glutamatergic synaptogenesis (54). Neuronal responses to ER-specific agonists could also be mediated by astrocyte ERs and astrocyte-derived neurotrophic factors.
Further analysis of E2 desensitization in neuronal responses (introductory text) may now consider direct involvement of astrocytes. A pathway analysis of ERs in both neurons and glia might reveal why some corticohippocampal circuits resist cell loss during aging and Alzheimer disease (1, 19, 55), eg, the vulnerable CA1 neurons were the only subtype to show E2 induction of progesterone receptors (56).
ERs in GFAP expression and neurotrophic activity
These findings extend the reciprocal relationships between GFAP expression and astrocyte neurotrophic activity (20) by linking the ERα to ERβ ratio to GFAP expression. Consistently across age groups and experimental conditions, elevated ERα increased GFAP expression and decreased neurite outgrowth. The astrocyte intermediate filament, GFAP has a role in the E2-mediated neurotrophic activity of astrocytes. In young astrocyte-neuron cocultures derived from whole cerebral cortex, E2 inhibited GFAP transcription through functional upstream estrogen response elements that can bind ERα (25).
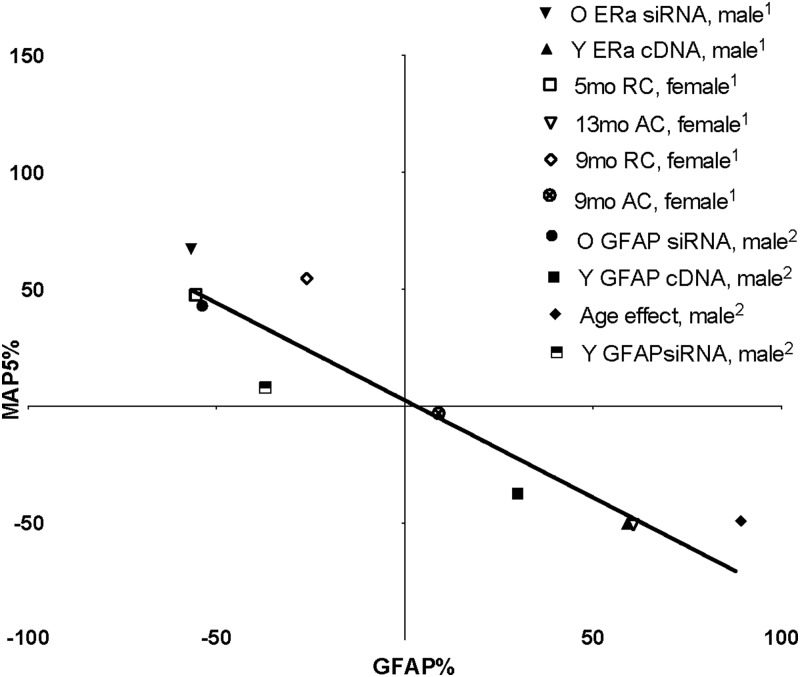
Links between GFAP levels and the astrocytic support of neurite growth were first reported by the group of Tardy and colleagues (21) with the wounding-in-a-dish model, in which neurite outgrowth with increased astrocyte laminin secretion was enhanced by antisense GFAP. Furthermore, we showed that E2 inhibited GFAP transcription after wounding and increased neurite outgrowth in association with increased extracellular laminin (22). In the present studies, the cultures had no astrocytes with enlarged reactive morphology, as induced by axotomy in vivo (8) or in vitro (20, 22). Transfection of young astrocytes with GFAP-cDNA inhibited neurite outgrowth and reduced secretion of laminins, whereas lowering GFAP by siRNA restored neurotrophic activity and extracellular laminin (20). Thus, an aging phenotype is induced in young astrocytes by experimentally increasing ERα (this study) or increasing GFAP (20). The inverse relationship of GFAP expression to E2-dependent astrocyte neurotrophic activity holds across a 2-fold range; present and past data are summarized in Figure 8.
Figure 8.
