Abstract
Torpor is a physiological state characterized by controlled lowering of metabolic rate and core body temperature, allowing substantial energy savings during periods of reduced food availability or harsh environmental conditions. The hypothalamus coordinates energy homeostasis and thermoregulation and plays a key role in directing torpor. We recently showed that mice lacking the orphan G protein-coupled receptor Gpr50 readily enter torpor in response to fasting and have now used these mice to conduct a microarray analysis of hypothalamic gene expression changes related to the torpor state. This revealed a strong induction of thioredoxin-interacting protein (Txnip) in the hypothalamus of torpid mice, which was confirmed by quantitative RT-PCR and Western blot analyses. In situ hybridization identified the ependyma lining the third ventricle as the principal site of torpor-related expression of Txnip. To characterize further the relationship between Txnip and torpor, we profiled Txnip expression in mice during prolonged fasting, cold exposure, and 2-deoxyglucose-induced hypometabolism, as well as in naturally occurring torpor bouts in the Siberian hamster. Strikingly, pronounced up-regulation of Txnip expression was only observed in wild-type mice when driven into torpor and during torpor in the Siberian hamster. Increase of Txnip was not limited to the hypothalamus, with exaggerated expression in white adipose tissue, brown adipose tissue, and liver also demonstrated in torpid mice. Given the recent identification of Txnip as a molecular nutrient sensor important in the regulation of energy metabolism, our data suggest that elevated Txnip expression is critical to regulating energy expenditure and fuel use during the extreme hypometabolic state of torpor.
To overcome periods of energetic constraint generated by diminished food supply and/or low ambient temperature, many small mammals and birds demonstrate controlled reductions in core body temperature (Tb) and metabolic rate, a phenomenon known as torpor. We previously reported that upon fasting, Gpr50−/− mice readily enter a state of torpor, characterized by hypometabolism and hypothermia (1). Gpr50 is the mammalian ortholog of the avian/amphibian melatonin receptor, Mel1c (2), and has been implicated in energy metabolism (3), neurite outgrowth (4), and affective disorders (5–7).
The hypothalamus is a major center of convergence and integration of signals that regulate energy homeostasis. It is also essential for expression of a regulated torpor response (8, 9). However, the neuroendocrine substrates that mediate torpor initiation, maintenance, and arousal remain poorly understood, and the mechanisms of metabolic fuel use during torpor are not extensively studied. During the onset of torpor, glucose provides the primary source of energy but is gradually replaced by an increasing reliance on lipid metabolism (10, 11). Decreased serum glucose levels are observed before spontaneous entrance into torpor, and animals remain hypoglycaemic throughout the torpor bout (10–14). In the final hours of torpor, glycolytic pathways are effectively replaced by lipid oxidation. However, in order to conserve energy, overall ATP-consuming processes are down-regulated, and only small reductions of serum lipids are observed (11, 15, 16).
In the current study, we use gene microarray analysis to examine transcript expression changes within the hypothalamus associated with torpor, using Gpr50−/− mice as a model. The most up-regulated gene in the hypothalamus during torpor was thioredoxin-interacting protein (Txnip), an endogenous negative regulator of the ubiquitously expressed antioxidant, thioredoxin (17). Txnip has recently emerged as an important regulator of cellular glucose and fatty acid (FA) metabolism, placed centrally in a mechanism whereby cells can integrate different signaling pathways to control fuel partitioning and use (18–21). Here, we demonstrate that increased Txnip expression is associated with torpor in 3 models: Gpr50−/− mice, female wild-type (WT) mice induced to enter torpor by prolonged fasting at reduced ambient temperature, and in the seasonal Siberian hamster during naturally occurring daily torpor. The consistent induction of Txnip suggests it is not simply due to fasting or decreased Tb but is a signal that may be required to deal with the unique energy demands experienced during torpor.
Results
Microarray analysis of gene expression during torpor in the murine hypothalamus
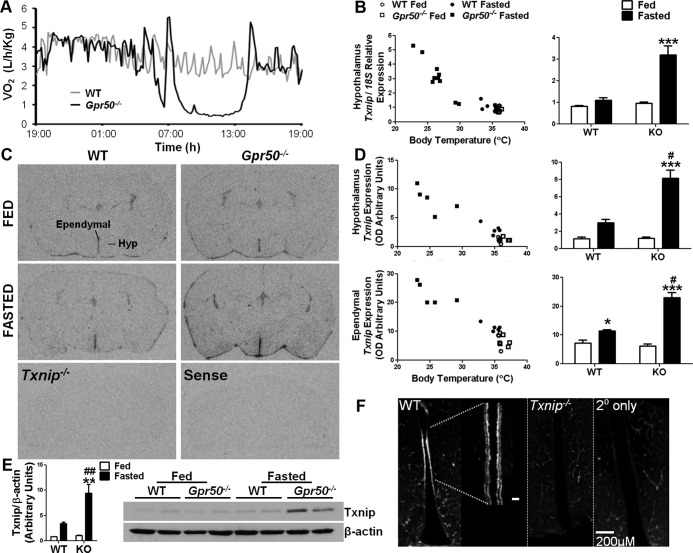
Fasting leads to reduced metabolic rate in mice. However, as we have previously reported (1), male Gpr50−/− mice readily enter a state of torpor upon fasting (Figure 1A). We therefore used these mice to profile gene expression changes in the hypothalamus associated with torpor. Hypothalamic blocks were collected at Zeitgeber time (ZT) 2 from WT and Gpr50−/− mice fed ad libitum or fasted (n = 4/group). Metabolic rates of animals were monitored in real time using indirect calorimetry, and only fasted Gpr50−/− mice exhibiting a stable torpor response, with no evidence of arousal before collection, were included in the microarray study. Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org, shows genes differentially expressed between fasted WT and Gpr50−/− mice (fold change > 1.2, q < .01), which are candidate torpor-regulated genes. Txnip and Hypoxanthine guanine phosphoribosyl transferase 1 (Hprt1) were further validated, because these were the transcripts most up-regulated and down-regulated, respectively, during torpor.
Figure 1.
