Abstract
Background
Because of its nutrients and anabolic hormones, cow’s milk may promote height growth, which in turn has been related to breast cancer risk. We prospectively investigated associations between dairy intakes and height growth.
Methods
A cohort of 5,101 girls from throughout the US completed annual surveys (1996-2001,2003) providing height, weight and past-year diet. At baseline, all were premenarchal, aged 9yr+, with no serious medical conditions. We studied three outcomes: annual height growth, peak growth velocity and adult height. Multivariate models estimated the effects of milk, cheese, yogurt, and energy on subsequent growth, adjusted for race/ethnicity, age, prior height and BMI. Other models studied fats and proteins.
Results
Premenarchal girls who drank >3servings/day of milk grew 0.11inch (p=0.02) more the following year than girls consuming <1serving/day. Yogurt (+0.13inch/cup; p=0.02), but not cheese or total calories, predicted height growth. In a separate model, dairy protein (+.034inch/10gm; p<0.001) predicted height growth. Larger peak velocities were seen among girls reporting, at baseline, more milk (>3glasses/day vs <1:+0.14inch, p=0.01)), more yogurt (+0.17inch/cup, p=0.02), and in a separate model, more dairy protein (+0.039inch/10gm; p=0.003). Baseline milk and dairy protein predicted taller adults. Dairy protein was more important than dairy fat, for all outcomes. Non-dairy animal protein and vegetable protein were never significant, nor were non-dairy animal fat and vegetable fat.
Conclusion
Of the foods/nutrients studied, dairy protein had the strongest association with height growth. These findings suggest that a factor in the non-lipid phase of milk, but not protein itself, has growth-promoting action in girls.
Keywords: girls, adolescent, puberty, growth velocity, peak height velocity, adult height, milk, cheese, yogurt, dietary fat, dietary protein, longitudinal
Introduction
Research on the impact of dietary intakes upon height growth in children, using longitudinal cohort studies, goes back decades. These original studies were interested in the relationship between malnutrition and inadequate physical growth. Although height has a genetic component, observed secular trends in growth during decades of dietary improvements (1) and studies of immigrant and refugee children whose growth rates, soon after their arrival in the US, matched or exceeded those of US white children (2) demonstrate the importance of the nutritional environment. There is renewed interest in the investigation of factors, including milk and other dairy products, which may promote height growth in today’s youth. Recent studies suggested that more rapid childhood height growth may be a factor in the development of cancer, especially breast cancer in women (3-5). Longitudinal studies have implicated milk specifically, rather than animal protein, as being associated with more rapid growth in the fetus and child (6-9).
In this study we investigated, in a large cohort of girls each having up to 8 years of follow-up, consumption of cow’s milk, cheese, yogurt, dietary protein and dietary fat in relation to 1-yr height growth, peak height growth velocity, and eventual adult height.
Materials and Methods
Subjects
Established in 1996, the Growing Up Today Study (GUTS) includes 8980 girls from all 50 states who are daughters of Nurses’ Health Study II (NHSII) participants (10). The study, approved by Human Subjects Committees at Harvard School of Public Health and Brigham and Women’s Hospital, is described elsewhere (11). Mothers provided informed consent, and their daughters assented by completing baseline questionnaires. The cohort, aged 9-14 years in 1996, returned follow-up questionnaires annually through 2001, and again in 2003. The girls’ response rate to one or more of these follow-ups after baseline was 96%, and over 80% of the girls remained in the study six or more years by providing data in 2001 and/or 2003.
Only girls who were premenarchal at baseline (n=5851) were eligible to be included in this analysis. Of these, 295 girls (5%) had a serious medical condition (reported by mother) that might affect growth and were therefore excluded entirely, leaving 5556 girls available for these analyses. The number of girls in each analysis below will depend upon the specific outcome and exposures studied. For example, some girls who do not provide an adult height will provide a peak growth velocity, or one or more annualized height growth increments, prior to menarche; other girls may not provide a peak velocity but will provide an adult height. Thus, the actual group of girls analyzed, and their sample sizes, will vary between models in our approach that uses all available data for studying each outcome.
Growth Data
Children reported their heights and weights on every survey. Our questionnaire provided specific measuring instructions but suggested they seek assistance; their mothers (nurses) biennially self-report their own heights and weights for NHSII. An analysis of NHANES III adolescents supported high validity for self-reported height and weight (12). We assessed relative weight status by computing body mass index (BMI= weight/height2, (kg/m2)). The validity of self-reported BMI was demonstrated by National Longitudinal Study of Adolescent Health analyses that found a high correlation between BMI computed from measured values and from self-reports by youth in grades 7-12 (13).
From the serial heights provided by each girl, we computed a series of annualized height growth increments, HTt-HTt-1 divided by the time interval (in years, to the month) between adjacent survey return dates. Whenever an annual survey or height was missing, we computed annualized height growth from surveys two years apart when possible. Seventy-eight percent of the cohort provided three, four, five or six annualized height growth increments. A total of 5101 girls (with no serious medical diagnoses) provided one or more annualized height growth increments, in which they were premenarchal when the interval began. After inspecting a girl’s series of annualized growth increments, we designated the largest of these as her peak height growth velocity (PHV; inches/yr). Each girl’s adult height was her greatest height attained after menses-onset; girls who provided no heights after menses began had missing adult height. As the median of the girls’ oldest observed ages was 17.8yrs, growth should be completed for most.