Reciprocal relationships between GFAP and neurite outgrowth (MAP5) including ER manipulations. 1, Present data; 2, prior findings (20) (r2 = 0.86, P < .001). Points represent values for GFAP and MAP5 normalized to control in each comparison, eg, age ▾(1) in upper left quadrant shows effect of siRNA for ERα that decreased GFAP by 57% and increased MAP5 by 67%.
In vivo, hypothalamic astrocytes also show age impaired response of GFAP to E2: in young female rats, GFAP was induced in the arcuate nucleus during the E2-induced (preovulatory) LH surge, whereas in middle-aged constant estrus rats, neither GFAP nor LH responded to E2 (57). In astrocytes from 24-month-old males, we showed here that GFAP repression by E2 was restored by experimentally decreasing ERα or increasing ERβ. However, the loss of E2-dependent neurotrophic activity was only partly restored, implying additional factors in addition to GFAP in age impairments of neurotrophic activity. In addition to GFAP, E2 directly regulates apoE, which is also required for E2-dependent neurite outgrowth (58, 59). Like GFAP expression in the present studies, apoE was induced by increased ERα and decreased by ERβ (60). Gene profiling of astrocytes for ER responses is needed for further study of steroidal-dependent glial neurotrophisms (61).
Persisting hyperestrogenic perturbations during ovarian senescence and E2 desensitization
The present in vitro glial models of aging showed the persistence of glial desensitization to E2 during 4 weeks in primary culture with greater than 1 population doubling. We suggest that the E2 desensitization of astrocytes cultured from middle-aged female rats is due to in vivo exposure to steroidal perturbations during ovarian senescence that may be described as hyperestrogenic. As the follicular pool becomes exhausted, there are increasing episodes of elevated plasma E2-P4, from the lengthening cycles with delayed luteal phases with elevated P4 and the ensuing constant estrus acyclicity when blood E2 is sustained for many days to weeks with low P4 (28–30). Extensive studies show that adult rodents given chronic exposure to unopposed estrogens as a model for constant estrus develop hypothalamic glial activation and impaired gonadotrophin regulation (62–65). As few as 6 weeks of low plasma E2 replacement in ovariectomized young mice caused premature hypothalamic impairments, as evaluated by subsequent transplantation of ovaries (66). These effects of chronic low plasma E2 were blocked by P4 implants (67). At a cell level, chronic E2 decreased axosomatic neuronal contacts (63) and prematurely activated astrocytes and microglia in the hypothalamic arcuate nucleus (62). Because the number of hypothalamic GnRH neurons was not decreased by chronic adult E2 treatment (68) or by normal aging (69), altered glial-neuron interactions, rather than neuron death, may be the main outcome of chronic E2. Conversely, ovariectomy for 6 months before the onset of cycle irregularity attenuated the impaired E2-negative feedback on LH in middle-aged mice (64, 70) and glial activation in the arcuate nucleus (71). Male rodent astrocyte changes during aging might also involve E2: chronic E2 activated arcuate nucleus astrocytes in both young male and female rats (62), whereas in male rats blood E2 may modestly increase during middle age (5, 72).
A precedent for persisting effects of estrogen exposure in vitro is the estrogen memory described for human hepatocarcinoma HepG2 cells, in which exposure to 20 nM E2 for 48 hours, but not 6 hours, induced a moderate-affinity E2-binding site that persisted 10 cell generations (73). This E2 binding site is unlikely to include the high-affinity ERα and ERβ. The concept of memory in physiological perturbations is also recognized in diabetes, in which the persistent induction of fibronectin, sirtuin 1, and other genes chronic hyperglycemia in vivo and in vitro (74–76) is described as metabolic memory (74). However, the term, “estrogen memory” (73), may not be appropriate for brain cells to avoid confusion with synaptic functions of learning and memory, in which astrocytes and microglia have emerging roles.