Txnip expression is increased in the hypothalamus of Gpr50−/− mice during torpor. (A) Representative recordings of oxygen consumption in Gpr50−/− (black line) and WT (gray line) mice subjected to a 24-hour fast, during which Gpr50−/− mice entered a state of deep torpor. (B) qRT-PCR analysis of Txnip expression in the hypothalamus of ad libitum-fed and fasted WT and Gpr50−/− mice (n = 5/group; Gpr50−/− mice n = 8) demonstrated significantly increased Txnip expression in fasted Gpr50−/− mice. (C) In situ hybridization studies on mouse brain demonstrated Txnip expression within the ependyma of the lateral ventricles and the choroid plexus. Within the hypothalamus, Txnip expression was pronounced in the ependymal cells lining the third ventricle (thirdV). No hybridization signal was observed in Txnip−/− mice or when sense riboprobe was used. (D) Quantification of the hybridization signal (n = 5/group) showed Txnip expression was similar between fed WT and Gpr50−/− mice in both the ependyma of the thirdV and in the whole hypothalamic area minus the ependymal area. Fasting caused a significant increase in Txnip expression in both genotypes within the ependyma of the thirdV, but the induction of expression was significantly higher in the fasted Gpr50−/− compared with WT fasted mice. Txnip levels showed a similar pattern of induction with fasting in the parenchyma of the hypothalamus. (E) Immunoblot analysis of hypothalamic lysates from ad libitum-fed and fasted WT and Gpr50−/− mice. Densitometric analysis showed significantly increased Txnip expression in fasted Gpr50−/− mice. Torpor was assessed by Tb at the time of killing. (F) Txnip immunoreactivity in the murine hypothalamus was limited to the ependymal cells lining the third ventricle and was lost in Txnip−/− mouse tissue and when no primary antibody was included. Blood vessels, including those of the median eminence, were labeled by the secondary antimouse antibody. Data shown are mean ± SEM; *P < .05, **P < .01, ***P < .001 fasted vs fed; #P < .05, ##P < .01, Gpr50−/− vs WT. Statistical significance was determined using 2-way ANOVA with Bonferroni's post hoc test. Txnip expressions of individual mice are also plotted against Tb at the time of tissue collection. KO, knockout (GPR50−/−).
The microarray study revealed an approximate 30-fold lower expression of the transcript encoding Hprt1 in Gpr50−/− mice compared with WT animals, regardless of feeding condition (Supplemental Table 1). This was confirmed by quantitative RT-PCR (qRT-PCR), demonstrating minimal expression of Hprt1 in the hypothalamus of Gpr50−/− mice (Supplemental Figure 1A). The magnitude of this repression, and the fact Hprt1 and Gpr50 are both X linked, suggested that the Hprt1 locus may have been disrupted during the original gene-trap targeting of the Gpr50 locus by DeltaGen (San Carlos, California) (22) and that its disrupted expression could contribute to the expression of torpor in these mice. To rule out this possibility, a second line of Gpr50−/− mice was obtained (Organon Pharmaceuticals, Motherwell, United Kingdom). Analysis of hypothalamic Hprt1 expression by qRT-PCR in this line of Gpr50−/− mice showed no difference compared with WT animals (Supplemental Figure 1B), indicating that reduced expression of Hprt1 in DeltaGen Gpr50−/− mice was not a consequence of the loss of Gpr50. Critically, both lines of Gpr50−/− mice exhibit torpor upon fasting, with a comparable depth and temporal profile (Supplemental Figure 1D). Furthermore, monitoring of targeted Hprt1 knockout mice (Hrpt1−/−) (23) by remote telemetry showed that the thermogenic response of these animals to fasting did not differ from that of WT littermates (Supplemental Figure 1C).
Txnip expression is increased in the hypothalamus during torpor
Microarray analysis of hypothalamic tissue revealed Txnip as the most increased transcript in fasted male Gpr50−/− mice (midtorpor bout) vs WT expression levels (Supplemental Table 1). No difference was detected by the array between ad libitum-fed WT and Gpr50−/− mice. This was confirmed by qRT-PCR in 2 lines of Gpr50−/− mice (Figure 1B and Supplemental Figure 1E). The association of Txnip expression with torpor was not dependent on the sex of the mice, because significantly increased Txnip expression was also observed in female Gpr50−/− mice in torpor (P < .01) (Supplemental Figure 2). Within the group of fasted females, 1 mouse did not enter, or had aroused from torpor before killing, and Txnip levels in the tissues of this individual remained comparable with those that were ad libitum fed. This suggests that the torpid state per se drives Txnip expression.
In situ hybridization on murine brain sections was employed to determine sites of altered Txnip expression. Within the hypothalamus, marked Txnip expression was observed in the ependymal cells lining the third ventricle, alongside general punctuate expression throughout the parenchyma. Elsewhere in the brain, Txnip localized to the lateral ventricles and choroid plexus (Figure 1C). No hybridization signal was observed in Txnip−/− mice or when sense riboprobe was used. Quantification of Txnip expression by measuring OD of autoradiographic films within the ependymal region of the third ventricle and the parenchyma of the hypothalamus (whole hypothalamic area minus the ependymal region), showed that Txnip expression was comparable between genotypes when mice were fed ad libitum (Figure 1D). Within the ependyma lining the third ventricle, fasting caused an increase in Txnip expression in both genotypes (mean OD [arbitrary units] WT fed: 7.16, WT fast: 11.35, P < .05; Gpr50−/− fed: 6.19, Gpr50−/− fast: 23.04, P < .001), but induction of Txnip in Gpr50−/− mice was more than double that observed in the WT mice (P < .05). A similar pattern was observed in the parenchyma of the hypothalamus. Strikingly, in all of our studies, the expression of Txnip reflected Tb at the time of collection, demonstrating increasing expression with decreasing Tb.
Paralleling changes in mRNA, Western blot analyses showed protein levels of Txnip were comparable between genotypes in the fed state, and in response to fasting, levels of Txnip significantly increased in the hypothalami of Gpr50−/− mice (n = 4/group, P < .01) (Figure 1E). Immunohistochemical analysis confirmed that Txnip protein in the murine hypothalamus was limited to the ependymal cells lining the third ventricle (Figure 1F). The morphology and anatomical location of the staining is suggestive of ciliated ependymal cells, rather than tanycytes (24, 25). Again, ependymal expression was lost in Txnip−/− mouse tissue, and immunoreactivity observed in blood vessels and the median eminence was due to nonspecific labeling by the antimouse IgG secondary antibody.
Txnip expression is globally responsive to altered energy status
Txnip demonstrates widespread peripheral expression (Supplemental Figure 3). Therefore, we assessed whether torpor-induced overexpression was specific to the hypothalamus by measuring Txnip transcript levels in metabolically important tissues in WT and Gpr50−/− mice. In WT mice, a significant change in Txnip expression with fasting was observed in brown adipose tissue (BAT) (P < .05) but not in the liver or white adipose tissue (WAT) (Figure 2). In contrast, Txnip expression was significantly increased by fasting in Gpr50−/− mice in all tissues studied (P < .01, WAT and BAT; P < .001, liver). Interestingly, as in the hypothalamus, Txnip reflected the Tb of Gpr50−/− animals at the time of tissue collection, showing higher expression with lower Tb. A similar pattern was observed in the liver, WAT, and BAT of female Gpr50−/− mice (Supplemental Figure 3). The global change in Txnip expression in torpid Gpr50−/− mice suggests that it is a necessary adaptation during torpor rather than a trigger per se for this hypometabolic phenotype.
Figure 2.
