Abstract
Female obesity is associated with insulin resistance, hyperandrogenemia, and reproductive dysfunction. We hypothesized that elevated free fatty acids (FFAs) might directly modulate pituitary gonadotropin production. FFAs caused a time- and dose-dependent increase in phosphorylation of the MAPKs p38MAPK, c-Jun N-terminal kinase (JNK)-1/2, and ERK1/2 in LβT2 gonadotrope cells. Furthermore, FFAs up-regulated Lhb mRNA expression acutely, an effect that was blocked by JNK inhibition, but suppressed Fshb mRNA expression, an effect that was independent of MAPK signaling. FFAs enhanced the activation of the MAPKs in the presence of GnRH, although the cotreatment did not alter Lhb induction but did eliminate the GnRH induction of Fshb. FFAs also suppressed activin-induced Fshb expression. Knockdown experiments showed that the FFA effect on the inflammatory kinases p38MAPK and JNK and on Lhb, but not Fshb, mRNA expression is mediated via toll-like receptor-2 and toll-like receptor-4 and was mimicked by lipopolysaccharide stimulation. In vivo, male C57BL/6 mice on a high-fat diet showed reduced FSH levels consistent with the suppression of Fshb seen in vitro. Histological analysis of the testes showed an increased number of abnormal seminiferous tubules. Female mice on a high-fat diet lacked the expected proestrus LH and FSH surge and exhibited an increase in the number of days at estrus and a reduced number of days at proestrus, and ovaries had significantly fewer corpora lutea. Taken together, our findings suggest that lipid excess can lead to reproductive defects in both male and female mice.
Obesity is a global epidemic. According to the World Health Organization, more than 1.4 billion adults are overweight and of these 500 million are obese (1). Of greater concern, more than 40 million children under the age of 5 years were obese in 2010. There are numerous reports on the deleterious effect of obesity on fertility. In women, obesity is often associated with polycystic ovary syndrome (PCOS), but it can independently lead to marked irregularities in menstrual cycles, abnormalities in the oocyte development, and an increased risk of miscarriages (2–5). Obesity in men leads to inferior sperm quality, reduced sperm quantity, and reduced levels of T, which all contribute to fertility defects (6–10). The mechanistic link is not clear, but obesity leads to abnormalities in metabolic homeostasis that may have adverse effects on reproductive fitness. The negative impact of maternal and paternal obesity on fertility, and on the health and development of offspring, has been confirmed in animals (11–20), but the neuroendocrine alterations underlying these impairments in fertility are not understood.
A possible link between obesity and reproductive dysfunction could be the enhanced mobilization of free fatty acids (FFAs) as a result of increased lipolysis (21). Plasma FFAs and insulin sensitivity are significantly linked in PCOS women (22), and it has been demonstrated that weight loss and a reduction in plasma FFAs leads to improved ovulatory frequency (23). Similarly, high-fat diet-induced obesity in mice increases plasma FFA levels, induces systemic inflammation and insulin resistance, and also causes reproductive abnormalities (24–26).
Recent studies suggest that insulin resistance in obesity could be mediated through the activation of toll-like receptors (TLRs) (27, 28), which are pattern recognition receptors that are evolutionarily conserved components of the innate immune system (29). TLRs activate inflammatory signaling cascades, leading to the activation of a number of kinases including c-Jun N-terminal kinase (JNK), p38MAPK, and inhibitor of κB kinase (IKK), which can cause cellular insulin resistance. As well as being expressed in immune cells, TLRs are also present in adipose tissue, liver, skeletal muscle, and other tissues (30–33). FFAs are known to bind and activate TLR2 and TLR4 signaling in many cell types (31). Because these same signaling pathways are activated by GnRH in gonadotrope cells, we speculated that FFAs might cause the dysregulation of gonatropin expression and impair fertility.
Materials and Methods
Materials
FFA cocktail (F7050, containing equimolar amounts of culture grade arachidonic acid, lauric acid, linoleic acid, myristic acid, and oleic acid) and BSA were purchased from Sigma (St Louis, Missouri). GnRH was from Dr A. F. Parlow (National Hormone and Pituitary Program, Harbor UCLA Medical Center, Torrance, California). The inhibitors including PD98059 (20 μM), SP600125 (10 μM), and PD169316 (5 μM) were from Calbiochem (La Jolla, California). Antibodies to phosphorylated and total p38, JNK, and ERK were from Cell Signaling Technology (Beverly, Massachusetts). β-Tubulin, horseradish peroxidase-linked secondary antibodies, and antibodies against TLR2 and TLR4 were purchased from Santa Cruz Biotechnology (Santa Cruz, California). Cell extracts were immunoblotted on polyvinyl difluoride membranes, blocked with 3% BSA in 0.1% Tween 20 in Tris-buffered saline, and probed with the given anitbodies at the dilutions recommended by the manufacturer and then visualized with a horseradish peroxidase-conjugated secondary antibody and chemiluminescence.
Cell culture and FFA treatment
LβT2 cells were maintained in monolayer cultures in DMEM supplemented with 10% FBS and antibiotics in a humidified 10% CO2 atmosphere at 37°C. After an overnight incubation, the cells were starved in media containing 0.1% endotoxin-free BSA. After 24 hours, an FFA cocktail was added at the indicated concentrations and times. GnRH was used at 100 nM, activin A (R&D Systems, Minneapolis, Minnesota) was used at 25 ng/mL, and lipopolysaccharide (LPS; Sigma) was used at 0.1, 10, or 100 ng/mL. Inhibitors of MAPK kinase (MEK), JNK, and p38MAPK (PD98059: 20 μM; SP600125: 5 μM; PD169316: 5 μM) were added 30 minutes before FFA (500 μM) treatment.
Real-time PCR
First-strand cDNA was synthesized using a high capacity cDNA synthesis kit and random hexamers (Applied Biosystems, Foster City, California). Samples were run in triplicate using the sequence-specific primers listed in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org under the following conditions: 95°C for 3 minutes and then 45 cycles of 94°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. Gene expression levels were calculated after normalization to the housekeeping gene, M36B, using the ΔΔcycle threshold method and expressed as relative mRNA levels compared with the control. The M36B gene did not vary with any of the treatments.
RNA interference knockdown
LβT2 cells were electroporated with a 1.0 μM small interfering RNA (siRNA) oligonucleotides to mouse TLR2, or TLR4 or with 1 μM of the control siRNA (Santa Cruz Biotechnology) using the XCell Gene Pulser (Bio-Rad Laboratories, Hercules, California). The cells were plated into 24-well plates, and 48 hours later the media were aspirated and replaced with DMEM containing 0.1% BSA for 24 hours before stimulation with FFAs.
High-fat diet feeding and measurement of estrous cycles
Eleven-week-old male (n = 12/group) and 13-week-old female (n = 10/group) C57BL/6 mice (Charles River Laboratories, Wilmington, Massachusetts) were fed either normal chow (NC: LabDiet, Cucamonga, California) or 60% high-fat diet (HFD: Research Diets D12492, New Brunswick, New Jersey). Mice were housed in a 12-hour light, 12-hour dark cycle, and weight was documented every 2 weeks. Female mice were evaluated for estrous cycles for 27 days before starting on diets and after 6, 12, and 24 weeks on diet by vaginal lavage. Smears were classified as di/metestrus, proestrus, and estrus based on cellular morphology. The first 6 days of the cycling were excluded from analysis to allow the mice to acclimatize to the procedure. All animal procedures were performed according the Guidelines for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of California, San Diego.