Given the evidence for persisting effects of E2 on rodent brains, one may ask whether these findings apply to humans. Unopposed estrogens are recognized risk factors in uterine cancer from the combination of modest E2 and low P4 in some hormone therapies (77, 78). However, effects of chronic E2 on the primate brain are not indicated by a study of young adult ovariectomized monkeys given 30 months of equine estrogens (Premarin), which did not find hypothalamic microglial activation or neuronal change (79). Several trials are evaluating benefits and hazards of unopposed estrogens to normative cognitive aging and risk of Alzheimer disease. Postmortem studies are needed to evaluate potential neuronal and glial responses to the chronic unopposed estrogens used in some regimens of hormone therapy for menopause.
Another open question is how ERα or ERβ in astrocytes or neurons respond to the various hormone therapies. In addition to the neurotrophic responses associated with the proportions of ERα and ERβ, mitochondria of neurons and mixed glia of neonatal rats have ERα or ERβ agonist specificity (80, 81). Future hormone therapy could consider lowering astrocyte ERα as well as the ERα to ERβ ratio to optimize glial neurotrophic support in normal aging and neurodegenerative conditions. Selective ER modulators (SERMs) could include brain cell specificities, eg, novel ERβ selective phyto-SERMs promote estrogen action in the brain without inducing estrogenic responses in reproductive tissues in the periphery (82). New phytoestrogens developed for cancer therapy show the possibility of manipulating ER heterodimers (48). The present findings that astrocyte ERα increases with age, in contrast to decreased ERα in rat hippocampal neurons (6), give a rationale for examining brain cell specificities of SERMs.
Other aging processes besides estrogen exposure may mediate the age increase of astrocyte ERα in male and female rats. In addition to its reproductive roles, ERα also regulates metabolic processes, including mitochondrial functions (80, 81) and inflammation in the brain and elsewhere (52, 82). Because aging in brain and other tissues involves mitochondrial dysfunction, oxidative stress, and inflammation, ERα could mediate multiple brain aging processes in both sexes that are independent of gonadal hormones, as well as those linked to hormonal perturbations.
Acknowledgments
Plasmids and reagents were generously given by Christian J. Pike (University of Southern California), Paul Micevych (University of California, Los Angeles), and Claudine Gaudon [Institut Génétique de Biologie Moléculaire et Cellulaire (Illkirch-Strasbourg, France)].
This work was supported by Grant P01 AG-026572 (to R.B., principal investigator), with projects of C.E.F and T.E.M.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AC
- acyclic
- apoE
- apolipoprotein E
- CE
- constant estrus
- CT
- cycle threshold
- E
- embryonic day
- E2
- estradiol
- ER
- estrogen receptor
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GFAP
- glial fibrillary acidic protein
- ICC
- immunocytochemistry
- IHC
- immunohistochemistry
- MAP5
- microtubule-associated protein 5
- P4
- progesterone
- RC
- regular cycler
- SERM
- selective ER modulator
- siRNA
- small interfering RNA
- STRAW
- Stages of Reproductive Aging Workshop.
