Abstract
There is substantial evidence that age-related ovarian failure in rats is preceded by abnormal responsiveness of the neuroendocrine axis to estrogen positive feedback. Because IGF-I seems to act as a permissive factor for proper GnRH neuronal response to estrogen positive feedback and considering that the hypothalamic content of IGF-I declines in middle-aged (M-A) rats, we assessed the effectiveness of long-term IGF-I gene therapy in the mediobasal hypothalamus (MBH) of M-A female rats to extend regular cyclicity and preserve ovarian structure. We used 3 groups of M-A rats: 1 group of intact animals and 2 groups injected, at 36.2 weeks of age, in the MBH with either a bicistronic recombinant adeno-associated virus (rAAV) harboring the genes for IGF-I and the red fluorescent protein DsRed2, or a control rAAV expressing only DsRed2. Daily vaginal smears were taken throughout the study, which ended at 49.5 weeks of age. We measured serum levels of reproductive hormones and assessed ovarian histology at the end of the study. Although most of the rats injected with the IGF-I rAAV had, on the average, well-preserved estrous cyclicity as well as a generally normal ovarian histology, the intact and control rAAV groups showed a high percentage of acyclic rats at the end of the study and ovaries with numerous enlarged cysts and scarce corpora lutea. Serum LH was higher and hyperprolactinemia lower in the treated animals. These results suggest that overexpression of IGF-I in the MBH prolongs normal ovarian function in M-A female rats.
There is substantial evidence that age-related ovarian failure in rats is preceded by abnormal responsiveness of the neuroendocrine system to estrogen positive feedback. Thus, middle-aged (M-A), eugonadal rats demonstrate increased FSH serum levels as well as delayed and attenuated LH surges despite normal estrous (E) cycle lengths (1). Changes in the positive feedback effects of estradiol on the LH surge in regularly cycling, M-A rats are not due to primary pituitary dysfunction (2, 3), or reduced estrogen receptor α or progestin receptor expression and/or binding in the hypothalamus (4–6) or the pituitary (7, 8). Instead, available data overwhelmingly point to an impaired hypothalamic response to estradiol positive, but not negative (9, 10), feedback as the etiology of the alterations in LH release typically observed in reproductively aging rodents (11). At the hypothalamic level, the age-related dysfunction of the LH surge is attributable neither to reduced numbers or abnormal morphology of GnRH neurons (12–15) nor to reduced GnRH peptide content, which remains unchanged or even increases with age (3). On the other hand, push-pull perfusion measurements of in vivo GnRH output from the mediobasal hypothalamus (MBH) of M-A rats suggest that GnRH peptide release is attenuated under estradiol positive feedback conditions (16). Moreover, GnRH mRNA may decrease (17), and fewer GnRH neurons express cFos (12, 18), a marker for GnRH neuron activation, in M-A compared with young rats (12, 18). Taken together, the available data suggest that decreased preovulatory GnRH release most likely reflects impaired GnRH neuronal activation under estrogen positive feedback conditions (19). There is solid evidence that M-A rats do not respond to estradiol positive feedback with appropriate modulation of excitatory and inhibitory hypothalamic neurotransmitter release (2, 18, 20–23), which in turn could cause reduced activation of GnRH neurons, reduced GnRH release, and an abnormal LH surge (18, 23). The initial deterioration of the modulation of excitatory and inhibitory hypothalamic neurotransmitter release in M-A female rats may stem from a reduction in the activity of permissive factors like IGF-I, whose content is reduced in the brains of M-A rats (17). Interestingly, it has been reported that intracerebroventricular infusion of IGF-I partially rescues the LH surge in M-A rats (3). IGF-I signaling, presumably in the hypothalamus, is necessary for estradiol positive feedback and may modulate the synthesis or release of kisspeptin or vasointestinal peptide and/or the expression of glutamate receptors (24, 25). Thus, it is possible that reduced hypothalamic IGF-I indirectly affects GnRH neuron activity by disrupting excitatory inputs mediated by glutamate and kisspeptin (26–28).
Besides its permissive role in the modulation of the GnRH system, IGF-I is known to be a powerful neurotrophic molecule, which appears to be part of the physiologic self-repair mechanisms of the adult brain (29). Furthermore, gene therapy for IGF-I has shown promising results in the brain of aging rats. Thus, IGF-I gene therapy in the MBH of senile female rats was highly effective to restore hypothalamic dopaminergic neuron number and correct the chronic hyperprolactinemia associated with depressed tuberoinfundibular dopaminergic neuron function in old female rats (30). In the same animal model, intracerebroventricular IGF-I gene therapy ameliorated the reduced motor performance of the senile animals (31, 32).
In the present study, we assessed the effectiveness of long-term IGF-I gene therapy in the MBH of cycling adult female rats to extend regular cyclicity and preserve ovarian structure when the animals progress through M-A.
Materials and Methods
Adeno-associated virus (AAV) vectors.
The recombinant AAV (rAAV) shuttle plasmids, pAAV-cytomegalovirus (CMV)-IGF-1b-internal ribosome entry site (ires)-Discosoma red fluorescent protein (DsRed) and pAAV-CMV-ires-DsRed, were made by cloning an expression cassette between the 2 inverted terminal repeats of an AAV2 shuttle plasmid using standard techniques. Both plasmids contain an expression cassette driven by a CMV promoter containing a β-globin intron enhancer. The 934-bp rat IGF-Ib sequence was obtained from Peter Rotwein (Oregon Health Sciences University). Both plasmids also contain an ires upstream of the cellular reporter gene DsRed2. The expression cassettes were confirmed by DNA sequencing and expression of DsRed2 in transfected HeLa cells before preparing high titer, helper-free rAAV. Both vectors were packaged as rAAV2/2 by the Children's Memorial Viral Vector Core following the protocol of Zolotukhin et al (33) with minor modifications. In brief, a shuttle plasmid and the pDG packaging plasmid (generously provided by Jürgen Kleinschmidt) (34) were used at a ratio of 1:3, respectively, for CaCl2 transfection into 293T cells. Cells were lysed 3 days after transfection by freeze-thawing in order to collect virus, and cellular debris was removed by centrifugation. The supernatant was treated with octyl-β-D-glucopyranoside and benzonase and then applied to a 15%–60% iodixanol discontinuous gradient. The 40% layer was further purified using a Mustang Q ion exchange membrane. A Centriplus 100,000 MS cut off membrane was used to concentrate virus, which was stored in PBS (pH 7.4), containing 5% sorbitol and 0.001% Pluronic F68. Quantitative RT-PCR was used to determine viral titers. Viral titers were: rAAV-IGF-I-ires-DsRed, 2.0 × 1012 vector genomes (vg)/mL; rAAV-ires-DsRed, 3.9 × 1012 vg/mL.