Dietary Intakes
We used a self-administered semi-quantitative food frequency questionnaire (FFQ), designed specifically for older children and adolescents (14). This FFQ for youth has good validity and reproducibility for children ages 9 through 18yr (14-15); the mean correlation for nutrients from the FFQ compared to three 24-hour recalls was r=0.54, comparable to the performance of a similar adult FFQ. Milk and dairy products, including yogurt and cheese, had particularly high validity among adult women (16). Another youth FFQ, similar to ours, provided estimates of milk and dairy food consumption by adolescent girls that correlated well with 7-day dietary records (17).
Our 1996 through 2001 annual surveys each inquired about the usual frequency of intake over the past year of milk, cheese, and yogurt; the white milk question indicated that the serving size was a “glass or with cereal”, for chocolate milk the serving was a “glass”, the cheese serving was “1 slice”, and a yogurt serving was “1 cup – not frozen”. We combined white and chocolate milk to get servings/day of dairy milk. Children also reported the fat content of the milk they usually drink (whole, 2%, 1%, or skim). We derived total dietary fat, dairy fat, vegetable fat, non-dairy animal fat (from meat/fish/eggs), total dietary protein, dairy protein, vegetable protein, non-diary animal protein (from meat/fish/eggs), and total energy intakes. Dairy fat and dairy protein were calculated from milk, butter, yogurt and cheese as whole foods and as ingredients in other foods. Regarding any girl who ever reported soy-milk intake, her soy-milk followup years were excluded from these analyses (0.62% of person-years), but her dairy milk intakes were retained in analyses.
Other Variables
At baseline, children reported their race/ethnic group by marking all (of six) options that applied to them. We assigned each child to one of 5 race/ethnic groups following US Census definitions, except we kept Asians as a separate group rather than pooled with ‘Other’. At baseline, each girl further reported her Tanner maturation stage, a validated self-rating of sexual maturity (18) that uses 5 illustrations for stage of pubic hair development. Annually, the girls reported whether their menstrual periods had yet begun. We computed each child’s age on each survey from dates of birth and questionnaire return.
Statistical Analyses
Our first series of analyses related dietary intakes to height growth during the following year. We studied only height increments for which the girl was premenarchal at the start of the time interval, because most growth occurs prior to onset of menses. We fitted linear regression models of annualized height growth on prior diet, adjusting for age (nonlinear: age (=age-11.5), age2 and age3), race/ethic group, prior height and prior relative BMI (girl’s BMI minus age-specific median BMI, from CDC growth charts (19)). Because each girl can have multiple 1-yr height growth increments, the assumption of independent observations as required by ordinary linear regression models is not met. Therefore we used mixed linear regression models (20) with estimation by SAS proc mixed (21) to take into account correlations among the repeated observations. We used continuous measures of dietary exposures (daily servings of milk (white and chocolate), cheese, yogurt, daily total energy, dietary fat and protein intakes) to model linear associations with height growth. In separate models, we used categorical white milk intake, to observe and estimate any nonlinear trends, as we were particularly interested in high intakes (>3 servings/day). Categorical chocolate milk was a separate variable in all models in which categorical white milk appeared. Protein and fat were studied as dietary totals (total protein, total fat) and also separately as dairy, non-dairy animal, and vegetable. Some models adjusted for total energy intake to address the possibility that girls consumed more dairy products because they were in a period of rapid growth; if they were eating more dairy products, as a result of growth-induced hunger, they’d likely be consuming more of other foods as well. In some models, prior year diet was replaced by the cumulative average of all prior intakes back to baseline.
Our second series of analyses investigated baseline dietary intakes and peak height growth velocity (PHV), and our third series of analyses similarly investigated baseline diet and eventual adult height. Regression models of these outcomes adjusted for race, nonlinear age, Tanner stage, height and relative BMI, all at baseline. We focused on baseline data because we wanted to assess the effect of diet recorded far in advance of the outcomes, so that reverse causation was not a plausible explanation for our findings. However, we also fit some further models that used cumulative early dietary intakes.
Our estimated regression model betas may be biased due to dairy-intake reporting errors, so we investigated the impact of dietary measurement error in two ways. First, we assessed whether conclusions changed when we instead used cumulative averages, for milk, cheese, yogurt and calories over multiple prior years, to reduce bias due to measurement error (22). Second, we used the validation study of individual foods on adult women (16) to obtain adjusted effect estimates that take into account measurement error (methods available from authors).