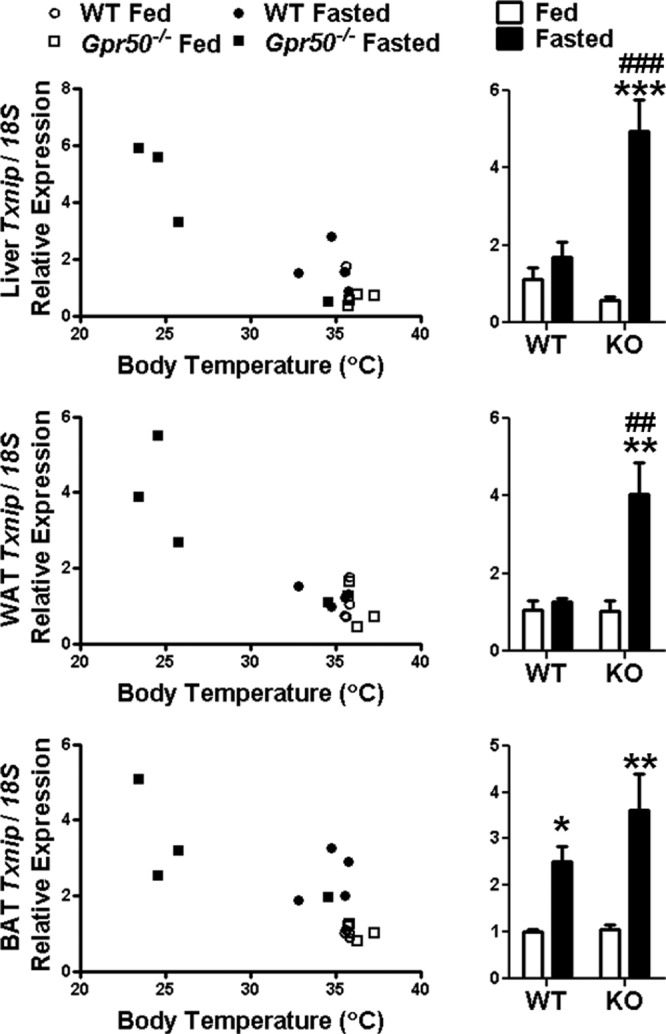
Peripheral expression of Txnip during torpor. qRT-PCR analysis of Txnip expression in peripheral tissues of fed and fasted WT and Gpr50−/− mice revealed that fasting did not alter Txnip expression in WT mice in liver or WAT but significantly increased expression in BAT. In Gpr50−/− mice, Txnip expression was significantly increased by fasting in all tissues. qRT-PCR data are normalized to mouse 18S rRNA control and fold change relative to WT fed animals. Data shown are mean ± SEM; **P < .01, ***P < .001 fasted vs fed; ##P < .01, ###P < .001 Gpr50−/− vs WT. Statistical significance was determined using 2-way ANOVA with Bonferroni's post hoc test. Txnip expressions of individual mice are also plotted against Tb at the time of tissue collection. KO, knockout (GPR50−/−).
We tested Txnip−/− mice during a 24-hour fast. Despite decreased Tb (Txnip−/−, 35.3 ± 0.2°C; WT, 37.0 ± 0.3°C; P < .01) and hypoglycemia (fasted Txnip−/− mean plasma glucose, 45.8 ± 2.7 mg/dL; WT, 90.5 ± 5.4 mg/dL; q < .001), mice did not enter torpor.
Driving Txnip response in WT mice
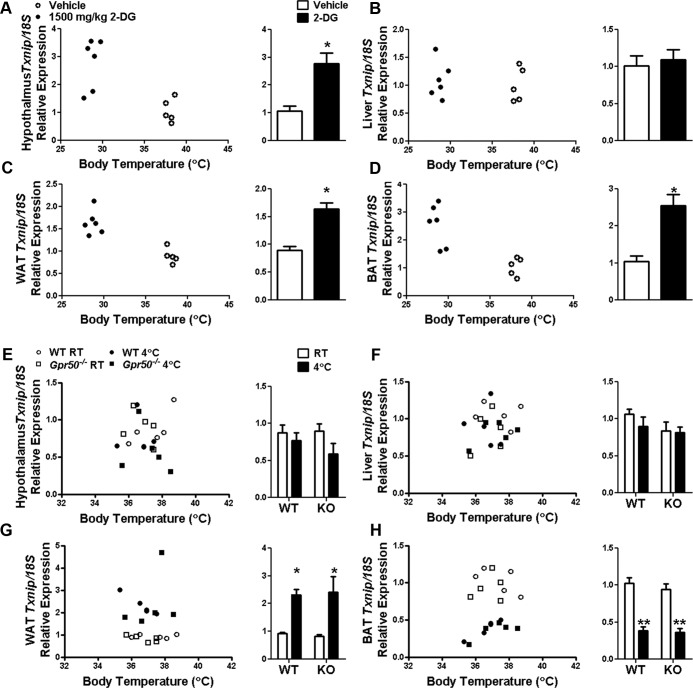
The above results indicate that Txnip is particularly responsive to the hypometabolic and hypothermic conditions of torpor. Torpor-like drops in metabolic rate and Tb in response to the glucose mimetic 2-deoxyglucose (2DG) have been reported in hamsters (26) and mice (1). Therefore, we tested whether increased Txnip expression could be driven in WT mice by 2DG. After 2DG treatment, we observed significantly increased Txnip expression in the hypothalamus, WAT, and BAT (P < .05) but not the liver (Figure 3, A–D). This lack of response in the liver to 2DG-induced hypothermia demonstrates that the induction of Txnip expression during fasting-induced torpor is not simply a passive response to Tb reduction.
Figure 3.
Tissue-specific regulation of Txnip expression after energetic challenge. qRT-PCR analysis of Txnip expression in the tissues of WT mice treated with 1500-mg/kg 2DG for 1 hour showed significantly increased Txnip expression in the hypothalamus (A), WAT (C), and BAT (D) but no change in the liver (B). qRT-PCR analysis of Txnip expression in the tissues of WT and Gpr50−/− mice exposed to 4°C for 2 hours or maintained at 20°C–22°C (RT, room temperature) revealed that although cold challenge did not alter the expression of Txnip in the hypothalamus or liver (E and F), expression was significantly increased in WAT (G) and decreased in BAT (H). Data are normalized to mouse 18S rRNA control and fold change relative to vehicle-treated animals (A–D) or RT-housed WT animals (E–H). Data shown are mean ± SEM; *P < .05, **P < .01 Student's t test or 2-way ANOVA with Bonferroni's post hoc test. Txnip expressions of individual mice are also plotted against Tb at the time of tissue collection. KO, knockout (GPR50−/−).
WT female mice will exhibit torpor in response to combined fasting and reduced ambient temperature (27, 28). The influence of low ambient temperature on expression of Txnip was therefore evaluated. WT and Gpr50−/− mice were acutely exposed to 4°C for 2 hours. Cold challenge did not alter Txnip expression in the murine hypothalamus or liver but significantly increased expression in WAT and significantly decreased expression in BAT of both genotypes (P < .05, WAT; P < .01, BAT) (Figure 3, E–H). Specific regulation of Txnip expression in these tissues during fasting-induced torpor and cold exposure may reflect the distinct metabolic activities of these tissues during these challenges.