In vitro studies
Cell cultures
The HEK293 human embryo kidney cell line was used to test the performance of rAAV-IGF-I-ires-DsRed in vitro. Cells were grown in Eagle's MEM, 16.8mM HEPES buffer (pH 7.0), 2mM glutamine, 0.1mM nonessential amino acids, 20-mg/L penicillin/streptomycin, 3.3-mg/L amphotericin B, 2.2-mg/L NaHCO3, and 10% (vol/vol) fetal bovine serum. They were grown at 37°C in a humidified atmosphere of 95% air-5% CO2. Cells were fed every 3-4 days and split when confluent.
Cell transduction protocol
Cells were plated on 12-well plates. When 70%–80% confluence was reached, the medium was replaced with fresh medium containing 4.8 × 109 vg/mL rAAV-IGF-I-ires-DsRed or 2.5 × 109 vg/mL rAAV-ires-DsRed. At appropriate times, cell supernatants were collected by gentle aspiration, 1-mL 0.1% Triton X-100 in PBS per well was added to cells, and they were scrapped off. Cell suspensions were freeze-thawed 3 times, centrifuged at 1000g for 10 minutes, and lysates collected for fluorescence determination by spectrofluorometry. Total IGF-I was measured in supernatants.
Animals and in vivo procedures
Female Sprague-Dawley rats aged 3, 8, 10, and 26 months were used. The animals were raised in our institution (Instituto de Investigaciones Bioquímicas de La Plata) and housed in a temperature-controlled room (22 ± 2°C) on a 12-hour light, 12-hour dark cycle (lights on from 7 am to 7 pm). Food and water were available ad libitum. All experiments with animals were performed according to the Animal Welfare Guidelines of National Institutes of Health (Instituto de Investigaciones Bioquímicas de La Plata's Animal Welfare Assurance A5647-01).
Stereotaxic injections
Rats were anesthetized with ketamine hydrochloride (40 mg/kg; ip) plus xylazine (8 mg/kg; im) and placed on a stereotaxic apparatus. In order to access the MBH, the tip of a 26-G needle fitted to a 10-μL syringe was brought to the following coordinates relative to the bregma: 3.0 mm posterior, 8.0 mm ventral, and 0.6 mm right and left (35).
Vaginal smears
Vaginal secretion was collected daily, between 11 am and 1 pm, with a plastic pipette filled with 20-μL normal saline (NaCl 0.9%) by inserting the tip into the rat vagina but not deeply. A drop of vaginal fluid was smeared on a glass slide, and the unstained material was observed under a light microscope, with a 40× phase-contrast objective. Three types of cells can be recognized: round and nucleated ones are epithelial cells, irregular ones without nucleus are cornified cells, and little round ones are leukocytes. The proportion among them was used for determination of the E cycle phases (36, 37), which are indicated as follows, proestrus (P), E, metestrus, diestrus (D), P entering E, and D entering P.
In M-A rats spending several days in a row in constant E (CE), a CE cycle was defined, for quantitation purposes, as a period of 5 consecutive days of vaginal smears showing only cornified cells. For instance, if an animal spent 13 days in a row in CE, they were counted as 13/5 = 2.6 CE cycles.
Experimental design for long-term IGF-I gene therapy in cycling females
Eight-month-old (34 wk) cycling females were allotted to a control or experimental group, thus forming 3 groups: intact control (intact), rAAV-DsRed-injected control (DsRed), and rAAV-IGF-I-ires-DsRed-injected experimental (IGF-I). Beginning at age 35.5 weeks, a small blood sample (0.3–0.4 mL) was taken (between 11 am and 1 pm) from the tail veins of all rats at appropriate intervals throughout the experiment. Serum was obtained and kept at −20°C for hormone assay. Vaginal smears were assessed daily from the beginning to the end of the study. At 36.2 weeks of age, the DsRed and IGF-I groups received bilateral 2.0-μL intrahypothalamic (MBH) injections containing 4 × 109 vg rAAV-DsRed or rAAV-IGF-I-ires-DsRed, respectively. The experiment was ended when the animals reached age 49.5 weeks.
Brain processing for fluorescence microscopy
Five DsRed and 5 IGF-I animals were placed under deep anesthesia and perfused with phosphate-buffered formaldehyde 4% (pH 7.4) fixative. Each brain was removed and serially cut into coronal sections 40 μm thick on a vibratome. Sections were placed on regular slides, mounted with Fluoromount G (Electron Microscopy Sciences, Hatfield, Pennsylvania), and observed under an Olympus BX51 fluorescence microscope (Olympus, Tokyo, Japan). Digital images were captured with an Olympus DP70 digital camera.