Intraperitoneal glucose tolerance test and insulin tolerance test
After 20-24 weeks on the diets, male and female mice were subjected to ip glucose- and insulin-tolerance tests. Mice were fasted for 6 hours and then injected ip with glucose (1 g/kg body weight) or insulin (0.75 U/kg body weight). Tail vein blood glucose was measured at 0, 15, 30, 45, 60, 90, and 120 minutes after injection using a glucometer (OneTouch; Bayer Healthcare, Tarrytown, New York). The area under the glucose curve (inverted in the case of the insulin tolerance tests) was calculated using the time 0 glucose value as baseline.
Hormone measurements
Previously collected human serum samples from saline- and lipid-infused healthy males (34) were analyzed in duplicate for LH and FSH using a human-specific RIA, with sensitivity and intra- and interassay coefficient of variation for LH of 0.2 mIU/mL, 5.4% and 8.0%, respectively, and for FSH of 0.2 mIU/mL, 3.0% and 4.6%, respectively (Siemens Medical Systems, Los Angeles, California). Briefly, each subject was admitted to the University of California, San Diego, Clinical Research Center, and studies were performed in the morning after a 10- to 12-hour overnight fast. Each subject underwent a 5-hour, 80 mU/m2·min hyperinsulinemic euglycemic clamp with or without an infusion of a triglyceride emulsion containing 21 g/L of glycerol as emulsifier (Intralipid 20%; Fresenius Kabi Clayton, Clayton, North Carolina) and heparin. The fatty acid composition of Intralipid is 52% linoleate, 13% palmitate, 22% oleate, 4.5% stearate, and 8.5% other. The Intralipid (60 mL/h) and heparin (900 U/h) infusions were started 2.5 h before the glucose clamp and continued during the clamp. The study was approved by the University of California, San Diego, Human Subjects Internal Review Board, and written informed consent was obtained from each subject. Blood was collected during the clamp for hormone measurements.
For the mouse studies, blood was collected from the tail vein. After 6 and 12 weeks on the diet, male mice and female mice in diestrus were bled at 1:00, 3:00, and 5:00 pm and the values averaged. After 24 weeks on the diet, the gonadotropin levels in females were measured at all stages of their estrous cycle. Diestrus and estrus samples were taken at 12:00 pm, and the proestrus samples were drawn between 5:45 and 6:15 pm. Plasma LH and FSH levels were measured by Luminex assay (catalog number RPT86K; Millipore Corp, Bedford, Massachusetts). Sensitivity of the assay is as follows LH: 4.9 pg/mL and FSH: 47.7 pg/mL with an intraassay coefficient of variation of 15%. For the GnRH stimulation test, tail vein blood was collected before and 10 minutes after ip injection of 1 μg/kg GnRH.
Tissue collection and histology
Testes were fixed in Bouin's solution for 6 hours and ovaries fixed in formalin for 48 hours followed by washing in 70% ethanol. Paraffin embedded sections (5 μm) were cut, dewaxed, and stained with hematoxylin and eosin. The tubules and follicles were analyzed in 5 sections taken every 10th section from each testis/ovary. Only healthy nonatretic follicles with visible oocytes were scored. Follicles were classified as primary, preantral, or antral as follows: primary follicles had an enlarged oocyte surrounded by a single layer of cuboidal granulosa cells, preantral follicles had an enlarged oocyte surrounded by 3 or layers of granulosa cells, and follicles with 5 or more layers of granulosa cells were considered antral follicles.
Statistical analysis
The in vitro data presented here were derived from at least 3 independent experiments. For the human data, the number of subjects was 12 per group. For the mouse HFD study, the number of subjects was 12 per group for male mice and 10 per group for female mice. Differences between groups are shown as mean ± SEM and analyzed for statistical significance using an ANOVA followed by a Tukey posttest. Data were tested for Gaussian distribution using D'Agostino-Pearson or Shapiro-Wilk normality tests. If non-Gaussian, data were log transformed before statistical analysis.
Results
FFAs activate the JNK, p38MAPK, and ERK pathways and increase Lhb mRNA but suppress Fshb mRNA in LβT2 cells
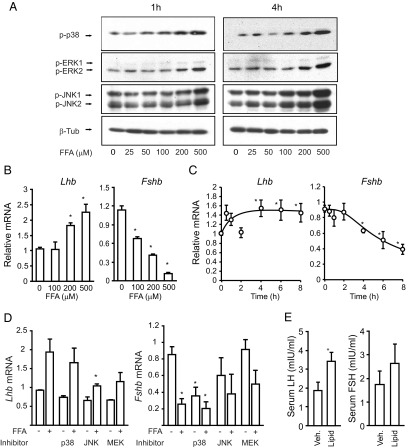
Because MAPK signaling has been implicated in the GnRH regulation of both Lhb and Fshb, we tested whether FFAs activate or inhibit the MAPKs in LβT2 immortalized gonadotrope cells. FFAs caused a dose- and time-dependent increase in phosphorylation of p38MAPK, JNK, and ERK (Figure 1A). Activation of the MAPKs can be observed at 1 hour and is sustained up to 4 hours. At the level of gonadotropin gene expression, FFA treatment for 8 hours caused a dose-dependent increase in Lhb expression at 200-500 μM and a progressive decrease in Fshb expression at 100-500 μM FFAs (Figure 1B). Time-course studies indicated an induction of Lhb and a suppression of Fshb at 4-8 hours (Figure 1C). All subsequent experiments used 500 μM FFA cocktail at 6-8 hours unless otherwise stated. Because the induction of Lhb at the mRNA level is modest, we evaluated the effect of FFAs on the Lhb primary transcript. FFAs caused a 30-fold induction of the primary transcript and a corresponding 100-fold induction of Egr1 mRNA, a regulator of Lhb (Supplemental Figure 1). We also noticed a 20-fold induction of Fos mRNA that was inconsistent with the decrease in Fshb, as we and others have published, that c-FOS is a positive regulator of Fshb expression (35). We were unable to measure primary transcript level for the Fshb gene due to its very low expression, but we were able to confirm that FFA treatment decreases Fshb promoter activity in a transient transfection consistent with the decrease in mRNA (Supplemental Figure 1).
Figure 1.
A, FFAs activate JNK, p38MAPK, and ERK pathways. LβT2 cells were treated with increasing concentrations of an FFA cocktail (25-500 μM) for increasing times (1 and 4 hours). Cell lysates were immunoblotted for phospho-p38MAPK, phospho-ERK, phospho-JNK, or β-Tubulin (β-Tub). B, FFAs cause a dose-dependent increase in Lhb mRNA and a decrease in Fshb mRNA. LβT2 cells were treated with 100, 200, or 500 μM FFAs for 8 hours, and Lhb and Fshb mRNA expression was measured by quantitative PCR. C, FFAs cause a time-dependent increase in Lhb mRNA and a decrease in Fshb mRNA. LβT2 cells were treated with FFAs (500 μM) for the indicated time points, and then Lhb and Fshb mRNA levels were measured by quantitative PCR. D, Cells were pretreated with the p38MAPK inhibitor PD169316 (5 μM), the MEK inhibitor PD98059 (20 μM), or the JNK inhibitor SP600125 (10 μM) for 30 minutes and then stimulated with 500 μM FFA for 8 hours and Lhb and Fshb mRNA expression measured by quantitative PCR. All quantitative PCR data represent mean ± SEM from 3 experiments, each repeated 3 times. E, Serum LH and FSH levels were measured in men undergoing acute intralipid infusion (n = 12/group). The control group received a saline vehicle infusion. *, P ≤ .05 vs untreated control.
Although FFAs have also been shown to activate the nuclear factor-κB (NfκB) pathway in other cells, there was no change in the phosphorylation of IKK or RelA (p65), indicating that the NfκB pathway is not activated in LβT2 cells (Supplemental Figure 2A). These cells do not express IKKβ (Supplemental Figure 2B), the major isoform signaling to NfκB activation (36, 37). Experiments using individual components of the FFA cocktail indicated that lauric acid, myristic acid, arachidonic acid, and linolenic acid, but not oleic acid, contributed to JNK activation (Supplemental Figure 2C).