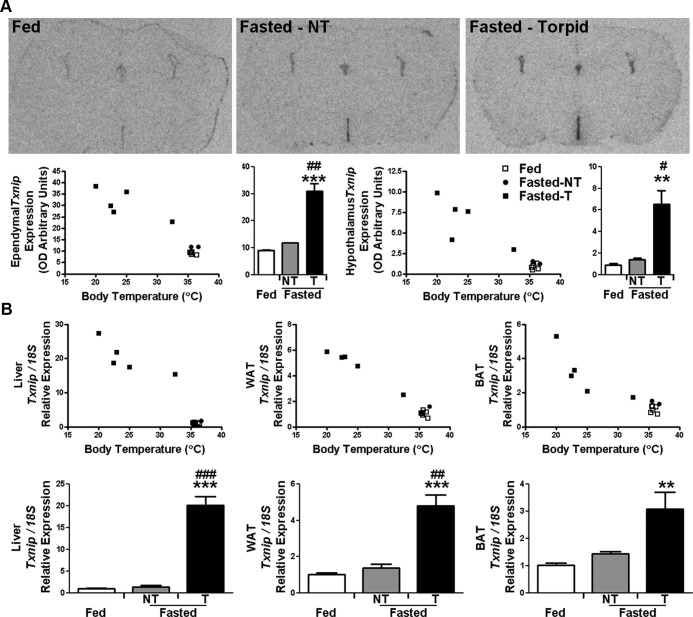
Transcript levels of Txnip were examined by in situ hybridization in the brains of female WT mice acclimated to 16°C ambient temperature for 2 days and then fasted for 48 hours to induce torpor. Of the 8 fasted females, 6 entered torpor as assessed by indirect calorimetry (oxygen consumption < 1000 ml/h·kg) at time of killing. Induction of torpor caused a profound elevation in Txnip expression within the ependymal cells lining the third ventricle, compared with ad libitum-fed and fasted mice that did not exhibit torpor (P < .001 torpid fasted vs fed; P < .01 torpid fasted vs normothermic fasted) (Figure 4A). A similar pattern was observed in the parenchyma of the hypothalamus. Examination of Txnip expression in liver, WAT, and BAT also revealed substantial increases in torpid mice, with no significant increases in normothermic fasted animals (torpid fasted vs fed: P < .001, liver and WAT and P < .01, BAT) (Figure 4B). The magnitude of increased Txnip expression in the tissues of WT mice induced to enter torpor was comparable with the levels demonstrated in torpid Gpr50−/− mice.
Figure 4.
Txnip expression is increased in the tissues of WT mice during torpor. (A) Txnip expression in the brain of ad libitum-fed and 48-hour fasted WT female mice housed at 16°C was examined by in situ hybridization (n = 6/group; 2 fasted normothermic [NT] mice). Induction of torpor by fasting caused a significant elevation in Txnip expression within the hypothalamus, compared with fed and NT fasted mice. Txnip expressions of individual mice are plotted against Tb at the time of tissue collection. (B) qRT-PCR analysis of Txnip expression in the peripheral tissues of ad libitum-fed and fasted WT female mice housed at 16°C again showed that induction of torpor by fasting caused a significant elevation of Txnip expression compared with ad libitum-fed and fasted NT mice. qRT-PCR data shown are normalized to mouse 18S rRNA control and fold change relative to WT fed animals. Data shown are mean ± SEM; **P < .01, ***P < .001 fasted vs fed; #P < .05, ##P < .01, ###P < .001 torpid vs normothermic. Statistical significance was determined using 2-way ANOVA with Bonferroni's post hoc test.
Hypothalamic Txnip expression is induced in the Siberian hamster during torpor
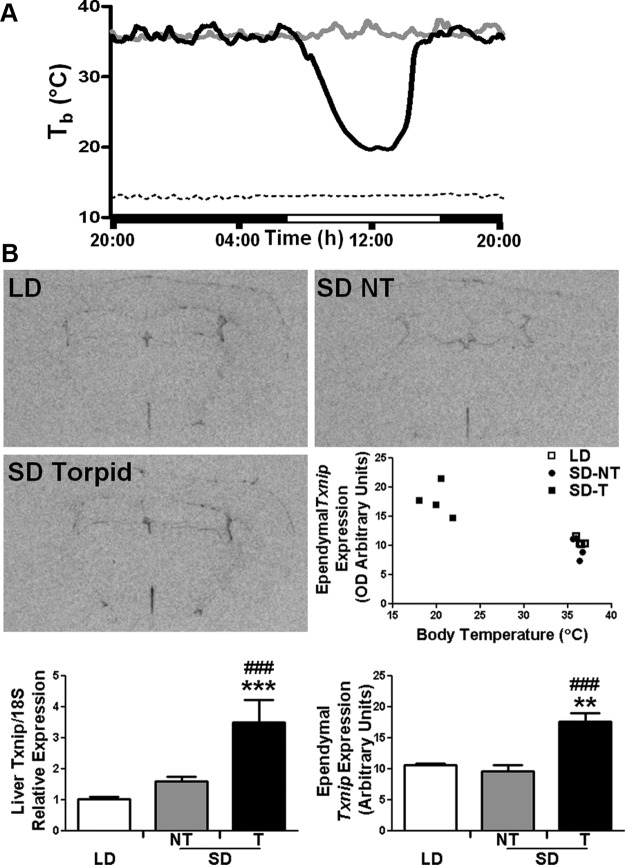
In the previous experiments, torpor was induced in mice by fasting, with Txnip expression showing similar changes in WT and Gpr50−/− mice driven into torpor. To assess whether increased Txnip is directly linked to torpor per se, we investigated a seasonal model of torpor, the Siberian hamster (29). Siberian hamster Txnip was cloned and used to generate riboprobe for in situ hybridization to measure expression in the brains of male animals housed on artificial long and natural winter (short) photoperiods, during which the hamsters exhibit spontaneous daily bouts of torpor (Figure 5A). Torpor in short photoperiod-housed hamsters was assessed by Tb at the time of killing. Txnip expression showed no difference in expression with photoperiod, with similar levels in the ependymal layer of the third ventricle of hamsters exposed to long days (LDs) and normothermic short-day (SD) animals (Figure 5B). However, torpid SD hamsters exhibited a significant elevation in Txnip expression within the ependyma lining the third ventricle (P < .01 SD torpid vs LD; P < .001 SD torpid vs SD normothermic). Txnip expression was also measured in the livers of these animals by qRT-PCR. As in the hypothalamus, there were no differences in expression between LD and normothermic SD animals. However, in torpid SD hamsters, Txnip expression was significantly increased (P < .001 SD torpid vs LD; P < .001 SD torpid vs SD normothermic).