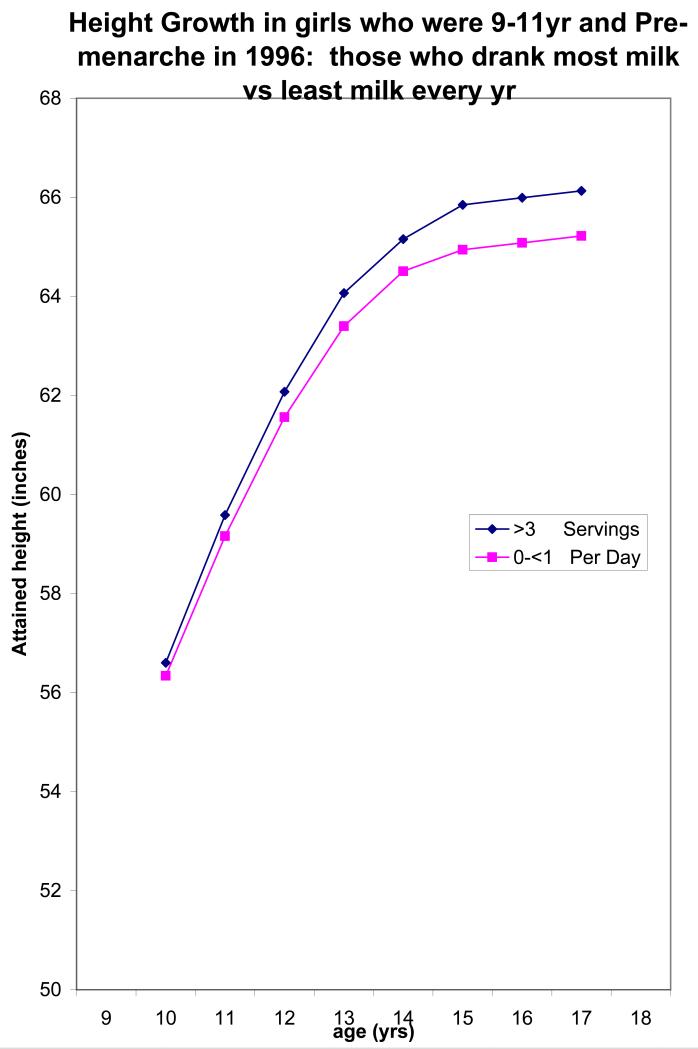
We illustrated the age-specific effects of drinking high amounts of dairy milk all through adolescence by focusing on those girls who were youngest at baseline. We fitted a model to white non-Hispanic girls who were aged 9, 10 or 11yr, and premenarchal, at baseline (n=3328). The dependent variable was adult height, and independent variables included dairy milk intakes (categorical) in 1996, in 1997, in 1998, in 1999 and in 2000, each a separate variable in the model, and baseline height, baseline age and baseline Tanner Stage. We used model estimates to prepare a figure that shows the height growth curves, from age 10 to adult, of the highest milk intake and lowest milk intake groups.
Results
Most participants, daughters of NHSII nurses, are white/non-Hispanic (95%). Table 1 shows means (unadjusted), within categories of white milk intake, of age, anthropometric measures and dietary intakes, at baseline. The largest proportion (37%) of girls reported 2 to 3 servings/day of white milk, and 11% reported >3 servings/day. Chocolate milk consumption was very low, with only 20% reporting more than 1 glass/week (not shown). Girls who, at baseline, consumed the most white milk (>3 servings/day) grew slightly more the following year (1996 to 1997), had slightly higher peak growth velocities, and became slightly taller adults (Table 1).
Table 1. Baseline characteristics of premenarchal girls by white dairy milk consumption. Girls with serious medical conditions and those who drank soy milk at baseline were excluded.
| Glasses of milk per day | ||||
|---|---|---|---|---|
| 0 - <1 | 1 | 2-3 | >3 | |
|
|
||||
| N | 1768 | 1126 | 2019 | 600 |
| Age (yr) | 11.3 | 11.3 | 11.1 | 11.3 |
| White race (%) Non-Hispanic |
94.3 | 94.3 | 96.5 | 97.3 |
| Height (inches) | 57.7 | 57.8 | 57.8 | 58.1 |
| BMI (kg/m2) | 18.4 | 18.1 | 18.0 | 18.2 |
| Height growth (inches) from 1996 to 1997 |
2.5 | 2.5 | 2.5 | 2.6 |
| Age at PHV (yr) | 12.6 | 12.6 | 12.5 | 12.6 |
| PHV (inches/yr) | 3.1 | 3.1 | 3.2 | 3.3 |
| Adult Height (inches) | 65.3 | 65.4 | 65.7 | 65.9 |
| White+Chocolate Milk Glasses/day |
0.6 | 1.2 | 2.8 | 4.3 |
| Calories/day | 1829 | 1978 | 2197 | 2360 |
| Cheese (slices/day) | 0.37 | 0.41 | 0.45 | 0.50 |
| Yogurt (cups/day) | 0.10 | 0.12 | 0.13 | 0.14 |
| Dairy Fat (gm/day) | 13.1 | 15.1 | 19.2 | 23.4 |
| Non-Dairy Animal Fat (gm/day) |
16.6 | 17.3 | 18.5 | 18.2 |
| Vegetable Fat (gm/day) | 34.1 | 35.6 | 36.5 | 36.9 |
| Dairy Protein (gm/day) | 15.0 | 20.5 | 33.5 | 46.2 |
| Non-Dairy Animal Prot (gm/day) |
25.8 | 27.2 | 29.1 | 28.1 |
| Vegetable Prot (gm/day) | 23.0 | 24.5 | 25.9 | 26.4 |
Associations between dietary intakes and following year height growth were studied in 5070 girls (analytic sample size after excluding person-yrs with soy milk intake or missing adjustment variables). Six models are summarized in Table 2. Large white milk intakes (>3 servings/day compared to <1/day) were significantly associated with greater height growth the following year (Models 1-3, Table 2). Yogurt consumption was also associated with greater height growth, but cheese and total energy intake (from all foods and beverages) were not (Models 3 and 4, Table 2). Total milk intake (white and chocolate), modeled as servings/day, was also associated with subsequent height growth (Model 4, Table 2). Our conclusions were identical when cumulative prior intakes of milk, cheese, yogurt, and calories were analyzed instead of the single prior year (not shown). Our conclusions were unchanged when we adjusted for total fat and total protein instead of total energy intake (not shown). An analysis of dairy fat, animal (excluding dairy) fat and vegetable fat together suggested that only dairy fat was associated with subsequent height growth (Model 5, Table 2). The analysis of sources of dietary protein similarly found that only dairy protein was associated with subsequent growth (Model 6, Table 2). Including dairy fat and dairy protein together in a model indicated that dairy protein (beta=+0.03/10gm, p=.015), rather than dairy fat (beta=+.012/10gm, p=.461), was the nutrient associated with height growth. When we included dairy protein in models along with milk, then milk was no longer significant (not shown), but keep in mind that milk and dairy protein are very highly correlated (r=+0.88 at baseline).