References
- 1. Foster TC. Role of estrogen receptor α and β expression and signaling on cognitive function during aging. Hippocampus. 2012;22:656–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ishunina TA, Swaab DF. Increased expression of estrogen receptor α and β in the nucleus basalis of Meynert in Alzheimer's disease. Neurobiol Aging. 2001;22:417–426 [DOI] [PubMed] [Google Scholar]
- 3. Yamaguchi N, Yuri K. Changes in oestrogen receptor-β mRNA expression in male rat brain with age. J Neuroendocrinol. 2012;24:310–318 [DOI] [PubMed] [Google Scholar]
- 4. Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor α (ERα) and estrogen receptor-β (ERβ) gene expression in specific regions of the rat brain. Mech Ageing Dev. 2002;123:593–601 [DOI] [PubMed] [Google Scholar]
- 5. Wu D, Lin G, Gore AC. Age-related changes in hypothalamic androgen receptor and estrogen receptor α in male rats. J Comp Neurol. 2009;512:688–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adams MM, Fink SE, Shah RA, et al. Estrogen and aging affect the subcellular distribution of estrogen receptor-α in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci USA. 2001;98:8071–8076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stone DJ, Rozovsky I, Morgan TE, et al. Effects of age on gene expression during estrogen-induced synaptic sprouting in the female rat. Exp Neurol. 2000;165:46–57 [DOI] [PubMed] [Google Scholar]
- 9. Han X, Aenlle KK, Bean LA, et al. Role of estrogen receptor α and β in preserving hippocampal function during aging. J Neurosci. 2013;33:2671–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spencer-Segal JL, Tsuda MC, Mattei L, et al. Estradiol acts via estrogen receptors α and β on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience. 2012;202:131–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pfaff D, Waters E, Khan Q, Zhang X, Numan M. Minireview: estrogen receptor-initiated mechanisms causal to mammalian reproductive behaviors. Endocrinology 2102;152:1209–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–3795 [DOI] [PubMed] [Google Scholar]
- 13. Azcoitia I, Sierra A, Garcia-Segura LM. Localization of estrogen receptor β-immunoreactivity in astrocytes of the adult rat brain. Glia. 1999;26:260–267 [PubMed] [Google Scholar]
- 14. Garcia-Ovejero D, Veiga S, García-Segura LM, Doncarlos LL. Glial expression of estrogen and androgen receptors after rat brain injury. J Comp Neurol. 2002;450:256–271 [DOI] [PubMed] [Google Scholar]
- 15. Finch CE. The neurobiology of middle-age has arrived. Neurobiol Aging. 2009;30:515–520 [DOI] [PubMed] [Google Scholar]
- 16. Kremsky I, Morgan TE, Hou X, Li L, Finch CE. Age changes in gene expression in primary mixed glia cultures from young vs. old rat cerebral cortex are modified by interactions with neurons. Brain Behav Immun. 2012;26:797–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kadish I, Thibault O, Blalock EM, et al. Hippocampal and cognitive aging across the lifespan: a bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J Neurosci. 2009;29:1805–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morgan TE, Xie Z, Goldsmith S, et al. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience. 1999;89:687–699 [DOI] [PubMed] [Google Scholar]
- 19. Burke SN, Barnes CA. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci. 2010;33:153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rozovsky I, Wei M, Morgan TE, Finch CE. Reversible age impairments in neurite outgrowth by manipulations of astrocytic GFAP. Neurobiol Aging. 2005;26:705–715 [DOI] [PubMed] [Google Scholar]
- 21. Costa S, Planchenault T, Charriere-Bertrand C, et al. Astroglial permissivity for neuritic outgrowth in neuron-astrocyte cocultures depends on regulation of laminin bioavailability. Glia. 2002;37:105–113 [DOI] [PubMed] [Google Scholar]
- 22. Rozovsky I, Wei M, Stone DJ, et al. Estradiol (E2) enhances neurite outgrowth by repressing glial fibrillary acidic protein expression and reorganizing laminin. Endocrinology. 2002;143:636–646 [DOI] [PubMed] [Google Scholar]