Figure 5.
Txnip expression is increased in the tissues of Siberian hamsters during torpor. (A) Representative Tb recordings of Siberian hamsters maintained under a SD photoperiod and temperature, during which some hamsters spontaneously exhibit daily torpor (black line) or remain normothermic (gray line). Dashed line shows ambient temperature. (B) Txnip expression in the brains of Siberian hamsters housed in different photoperiods (LDs vs SDs, during which hamsters entered torpor [T] or remained normothermic [NT]), was examined by in situ hybridization (n = 4/group). Induction of torpor in SD-housed hamsters caused significant elevation in Txnip expression within the ependymal layer of cells lining the third ventricle, compared with LD-housed and NT-SD animals. Txnip expression in liver was also measured by qRT-PCR, and as in the hypothalamus, Txnip was significantly increased in torpid vs LD and NT-SD animals. Data shown are mean ± SEM; **P < .01, ***P < .001 LD vs SD; #P < .05, ##P < .01, ###P < .001 SD torpid vs NT-SD. Statistical significance was determined using 2-way ANOVA with Bonferroni's post hoc test.
Discussion
The present study revealed a strong association of Txnip with the expression of torpor. Txnip was markedly up-regulated in the tissues of 2 murine models of torpor: Gpr50−/− and female WT mice, driven into torpor by fasting alone or prolonged fasting at reduced ambient temperature, respectively. Txnip expression was also increased in the hypothalamus of an additional species, the Siberian hamster, during naturally occurring seasonal torpor. That a similar induction of Txnip is observed in different species is intriguing and suggests a fundamental role for this protein in the animal's ability to use torpor. Up-regulation of Txnip occurred only in animals (mice or hamsters) during the torpor bout, with basal levels maintained in normothermic animals under torpor-promoting conditions.
Within the hypothalamus, Txnip is strongly expressed in the ependymal layer of cells lining the third ventricle. The proposed function of Txnip as a molecular nutrient sensor, capable of directing fuel use, means that it is ideally placed to influence hypothalamic circuits that gate metabolic and thermogenic responses to changing energy status. A pivotal role for hypothalamic Txnip in the regulation of whole-body energy homeostasis has previously been highlighted (21, 30). These studies show that selective modulation of Txnip expression in the murine medio-basal hypothalamus altered adipose tissue metabolism, fuel partitioning, and glucose homeostasis, adjusting overall metabolic rate (increased Txnip levels down-regulated energy expenditure; decreased Txnip levels promoted energy expenditure). Therefore, increased hypothalamic Txnip expression during torpor could be crucial to reduce whole-body energy expenditure (and Tb), necessary to perpetuate the torpid state. It must be noted that Blouet and Schwartz (21) and Blouet et al (30) report neuronal localization of Txnip in their studies. We do not observe similar profiles, despite using the same antibody, but critically, we confirm specificity of ependymal Txnip expression using Txnip−/− mice. In true hibernators, animals enter a prolonged deep torpor over several days (Tb ∼ 5°C), interspersed with brief arousal periods to normothermy of just a few hours. A recent study has used RNA-sequencing to profile gene expression changes in hibernating ground squirrels, contrasting hypothalamic transcript changes during deep torpor and interbout arousals (31). This revealed significant up-regulation of Txnip in the hypothalamus during torpor, which declined during the interbout period of normothermy. These data from a natural hibernator further support the hypothesis that Txnip is a central feature of metabolic homeostasis within the brain during prolonged periods of hypothermia.
As well as playing a key role in regulating cellular metabolism, Txnip is a multifunctional protein, which is induced by diverse stress stimuli, and impacts on a variety of cellular processes, including cell proliferation, redox regulation, and inflammatory responses (32–34). As previously shown (21), Txnip expression is activated by fasting in WT mice. However, it is induced to a much greater extent in torpid animals. Because mice lacking Txnip die during a prolonged fast (35), Txnip may offer some protective function during hypometabolism that is critical to the ability of mice to use or survive the extreme hypometabolic state experienced during torpor.
Txnip is intimately linked to glycolysis and mitochondrial oxidative phosphorylation through the glucose-sensing transcriptional complex MondoA:max-like protein X (Mlx) (36, 37). This heterodimeric complex is activated by intracellular glucose flux and regulates metabolic targets involved in glycolysis and lipogenesis by binding to target gene promoters containing carbohydrate-response elements (38). Under conditions of decreased glycolysis, or in response to accumulating glycolytic metabolites (particularly glucose-6-phosphate [G6P]), MondoA:Mlx translocates to the nucleus and drives the transcription of Txnip, which feeds back to reduce cellular glucose uptake, enabling cells to efficiently regulate glucose homeostasis (36). During torpor, levels of metabolic activity are globally suppressed (39), and plasma glucose levels are reduced (11, 40), which implies a decrease in cellular glycolytic rate. In such a state, Txnip induction would feed back to inhibit unnecessary glucose influx. Further, during torpor, animals switch from using glucose as a fuel source and instead rely on the metabolism of FAs (11). Alongside inhibiting cellular glucose uptake and metabolism, Txnip promotes FA use (35). Therefore, its elevated expression during torpor may be crucial to resetting cellular metabolism. Here, we show that although Txnip−/− mice become profoundly hypoglycaemic in response to 24 hours of fasting, with plasma glucose levels falling below those demonstrated in fasted Gpr50−/− mice (1), they fail to use torpor as an energy saving strategy.
The tissue-specific regulation of Txnip expression in response to various physiological challenges reflects the relationship between Txnip and cellular metabolism. Treatment of WT mice with 2DG increased expression of Txnip in all tissues studied, with the exception of the liver, where levels of Txnip remained unchanged. 2DG is a stable glucose analog, which upon transport into cells becomes phosphorylated by hexokinase but cannot be further metabolized. Accumulating 2DG6P inhibits glycolytic enzymes via negative feedback mechanisms (41), blocking glycolysis, and presumably activating MondoA:Mlx-mediated Txnip expression. However, liver possesses significant glucose-6-phosphatase activity and so trapped 2DG6P can be dephosphorylated and returned to the blood (42), relieving inhibition of glycolytic enzymes. Further, glucokinase, a variant of hexokinase found in the liver, is not subject to product inhibition by G6P (or 2DG6P) (43), so it will continue to metabolize glucose, preventing accumulation of glycolytic intermediates and MondoA:Mlx-driven Txnip transcription.