Histologic and histomorphometric assessment of ovaries
Ovaries were removed, fixed in 4% formaldehyde, dehydrated, and embedded in paraffin. Four-micrometer-thick serial sections, cut following the organ's major axis, were stained with hematoxylin and eosin. Micrographs of ovarian sections were taken with a digital camera Canon MC30 attached to an Olympus CX31 microscope. The number of mature and developing follicles, corpora lutea (CL), and cysts per ovarian section was determined using 6 images per gonad (which were at least 100 μm apart) taken with a 4× objective. The assessment of the ovaries was done by 2 blind observers (C.G.B. and M.A.F.). The criteria for histologically grading the ovaries were based on previous studies in young rats (38, 39) and adapted for the ovarian features of M-A rats. The grades are as follows:
Grade 1, corresponds to ovaries with an average of 1 or more large cysts, less than 1 CL and less than 1 mature or growing follicle per section;
Grade 2, corresponds to ovaries showing small or medium-sized but not large cysts, between 1 and less than 2.5 CL and between 1 and 1.5 mature or growing follicles per section;
Grade 3, corresponds to ovaries without medium-sized or large cysts and with an average of 2.5 or more CL as well as more than 1.5 mature or growing follicles.
Hormone assays
IGF-I assay
IGF-I was extracted from serum by acid-ethanol cryoprecipitation and was radioimmunoassayed (RIA) as previously described (30) using antibody UB2-495 distributed by Dr A. F. Parlow (Pituitary Hormones and Antisera Center, Harbor-University of California, Los Angeles (UCLA) Medical Center, Torrance, California). Recombinant human IGF-I (Cell Sciences, Inc, Canton, Massachusetts) was used as tracer and unlabeled ligand. Cell supernatants were homogenized in PBS, and IGF-I was extracted by acid-ethanol cryoprecipitation and quantified by RIA. The sensitivity and intraassay coefficient of variation (CV) for IGF-I was 2.4 ng/mL and 11%, respectively.
Pituitary hormone assays
Serum prolactin (PRL) and LH were measured by specific RIA using the rat materials provided by Dr A. F. Parlow. Serum PRL and LH were expressed in terms of National Hormone and Pituitary Program rat prolactin reference preparation 3, and rat luteinizing hormone reference preparation 3, respectively. The sensitivity and intraassay CV for LH and PRL was 0.6 ng/mL, 14% and 2.3 ng/mL, 13%, respectively.
Steroid hormone assays
Serum progesterone (P4) levels were measured by solid phase RIA using a commercial kit (Coat-A-Count, DPC; Diagnostic Products Corp, Los Angeles, California). Serum β-estradiol (E2) was measured using a liquid phase commercial RIA kit (Estradiol Ultrasensitive DSL 4800; Diagnostic Systems Laboratories, Webster, Texas). The sensitivity and intrassay CV for E2 and P4 were 4.1 pg/mL, 8% and 27 ng/mL, 5%, respectively.
Statistical analysis
Data are expressed as mean ± SEM, unless otherwise indicated. For multiple experimental groups, statistical comparisons were performed by 1-way ANOVA followed by the Tukey pos hoc test when the ANOVA was significant. For comparisons between pairs of means the Student's t test was used when the SDs did not differ significantly. Otherwise, the Welch's approximate t estimator was used.
Results
Characterization of E cyclicity of female rats at different ages
E cycle patterns throughout the lifespan
Preliminary characterization in our female rat colony of E cycle patterns from youth through very old age showed that transition from regular to irregular cyclicity takes place at around 9 months of age and is followed by a prevalence of CE status from age 10 to 18-20 months. Thus, 3-month-old females typically show 4- to 5-day E cycles characterized by 1 day or less of P (in some cycles, P was so short that when vaginal smear was performed the cell proportion was consistent with P entering E or sometimes just E), 1 day of E, and 2-3 days of metestrus and/or D (data not shown). Ten-month-old females typically show a prevalence of lengthy CE periods with interspersed irregular cycles (data not shown).
In vitro DsRed and IGF-I gene transfer
Both rAAV-DsRed and rAAV-IGF-I-ires-DsRed induced strong red fluorescence in HEK293 cell cultures (data not shown). rAAV-IGF-I-ires-DsRed showed a strong overexpression of IGF-I when compared with nontreated cells or cells incubated with rAAV-DsRed (Supplemental Figure 1, graph, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). IGF-I concentration in the supernatants peaked on day 2 after vector addition and remained steady afterwards. No cytopathic effect was detected in either control or experimental cells at the vector concentrations used.
IGF-I gene therapy in the MBH
Expression of transgenic DsRed in the MBH
Both rAAV-DsRed and rAAV-IGF-I-ires-DsRed induced strong red fluorescence in the MBH of M-A females from postvector injection day 3 until the end of the treatment at postvector injection day 93 (Supplemental Figure 1, images). As expected, most of the transduced cells were neurons and in most of the animals were distributed radiating from the needle track within the MBH. Vector diffusion did not extend beyond this region. Visual comparison of DsRed expression in different animals revealed some interanimal variability concerning the location where the vector was injected within the MBH, but around each needle track, the number of fluorescent neurons appeared comparable between sides and among animals.
Effect of long-term hypothalamic IGF-I gene therapy on E cycles in M-A rats
Injection of rAAV-IGF-I-ires-DsRed in the MBH of 36.2-week-old females had a favorable impact, when compared with rAAV-DsRed-injected or intact animals, on the average regularity of the E cycle patterns assessed during 13.3-week postvector injection (Supplemental Figure 2). As expected, stereotaxic surgery induced a slight loss in body weight that lasted for around 10 days in both DsRed and IGF-I rats. Subsequently, animals in the 3 groups gained weight at the same rate.
In quantitative terms, the average number of regular cycles per week before and after IGF-I vector injection was not significantly different. In contrast, intact rats or those receiving the DsRed control vector showed significantly lower numbers of regular cycles per week during the posttreatment period as compared with the pretreatment period (Figure 1). The frequency of irregular cycles in the 3 groups was comparable before and after treatment, whereas the number of CE cycles per week in the intact and DsRed, but not in the IGF-I animals, was significantly higher after than before treatment (Figure 1).
Figure 1.