TABLE 2. Dairy intakes preceding one-yr height growth in premenarchal girls. All linear mixed regression models are adjusted#. Estimated betas are per daily serving, or other shown daily quantity. Models 1 to 3 compare servings/day of milk to lowest intake (<1 serving/day) group. Sample sizes range from 5070 girls (Model 1) to 5024 girls (Model 4).
| Height growth difference (inches) over 1-yr | ||||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|
|
||||||
| Milk | Milk | Milk | Milk | Per 10gm: | Per 10gm: | |
| >3 vs <1 | >3 vs <1 | >3 vs <1 | (white + choc) | Dairy Fat | Dairy Protein | |
| Beta | +0.120 | +0.112 | +0.108 | +.024 | +.033 | +.034 |
| (P-value) | (0.003) | (0.007) | (0.017) | (0.019) | (0.016) | (0.001) |
| 2-3vs<1 | Cheese | Cheese | Cheese | Animal* Fat | Animal* Protein | |
| Beta | +0.029 | +.014 | +.004 | +.004 | −0.015 | −0.010 |
| (P-value) | (.296) | (0.502) | (0.866) | (0.855) | (0.395) | (0.280) |
| 1 vs <1 | Yogurt | Yogurt | Yogurt | Vegetable Fat | Vegetable Protein | |
| Beta | +0.004 | +0.094 | +.133 | +.126 | +.011 | +.015 |
| (P-value) | (.985) | (0.061) | (0.019) | (0.027) | (0.337) | (0.353) |
| Calories/100kcal | Calories/100kcal | |||||
| Beta | +.002 | +.002 | ||||
| (P-value) | (0.293) | (.284) | ||||
Adjusted for race, age, age2 , age3 , prior height, and prior relative BMI. Models 1-3 include categorical white milk (results shown for >3 vs <1 glasses/day) and categorical chocolate milk (not shown). Model 4 includes white plus chocolate milk (glasses/day, continuous). Cheese serving is 1 slice, yogurt is 1 cup.
Dairy intakes are excluded from Animal Fat and Animal Protein grams.
The mean age at peak height growth velocity was 12.6yr (sd=1.4), and the mean PHV was 3.2 (sd=1.3) inches/yr. This mean PHV is similar to that reported on two earlier female cohorts (whites: 3.1 inch/yr (23)) (whites: 3.2 inch/yr, Blacks: 3.3 inch/yr (24)), in which heights of children were measured by study personnel, rather than self-reported as in our study. Occurrence of PHV at younger ages was associated with more growth during the peak year; for instance, among those whose peak growth occurred at age 9, they grew, on average, 4.1 inches over 1yr, whereas those with peak growth at age 14 grew only 2.2 inches during the year. This age-dependent pattern for PHV is comparable to that observed in a longitudinal cohort of US girls (25) followed during the 1970’s. We have 5022 GUTS cohort girls for studying associations between baseline diet and peak height growth velocity (Table 3), after excluding those who drank soy milk at baseline and those with missing adjustment variables. Higher milk and yogurt intakes at baseline predicted larger peak velocities (Models 3 and 4, Table 3); conclusions were identical when we instead analyzed cumulative prior intakes (not shown). Baseline dairy protein (Model 6, Table 3) predicted larger peak height growth velocities. Conclusions regarding milk and yogurt were similar when we adjusted for total fat and total protein, instead of energy, but milk was no longer significant when dairy protein was included in the same model (not shown).
TABLE 3. Baseline diet of premenarchal girls and Peak Height Growth Velocity.All models are adjusted#. Estimated betas are per daily serving, or other shown daily quantity.Models 1 to 3 compare servings/day of milk to lowest intake (<1 serving/day) group. Sample sizes range from 5022 girls (Model 1) to 4975 girls (Model 4).
| PHV difference (inches over 1 yr) | ||||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|
|
||||||
| Milk | Milk | Milk | Milk | Per 10gm: | Per 10gm: | |
| >3 vs <1 | >3 vs <1 | >3 vs <1 | (white + choc) | Dairy Fat | Dairy Protein | |
| Beta | +0.165 | +.159 | +.140 | +.039 | +.007 | +.039 |
| (P-Value) | (.003) | (0.004) | (0.014) | (0.003) | (0.705) | (0.003) |
| 2-3vs<1 | Cheese | Cheese | Cheese | Animal* Fat | Animal* Protein | |
| Beta | +0.039 | +.001 | −.008 | −.007 | −0.033 | −0.012 |
| (P-value) | (.314) | (0.960) | (0.765) | (.794) | (0.151) | (0.352) |
| 1 vs <1 | Yogurt | Yogurt | Yogurt | Vegetable Fat | Vegetable Protein | |
| Beta | −0.074 | +0.138 | +.169 | +.167 | +.028 | +.026 |
| (P-value) | (.099) | (0.057) | (0.024) | (0.026) | (0.068) | (0.222) |
| Calories/100kcal | Calories/100kcal | |||||
| Beta | +0.002 | +.001 | ||||
| (P-value) | (0.498) | (0.603) | ||||
Adjusted for race, age, age2 , age3 , Tanner Stage, height, and relative BMI, all at baseline. Models 1-3 include categorical white milk (results shown for >3 vs <1 glasses/day) and categorical chocolate milk (not shown). Model 4 shows results for white + chocolate milk (glasses/day, continuous). Cheese serving is 1 slice/day, yogurt serving is one cup/day.