The tight link between Txnip and cellular metabolism is further demonstrated by tissue-specific regulation of its expression in response to cold challenge. In mice acutely exposed to low ambient temperature, levels of Txnip remained unchanged in the hypothalamus and liver but were significantly increased in WAT and decreased in BAT. During cold exposure, sympathetic nervous system drive to both adipose tissues is increased, leading to elevated lipolysis in WAT and nonshivering thermogenesis in BAT. Thus, increased oxidative phosphorylation in WAT and an accompanied decrease in glycolytic flux presumably leads to activation of MondoA:Mlx-driven Txnip expression, which reciprocally functions to limit glucose uptake. On the other hand, in BAT, nonshivering thermogenesis sees energy production uncoupled from oxidative phosphorylation, and cells rely on increased glycolytic rates to meet the cell's energy requirements (44), conversely resulting in reduced MondoA:Mlx-driven Txnip transcription, leading to increased glucose uptake by BAT. Specific induction of Txnip in BAT of WT mice during fasting may again reflect metabolic activity within this tissue. Fasting reduces sympathetic drive to BAT to prevent energy wasteful heat production and results in decreased glucose uptake and reduced rates of glycolysis (45), likely driving Txnip expression, which then further restricts glucose uptake. Indeed, it may be imperative to induce Txnip expression in BAT early during a fast as a means to limit glucose uptake, glycolysis, and thermogenic activities of this tissue. Regulation of glucose uptake and metabolic output of tissues will also be pivotal to direct the rapid changes in metabolic rate and temperature during entry into and arousal from torpor. Changes in Txnip expression during the torpor bout could be an essential mechanism through which these precise metabolic shifts are controlled.
In summary, we have shown Txnip to be consistently up-regulated during fasting-induced and natural torpor. Txnip expression is switched on during fasting and increased substantially during the pronounced hypometabolism of torpor. Together with recent studies highlighting the influence of Txnip on energy balance, our results suggest that hypothalamic Txnip expression is an important pathway for directing whole-body metabolism in response to changing energy status. Further, augmented Txnip expression in peripheral tissues in response to torpor may constitute an essential protective measure to deal with major shifts in energy supply and fuel type.
Materials and Methods
Animal maintenance
Experimental procedures were licensed under the Animals Act, 1986, and local animal welfare committee. Gpr50−/− mice were generated by DeltaGen and obtained via AstraZeneca (Alderley Park, Cheshire, United Kingdom). A second colony of Gpr50−/− mice was obtained from Organon Pharmaceuticals, although their use was limited to studies shown in Supplemental Figure 1. Adult male mice maintained in a 12-hour light, 12-hour dark lighting schedule and housed at an ambient temperature of 20°C–22°C were used for all experiments, unless stated otherwise. Standard rodent chow and water were supplied ad libitum, except during fasting periods, in which all food was removed (for experiments requiring the induction of torpor in Gpr50−/− mice, WT and Gpr50−/− mice were typically fasted for approximately 16 h before killing; for studies shown in Figure 3, WT female mice were fasted for 48 h). Tissue was collected at ZT 2 (selected because fasted Gpr50−/− mice are in deep torpor at this time).
Hprt1−/− mice were derived from a line carrying a deletion mutation of the first 2 exons of the Hprt1 gene (23) and maintained congenically with C57BL/6J mice (The Jackson Laboratory, Bar Harbor, Maine) for more than 20 generations. Animals were maintained on a 14-hour light, 10-hour dark cycle with food and water ad libitum, except during fasting periods when all food was removed for 24 hours.
Generation of Txnip−/− mice was described previously (20). Progeny were backcrossed to C57BL/6 mice for ten generations. Animals were kept in a 12-hour light, 12-hour dark cycle and fed standard rodent chow ad libitum unless otherwise stated.
Adult male Siberian hamsters (Phodopus sungorus), wild trapped from Siberia, were subsequently bred at the University of Veterinary Medicine Hanover. Winter phenotype hamsters were housed outdoors, exposed to natural SD photoperiod and temperature (∼8 h light, 4.9 ± 0.4°C). A parallel set of hamsters were maintained indoors under an artificial LD light cycle (16 h light, 8 h dark) and ambient temperature (∼18°C). All animals were individually housed and given ad libitum access to food and water, tissue was collected at ZT 5.
Indirect calorimetry, 2DG administration, and cold chamber
Metabolic rate was measured using indirect calorimetric cages (Columbus Instruments, Columbus, Ohio). 2DG (1500 mg/kg; Sigma, Gillingham, United Kingdom) or saline vehicle was administered as a bolus injection, ip for 1 hour, during which time food was removed from the cages. Tb was measured using a rectal thermometer at the time of killing. For cold challenge, mice were individually housed in a modified upright 1200 L industrial fridge (Polar Refrigeration, Northampton, United Kingdom), adapted to include an Akor ventilation unit (Akor Systems, Rosny sous Bois, France) to allow an internal-external air exchange to meet home office requirements, and exposed to 4°C for 2 hours.
Micorarray procedures
Coronal sections of whole brain were cut using a vibratome (Campden Instruments, Lafayette, Indiana) up until the level of the suprachiasmatic nucleus. A 1.5-mm slice was then taken to include the whole hypothalamus, ending at the caudal edge of the dorsomedial nucleus. Blocks were trimmed to remove tissue dorsal to the hypothalamus and laterally adjacent to the optic tract. Tissue was homogenized in TRIzol reagent (Invitrogen, Carlsbad, California) using lysing matrix D tubes (MP Biomedicals, Solon, Ohio), and total RNA was isolated according to the manufacturer's specifications. cDNA was produced, labeled, and hybridized using Affymetrix Mouse Exon 1.0 ST Array (Genomic Technologies Core Facility, University of Manchester, Manchester, United Kingdom). Microarray data were processed and analyzed using Partek Genomics Solution (version 6.5). Probesets were quantile normalized, and robust multichip average background correction was applied. Exons were summarized to genes by calculating the mean of the exons. Differential expression was calculated by 2-way ANOVA, and correction for false discovery rates was done using the method of q value (Bioinformatics Core Facility, University of Manchester).
In situ hybridization and quantitative real-time PCR
Brains were frozen whole (in situ) or tissue blocks immediately dissected and frozen (qRT-PCR). For in situ, brains were sectioned (12 μm) using a cryostat freezing microtome. Rostral to caudal coronal sections of the entire hypothalamus were taken from each animal starting from the emergence of the suprachiasmatic nucleus to the end of the dorsomedial nucleus. Representative sections throughout the hypothalamus from each animal were then analyzed to ensure inclusion of all anatomical levels. Primers used for generating in situ riboprobes were forward (F), 5′-GCCGCTCGAGCATGAGGCCTGGAAACAAAT-3′ and reverse (R), 5′-CGGCGAATTCGCCATTGGCAAGGTAAGTGT-3′; and amplifying mouse transcripts by qRT-PCR were Txnip F, 5′-CATGAGGCCTGGAAA-CAAAT-3′ and R, 5′-ACTGGTGCCATTAGGTCAGG-3′; Hprt1 Mm_Hprt1_1_SG (QIAGEN, Valencia, California), 18S F, 5′-TCCGACCATAAACGATGCCGACT-3′ and R, 5′-TCCTGGTGGTGCCCTTCCGTCAAT-3′. Murine and Siberian hamster Txnip probes were cloned into pcDNA3, synthesized in the presence of 33P-uridine triphosphate (MP Biomedicals), and hybridization visualized by film autoradiography (Kodak BioMax MR film; Kodak, New York, New York). OD was determined using 5-10 sections per animal. qRT-PCR was performed using the SYBR Green PCR Master Mix (QIAGEN) and an Applied Biosystems 7500 Real-Time PCR system (Applied Biosystems, Foster City, California). Mouse housekeeping gene 18S rRNA was used as an internal control.