JNK and MEK are required for FFA-induced Lhb mRNA expression, but FFA-induced suppression of Fshb mRNA is independent of MAPKs
We then used a panel of kinase inhibitors to test the involvement of the MAPKs in gonadotropin expression. Inhibition of the JNK and MEK pathways reduces the FFA induction of Lhb mRNA, but inhibition of p38MAPK had no effect (Figure 1D). Inhibition of p38MAPK suppresses basal Fshb expression, as we have published previously, so it was not possible to test the involvement of this kinase in FFA signaling; however, there is no further suppression by FFAs, so the pathways are not additive. Because inhibition of p38MAPK suppresses Fshb, we can conclude that the suppression by FFA is not likely mediated by p38MAPK activation. Furthermore, the suppression of Fshb mRNA was unchanged by the inhibition of JNK or MEK (Figure 1D). These data indicate that FFA induction of Lhb is mediated via the JNK/MEK pathways, but the suppression of Fshb is independent of the MAPKs.
Acute infusion of intralipid increases serum LH in men
To test whether acute lipid exposure would alter circulating gonadotropin levels in humans, we turned to samples from a previous study in which men received an infusion of 20% intralipid and heparin during a euglycemic hyperinsulinemic glucose clamp study (34). This manipulation elevated the plasma nonesterified fatty acid, caused a state of insulin resistance, and reduced whole-body glucose disposal. The acute lipid infusion also caused an increase in the plasma LH levels (Figure 1E) with no significant change in the FSH levels.
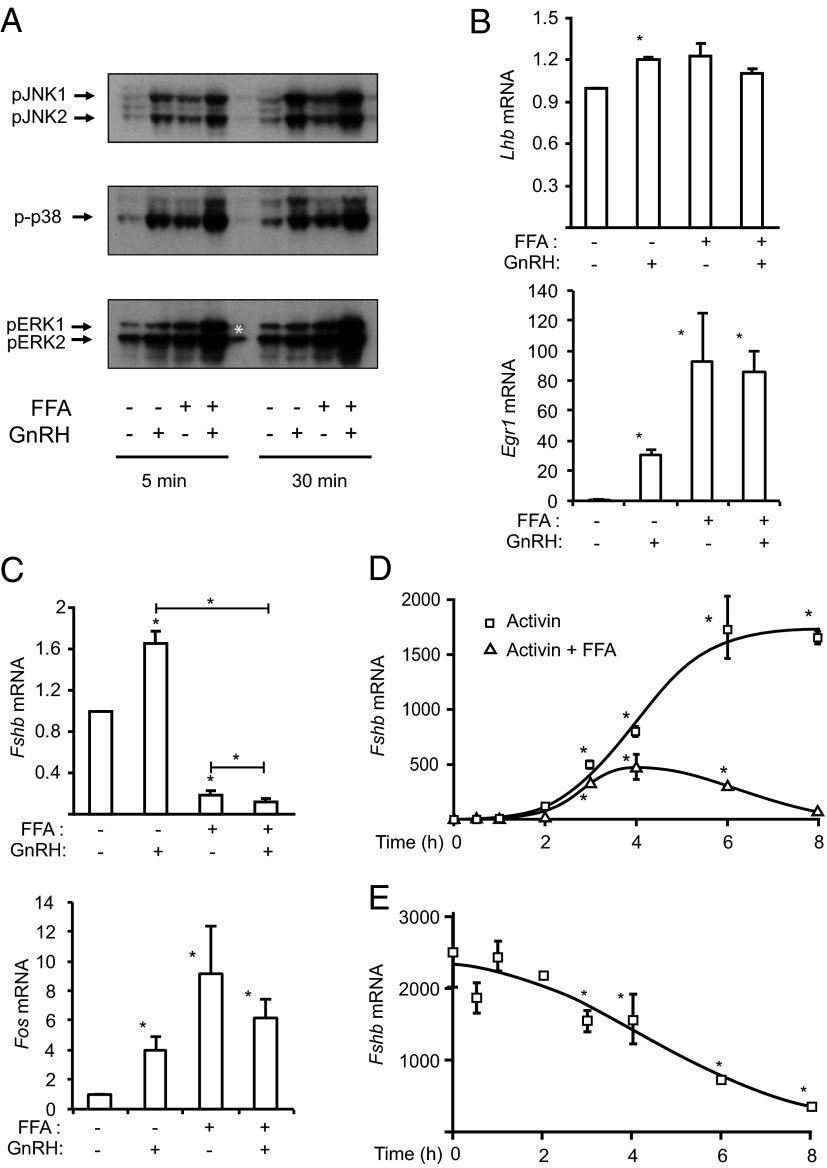
FFA cotreatment enhances the GnRH induction of the MAPKs and reverses the GnRH induction of Fshb mRNA
Previously we and others have reported that the 3 MAPK families (ERK, p38MAPK, and JNK) are activated by GnRH in LβT2 cells and primary pituitary gonadotropes (38). To investigate the effects of FFAs on GnRH-induced MAPK signaling, we treated cells with FFAs (500 μM) for 4 hours and added GnRH (100 nM) for the last 5 and 30 minutes before harvest. Both GnRH and FFA treatments induce the MAPKs as expected, and cotreatment leads to an additive induction of MAPK phosphorylation (Figure 2A). To investigate the effects of cotreatment on gonadotropin gene induction, cells were cotreated with FFA and GnRH for 8 hours. Both GnRH and FFA modestly increased Lhb mRNA and the cotreatment had no additive effect (Figure 2B). Similar effects were seen for induction of early growth response protein 1 (Egr1) mRNA. Although GnRH induced and FFA repressed Fshb mRNA, cotreatment with both agonists eliminated the GnRH induction and led to a further significant down-regulation of Fshb mRNA expression (Figure 2C). Again, Fos mRNA was induced by both FFAs and GnRH so does not explain the repression of Fshb.
Figure 2.
FFA treatment modulates the GnRH and activin effects on Lhb and Fshb mRNA. A, GnRH and FFAs have additive effects on MAPK signaling. LβT2 cells were treated with 500 μM FFAs for 4 hours, and 100 nM GnRH was added for the last 5 or 30 minutes. Whole-cell lysates were immunoblotted for phospho-JNK, phospho-p38MAPK, and phospho-ERK as before. The asterisk indicates where the sample spilled over from the adjacent well during loading. B, FFA and GnRH do not have additive effects on Lhb gene induction. LβT2 cells were treated with 500 μM FFAs and 100 nM GnRH for 8 hours. Lhb and Egr1 mRNA expressions were measured by quantitative PCR. C, FFA and GnRH effects on Fshb gene expression. LβT2 cells were treated with 500 μM FFAs and 100 nM GnRH for 8 hours. Fshb and Fos mRNA expressions were measured by quantitative PCR. D, FFAs suppress activin-induced Fshb expression. LβT2 cells were treated with 500 μM FFA, 25 ng/mL activin A, or both for increasing times (0-8 hours). Fshb mRNA expression was measured by quantitative PCR. E, LβT2 cells were pretreated with 25 ng/mL activin overnight and then with 500 μM FFAs for increasing times (0-8 hours). Fshb mRNA expression was measured by quantitative PCR. Quantitative PCR data represent mean ± SEM from 3 experiments, each repeated 3 times. *, P ≤ .05 vs untreated control or between indicated groups.