Dairy intakes are excluded from Animal Fat and Animal Protein grams.
The mean adult height was 65.5 (sd 2.8) inches. We had 4870 girls for the analysis of early diet and adult height, after excluding girls who drank soy milk at baseline or who had missing adjustment variables. Those who reported higher baseline milk intakes became taller adults (Table 4: Models 1-4). Analysis of cumulative prior diet provided the same conclusions (not shown). Replacing calories in Models 3 and 4 with total fat and total protein intake also did not change these findings (not shown). Dairy fat (Model 5) and dairy protein (Model 6) at baseline both predicted taller adults. Dairy protein (beta=+0.072/10gm, p=0.008) was more important than dairy fat (beta =-0.009/10gm, p=0.806) when modeled simultaneously (model not shown). When dairy protein was added to Models 1 and 2, milk was no longer significant (not shown).
TABLE 4. Baseline diet of premenarchal girls and eventual Adult Height. All models are adjusted#. Estimated betas are per daily serving, or other shown daily quantity. Models 1 to 3 compare servings/day of milk to lowest intake (<1 serving/day) group. Sample sizes range from 4870 girls (Model 1) to 4829 girls (Model 4).
| Adult Height Difference (inches) | ||||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|
|
||||||
| Milk | Milk | Milk | Milk | Per 10gm: | Per 10gm: | |
| >3 vs <1 | >3 vs <1 | >3 vs <1 | (white+choc) | Dairy Fat | Dairy Protein | |
| Beta | +0.318 | +.317 | +.297 | +.076 | +.066 | +.068 |
| (P-Value) | (.001) | (0.001) | (0.003) | (0.001) | (0.040) | (0.003) |
| 2-3vs<1 | Cheese | Cheese | Cheese | Animal* Fat | Animal* Protein | |
| Beta | +0.198 | −.001 | −.005 | −.009 | −0.013 | −0.025 |
| (P-value) | (.003) | (0.989) | (0.915) | (.845) | (0.748) | (0.265) |
| 1 vs <1 | Yogurt | Yogurt | Yogurt | Vegetable Fat | Vegetable Protein | |
| Beta | +0.024 | −0.043 | −0.055 | −0.040 | −.020 | +.025 |
| (P-value) | (.757) | (0.735) | (0.668) | (0.755) | (0.451) | (0.495) |
| Calories/100kcal | Calories/100kcal | |||||
| Beta | +0.001 | +.000 | ||||
| (P-value) | (0.806) | (0.941) | ||||
Adjusted for race, age, age2 , age3 , Tanner Stage, height, and relative BMI, all at baseline. Models 1-3 include categorical white milk (results shown for >3 vs <1 glasses/day) and categorical chocolate milk (not shown). Model 4 shows results for white + chocolate milk (glasses/day, continuous). Cheese serving is one slice/day, and yogurt is one cup/day.
Dairy intakes are excluded from Animal Fat and Animal Protein grams.
Dairy-intake reporting errors are likely non-differential with respect to subsequent height growth. We demonstrated above, for all three outcomes, that conclusions were identical when we instead used cumulative averages for milk, cheese, yogurt and calories over multiple prior years, suggesting minimal bias in our estimated betas due to dietary measurement error. We also obtained adjusted estimates that take into account measurement error. These adjusted betas for milk were 2.5 times larger than those shown in Tables 2, 3 and 4 (Model 4). For example, the Table 2 Model 4 estimated milk effect of +0.024 inch/serving becomes +0.06 after correction for measurement error. The corrected milk beta for adult height (Table 4, Model 4) is 0.19inch/serving, so that a girl who at baseline reported 4 servings/day is expected to be nearly 0.80 inch taller in adulthood.
Figure 1 illustrates the long term impact of drinking >3 servings/day of milk from age 10 years to adulthood, relative to girls who consistently drink <1 serving/day throughout. Also shown is the existing height difference at age 10yr, between the high and low milk intake groups. We believe these baseline differences in height exist because girls who consumed high amounts of milk had probably been doing so for years prior to entering our study, and conversely for those who drank little or no milk at baseline. The average net difference in eventual adult height is nearly 1 inch, although with consideration of measurement error this may be closer to 2 inches.
Figure 1.
Illustrated difference in attained height growth curves between girls who consistently drink >3 servings/day and those who consistently drink <1 serving/day. Curve differences were estimated from models fit to N=3328 girls (White, not-Hispanic) who were aged 9-11 years and premenarchal at baseline (1996). Curves reflect the pre-existing height difference, between milk groups, at baseline.
Discussion
In this large cohort of girls, 80% of whom were followed at least 6 years, we found that intakes of dairy milk, yogurt, and dairy protein were positively associated with height growth during the following year; baseline intakes of these were also associated with peak height growth velocity. Baseline milk and dairy protein were similarly associated with eventual adult height. We found no evidence that cheese intakes were relevant to any of our growth outcomes. Analyzing cumulative prior dietary intakes provided the same conclusions (as described above) for all outcomes and all exposures. When modeled together, dairy protein, but not dairy fat, was associated with later height growth. We also considered models (not shown) that included milk type (whole, 2%, 1%, skim) along with milk quantity, and these models likewise did not support a role for dairy fat. Of particular interest is that dairy protein was associated with subsequent height growth, but non-dairy animal protein and vegetable protein were not significant in any of our models, suggesting that protein itself is not the growth promoting factor. The protein in milk may be a marker for other factors in the non-lipid component of milk, since adding dairy protein to models of milk intake greatly diminished the milk association. By analyzing dietary intakes reported prior to height growth outcomes, and by including total energy intake and/or multiple dietary factors within some models, the likelihood is enhanced that the associations we observed are causal.