Immunohistochemistry
Brains were removed and fixed for 48 hours in 4% paraformaldehyde, equilibrated to sucrose, and frozen for sectioning. Sections (20 μm) were blocked with serum followed by incubation with mouse anti-Txnip (1:200, JY2; MBL International, Woburn, Massachusetts). Tissue was then incubated with fluorescein isothiocyanate-conjugated donkey IgG secondary antibody (1:400; Jackson ImmunoLabs, Suffolk, United Kingdom), before being mounted onto slides and coverslipped using Vectashield Hard Set Mounting Medium with 4′,6-diamidino-2-phenylindole. Fluorescent images were collected using an Olympus BX51 upright widefield microscope (Olympus, New York, New York) and a Coolsnap ES camera (Photometrics, Tucson, Arizona).
Immunoblot analysis
Hypothalamic blocks were homogenized in Tissue Protein Extraction reagent (Thermo Scientific, Rockford, Illinois) using lysing matrix D tubes (MP Biomedicals). Protein concentration was measured with a BCA protein quantification kit (Pierce Biotechnology, Rockford, Illinois). Eighty micrograms of protein were subject to SDS-PAGE using Mini-PROTEAN 3 apparatus (Bio-Rad, Hercules, California), according to manufacturer's instructions, before transfer to nitrocellulose membrane using an iBlot gel transfer system (Invitrogen). Immunoblots were incubated with mouse anti-Txnip (1:1000, JY2; MBL International) or mouse α-β-actin monoclonal antibody (1:5000; Abcam, Cambridge, Massachusetts), followed by horseradish peroxidase-linked sheep antimouse IgG (GE Healthcare, Princeton, New Jersey). Immunoblots were imaged using the enhanced chemiluminescence Amersham advanced detection system (GE Healthcare) and quantitated using SigmaScan software.
Statistical analysis
Data are presented as mean ± SEM. The statistical test used is specified in each data section. Student's t test was used when only two groups were studied; one-way ANOVA was used when more than two groups were studied and only one factor investigated, followed by post hoc analysis; two-way ANOVA was used when more than two factors were analyzed, followed by post hoc analysis.
Supplementary Material
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) (United Kingdom). L.H. was supported by a studentship funded by the BBSCR and Astraeneca. S.H. was funded by the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 1970
- BAT
- brown adipose tissue
- 2DG
- 2-deoxyglucose
- F
- forward
- FA
- fatty acid
- G6P
- glucose-6-phosphate
- Hprt1
- Hypoxanthine guanine phosphoribosyl transferase 1
- LD
- long day
- Mlx
- Max-like protein x
- qRT-PCR
- quantitative RT-PCR
- R
- reverse
- SD
- short day
- Tb
- body temperature
- Txnip
- thioredoxin-interacting protein
- WAT
- white adipose tissue
- WT
- wild type
- ZT
- Zeitgeber time.
References
- 1. Bechtold DA, Sidibe A, Saer BR, et al. A role for the melatonin-related receptor GPR50 in leptin signaling, adaptive thermogenesis, and torpor. Curr Biol. 2012;22:70–77 [DOI] [PubMed] [Google Scholar]
- 2. Dufourny L, Levasseur A, Migaud M, et al. GPR50 is the mammalian ortholog of Mel1c: evidence of rapid evolution in mammals. BMC Evol Biol. 2008;8:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ivanova EA, Bechtold DA, Dupré SM, et al. Altered metabolism in the melatonin-related receptor (GPR50) knockout mouse. Am J Physiol Endocrinol Metab. 2008;294:E176–E182 [DOI] [PubMed] [Google Scholar]
- 4. Grünewald E, Kinnell HL, Porteous DJ, Thomson PA. GPR50 interacts with neuronal NOGO-A and affects neurite outgrowth. Mol Cell Neurosci. 2009;42:363–371 [DOI] [PubMed] [Google Scholar]
- 5. Thomson PA, Wray NR, Thomson AM, et al. Sex-specific association between bipolar affective disorder in women and GPR50, an X-linked orphan G protein-coupled receptor. Mol Psychiatry. 2005;10:470–478 [DOI] [PubMed] [Google Scholar]
- 6. Macintyre DJ, McGhee KA, Maclean AW, et al. Association of GPR50, an X-linked orphan G protein-coupled receptor, and affective disorder in an independent sample of the Scottish population. Neurosci Lett. 2010;475:169–173 [DOI] [PubMed] [Google Scholar]
- 7. Delavest M, Even C, Benjemaa N, Poirier MF, Jockers R, Krebs MO. Association of the intronic rs2072621 polymorphism of the X-linked GPR50 gene with affective disorder with seasonal pattern. Eur Psychiatry. 2012;27:369–371 [DOI] [PubMed] [Google Scholar]
- 8. Ruby NF. Paraventricular nucleus ablation disrupts daily torpor in Siberian hamsters. Brain Res Bull. 1995;37:193–198 [DOI] [PubMed] [Google Scholar]
- 9. Ruby NF, Ibuka N, Barnes BM, Zucker I. Suprachiasmatic nuclei influence torpor and circadian temperature rhythms in hamsters. Am J Physiol. 1989;257:R210–R215 [DOI] [PubMed] [Google Scholar]
- 10. Nestler JR. Relationships between respiratory quotient and metabolic rate during entry to and arousal from daily torpor in deer mice (Peromyscus maniculatus). Physiol Zool. 1990;63:504–515 [Google Scholar]
- 11. Heldmaier G, Klingenspor M, Werneyer M, Lampi BJ, Brooks SP, Storey KB. Metabolic adjustments during daily torpor in the Djungarian hamster. Am J Physiol. 1999;276:E896–E906 [DOI] [PubMed] [Google Scholar]
- 12. Nestler JR. Metabolic substrate change during daily torpor in deer mice. Canad J Zool. 1991;69:322–327 [Google Scholar]
- 13. Dark J, Lewis DA, Zucker I. Hypoglycemia and torpor in Siberian hamsters. Am J Physiol. 1999;276:R776–R781 [DOI] [PubMed] [Google Scholar]
- 14. Atgie C, Nibbelink M, Ambid L. Sympathoadrenal activity and hypoglycemia in the hibernating garden dormouse. Physiol Behav. 1990;48:783–787 [DOI] [PubMed] [Google Scholar]
- 15. Brown JC, Gerson AR, Staples JF. Mitochondrial metabolism during daily torpor in the dwarf Siberian hamster: role of active regulated changes and passive thermal effects. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1833–R1845 [DOI] [PubMed] [Google Scholar]
- 16. Brown JC, Staples JF. Mitochondrial metabolism during fasting-induced daily torpor in mice. Biochim Biophys Acta. 2010;1797:476–486 [DOI] [PubMed] [Google Scholar]