FFAs inhibit the activin induction of the Fshb mRNA
Activin, rather than GnRH, is the primary driver of Fshb expression, so we examined the effect of FFAs on activin-induced Fshb expression. The LβT2 cells produce endogenous activin that maintains basal Fshb expression via an autocrine induction. So the suppression of basal Fshb mRNA could reflect interference with this autocrine loop. To test this, we measured expression of activin and inhibin subunits, the activin receptors, follistatin and the p120 inhibin-binding protein Igsf1 after FFA treatment. FFA treatment leads to a repression of Inha but an induction of Inhba, which would be predicted to increase the activin/inhibin ratio. In contrast, FFAs repress Acvr1a and Acvr1b and induces Fst but decreases Igsf1 that would be consistent with decreased activin signaling (Supplemental Figure 3).
To test whether alterations in this endogenous autocrine loop might underlie the FFA suppression, we treated cells with a maximal concentration of exogenous activin to compensate for any changes in endogenous production. Cotreatment with activin and FFAs severely reduced but did not eliminate the induction of the Fshb mRNA (Figure 2D), suggesting the FFAs could prevent the induction by exogenous activin. In a different experiment, Fshb mRNA was maximally induced by pretreatment with activin overnight before treatment with FFAs. Here again, FFAs caused a time-dependent decrease in Fshb mRNA levels over 8 hours, indicating that FFAs could reverse the activin effect (Figure 2E). Treatment of cells with recombinant follistatin to block endogenous activin completely eliminated basal Fshb expression, so we were unable to test for FFA effects (Supplemental Figure 4A). These results show that FFAs are able to reduce activin induction of Fshb, even in the presence of maximal doses of activin. We have shown that the ratio of transcriptional activators to repressors is important for the induction of the Fshb gene, so we measured the mRNA levels of Smad2, Smad7, Tgif, and Crem. Both of the corepressors, Tgif and Crem, are induced by FFA treatment, and Smad2 is decreased, which is consistent with the decreased activin induction of the Fshb promoter (Supplemental Figure 4B).
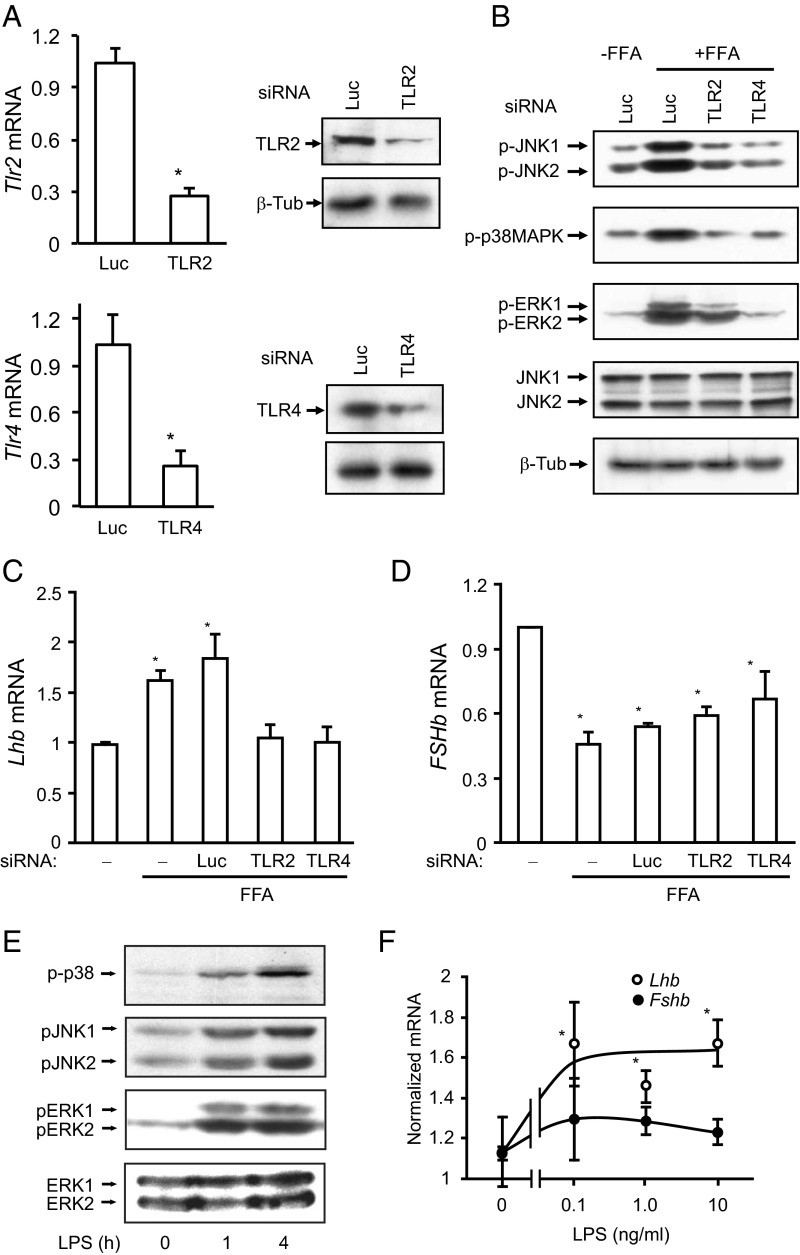
FFA effect on Lhb, but not Fshb, mRNA expression is mediated via TLRs
Because FFAs impair insulin signaling via activation of TLR2 and TLR4 (31), we knocked down TLR2 and TLR4 using siRNA, obtaining a 65%-75% decrease in TLR2 or TLR4 protein and mRNA (Figure 3A). We then showed that FFA activation of JNK, p38MAPK, and ERK was suppressed (Figure 3B). At the level of gonadotropin gene expression, knockdown of either TLR2 or TLR4 abolished the FFA induction of Lhb (Figure 3C), but the FFA suppression of Fshb was unchanged (Figure 3D). Taking a different approach, we stimulated TLR signaling using the bacterial product LPS. As expected, LPS activated JNK, p38MAPK, and ERK in a time-dependent manner (Figure 3E) and enhanced Lhb mRNA expression but did not repress Fshb mRNA levels (Figure 3F). Therefore, the FFA effects on Lhb expression appear to be mediated by activation of the classical TLR pathways, but the repression of Fshb is TLR2/4 independent. FFAs have also been shown to signal through G protein-coupled receptor (GPR) 40. Treatment with a GPR40 antagonist did not alter the FFA repression of Fshb in our system (Supplemental Figure 5A), although expression of GPR40, and two related receptors, GPR119 and GPR120, is induced by FFAs (Supplemental Figure 5, B–D).
Figure 3.
FFA activation of MAPKs and induction of Lhb is mediated via TLRs. A, Quantification of knockdown of TLR2 and TLR4 in LβT2 cells. Expression of TLR2 and TLR4 was measured 72 hours after electroporation by quantitative PCR and immunoblotting. *, P < .05 vs control siRNA against luciferase (Luc). B, Knockdown of TLR2 and TLR4 impairs FFA activation of p38MAPK, JNK, and ERK. Forty-eight hours after electroporation, the knockdown cells were starved in media containing 0.1% BSA and then stimulated with 500 μM FFAs for 4 hours. Cell lysates were immunoblotted for phospho-JNK, phospho-p38MAPK, phospho-ERK, JNK, and β-tubulin (β-Tub) as before. C, Knockdown of either TLR2 or TLR4 abolished FFA induction of Lhb expression. Knockdown cells were starved in medium containing 0.1% BSA and then stimulated with 500 μM FFAs and 100 nM GnRH for 8 hours. Lhb mRNA expression was measured by quantitative PCR. D, FFA suppression of Fshb mRNA is TLR independent. Fshb mRNA expression was measured by quantitative PCR. *, P < .05 vs untreated control. E, LPS stimulates p38MAPK, JNK, and ERK in LβT2 cells. LβT2 cells were starved and treated with 100 ng/mL LPS or ethanol for 1 and 4 hours. Whole-cell lysates were immunoblotted for phospho-p38MAPK, phospho-JNK, phospho-ERK, or total ERK. The blots are representatives of 3 experiments. F, LPS increases Lhb mRNA expression. Cells were treated with 0.1, 10, or 100 ng/mL LPS for 8 hours. Lhb and Fshb mRNA expressions were measured by quantitative PCR. *, P < .05 vs untreated.