Note that yogurt was a significant predictor of following year height growth and of PHV, but not of adult height. We believe this is due to two factors: adult height is a much later outcome than annual height growth or PHV, and correlations between baseline yogurt and later yogurt intakes were far lower than the correlations between baseline milk (or cheese) and later year intakes. The correlation between 1996 and 1997 total milk was 0.55, but only 0.36 between 1996 and 1997 yogurt intakes.
Our findings are generally consistent with earlier longitudinal studies. Adolescent boys who consumed the most total protein had higher height growth velocity curves than low-protein consumers (26), and similarly for preschool boys and girls (27). Girls who consumed more (energy adjusted) animal protein two years before peak growth had higher peak velocities (23). Unfortunately, those studies did not separate dairy from other animal protein. From NHANES data, childhood milk consumption was positively associated with adolescent and adult height (9). New Zealand children with a history of cow milk avoidance were shorter (28). A school-based milk intervention trial in Chinese girls demonstrated benefits for bone growth and also significantly greater height growth over 2 years (8). A longitudinal study of 92 Japanese children found greater 3-yr height growth among children who drank more milk (7). Our findings were also consistent with a pregnancy study in which infant birth length was related to maternal milk intake; interestingly, maternal dairy protein intake was linked to birth-weight, but dairy fat, cheese, and non-dairy protein were not (6).
The mechanism whereby dairy consumption promotes height growth in girls needs to be understood. Recent trials of Mongolian children and Boston children found that drinking milk increased somatotropic hormone (GH, IGF-1 and IGFBP-3) concentrations in pre-pubertal children, and the Mongolian children experienced rapid linear growth during the month-long milk intervention (29). In a randomized trial, milk supplementation raised IGF-1 in 12yr old girls (30). Other intervention trials similarly found increased blood concentrations of IGF-1 associated with milk intake (31-32). In a cross-sectional study of 2yr olds, milk intake was positively associated with higher concentrations of IGF-1 in blood and with taller height (33), though another cross-sectional analysis of 7-8 year-old girls found no associations among milk/dairy intakes, IGF-1, or height (34). The reason that our study demonstrated positive associations with milk but not with cheese may be that fermentation of cheese alters the biologic activity of IGF-1 in dairy products (35).
A major strength of this analysis was the longitudinal design of our study, in which height and weight measurements and dietary intakes were obtained annually on a large cohort of girls from all over the US. Longitudinal observational studies such as ours cannot determine causality as validly as randomized controlled trials, but our study design is superior to cross-sectional studies, where associations may represent reverse causality. Though we controlled for potential confounders in our models, some residual and unmeasured confounding may remain. We cannot exclude the possibility of incomplete adjustment of some covariates, or confounding through variables not considered. Because we included total energy intake in some models, and other models included all sources of protein simultaneously, or all sources of dietary fat simultaneously, we have minimized the possibility that higher dairy intakes were the result of rapidly growing (hungry) children, rather than the cause. Although our cohort is not representative of US girls, associations among factors within our cohort should still be valid. Because all participants are daughters of nurses, this reduces confounding by socioeconomic and other unmeasured factors, as well as enhances the accuracy of the information they provide. But the racial and ethnic makeup of our cohort (95% white/NH) limits the generalization of our findings.
The major limitation of our study was the necessity to collect data by self-report on mailed questionnaires, but with our large, geographically dispersed cohort, alternatives were not feasible. Errors in reporting dairy foods and beverages are likely to be non-differential with respect to subsequent height growth, resulting in underestimates of true associations. A food-based validation study of dietary questionnaires (in adult female nurses) found high validity for dairy products and most beverages, including milk (16). Still, we addressed the diet measurement error issue in two ways, by fitting models using cumulative averages of prior dietary intakes to reduce bias due to measurement error (22), and by using the validation study of individual foods (16) to further assess how measurement error may have biased our estimates.
This work is significant because studies in the past decade have suggested that adult height (36), height growth during ages 8-14yr (3), during ages 4-7 and 11-15yr (4), and peak height growth velocity (5), are associated with breast cancer risk. Whether the rapid growth itself or other factors, such as dietary intakes or hormones that may promote growth, are cancer initiators/promoters is unknown. The small number of cohort studies collecting data on childhood milk intake did not consistently support any association between early dairy consumption and adult breast cancer, though most of these studies involved diets recalled later in adulthood (37-40). Studies of dairy products consumed in adulthood, when there are no implications regarding height growth, had very mixed results regarding associations with breast cancer; the most recent review of prospective observational studies concluded there was no consistent link (41). We cannot here directly assess risk for breast cancer, because our cohort is still too young, but we were able to consider dietary factors that may promote more rapid pre-pubertal height growth, which may be a critical period for the development of breast cancer or other adult diseases.
These longitudinal analyses of three different height growth outcomes provided evidence that dairy milk and yogurt intakes and dairy protein consumption, but not protein from other sources (non-dairy animal and vegetable) or dietary fat or cheese, promote height growth. These findings suggest that a factor in the non-lipid phase of milk, but not protein itself, has growth promoting activity in pre-pubertal girls.