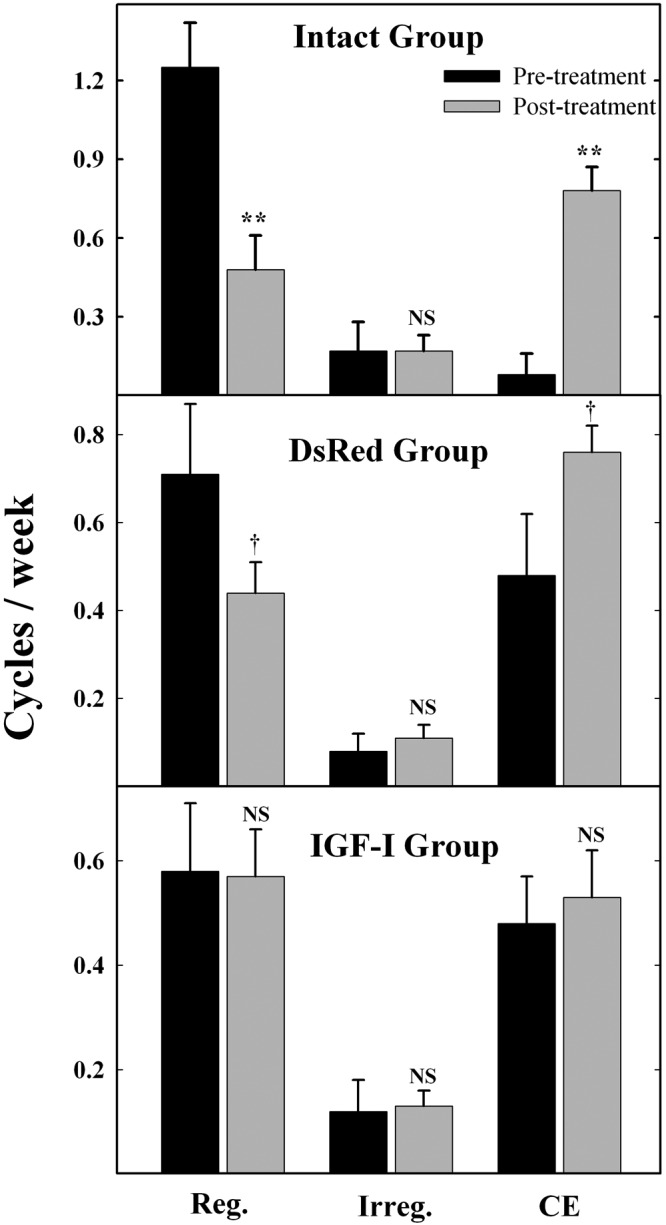
Effect of long-term MBH IGF-I gene therapy on the frequency of regular, irregular, and CE cycles in M-A female rats. Rats had their vaginal cytology assessed daily from 34.5 to 49.5 weeks of age. Vector injection in the MBH was performed on week 36.2 (except in the intact animals), and the number of regular, irregular, and CE cycles per week was calculated for the pretreatment period (wk 34.5-36.2) and the posttreatment period (wk 36.2-49.5). N was 6 for the intact group and 13 for both the DsRed and the IGF-I groups. Asterisks indicate significant (*P < .05) or highly significant (**P < .01) differences vs respective preinjection control for a 2-tailed t test for equal SD. Dagger indicates significant difference for a 1-tailed t test for significantly different SDs. Reg, regular cycling rats; Irreg., irregular cycling rats; NS, nonsignificant.
Effect of long-term IGF-I gene therapy on ovarian histology in M-A rats
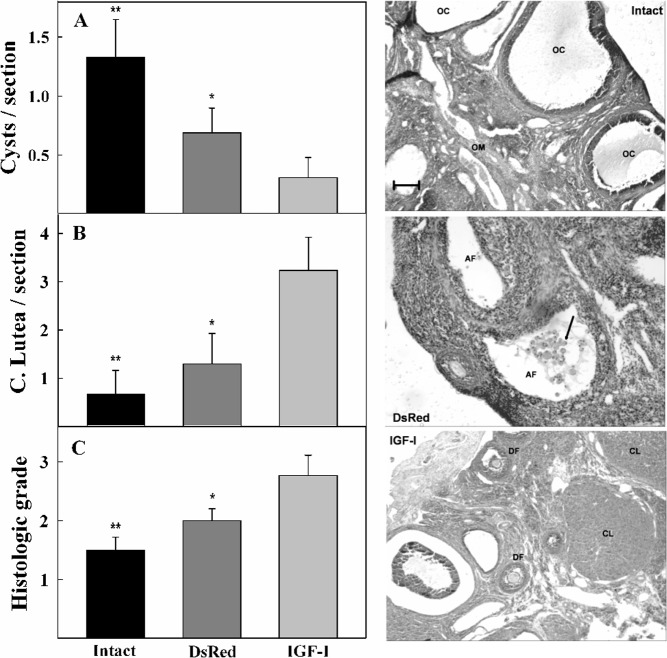
At the end of the study, when rats were aged 49.5 weeks, animals were killed and gonads histologically assessed. The ovaries of intact and DsRed M-A rats weighed significantly (P < .01) less than those of intact young (3 mo) counterparts. Interestingly, the weight of the ovaries from IGF-I-treated animals did not differ significantly from those of intact young rats (data not shown). The ovaries of both intact and DsRed, M-A females showed, on the average, clear structural alterations, the most conspicuous of which was the presence of large follicular cysts (Figure 2, upper and middle images, respectively), although the extent of these alterations varied substantially among animals even within the same group. In the females from the IGF-I group, the ovaries had, on the average, substantially milder structural alterations, and most of them showed follicles in all developmental stages as well as normal CL (Figure 2, bottom image). Histomorphometric analysis revealed a significantly lower incidence of follicular cysts in the ovaries of the IGF-I group (Figure 2A). The number of CL was significantly lower in the intact and DsRed groups than in the IGF-I animals (Figure 2B), whereas the number of atretic, growing, and mature follicles was not significantly different among groups (data not shown). The histologic grade, an index of the structural integrity of the ovaries, was significantly higher in the IGF-I group than in the intact and DsRed rats (Figure 2C). The rats with histologic grade 3 had a significantly better preserved regularity of E cycles than the animals with histologic grade 1 (data not shown).
Figure 2.