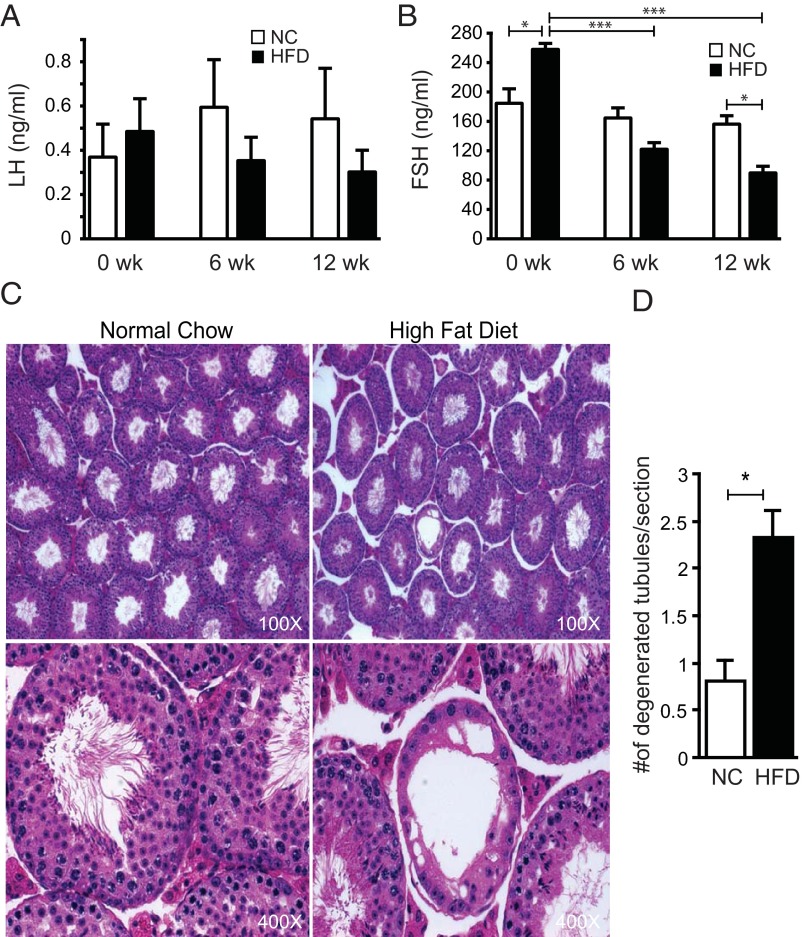
Chronic high-fat feeding causes obesity, insulin resistance, glucose intolerance, and reproductive defects
To test the effect of chronic exposure to FFAs, we used a HFD-induced model of obesity in mice. C57BL/6 mice were placed on a 60% HFD or a control low-fat diet (NC) for 24 weeks. As expected, both male and female mice gained significant weight on the HFD (Supplemental Figure 6A). At the end of the diet, both male and female HFD mice were insulin resistant as assessed by an insulin tolerance test (Supplemental Figure 6B) and glucose tolerance test (Supplemental Figure 6C).
Male mice on the HFD did not show a difference in plasma LH at 6 or 12 weeks (Figure 4A), but FSH levels were progressively reduced after 6 and 12 weeks (Figure 4B). Although the mice were randomized into the treatment groups, the group of mice in the HFD group had slightly higher basal FSH levels, but their FSH levels dropped significantly after 6 and 12 weeks on the HFD (50% and 65%, respectively). Furthermore, after 12 weeks mice had significantly lower FSH levels than controls irrespective of their basal levels (Figure 4B). Histological examination of testicular sections (Figure 4C) showed occasional tubules with abnormal architecture, incomplete maturation and no mature spermatids within the lumen of the tubule in mice fed the HFD. In some tubules, degeneration was evident with vacuolated cytoplasm and clumped nuclei, depletion of germ cells, and flattening of Sertoli cells. Quantification of multiple sections from multiple animals indicated a significant increase in tubular defects in mice on the HFD (Figure 4D). Pituitary Lhb and Fshb mRNA levels were unchanged but hypothalamic GnRH tended to be lower (Supplemental Figure 7).
Figure 4.
HFD male mice have reduced FSH levels and more abnormal tubules. A and B, Plasma LH and FSH levels from male mice on NC (n = 9) and an HFD (n = 12) measured before diets (0 week) or after 6 and 12 weeks on diets. C, Representative sections from NC and HFD testes at ×100 and ×400 magnification. D, Quantification of the number of abnormal tubules per testis section from NC (n = 38) and HFD (n = 44) mice. Asterisks show statistical significance between indicated columns: *, P < .05; ***, P < .001.
Female mice on an HFD have irregular estrous cycles
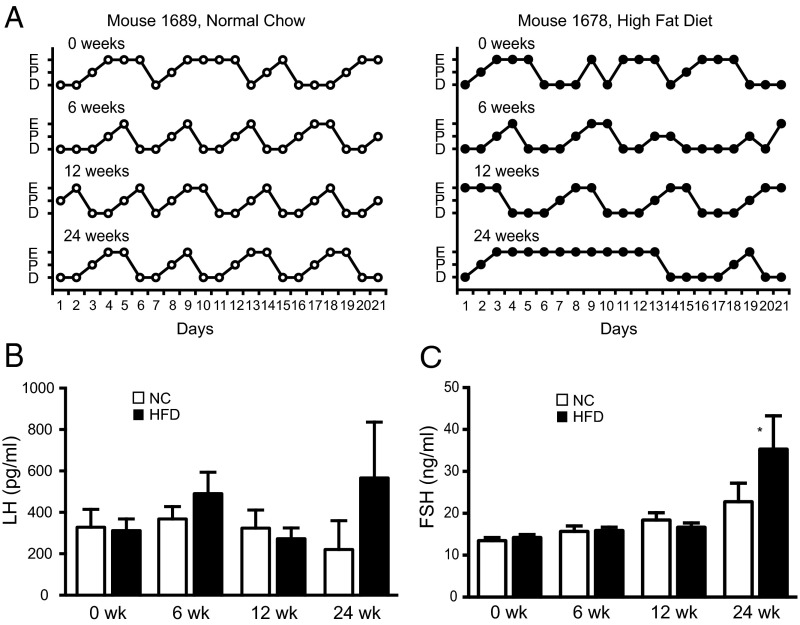
Female mice on normal chow showed regular 4- to 5-day estrous cycles, but mice on the HFD showed fewer regular cycles with prolonged estrus (Table 1). Representative estrous cycle data for 2 mice are shown (Figure 5A, complete cycling data for all mice are included in Supplemental Figure 8). For the mice on NC, there was a progressive increase in the number of days of estrus over time, which became significant when comparing mice after 24 weeks with 6 weeks (P < .05). In contrast, after 6 and 12 weeks of the HFD, the number of days of proestrus and the number of cycles were decreased, but the days of estrus were increased. The number of cycles was also significantly less at 12 weeks than before the diets or at 6 weeks (P < .05), suggesting a progressive defect. After 24 weeks on the HFD, the number of cycles and the number of days of proestrus were again reduced compared with the mice on NC and also with the number of cycles before the diets and at week 6 on HFD (P < .05), but the number of days of estrus was not different between the HFD and NC. There was no difference in the number of days of diestrus at any time.
Table 1.