- 23. Gatson JW, Simpkins JW, Yi KD, Idris AH, Minei JP, Wigginton JG. Aromatase is increased in astrocytes in the presence of elevated pressure. Endocrinology. 2011;152:207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stone DJ, Song Y, Anderson CP, Krohn KK, Finch CE, Rozovsky I. Bidirectional transcription regulation of glial fibrillary acidic protein by estradiol in vivo and in vitro. Endocrinology. 1998;139:3202–3209 [DOI] [PubMed] [Google Scholar]
- 26. Lewis DK, Woodin HR, Sohrabji F. Astrocytes from acyclic female rats exhibit lowered capacity for neuronal differentiation. Aging Cell. 2008;7:836–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paris JJ, Walf AA, Frye CA. II. Cognitive performance of middle-aged female rats is influenced by capacity to metabolize progesterone in the prefrontal cortex and hippocampus. Brain Res. 2011;1379:149–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nelson JF, Felicio LS, Osterburg HH, Finch CE. Altered profiles of estradiol and progesterone associated with prolonged estrous cycles and persistent vaginal cornification in aging C57BL/6J mice. Biol Reprod. 1981;24:784–794 [DOI] [PubMed] [Google Scholar]
- 29. Finch CE, Felicio LS, Mobbs CV, Nelson JF. Ovarian and steroidal influences on neuroendocrine aging processes in female rodents. Endocr Rev. 1984;5:467–497 [DOI] [PubMed] [Google Scholar]
- 30. Lu JK, Damassa DA, Gilman DP, Judd HL, Sawyer CH. Differential patterns of gonadotropin responses to ovarian steroids and to LH-releasing hormone between constant-estrous and pseudopregnant states in aging rats. Biol Reprod. 1980;23:345–351 [DOI] [PubMed] [Google Scholar]
- 31. Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Finch CE. The menopause and aging: a comparative perspective. J Steroid Biochem Mol Biol. (Special Issue on Menopause); doi:10.1016/j.jsbmb.2013.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prior JC. Ovarian aging and the perimenopausal transition: the paradox of endogenous ovarian hyperstimulation, Endocrine. 2005;26:297–300 [DOI] [PubMed] [Google Scholar]
- 34. O'Connor KA, Ferrell R, Brindle E, Trumble B, Shofer J, Holman DJ, Weinstein M. Progesterone and ovulation across stages of the transition to menopause, Menopause. 2009;16:1178–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Downs JL, Wise PM. The role of the brain in female reproductive aging. Mol Cell Endocrinol. 2009;299:32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 2004;292:2991–2996 [DOI] [PubMed] [Google Scholar]
- 37. Gosden RG, Laing SC, Felicio LS, Nelson JF, Finch CE. Imminent oocyte exhaustion and reduced follicular recruitment mark the transition to acyclicity in aging C57BL/6J mice. Biol Reprod. 1983;28:255–260 [DOI] [PubMed] [Google Scholar]
- 38. Finch CE, Holmes DJ. Ovarian aging in developmental and evolutionary contexts. Ann NY Acad Sci. 2010;1204:82–94 [DOI] [PubMed] [Google Scholar]
- 39. Gee DM, Flurkey K, Finch CE. Aging and the regulation of luteinizing hormone in C57BL/6J mice. Impaired elevations after ovariectomy and spontaneous elevations at advanced ages. Biol Reprod. 1983;28:598–607 [DOI] [PubMed] [Google Scholar]
- 40. Diaz Brinton R. Minireview: translational animal models of human menopause: challenges and emerging opportunities. Endocrinology. 2012;153:3571–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rozovsky I, Finch CE, Morgan TE. Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol Aging.1998;19:97–103 [DOI] [PubMed] [Google Scholar]
- 42. Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Al-Bader MD, Malatiali SA, Redzic ZB. Expression of estrogen receptor α and β in rat astrocytes in primary culture: effects of hypoxia and glucose deprivation. Physiol Res. 2011;60:951–960 [DOI] [PubMed] [Google Scholar]
- 44. Sakuma S, Tokuhara D, Hattori H, Matsuoka O, Yamano T. Expression of estrogen receptor α and β in reactive astrocytes at the male rat hippocampus after status epilepticus. Neuropathology. 2009;29:55–62 [DOI] [PubMed] [Google Scholar]