(Right panels) Histology of representative ovaries from control and experimental 49.5-week-old M-A rats submitted to long-term IGF-I gene therapy in the MBH. (Upper panel) Ovarian section from an intact animal. It was assigned grade 1 and shows large ovarian cysts (OC) but no CL or mature follicles (MF). (Middle panel) Ovarian section from a DsRed rat. It was assigned grade 2 and shows numerous atretic follicles (AF) but no CL or MF. Apoptotic cells can be observed in the follicular space (arrow). Interstitial connective tissue is abundant and highly cellular. (Bottom panel) Ovarian section from an animal submitted to IGF-I gene therapy. It was assigned grade 3 and shows developing follicles (DF) and CL of normal aspect as well as some AF. Scale bar, 300 μm; OM, ovarian medulla. (Left panels) Histomorphometric assessment of the ovaries of control and rAAV-IGF I-treated MA rats. The number of follicular cysts (A), and CL (B) per section, and the histologic grade (C) was assessed in the intact (n = 6), DsRed (n = 13), and IGF-I (n = 13) groups. Asterisks refer to differences vs the IGF-I group (ANOVA followed by the Tukey's test); **P < .01 and *P < .05.
Effect of MBH IGF-I gene therapy on serum hormone levels in M-A rats
Pituitary weight was comparable in the 3 experimental groups at 49 weeks of age (data not shown). Hormones were measured before treatment (wk 35–36) and at the end of the study (wk 48–49). Serum PRL increased significantly in the posttreatment period only in the intact and DsRed groups. The IGF-I group showed a trend towards an increase, but it did not attain significance (Figure 3, middle panel). Serum LH increased with age and after vector injection, but this change was significant only in the DsRed and IGF-I groups (Figure 3, bottom panel). Serum E2 was affected neither by age nor by the treatment (Figure 3, upper panel). Serum P4 levels were measured at the end of the study only, and no significant differences were detected among the 3 experimental groups (data not shown).
Figure 3.
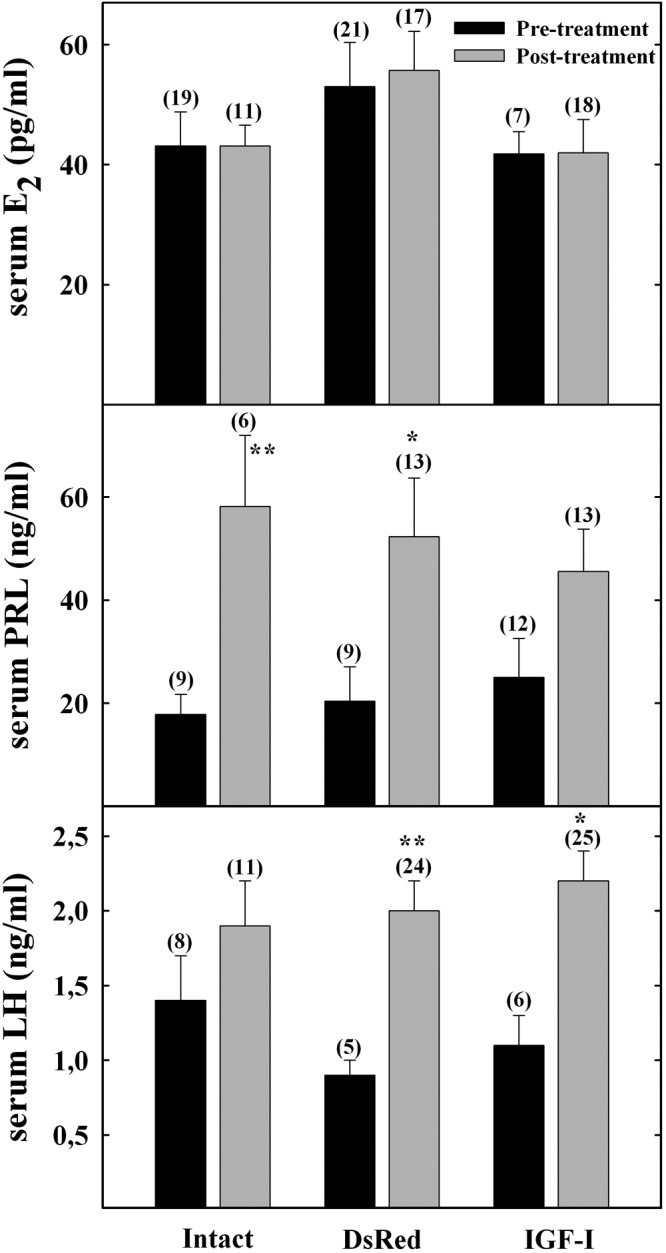
Serum hormone levels in M-A rats before and after IGF-I gene therapy. Blood samples were collected from the tail veins at the beginning (wk 35–36, pretreatment) and at the end (wk 48–49, posttreatment) of the study. Numbers in parentheses above columns indicate the number of samples assessed. Column height and bar above represents mean and SEM, respectively of each data point. *P < .05 and **P < .01 vs respective pretreatment value.
Discussion
Although the sequence of changes that take place during reproductive aging in female rats is qualitatively similar in most strains, the timing is likely to differ among strains and in different laboratory environments. This is why we considered it necessary to characterize the chronology of reproductive changes in our female rat colony before attempting to implement long-term protective gene therapy in cycling M-A animals. In qualitative terms, the age changes in vaginal cytology observed in our Sprague-Dawley females are in agreement with early reports in Long-Evans rats. Thus, in Long-Evans females, the vaginal smears show regular 4- to 5-day cycles from 2 to 10 months of age, transitioning to irregular cycles and persistent vaginal cornification (CE) during the next 2 months (40, 41). In our M-A females, this transition takes place earlier. Based on the above results, we started hypothalamic IGF-I gene therapy at 36.2 weeks of age, when a significant proportion of our rats was still cycling regularly. The bicistronic vector used allowed us to visually monitor transgene expression in the hypothalamus at different times after virus injection.