Number of Days at Proestrus, Estrus, and Diestrus and the Total Number of Cycles Over 21 Days of Monitoring
| Stage | Before Diets |
After 6 Weeks |
After 12 Weeks |
After 24 Weeks |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | HFD | P Value | NC | HFD | P Value | NC | HFD | P Value | NC | HFD | P Value | |
| Proestrus | 3.4 ± 1.0 | 3.2 ± 1.0 | 0.7 | 4.1 ± 0.7 | 3.3 ± 0.3 | 0.03 | 3.4 ± 1.0 | 2.2 ± 0.8 | 0.01 | 3.7 ± 0.5 | 2.0 ± 0.8 | 0.001 |
| Estrus | 7.4 ± 1.4 | 6.5 ± 2.4 | 0.3 | 4.8 ± 1.4 | 7.1 ± 1.9 | 0.01 | 6.3 ± 1.2 | 9.0 ± 2.6 | 0.01 | 8.1 ± 2.3 | 9.0 ± 2.7 | 0.4 |
| Diestrus | 10.2 ± 1.4 | 11.4 ± 1.9 | 0.1 | 11.9 ± 1.6 | 10.6 ± 1.6 | 0.1 | 10.8 ± 0.2 | 9.6 ± 1.9 | 0.2 | 9.2 ± 2.1 | 10.6 ± 2.8 | 0.3 |
| Total cycles | 3.2 ± 0.4 | 2.8 ± 0.2 | 0.4 | 4.1 ± 0.2 | 3.3 ± 0.2 | 0.02 | 3.8 ± 0.3 | 2.0 ± 0.3 | 0.001 | 3.4 ± 0.2 | 1.5 ± 0.4 | 0.001 |
Values are mean ± SEM. P value indicates statistical significance for HFD vs NC.
Figure 5.
HFD female mice show altered estrous cycles. A, Representative estrous cycles for mouse 1689 on NC and mouse 1678 on a HFD. Mice were cycled for 21 days before diets (0 week), or starting at 6, 12, and 24 weeks on diets. E, P, and D represent estrus, proestrus, and diestrus/metestrus. B and C, Diestrous LH and FSH after 6, 12, and 24 weeks on an HFD or NC. Plasma LH and FSH were measured by a Luminex assay (Millipore). Asterisks show statistical significance between diets: *, P < .05.
Female mice on HFD are more responsive to GnRH stimulation and lack the afternoon LH surge at proestrus
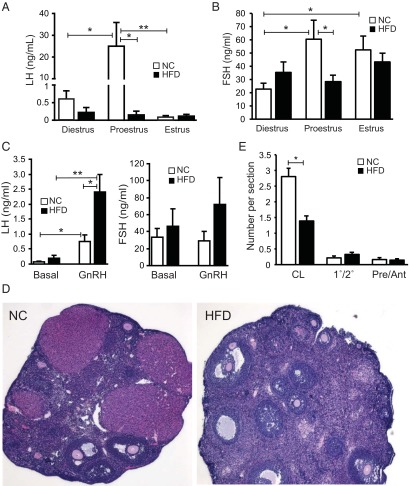
Diestrus LH levels were monitored over time and were not different before the diets or after 6, 12, or 24 weeks of the HFD, but FSH levels were slightly elevated at 24 weeks (Figure 5, B and C). At the end of the 24-week HFD feeding study, a comprehensive analysis of gonadotropin levels throughout the estrous cycle was undertaken. LH levels were unchanged at diestrus, similar to the 6- and 12-week values, and were also unchanged at estrus, but HFD-fed mice lacked the endogenous LH surge on the afternoon of proestrus (Figure 6A), whereas the mice on NC had the expected LH surge rising greater than 50-fold (25 ng/mL from <0.5 ng/mL). The mice on NC showed an elevation of FSH at both proestrus and estrus compared with diestrus, consistent with the known pattern of gonadotropin secretion in rodents. FSH levels were unaltered at diestrus and estrus between the HFD and NC groups but were significantly reduced in the HFD group on the afternoon of proestrus (Figure 6B). Because the LH surge was impaired, we assessed whether the pituitaries in the HFD mice were sensitive to GnRH stimulation. Basal LH levels were indistinguishable as observed before for mice on NC and the HFD, and injection of GnRH increased the LH levels in both the NC and HFD groups (Figure 6C). The absolute LH response to GnRH was increased 3-fold in the HFD group compared with NC, indicating that the pituitaries in the HFD group have an exaggerated response to GnRH. The FSH levels did not change after the short-term GnRH stimulation in either of the groups.
Figure 6.
HFD female mice lack the afternoon LH surge at proestrus. A and B, Plasma LH and FSH levels after 24 weeks on an HFD measured at the morning of diestrus, the afternoon of proestrus (6:00 pm) and the morning of estrus. Values are mean ± SEM for n = 10/group. C, GnRH-stimulated LH and FSH levels. Mice were stimulated with 1 μg/kg of GnRH and blood was drawn after 10 minutes. Values were compared with basal values obtained before the GnRH stimulation. Values are mean ± SEM for n = 8/group. Asterisks show statistical significance between indicated columns: *, P < .05; **, P < .01. D, Representative ovarian sections from NC and HFD mice. E, Quantification of the number of corpora lutea (CL), primary and secondary follicles (1°/2°), or preantral and antral follicles (Pre/Ant) per section from each group (NC, n = 56; HFD, n = 77). Asterisks show statistical significance between indicated columns: *, P < .05.
The impaired proestrus LH and FSH surge suggested a potential ovulation defect, so we examined the ovaries in mice killed at diestrus at the end of the study (Figure 6D). Histological analysis of the ovaries revealed a significantly reduced number of corpora lutea per section of ovary from mice on the HFD (Figure 6E), but there was no significant difference in the number of primary/secondary follicles or preantral/antral follicles. Despite the reduced number of corpora lutea, there were no differences in plasma progesterone and estradiol levels in mice on the HFD (Supplemental Figure 9, A and B). We also measured pituitary gonadotropin mRNA and did not see a difference in pituitary Lhb or hypothalamic Gnrh mRNA expression in the HFD group, but pituitary Fshb tended to be higher (Supplemental Figure 9, C–E).
Discussion
Because plasma FFA levels are significantly elevated in PCOS patients (39, 40), we hypothesized that exposure to FFAs may negatively impact reproduction through direct effects at the pituitary to regulate gonadotropin synthesis. We and others have shown that GnRH activates the inflammatory kinases JNK and p38MAPK in primary and immortalized pituitary gonadotropes, and it is also known that FFAs activate these same pathways in other cells, so we tested our hypothesis initially in the LβT2 pituitary gonadotrope cell line. We showed that FFAs activate JNK, p38MAPK, and ERK signaling in these cells and induce Egr1 and Lhb. Fos is also induced, but paradoxically Fshb expression is suppressed due to inhibition of the activin induction. Cotreatment of FFAs and GnRH led to enhanced MAPK signaling, but we did not observe any additive or synergistic effects on Lhb induction. In contrast, the cotreatment eliminated the GnRH induction of Fshb, probably because FFAs inhibit the activin induction of Fshb, and it has been published that autocrine activin induction is required for the GnRH activation of Fshb (41). We demonstrated that the induction of Lhb is mediated via TLR2/4 induction of JNK and ERK signaling and is mimicked by LPS, but the repression of Fshb is MAPK and TLR independent. We also showed that acute lipid infusion in men increases serum LH, consistent with the acute elevation of Lhb seen in vitro, but we did not see a reduction in FSH, perhaps because FSH has a longer half-life in circulation that may mask acute changes.
We then looked at chronic exposure to elevated FFAs using the well-characterized, HFD-induced obesity model in C57BL/6 mice. We found that both male and female mice gain significant weight and become insulin resistant and glucose intolerant as expected. Consistent with our in vitro data, male mice have reduced FSH levels on HFD and show more seminiferous tubules possessing abnormal architecture with incomplete maturation and no mature spermatids in the lumen of the tubules. It is possible that the impaired spermatogenesis is due to the reduced FSH because FSH immunoneutralization in male rats leads to an increase in germ cell apoptosis (42) and the Fshr or Fshb knockout mice have reduced Sertoli and germ cells (43–45).