- 45. Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Hajian H, Finch CE. Astrocytes and microglia respond to estrogen with increased apoE mRNA in vivo and in vitro. Exp Neurol. 1997;143:313–318 [DOI] [PubMed] [Google Scholar]
- 46. Wong AM, Rozovsky I, Arimoto JM, Du Y, Wei M, Morgan TE, Finch CE. Progesterone influence on neurite outgrowth involves microglia. Endocrinology. 2009;150:324–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bali N, Brinton RD, Morgan TE, Finch CE. Microglial Pgrmc1 mediates progesterone antagonism of E2 mediated neurite outgrowth through soluble factors. Soc Neurosci Abstr. 2012;188.13 [Google Scholar]
- 48. Powell E, Shanle E, Brinkman A, et al. Identification of estrogen receptor dimer selective ligands reveals growth-inhibitory effects on cells that co-express ERα and ERβ. PLoS One. 2012;7:e30993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gottfried-Blackmore A, Croft G, McEwen BS, Bulloch K. Transcriptional activity of estrogen receptors ERα and ERβ in the EtC. 1 cerebellar granule cell line. Brain Res. 2007;1186:41–44 [DOI] [PubMed] [Google Scholar]
- 50. Hall JM, McDonnell DP. The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578 [DOI] [PubMed] [Google Scholar]
- 51. McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci. 2012;126:4–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brown CM, Mulcahey TA, Filipek NC, Wise PM. Production of proinflammatory cytokines and chemokines during neuroinflammation: novel roles for estrogen receptors α and β. Endocrinology. 2010;151:4916–4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu F, Day M, Muñiz LC, et al. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343 [DOI] [PubMed] [Google Scholar]
- 54. Jelks KB, Wylie R, Floyd CL, McAllister AK, Wise P. Estradiol targets synaptic proteins to induce glutamatergic synapse formation in cultured hippocampal neurons: critical role of estrogen receptor-α. J Neurosci. 2007;27:6903–6913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Geinisman Y, DeToledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444 [DOI] [PubMed] [Google Scholar]
- 56. Bali N, Arimoto JM, Iwata N, et al. Differential responses of progesterone receptor membrane component-1 (Pgrmc1) and the classical progesterone receptor (Pgr) to 17β-estradiol and progesterone in hippocampal subregions that support synaptic remodeling and neurogenesis. Endocrinology. 2012;153:759–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Anderson CP, Rozovsky I, Stone DJ, Song Y, Lopez LM, Finch CE. Aging and increased hypothalamic glial fibrillary acid protein (GFAP) mRNA in F344 female rats. Dissociation of GFAP inducibility from the luteinizing hormone surge. Neuroendocrinology. 2002;76:121–130 [DOI] [PubMed] [Google Scholar]
- 58. Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Finch CE. Increased synaptic sprouting in response to estrogen via an apolipoprotein E-dependent mechanism: implications for Alzheimer's disease. J Neurosci. 1998;18:3180–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Struble RG, Cady C, Nathan BP, McAsey M. Apolipoprotein E may be a critical factor in hormone therapy neuroprotection. Front Biosci. 2008;13:5387–5405 [DOI] [PubMed] [Google Scholar]
- 60. Wang JM, Irwin RW, Brinton RD. Activation of estrogen receptor α increases and estrogen receptor β decreases apolipoprotein E expression in hippocampus in vitro and in vivo. Proc Natl Acad Sci USA. 2006;103:16983–16988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Arevalo MA, Santos-Galindo M, Acaz-Fonseca E, Azcoitia I, Garcia-Segura LM. Gonadal hormones and the control of reactive gliosis. Horm Behav. 2013;63:216–221 [DOI] [PubMed] [Google Scholar]
- 62. Brawer JR, Schipper H, Naftolin F. Ovary-dependent degeneration in the hypothalamic arcuate nucleus. Endocrinology. 1980;107:274–279 [DOI] [PubMed] [Google Scholar]