The average number of regular and CE cycles per week was taken as an index of reproductive capacity, the former being high in rats with regular ovulatory activity and the latter being high in anovulatory rats (42). The numerous medium and large ovarian follicular cysts and scarce CL shown by the intact and DsRed rats at the end of the study constitute a morphology consistent with the sustained secretion of FSH, estradiol, and estrone and the low P4 serum concentrations known to exist in anovulatory M-A rats (41, 43–45). In contrast, the rats submitted to IGF-I gene therapy showed substantially better preserved ovaries.
Taken together, the well-preserved histologic features generally observed in the ovaries from the rats submitted to IGF-I gene therapy and the vaginal cytology data from the same animals strongly suggest that the above intervention delayed the onset of anovulation in M-A females. Additionally, the hormone data indicate that the treatment increased serum LH levels and attenuated the progressive hyperprolactinemia that develops during aging in female rats (46).
We conclude that long-term overexpression of IGF-I in the MBH of female rats, started before they transition to the CE stage, prolongs normal hypothalamo-ovarian function. Although the mechanism by which this was achieved remains unknown, some of the studies reviewed above point to IGF-I as a permissive factor necessary for a proper functioning of the positive feedback of E2 on GnRH release from GnRHergic terminals into the median eminence portal system. This idea is consistent with the firm evidence that IGF-I and estrogens act synergistically in the brain both on reproductive function and in neuroprotection. In fact, an extensive colocalization has been documented for estrogen and IGF-I receptors both in neurons and astrocytes (47, 48). This colocalization suggests a cooperative cross talk between the 2 receptors (49).
Because IGF-I content is reduced in the hypothalamus of M-A rats (17), it could be hypothesized that by preventing the reduction of IGF-I levels in the MBH of our M-A rats, we preserved the positive feedback of E2 on GnRH release. This in turn may not only have maintained normal ovulatory cycles, but it may also have prevented or at least delayed the progressive disruption that typically occurs in ovarian steroid secretion in M-A rats (45, 50). The progressive delay and amplitude reduction in P LH surges (and the increased FSH secretion) in M-A female rats are thought to lead to persistently sustained ovarian E2 secretion, which over time exerts an inhibitory action on the steroid positive feedback mechanism on hypothalamic GnRH release (51, 52), thus further inhibiting the preovulatory LH surge. In any case, IGF-I is probably one of many permissive molecules contributing to a proper functioning of the positive steroid feedback mechanism on GnRH. Thus, hypothalamic IGF-I overexpression by itself should not be expected to indefinitely prolong regular cyclicity in M-A rats. As time passes, the hypothalamic expression of other permissive factors may also drop below critical levels, and the positive steroid feedback mechanism on GnRH driving preovulatory LH surges will eventually fail even in rats undergoing hpothalamic IGF-I gene therapy. Because the pituitary gland and the ovaries of old female rats transplanted into young hypophysectomized/ovariectomized recipients can sustain vaginal cyclicity (53), it appears that the hypothalamus is the critically age-sensitive component of the hypothalamo-pituitary-ovarian axis in rats. Therefore, the implementation of hypothalamic multigene therapy for an appropriate set of permissive factors may allow extending normal ovarian function of M-A female rats well into old age.
In rats, reproductive senescence occurs in midlife, whereas in some nonhuman primates, such as rhesus monkeys, this process occurs much later in life (54). Despite this and other interspecies differences in reproductive aging, understanding the basic mechanisms that trigger age changes in the rodent reproductive hypothalamus is likely to shed light on hypothalamic aging in primates, including humans.
Supplementary Material
Acknowledgments
We thank Ms Yolanda E. Sosa for technical assistance and Dr Allison Ebert for pilot data confirming that the AAV-IGFI-ires-DsRed shuttle plasmid expresses IGF-I peptide. C.G.B. and R.G.G. are Consejo Nacional de Investigaciones Científicas y Técnicas career researchers.
This work was supported in part by the National Institute on Aging and the Fogarty International Center, National Institutes of Health, Grant R01AG029798-3, the Agencia Nacional de Promoción Científica y Tecnológica Grant PICT08-369, and the Consejo Nacional de Investigaciones Científicas y Técnicas Grant PIP2378 (to R.G.G.).
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 1965
- AAV
- adeno-associated virus
- CE
- constant E
- CL
- corpora lutea
- CMV
- cytomegalovirus
- CV
- coefficient of variation
- D
- diestrus
- DsRed
- Discosoma red fluorescent protein
- E2
- β-estradiol
- E
- estrus
- ires
- internal ribosome entry site
- M-A
- middle aged
- MBH
- mediobasal hypothalamus
- P
- proestrus
- P4
- progesterone
- PRL
- prolactin
- rAAV
- recombinant AAV
- RIA
- radioimmunoassay
- vg
- vector genomes.