Acknowledgments
All five authors contributed to the collection of data from the GUTS cohort over the years. Drs. Berkey, Colditz and Willett conceived the hypotheses investigated in this manuscript. All authors participated in the design of this particular study; Dr. Berkey performed the analyses and drafted the manuscript. Funding was obtained by Dr. Colditz. All authors contributed to the interpretation of the data and writing/revision of the manuscript. The authors have no conflicts of interest to disclose.
Funding: Supported by grant DK46834 from the National Institutes of Health (Bethesda, MD) and by a grant from The Breast Cancer Research Foundation (NYC, NY).
References
- 1.Murata M. Secular trends in growth and changes in eating patterns of Japanese children. Am J Clin Nutr. 2000;72:1379S–1383S. doi: 10.1093/ajcn/72.5.1379s. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher LB, Pawson IG, Kretchmer N. Growth of immigrant children in the newcomer schools of San Francisco. Pediatrics. 1987;80:861–8. [PubMed] [Google Scholar]
- 3.Ahlgren M, Melbye M, Wohlfahrt J, Sorensen TI. Growth patterns and the risk of breast cancer in women. NEJM. 2004;351:1619–26. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- 4.De Stavola BL, dos Santos Silva I, McCormack V, Hardy RJ, Kuh DJ, Wadsworth ME. Childhood growth and breast cancer. Am J Epidemiol. 2004;159:671–682. doi: 10.1093/aje/kwh097. [DOI] [PubMed] [Google Scholar]
- 5.Berkey CS, Frazier AL, Gardner JD, Colditz GA. Adolescence and breast carcinoma risk. Cancer. 1999;85:2400–9. doi: 10.1002/(sici)1097-0142(19990601)85:11<2400::aid-cncr15>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Olsen SF, Halldorsson TI, Willett WC, et al. NUTRIX Consortium Milk consumption during pregnancy is associated with increased infant size at birth: prospective cohort study. Am J Clin Nutr. 2007;86:1104–10. doi: 10.1093/ajcn/86.4.1104. [DOI] [PubMed] [Google Scholar]
- 7.Okada T. Effect of cow milk consumption on longitudinal height gain in children. Letter to Editor. Am J Clin Nutr. 2004;80:1088–9. doi: 10.1093/ajcn/80.4.1088. [DOI] [PubMed] [Google Scholar]
- 8.Du X, Zhu K, Trube A, et al. School-milk intervention trial enhances growth and bone mineral accretion in Chinese girls aged 10-12 years in Beijing. Br J Nutr. 2004;92:159–68. doi: 10.1079/BJN20041118. [DOI] [PubMed] [Google Scholar]
- 9.Wiley AS. Does milk make children grow? Relationships between milk consumption and height in NHANES 1999-2002. Am J Human Biol. 2005;17:425–41. doi: 10.1002/ajhb.20411. [DOI] [PubMed] [Google Scholar]
- 10.Colditz GA, Hankinson SE. The Nurses’ Health Study: Lifestyle and health among women. Nat Rev Cancer. 2005;5:388–96. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 11.Berkey CS, Rockett HRH, Gillman MW, Colditz GA. One-year changes in activity and in inactivity among 10- to 15-year old boys and girl. Relationship to change in BMI. Pediatrics. 2003;111:836–843. doi: 10.1542/peds.111.4.836. [DOI] [PubMed] [Google Scholar]
- 12.Strauss RS. Comparison of measured and self-reported weight and height in a cross-sectional sample of young adolescents. Int J Obes Relat Metab Disord. 1999;23:904–908. doi: 10.1038/sj.ijo.0800971. [DOI] [PubMed] [Google Scholar]
- 13.Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 2000;106:52–58. doi: 10.1542/peds.106.1.52. [DOI] [PubMed] [Google Scholar]
- 14.Rockett HR, Wolf AM, Colditz GA. Development and reproducibility of a food frequency questionnaire to assess diets of older children and adolescents. J Am Diet Assoc. 1995;95:336–340. doi: 10.1016/S0002-8223(95)00086-0. [DOI] [PubMed] [Google Scholar]
- 15.Rockett HRH, Breitenbach M, Frazier AL, et al. Validation of a Youth/Adolescent Food Frequency Questionnaire. Prev Med. 1997;26:808–816. doi: 10.1006/pmed.1997.0200. [DOI] [PubMed] [Google Scholar]
- 16.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–67. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 17.Phillips SM, Bandini LG, Cyr H, Colclough-Douglas S, Naumova E, Must A. Dairy food consumption and body weight and fatness studied longitudinally over the adolescent period. Int J Obes Relat Metab Disord. 2003;27:1106–13. doi: 10.1038/sj.ijo.0802370. [DOI] [PubMed] [Google Scholar]
- 18.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–80. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 19.Kuczmarksi RJ, Ogden CL, Grummer-Strawn LM, et al. 2000 CDC growth charts: United States. National Center for Health Statistics/CDC; [Accessed April 30, 2004]. 2000. Web site. www.cdc.gov/growthcharts. [Google Scholar]