The female C57BL/6 mice on the HFD exhibit prolonged estrus and decreased proestrus and in addition lack the expected LH and FSH surge on the afternoon of proestrus. The pituitaries in the HFD-fed female mice are sensitive to GnRH stimulation and show an exaggerated response to exogenous GnRH, so we concluded that the lack of LH surge is due to a lack of endogenous hypothalamic GnRH drive rather than a defect at the pituitary. The exaggerated response to GnRH, the prolonged estrus, and the decreased proestrus are consistent with another study of mixed background CD1/129SvJ/C57BL6 mice (26). We did not find an additive or synergistic effect with GnRH and FFA treatment in the in vitro gonadotrope cell system, yet obesity sensitizes the pituitary to GnRH in vivo. This discrepancy is likely explained by the short-term, acute treatment in vitro compared with the chronic obesity in vivo. Indeed in the previous published study, the authors found that pituitary GnRH receptor expression was elevated after 12 weeks on an HFD, likely explaining the enhanced sensitivity (26). We also found that ovaries from mice on the HFD have significantly less corpora lutea, consistent with a defect in ovulation, but follicle maturation appears intact.
The lack of endogenous gonadotropin surge coupled with the enhanced pituitary sensitivity to exogenous GnRH suggests the origin of the defect in the obese female C57BL/6 mice is likely hypothalamic rather than pituitary. Hypothalamic infertility has also been seen in the DBA/2J mouse when placed on a 24% fat diet (25). In this study, the infertility was associated with hyperleptinemia, decreased hypothalamic Lepr expression, and increased Npy expression but not with insulin resistance. Thus, the DBA/2J mouse represents a model of obesity-dependent reproductive dysfunction in the absence of significant insulin resistance. In contrast, obesity in the C57BL/6 mouse more closely mirrors human obesity and its associated insulin resistance. How might these central effects be mediated? Kisspeptin is essential for activation of GnRH neurons, and HFD feeding in DBA/2J mice decreases kisspeptin expression in both the arcuate and rostral periventricular region of the third ventricle and, more intriguingly, also reduces the number of kisspeptin neurons in the rostral periventricular region of the third ventricle (24). This latter group of neurons is thought to participate in the generation of the LH surge under positive feedback by estrogen in female mice and is lacking in males, so loss of this population would be consistent with the reduced LH surge that we observed.
HFD-induced obesity also leads to central leptin resistance by decreasing the expression of both isoforms of the leptin receptor and reducing signal transducer and activator of transcription-3 signaling (46), but leptin does not appear to activate kisspeptin neurons directly but acts via neurons in the forebrain (47). Consistent with an indirect effect, deletion of the leptin receptor in either GnRH or Kiss1 neurons does not impair fertility (47, 48); however, lesions in the ventral premammillary nucleus in rats and mice, which controls sexual maturation, impaired puberty and fertility (49). Support for the idea of central leptin resistance comes from studies of the db/db mouse because leptin receptor deficiency prevents the activation of GnRH and kisspeptin neurons during an artificial steroid-induced surge in mice (24) and the leptin-resistant ovariectomized obese Zucker rat has decreased LH pulse amplitude but not frequency (50). Whether such leptin resistance in selected neuronal populations is responsible for the impaired fertility due to diet-induced, insulin-resistant obesity in C57BL/6 mice remains to be determined.
In summary, we demonstrated that FFAs activate TLR-mediated inflammatory signaling in LβT2 cells, leading to the induction of Lhb and that FFAs suppress Fshb expression but in a TLR-independent manner. We also showed that HFD-induced obesity in mice leads to the suppression of FSH in males and the disruption of the proestrous LH and FSH surge in females.
Supplementary Material
Acknowledgments
We thank Dr Devendra Mistry for help with the Luminex assay and Dr Shunichi Shimasaki, Dr Karel De Gendt, and Ms Leslie Carter for helpful suggestions/discussions and analysis of the histochemical data.
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Infertility Research (to N.J.G.W., J.M.O.) and by National Institutes of Health Grants DK033651, DK074868, HD047400, DK007494, DK090962, DK063491, CA023100, and CA155435 and by Veterans Affairs Merit Award BX000130. N.J.G.W. and J.M.O. are faculty members of the University of California, San Diego, Biomedical Sciences Graduate Program.
Current address for S.S.: Roche Diagnostics, San Diego, California.
Disclosure Summary: The authors have nothing to disclose.
Note Added in Proof
After acceptance of this paper the authors realized they did not include a relevant reference by Garrel et al (51).
Footnotes
- Egr1
- early growth response protein 1
- FFA
- free fatty acid
- GPR
- G protein-coupled receptor
- HFD
- high-fat diet
- IKK
- inhibitor of κB kinase
- JNK
- c-Jun N-terminal kinase
- LPS
- lipopolysaccharide
- MEK
- MAPK kinase
- NC
- normal chow
- NfκB
- nuclear factor-κB
- PCOS
- polycystic ovary syndrome
- siRNA
- small interfering RNA
- TLR
- toll-like receptor.
References
- 1. World Health Organization Obesity and overweight—fact sheet number 311. Washington, DC: World Health Organization; 2012 [Google Scholar]
- 2. Bjercke S, Dale PO, Tanbo T, Storeng R, Ertzeid G, Abyholm T. Impact of insulin resistance on pregnancy complications and outcome in women with polycystic ovary syndrome. Gynecol Obstet Invest. 2002;54:94–98 [DOI] [PubMed] [Google Scholar]
- 3. Chang RJ. The reproductive phenotype in polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2007;3:688–695 [DOI] [PubMed] [Google Scholar]
- 4. Norman JE. The adverse effects of obesity on reproduction. Reproduction. 2010;140:343–345 [DOI] [PubMed] [Google Scholar]
- 5. Ramsay JE, Greer I, Sattar N. ABCs of obesity. Obesity and reproduction. BMJ. 2006;333:1159–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Niederberger C. Re: Functional relationship between obesity and male reproduction: from humans to animal models. J Urol. 2012;188:553–554 [DOI] [PubMed] [Google Scholar]
- 7. Teerds KJ, de Rooij DG, Keijer J. Functional relationship between obesity and male reproduction: from humans to animal models. Hum Reprod Update. 2011;17:667–683 [DOI] [PubMed] [Google Scholar]
- 8. Cabler S, Agarwal A, Flint M, du Plessis SS. Obesity: modern man's fertility nemesis. Asian J Androl. 2010;12:480–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shayeb AG, Harrild K, Mathers E, Bhattacharya S. An exploration of the association between male body mass index and semen quality. Reprod Biomed Online. 2011;23:717–723 [DOI] [PubMed] [Google Scholar]
- 10. Du Plessis SS, Cabler S, McAlister DA, Sabanegh E, Agarwal A. The effect of obesity on sperm disorders and male infertility. Nat Rev Urol. 2010;7:153–161 [DOI] [PubMed] [Google Scholar]
- 11. Ghanayem BI, Bai R, Kissling GE, Travlos G, Hoffler U. Diet-induced obesity in male mice is associated with reduced fertility and potentiation of acrylamide-induced reproductive toxicity. Biol Reprod. 2010;82:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang AS, Dale AN, Moley KH. Maternal diabetes adversely affects preovulatory oocyte maturation, development, and granulosa cell apoptosis. Endocrinology. 2005;146:2445–2453 [DOI] [PubMed] [Google Scholar]