References
- 1. Wise PM. Alterations in proestrous LH, FSH, and prolactin surges in middle-aged rats. Proc Soc Exp Biol Med. 1982;169:348–354 [DOI] [PubMed] [Google Scholar]
- 2. Neal-Perry G, Lebesgue D, Lederman M, Shu J, Zeevalk GD, Etgen AM. The excitatory peptide kisspeptin restores the luteinizing hormone surge and modulates amino acid neurotransmission in the medial preoptic area of middle-aged rats. Endocrinology. 2009;150:3699–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Todd BJ, Merhi ZO, Shu J, Etgen AM, Neal-Perry GS. Hypothalamic insulin-like growth factor-I receptors are necessary for hormone-dependent luteinizing hormone surges: implications for female reproductive aging. Endocrinology. 2010;151:1356–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chakraborty TR, Hof PR, Ng L, Gore AC. Stereologic analysis of estrogen receptor α (ERα) expression in rat hypothalamus and its regulation by aging and estrogen. J Comp Neurol. 2003;466:409–421 [DOI] [PubMed] [Google Scholar]
- 5. Funabashi T, Kimura F. Effects of estrogen and estrogen receptor messenger RNA levels in young and middle-aged female rats: comparison of medial preoptic area and mediobasal hypothalamus. Acta Biol Hung. 1994;45:223–231 [PubMed] [Google Scholar]
- 6. Funabashi T, Kleopoulos SP, Brooks PJ, et al. Changes in estrogenic regulation of estrogen receptor α mRNA and progesterone receptor mRNA in the female rat hypothalamus during aging: an in situ hybridization study. Neurosci Res. 2000;38:85–92 [DOI] [PubMed] [Google Scholar]
- 7. Rubin BS, Fox TO, Bridges RS. Estrogen binding in nuclear and cytosolic extracts from brain and pituitary of middle-aged female rats. Brain Res. 1986;383:60–67 [DOI] [PubMed] [Google Scholar]
- 8. Zheng W, Jimenez-Linan M, Rubin BS, Halvorson LM. Anterior pituitary gene expression with reproductive aging in the female rat. Biol Reprod. 2007;76:1091–1102 [DOI] [PubMed] [Google Scholar]
- 9. Neal-Perry GS, Zeevalk GD, Santoro NF, Etgen AM. Attenuation of preoptic area glutamate release correlates with reduced luteinizing hormone secretion in middle-aged female rats. Endocrinology. 2005;146:4331–4339 [DOI] [PubMed] [Google Scholar]
- 10. Huang HH, Marshall S, Meites J. Capacity of old versus young female rats to secrete LH, FSH and prolactin. Biol Reprod. 1976;14:538–543 [DOI] [PubMed] [Google Scholar]
- 11. Neal-Perry G, Nejat E, Dicken C. The neuroendocrine physiology of female reproductive aging: an update. Maturitas. 2010;67:34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rubin BS, Lee CE, King JC. A reduced proportion of luteinizing hormone (LH)-releasing hormone neurons express Fos protein during the preovulatory or steroid-induced LH surge in middle-aged rats. Biol Reprod. 1994;51:1264–1272 [DOI] [PubMed] [Google Scholar]
- 13. Hoffman GE, Finch CE. LHRH neurons in the female C57BL/6J mouse brain during reproductive aging: no loss up to middle age. Neurobiol Aging. 1986;7:45–48 [DOI] [PubMed] [Google Scholar]
- 14. Krajnak K, Rosewell KL, Wise PM. Fos-induction in gonadotropin-releasing hormone neurons receiving vasoactive intestinal polypeptide innervation is reduced in middle-aged female rats. Biol Reprod. 2001;64:1160–1164 [DOI] [PubMed] [Google Scholar]
- 15. Rubin BS, Lee CE, Ohtomo M, King JC. Luteinizing hormone-releasing hormone gene expression differs in young and middle-aged females on the day of a steroid-induced LH surge. Brain Res. 1997;770:267–276 [DOI] [PubMed] [Google Scholar]
- 16. Rubin BS, Bridges RS. Alterations in luteinizing hormone-releasing hormone release from the mediobasal hypothalamus of ovariectomized, steroid-primed middle-aged rats as measured by push-pull perfusion. Neuroendocrinology. 1989;49:225–232 [DOI] [PubMed] [Google Scholar]
- 17. Miller BH, Gore AC. Alterations in hypothalamic insulin-like growth factor-I and its associations with gonadotropin releasing hormone neurones during reproductive development and ageing. J Neuroendocrinol. 2001;13:728–736 [DOI] [PubMed] [Google Scholar]
- 18. Le WW, Wise PM, Murphy AZ, Coolen LM, Hoffman GE. Parallel declines in Fos activation of the medial anteroventral periventricular nucleus and LHRH neurons in middle-aged rats. Endocrinology. 2001;142:4976–4982 [DOI] [PubMed] [Google Scholar]
- 19. Wise PM, Smith MJ, Dubal DB, et al. Neuroendocrine modulation and repercussions of female reproductive aging. Recent Prog Horm Res. 2002;57:235–256 [DOI] [PubMed] [Google Scholar]
- 20. Neal-Perry GS, Zeevalk GD, Shu J, Etgen AM. Restoration of the luteinizing hormone surge in middle-aged female rats by altering the balance of GABA and glutamate transmission in the medial preoptic area. Biol Reprod. 2008;79:878–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jarry H, Wise PM, Leonhardt S, Wuttke W. Effects of age on GABA turnover rates in specific hypothalamic areas in female rats. Exp Clin Endocrinol Diabetes. 1999;107:59–62 [DOI] [PubMed] [Google Scholar]
- 22. Brann DW, Mahesh VB. The aging reproductive neuroendocrine axis. Steroids. 2005;70:273–283 [DOI] [PubMed] [Google Scholar]
- 23. Krajnak K, Kashon ML, Rosewell KL, Wise PM. Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA but not arginine vasopressin mRNA in the suprachiasmatic nuclei of female rats. J Neurosci. 1998;18:4767–4774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Le Grevès M, Le Grevès P, Nyberg F. Age-related effects of IGF-1 on the NMDA-, GH- and IGF-1-receptor mRNA transcripts in the rat hippocampus. Brain Res Bull. 2005;65:369–374 [DOI] [PubMed] [Google Scholar]
- 25. Sonntag WE, Bennett SA, Khan AS, A, et al. Age and insulin-like growth factor-1 modulate Nmethyl-D-aspartate receptor subtype expression in rats. Brain Res Bull. 2000;51:331–338 [DOI] [PubMed] [Google Scholar]