- 20.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 21.SAS Institute Inc . SAS/STAT Software: Changes and Enhancements Through Release 6.12. Proc Mixed. SAS Institute Inc; Cary, NC: 1997. [Google Scholar]
- 22.Willett WC. Nutritional Epidemiology. Oxford University Press; New York, NY: 1998. [Google Scholar]
- 23.Berkey CS, Gardner J, Frazier AL, Colditz GA. Childhood diet and body size related to menarche and adolescent growth in girls. Am J Epidemiol. 2000;152:446–52. doi: 10.1093/aje/152.5.446. [DOI] [PubMed] [Google Scholar]
- 24.Berkey CS, Wang X, Dockery DW, Ferris BG., Jr. Adolescent height growth in a US population. Ann Hum Biol. 1994;21:435–42. doi: 10.1080/03014469400003452. [DOI] [PubMed] [Google Scholar]
- 25.Berkey CS, Dockery DW, Wang X, Wypij D, Ferris BG., Jr. Longitudinal height velocity standards for US adolescents. Stat Med. 1993;12:403–14. doi: 10.1002/sim.4780120321. [DOI] [PubMed] [Google Scholar]
- 26.Berkey CS, Laird NM, Valadian I, Gardner J. The analysis of longitudinal growth data with covariates. In: Tanner JM, editor. Auxology ’88: Perspectives in the Science of Growth and Development. Smith-Gordon; London: 1989. pp. p31–40. [Google Scholar]
- 27.Berkey CS, Laird NM. Nonlinear growth curve analysis: estimating the population parameters. Ann Hum Biology. 1986;13:111–128. doi: 10.1080/03014468600008261. [DOI] [PubMed] [Google Scholar]
- 28.Black RE, Williams SM, Jones IE, Goulding A. Children who avoid drinking cow milk have low dietary calcium intakes and poor bone health. Am J Clin Nutr. 2002;76:675–80. doi: 10.1093/ajcn/76.3.675. [DOI] [PubMed] [Google Scholar]
- 29.Rich-Edwards JW, Ganmaa D, Pollak MN, et al. Milk consumption and the prepubertal somatotropic axis. Nutrition Journal. 2007;6:28. doi: 10.1186/1475-2891-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cadogan J, Eastell R, Jones N, Barker ME. Milk intake and bone mineral acquisition in adolescent girls: randomized, controlled intervention trial. BMJ. 1997;315:1255–60. doi: 10.1136/bmj.315.7118.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu K, Du X, Cowell CT, et al. Effects of school milk intervention on cortical bone accretion and indicators relevant to bone metabolism in Chinese girls aged 10-12 y in Beijing. Am J Clin Nutr. 2005;81:1168–75. doi: 10.1093/ajcn/81.5.1168. [DOI] [PubMed] [Google Scholar]
- 32.Hoppe C, Molgaard C, Juul A, Michaelsen KF. High intakes of skimmed milk, but not meat, increase serum IGF-1 and IGFBP-3 in eight-year-old boys. Eur J Clin Nutr. 2004;58:1211–16. doi: 10.1038/sj.ejcn.1601948. [DOI] [PubMed] [Google Scholar]
- 33.Hoppe C, Udam TR, Lauritzen L, Molgaard C, Juul A, Michaelsen KF. Animal protein intake, serum insulin-like growth factor I, and growth in healthy 2.5-yr-old Danish children. Am J Clin Nutr. 2004;80:447–452. doi: 10.1093/ajcn/80.2.447. [DOI] [PubMed] [Google Scholar]
- 34.Rogers I, Emmett P, Gunnell D, Dunger D, Holly J, ALSPAC Study Team Milk as a food for growth? The insulin-like growth factors link. Public Health Nutrition. 2006;9:359–368. doi: 10.1079/phn2006853. [DOI] [PubMed] [Google Scholar]
- 35.Kang SH, Kim JU, Imm JY, Oh S, Kim SH. The effects of dairy processes and storage on insulin-like growth factor-1 (IGF-1) content in milk and in model IGF-1-fortified dairy products. J Dairy Sci. 2006;89:402–9. doi: 10.3168/jds.S0022-0302(06)72104-X. [DOI] [PubMed] [Google Scholar]
- 36.Gunnell D, Okasha M, Smith GD, Oliver SE, Sandhu J, Holly JM. Height, leg length, and cancer risk: a systematic review. Epidemiol Rev. 2001;23:313–42. doi: 10.1093/oxfordjournals.epirev.a000809. [DOI] [PubMed] [Google Scholar]
- 37.Frazier AL, Li L, Cho E, Willett WC, Colditz GA. Adolescent diet and risk of breast cancer. Cancer Causes Control. 2004;15:73–82. doi: 10.1023/B:CACO.0000016617.57120.df. [DOI] [PubMed] [Google Scholar]
- 38.Hjartaker A, Laake P, Lund E. Childhood and adult milk consumption and risk of premenopausal breast cancer in a cohort of 48,844 women – the Norwegian women and cancer study. Int J Cancer. 2001;93:888–93. doi: 10.1002/ijc.1409. [DOI] [PubMed] [Google Scholar]
- 39.van der Pols JC, Bain C, Gunnell D, Smith GD, Frobisher C, Martin RM. Childhood dairy intake and adult cancer risk: 65-yr followup of the Boyd Orr cohort. Am J Clin Nutr. 2007;86:1722–9. doi: 10.1093/ajcn/86.5.1722. [DOI] [PubMed] [Google Scholar]
- 40.Michels KB, Rosner BA, Chumlea WC, Colditz GA, Willett WC. Preschool diet and adult risk of breast cancer. Int J Cancer. 2006;118:749–54. doi: 10.1002/ijc.21407. [DOI] [PubMed] [Google Scholar]
- 41.Michels KB, Mohllajee AP, Roset-Bahmanyar E, Beehler GP, Moysich KB. Diet and breast cancer: a review of the prospective observational studies. Cancer. 2007;109(12 Suppl):2712–49. doi: 10.1002/cncr.22654. [DOI] [PubMed] [Google Scholar]