- 63. Garcia-Segura LM, Baetens D, Naftolin F. Synaptic remodelling in arcuate nucleus after injection of estradiol valerate in adult female rats. Brain Res. 1986;366:131–136 [DOI] [PubMed] [Google Scholar]
- 64. Mobbs CV, Finch CE. Estrogen-induced impairments as a mechanism in reproductive senescence of female C57BL/6J mice. J Gerontol. 1992;47:B48–B51 [DOI] [PubMed] [Google Scholar]
- 65. Hung AJ, Stanbury MG, Shanabrough M, Horvath TL, Garcia-Segura LM, Naftolin F. Estrogen, synaptic plasticity and hypothalamic reproductive aging. Exp Gerontol. 2003;38:53–59 [DOI] [PubMed] [Google Scholar]
- 66. Kohama SG, Anderson CP, Osterburg HH, May PC, Finch CE. Oral administration of estradiol to young C57BL/6J mice induces age-like neuroendocrine dysfunctions in the regulation of estrous cycles. Biol Reprod. 1989;4:227–232 [DOI] [PubMed] [Google Scholar]
- 67. Kohama SG, Anderson CP, Finch CE. Progesterone implants extend the capacity for 4-day estrous cycles in aging C57BL/6J mice and protect against acyclicity induced by estradiol. Biol Reprod. 1989;41:233–244 [DOI] [PubMed] [Google Scholar]
- 68. Kohama SG, Brown SA, Finch CE, McNeill TH. Chronic estradiol administration did not cause loss of hypothalamic LHRH or TIDA neurons in young or middle-aged C57BL/6J mice. Brain Res. 1992;574:341–334 [DOI] [PubMed] [Google Scholar]
- 69. Hoffman GE, Finch CE. LHRH neurons in the female C57BL/6J mouse brain during reproductive aging: no loss up to middle age. Neurobiol Aging. 1986;7:45–48 [DOI] [PubMed] [Google Scholar]
- 70. Mobbs CV, Cheyney D, Sinha YN, Finch CE. Age-correlated and ovary-dependent changes in relationships between plasma estradiol and luteinizing hormone, prolactin, and growth hormone in female C57BL/6J mice. Endocrinology. 1985;116:813–820 [DOI] [PubMed] [Google Scholar]
- 71. Schipper H, Brawer JR, Nelson JF, Felicio LS, Finch CE. Role of the gonads in the histologic aging of the hypothalamic arcuate nucleus. Biol Reprod. 1981;25:413–419 [DOI] [PubMed] [Google Scholar]
- 72. Rosario ER, Chang L, Beckett TL, et al. Age-related changes in serum and brain levels of androgens in male Brown Norway rats. Neuroreport. 2009;20:1534–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tam SP, Haché RJ, Deeley RG. Estrogen memory effect in human hepatocytes during repeated cell division without hormone. Science. 1986;234:1234–1237 [DOI] [PubMed] [Google Scholar]
- 74. Roy S, Sala R, Cagliero E, Lorenzi M. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci USA. 1990;87:404–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zheng Z, Chen H, Li J, et al. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes. 2012;61:217–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mobbs CV, Mastaitis JW, Zhang M, Isoda F, Cheng H, Yen K. Secrets of the lac operon. Glucose hysteresis as a mechanism in dietary restriction, aging and disease. Interdiscip Top Gerontol. 2007;35:39–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pike MC. Age-related factors in cancers of the breast, ovary, and endometrium. J Chronic Dis. 1987;40(suppl 2):59S–69S [DOI] [PubMed] [Google Scholar]
- 78. Hale GE, Hughes CL, Cline JM. Endometrial cancer: hormonal factors, the perimenopausal “window of risk,” and isoflavones. J Clin Endocrinol Metab. 2002;87:3–15 [DOI] [PubMed] [Google Scholar]
- 79. Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab. 1999;84:2111–2118 [DOI] [PubMed] [Google Scholar]
- 80. Irwin RW, Yao J, To J, Hamilton RT, Cadenas E, Brinton RD. Selective oestrogen receptor modulators differentially potentiate brain mitochondrial function. J Neuroendocrinol. 2012;24:236–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Petrović S, Veličković N, Stanojević I, et al. Inhibition of mitochondrial Na+-dependent Ca2+ efflux by 17β-estradiol in the rat hippocampus. Neuroscience. 2011;192:195–204 [DOI] [PubMed] [Google Scholar]
- 82. Zhang QG, Raz L, Wang R, et al. Estrogen attenuates ischemic oxidative damage via an estrogen receptor α-mediated inhibition of NADPH oxidase activation. J Neurosci. 2009;29:13823–13836 [DOI] [PMC free article] [PubMed] [Google Scholar]