- 26. Hiney JK, Srivastava VK, Pine MD, Les Dees W. Insulin-like growth factor-I activates KiSS-1 gene expression in the brain of the prepubertal female rat. Endocrinology. 2009;150:376–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lara JI, Lorenzo MJ, Cacicedo L, et al. Induction of vasoactive intestinal peptide gene expression and prolactin secretion by insulin-like growth factor I in rat pituitary cells: evidence for an autoparacrine regulatory system. Endocrinology. 1994;135:2526–2532 [DOI] [PubMed] [Google Scholar]
- 28. Servoss SJ, Lee SJ, Gibney G, Gozes I, Brenneman DE, Hill JM. IGF-I as a mediator of VIP/activity-dependent neurotrophic factor-stimulated embryonic growth. Endocrinology. 2001;142:3348–3353 [DOI] [PubMed] [Google Scholar]
- 29. Carro E, Torres-Aleman I. Serum insulin growth factor I in brain function. IKeio J Med. 2006;55:59–63 [DOI] [PubMed] [Google Scholar]
- 30. Hereñú CB, Cristina C, Rimoldi OJ, et al. Restorative effect of insulin-like growth factor-I gene therapy in the hypothalamus of senile rats with dopaminergic dysfunction. Gene Therapy. 2007;14:237–245 [DOI] [PubMed] [Google Scholar]
- 31. Hereñú CB, Sonntag WE, Morel GR, Portiansky EL, Goya RG. The ependymal route for insulin-like growth factor-1 gene therapy in the brain. Neuroscience. 2009;163:442–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nishida F, Morel GR, Hereñú CB, Schwerdt JI, Goya RG, Portiansky EL. Restorative effect of intracerebroventricular insulin-like growth factor-I gene therapy on motor performance in aging rats. Neuroscience. 2011;177:195–206 [DOI] [PubMed] [Google Scholar]
- 33. Zolotukhin S, Potter M, Zolotukhin I, et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–167 [DOI] [PubMed] [Google Scholar]
- 34. Grimm D, Kern A, Rittner K, Kleinschmidt JA. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther. 1998;9:2745–2760 [DOI] [PubMed] [Google Scholar]
- 35. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, California: Academic Press; 1998 [Google Scholar]
- 36. Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614 [DOI] [PubMed] [Google Scholar]
- 37. Astwood EB. Changes in the weight and water content of the uterus of the normal adult rat. Am J Physiol. 1939;126:162–170 [Google Scholar]
- 38. Yoshida M, Sanbuissyo A, Hisada S, Takahashi M, Ohno Y, Nishikawa A. Morphological characterization of the ovary under normal cycling in rats and its viewpoints of ovarian toxicity detection. J Toxicol Sci. 2009;34(suppl 1):189–197 [DOI] [PubMed] [Google Scholar]
- 39. Røste LS, Taubøll E, Berner A, Isojärvi JI, Gjerstad L. Valproate, but not lamotrigine, induces ovarian morphological changes in Wistar rats. Exp Toxicol Pathol. 2001;52:545–552 [DOI] [PubMed] [Google Scholar]
- 40. Huang HH, Meites J. Reproductive capacity of aging female rats. Neuroendocrinology. 1975;17:289–295 [DOI] [PubMed] [Google Scholar]
- 41. Lu KH, Hopper BR, Vargo TM, Yen SSC. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol Reprod. 1979;21:193–203 [DOI] [PubMed] [Google Scholar]
- 42. Aschheim P. Repetitive pseudopregnancy in senile rats [in French] CR. Acad Sci. 1961;253:1988–1990 [PubMed] [Google Scholar]
- 43. Huang HH, Steger RW, Bruni JF, Meites J. Patterns of sex steroid and gonadotropin secretion in aging female rats. Endocrinology. 1978;103:1855–1859 [DOI] [PubMed] [Google Scholar]
- 44. Lu JKH, Kledzik GS. Chronological changes in ovarian function and morphology in aging rats and their relation to neuroendocrine responses. In: Schwartz NB, Hunzicker-Dunn M, eds. Dynamics of Ovarian Function. New York, New York: Raven Press; 1981:291–296 [Google Scholar]
- 45. Lu JKH. Changes in ovarian function and gonadotropin and prolactin secretion in aging female rats. In: Meites J, ed. Neuroendocrinology of Aging. New York, New York: Plenum Press; 1983:103–122 [Google Scholar]
- 46. Goya RG, Lu JKH, Meites J. Gonadal function in aging rats and its relation to pituitary and mammary pathology. Mech Age Dev. 1990;56:77–88 [DOI] [PubMed] [Google Scholar]
- 47. Quesada A, Romeo HE, Micevych P. Distribution and localization patterns of estrogen receptor-β and insulin-like growth factor-1 receptors in neurons and glial cells of the female rat substantia nigra: localization of ERβ and IGF-1R in substantia nigra. J Comp Neurol. 2007;503:198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cardona-Gómez GP, DonCarlos L, Garcia-Segura LM. Insulin-like growth factor I receptors and estrogen receptors colocalize in female rat brain. Neuroscience. 2000;99:751–760 [DOI] [PubMed] [Google Scholar]
- 49. Méndez P, Wandosell F, Garcia-Segura LM. Cross-talk between estrogen receptors and insulin-like growth factor-I receptor in the brain: cellular and molecular mechanisms. Front. Neuroendocr. 2006;27:391–403 [DOI] [PubMed] [Google Scholar]
- 50. Nass TE, LaPolt PS, Judd HL, Lu JK. Alterations in ovarian steroid and gonadotrophin secretion preceding the cessation of regular oestrous cycles in ageing female rats. J Endocrinol. 1984;100:43–50 [DOI] [PubMed] [Google Scholar]
- 51. Lu KH, Huang HH, Chen HT, Kurcz M, Mioduskewski R, Meites J. Positive feedback by estrogen and progesterone on LH release in old and young rats. Proc Soc Exp Biol Med. 1977;154:82–85 [DOI] [PubMed] [Google Scholar]
- 52. Lu KH, Gilman DP, Meldrum DR, Judd HL, Sawyer CH. Relationship between circulating estrogens and the central mechanisms by which ovarian steroids stimulate luteinizing hormone secretion in aged and young female rats. Endocrinology. 1981;108:836–841 [DOI] [PubMed] [Google Scholar]
- 53. Peng MT, Huang HH. Aging of hypothalamic-pituitary-ovarian function in the rat. Fertil Steril. 1972;23:535–542 [DOI] [PubMed] [Google Scholar]
- 54. Yin W, Gore AC. Neuroendocrine control of reproductive aging: roles of GnRH neurons. Reproduction. 2006;131:403–414 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.