- 17. Nishiyama A, Matsui M, Iwata S, et al. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem. 1999;274:21645–21650 [DOI] [PubMed] [Google Scholar]
- 18. Sheth SS, Castellani LW, Chari S, et al. Thioredoxin-interacting protein deficiency disrupts the fasting-feeding metabolic transition. J Lipid Res. 2005;46:123–134 [DOI] [PubMed] [Google Scholar]
- 19. Chutkow WA, Birkenfeld AL, Brown JD, et al. Deletion of the α-arrestin protein Txnip in mice promotes adiposity and adipogenesis while preserving insulin sensitivity. Diabetes. 2010;59:1424–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hui ST, Andres AM, Miller AK, et al. Txnip balances metabolic and growth signaling via PTEN disulfide reduction. Proc Natl Acad Sci USA. 2008;105:3921–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blouet C, Schwartz GJ. Nutrient-sensing hypothalamic TXNIP links nutrient excess to energy imbalance in mice. J Neurosci. 2011;31:6019–6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barrett P, Ivanova E, Graham ES, et al. Photoperiodic regulation of cellular retinol binding protein, CRBP1 [corrected] and nestin in tanycytes of the third ventricle ependymal layer of the Siberian hamster. J Endocrinol. 2006;191:687–698 [DOI] [PubMed] [Google Scholar]
- 23. Hooper M, Hardy K, Handyside A, Hunter S, Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987;326:292–295 [DOI] [PubMed] [Google Scholar]
- 24. Mathew TC. Regional analysis of the ependyma of the third ventricle of rat by light and electron microscopy. Anat Histol Embryol. 2008;37:9–18 [DOI] [PubMed] [Google Scholar]
- 25. Rodríguez EM, Blázquez JL, Pastor FE, et al. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol. 2005;247:89–164 [DOI] [PubMed] [Google Scholar]
- 26. Dark J, Miller DR, Zucker I. Reduced glucose availability induces torpor in Siberian hamsters. Am J Physiol. 1994;267:R496–R501 [DOI] [PubMed] [Google Scholar]
- 27. Swoap SJ, Gutilla MJ, Liles LC, Smith RO, Weinshenker D. The full expression of fasting-induced torpor requires β3-adrenergic receptor signaling. J Neurosci. 2006;26:241–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oelkrug R, Heldmaier G, Meyer CW. Torpor patterns, arousal rates, and temporal organization of torpor entry in wildtype and UCP1-ablated mice. J Comp Physiol B. 2011;181:137–145 [DOI] [PubMed] [Google Scholar]
- 29. Ruf T, Klingenspor M, Preis H, Heldmaier G. Daily torpor in the Djungarian hamster (Phodopus sungorus): interactions with food intake, activity, and social behaviour. J Comp Physiol B. 1991;160:609–615 [Google Scholar]
- 30. Blouet C, Liu SM, Jo YH, Chua S, Schwartz GJ. TXNIP in Agrp neurons regulates adiposity, energy expenditure, and central leptin sensitivity. J Neurosci. 2012;32:9870–9877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwartz C, Hampton M, Andrews MT. Seasonal and regional differences in gene expression in the brain of a hibernating mammal. PLoS One. 2013;8:e58427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim SY, Suh HW, Chung JW, Yoon SR, Choi I. Diverse functions of VDUP1 in cell proliferation, differentiation, and diseases. Cell Mol Immunol. 2007;4:345–351 [PubMed] [Google Scholar]
- 33. Zhou J, Chng WJ. Roles of thioredoxin binding protein (TXNIP) in oxidative stress, apoptosis and cancer. Mitochondrion. 2013;13(3):163–169 [DOI] [PubMed] [Google Scholar]
- 34. Patwari P, Lee RT. An expanded family of arrestins regulate metabolism. Trends Endocrinol Metab. 2012;23:216–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oka S, Liu W, Masutani H, et al. Impaired fatty acid utilization in thioredoxin binding protein-2 (TBP-2)-deficient mice: a unique animal model of Reye syndrome. FASEB J. 2006;20:121–123 [DOI] [PubMed] [Google Scholar]
- 36. Stoltzman CA, Peterson CW, Breen KT, Muoio DM, Billin AN, Ayer DE. Glucose sensing by MondoA:Mlx complexes: a role for hexokinases and direct regulation of thioredoxin-interacting protein expression. Proc Natl Acad Sci USA. 2008;105:6912–6917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu FX, Chai TF, He H, Hagen T, Luo Y. Thioredoxin-interacting protein (Txnip) gene expression: sensing oxidative phosphorylation status and glycolytic rate. J Biol Chem. 2010;285:25822–25830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Havula E, Hietakangas V. Glucose sensing by ChREBP/MondoA-Mlx transcription factors. Semin Cell Dev Biol. 2012;23:640–647 [DOI] [PubMed] [Google Scholar]
- 39. Staples JF, Brown JC. Mitochondrial metabolism in hibernation and daily torpor: a review. J Comp Physiol B. 2008;178:811–827 [DOI] [PubMed] [Google Scholar]
- 40. Nestler JR. Tissue-specific metabolism during normothermy and daily torpor in deer mice (Peromyscus maniculatus). J Exp Zool. 1992;261:406–413 [DOI] [PubMed] [Google Scholar]
- 41. Ralser M, Wamelink MM, Struys EA, et al. A catabolic block does not sufficiently explain how 2-deoxy-D-glucose inhibits cell growth. Proc Natl Acad Sci USA. 2008;105:17807–17811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Young P, Cawthorne MA, Levy AL, Wilson K. Reduced maximum capacity of glycolysis in brown adipose tissue of genetically obese, diabetic (db/db) mice and its restoration following treatment with a thermogenic β-adrenoceptor agonist. FEBS Lett. 1984;176:16–20 [DOI] [PubMed] [Google Scholar]
- 43. Massa ML, Gagliardino JJ, Francini F. Liver glucokinase: an overview on the regulatory mechanisms of its activity. IUBMB Life. 2011;63:1–6 [DOI] [PubMed] [Google Scholar]
- 44. Inokuma K, Ogura-Okamatsu Y, Toda C, Kimura K, Yamashita H, Saito M. Uncoupling protein 1 is necessary for norepinephrine-induced glucose utilization in brown adipose tissue. Diabetes. 2005;54:1385–1391 [DOI] [PubMed] [Google Scholar]
- 45. Shimizu Y, Nikami H, Saito M. Sympathetic activation of glucose utilization in brown adipose tissue in rats. J Biochem. 1991;110:688–692 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.