- 13. Elmes MJ, Tan DS, Cheng Z, Wathes DC, McMullen S. The effects of a high-fat, high-cholesterol diet on markers of uterine contractility during parturition in the rat. Reproduction. 2011;141:283–290 [DOI] [PubMed] [Google Scholar]
- 14. Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151:4039–4046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McKnight JR, Satterfield MC, Li X, et al. Obesity in pregnancy: problems and potential solutions. Front Biosci (Elite Ed). 2011;3:442–452 [DOI] [PubMed] [Google Scholar]
- 16. Oben JA, Mouralidarane A, Samuelsson AM, et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol. 2010;52:913–920 [DOI] [PubMed] [Google Scholar]
- 17. Shalev U, Tylor A, Schuster K, Frate C, Tobin S, Woodside B. Long-term physiological and behavioral effects of exposure to a highly palatable diet during the perinatal and post-weaning periods. Physiol Behav. 2010;101:494–502 [DOI] [PubMed] [Google Scholar]
- 18. Shankar K, Kang P, Harrell A, et al. Maternal overweight programs insulin and adiponectin signaling in the offspring. Endocrinology. 2010;151:2577–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bakos HW, Mitchell M, Setchell BP, Lane M. The effect of paternal diet-induced obesity on sperm function and fertilization in a mouse model. Int J Androl. 2011;34:402–410 [DOI] [PubMed] [Google Scholar]
- 20. Mitchell M, Bakos HW, Lane M. Paternal diet-induced obesity impairs embryo development and implantation in the mouse. Fertil Steril. 2011;95:1349–1353 [DOI] [PubMed] [Google Scholar]
- 21. Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007;10:142–148 [DOI] [PubMed] [Google Scholar]
- 22. Macut D, Panidis D, Glisic B, et al. Lipid and lipoprotein profile in women with polycystic ovary syndrome. Can J Physiol Pharmacol. 2008;86:199–204 [DOI] [PubMed] [Google Scholar]
- 23. Norman RJ, Noakes M, Wu R, Davies MJ, Moran L, Wang JX. Improving reproductive performance in overweight/obese women with effective weight management. Hum Reprod Update. 2004;10:267–280 [DOI] [PubMed] [Google Scholar]
- 24. Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011;152:1541–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tortoriello DV, McMinn J, Chua SC. Dietary-induced obesity and hypothalamic infertility in female DBA/2J mice. Endocrinology. 2004;145:1238–1247 [DOI] [PubMed] [Google Scholar]
- 26. Brothers KJ, Wu S, DiVall SA, et al. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab. 2010;12:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fresno M, Alvarez R, Cuesta N. Toll-like receptors, inflammation, metabolism and obesity. Arch Physiol Biochem. 2011;117:151–164 [DOI] [PubMed] [Google Scholar]
- 28. Kanczkowski W, Ziegler CG, Zacharowski K, Bornstein SR. Toll-like receptors in endocrine disease and diabetes. Neuroimmunomodulation. 2008;15:54–60 [DOI] [PubMed] [Google Scholar]
- 29. Kannaki TR, Shanmugam M, Verma PC. Toll-like receptors and their role in animal reproduction. Anim Reprod Sci. 2011;125:1–12 [DOI] [PubMed] [Google Scholar]
- 30. Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336 [DOI] [PubMed] [Google Scholar]
- 31. Nguyen MT, Satoh H, Favelyukis S, et al. JNK and tumor necrosis factor-α mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280:35361–35371 [DOI] [PubMed] [Google Scholar]
- 32. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science. 2001;293:1673–1677 [DOI] [PubMed] [Google Scholar]
- 34. Kruszynska YT, Worrall DS, Ofrecio J, Frias JP, Macaraeg G, Olefsky JM. Fatty acid-induced insulin resistance: decreased muscle PI3K activation but unchanged Akt phosphorylation. J Clin Endocrinol Metab. 2002;87:226–234 [DOI] [PubMed] [Google Scholar]
- 35. Mistry DS, Tsutsumi R, Fernandez M, et al. Gonadotropin-releasing hormone pulse sensitivity of follicle-stimulating hormone-β gene is mediated by differential expression of positive regulatory activator protein 1 factors and corepressors SKIL and TGIF1. Mol Endocrinol. 2011;25:1387–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science. 1999;284:321–325 [DOI] [PubMed] [Google Scholar]
- 37. Tanaka M, Fuentes ME, Yamaguchi K, et al. Embryonic lethality, liver degeneration, and impaired NF-κB activation in IKK-β-deficient mice. Immunity. 1999;10:421–429 [DOI] [PubMed] [Google Scholar]
- 38. Zhang H, Bailey JS, Coss D, et al. Activin modulates the transcriptional response of LβT2 cells to gonadotropin-releasing hormone and alters cellular proliferation. Mol Endocrinol. 2006;20:2909–2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Evans DJ, Hoffmann RG, Kalkhoff RK, Kissebah AH. Relationship of androgenic activity to body fat topography, fat cell morphology, and metabolic aberrations in premenopausal women. J Clin Endocrinol Metab. 1983;57:304–310 [DOI] [PubMed] [Google Scholar]
- 40. Holte J, Bergh T, Berne C, Lithell H. Serum lipoprotein lipid profile in women with the polycystic ovary syndrome: relation to anthropometric, endocrine and metabolic variables. Clin Endocrinol (Oxf). 1994;41:463–471 [DOI] [PubMed] [Google Scholar]
- 41. Pernasetti F, Vasilyev VV, Rosenberg SB, et al. Cell-specific transcriptional regulation of follicle-stimulating hormone-β by activin and gonadotropin-releasing hormone in the LβT2 pituitary gonadotrope cell model. Endocrinology. 2001;142:2284–2295 [DOI] [PubMed] [Google Scholar]
- 42. Shetty J, Marathe GK, Dighe RR. Specific immunoneutralization of FSH leads to apoptotic cell death of the pachytene spermatocytes and spermatogonial cells in the rat. Endocrinology. 1996;137:2179–2182 [DOI] [PubMed] [Google Scholar]
- 43. Grover A, Sairam MR, Smith CE, Hermo L. Structural and functional modifications of Sertoli cells in the testis of adult follicle-stimulating hormone receptor knockout mice. Biol Reprod. 2004;71:117–129 [DOI] [PubMed] [Google Scholar]
- 44. Sairam MR, Krishnamurthy H. The role of follicle-stimulating hormone in spermatogenesis: lessons from knockout animal models. Arch Med Res. 2001;32:601–608 [DOI] [PubMed] [Google Scholar]
- 45. Wreford NG, Rajendra Kumar T, Matzuk MM, de Kretser DM. Analysis of the testicular phenotype of the follicle-stimulating hormone β-subunit knockout and the activin type II receptor knockout mice by stereological analysis. Endocrinology. 2001;142:2916–2920 [DOI] [PubMed] [Google Scholar]
- 46. Townsend KL, Lorenzi MM, Widmaier EP. High-fat diet-induced changes in body mass and hypothalamic gene expression in wild-type and leptin-deficient mice. Endocrine. 2008;33:176–188 [DOI] [PubMed] [Google Scholar]
- 47. Quennell JH, Mulligan AC, Tups A, et al. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology. 2009;150:2805–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Donato J, Jr, Cravo RM, Frazao R, et al. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121:355–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Donato J, Jr, Frazao R, Fukuda M, Vianna CR, Elias CF. Leptin induces phosphorylation of neuronal nitric oxide synthase in defined hypothalamic neurons. Endocrinology. 2011;151:5415–5427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Todd BJ, Ladyman SR, Grattan DR. Suppression of pulsatile luteinizing hormone secretion but not luteinizing hormone surge in leptin resistant obese Zucker rats. J Neuroendocrinol. 2003;15:61–68 [DOI] [PubMed] [Google Scholar]
- 51. Garrel G, Simon V, Denoyelle C, et al. Unsaturated fatty acids stimulate LH secretion via novel PKCε and -θ in gonadotrope cells and inhibit GnRH-induced LH release. Endocrinology. 2011;152:3905–3916 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.