Abstract
Objectives
To present and discuss a high-performance negative depletion method for the isolation of circulating tumor cells (CTCs) in the blood of patients with head and neck cancer and to determine the correlation between the presence of CTCs and early clinical outcome in these patients.
Design
Prospective clinical follow-up study of patients with squamous cell carcinoma of the head and neck (SCCHN) undergoing surgical intervention, who had peripheral blood examined for the presence of CTCs.
Patients
The study population comprised 48 patients diagnosed as having SCCHN and undergoing surgical intervention.
Intervention
A negative depletion process to isolate and quantify CTCs from the blood of patients with SCCHN using immunomagnetic separation was developed and validated. Immunostaining for cytokeratin was performed on the enriched samples to determine the number of CTCs extracted from each patient’s blood sample. Correlation of the presence of CTCs, tumor stage, nodal status, clinical characteristics, and outcome was made.
Main Outcome Measure
Disease-free survival.
Results
Our initial data, that have a mean follow-up of 19.0 months, suggest that patients with no detectable CTCs per milliliter of blood had a significantly higher probability of disease-free survival (P=.01). There was no correlation between the presence of CTCs with regard to age, sex, tumor site, stage, or nodal involvement.
Conclusions
Our enrichment technology, based on the removal of normal cells, has been used on the peripheral blood of patients with head and neck cancer for which follow-up data were collected. If no CTCs were present, a statistically significant improved disease-free survival was observed in SCCHN. A blood test with such a prognostic capability could have important implications in the treatment of patients with head and neck cancer.
In 2009, According to the National Cancer Institute, 48 010 new cases of oral cavity, pharyngeal, and laryngeal cancer were estimated to occur in the United States; approximately 11 260 deaths were attributed to these cases. It has been established that squamous cell carcinomas make up greater than 95% of these cases.1,2 For all stages combined, the 5-year survival rate is approximately 50%,3 and this rate has not changed significantly in the last 3 decades. Treatment failure in patients with squamous cell carcinoma of the head and neck (SCCHN) can include local recurrence, regional recurrence (cervical lymph nodes), distant metastasis, or development of a second primary cancer. Despite intensive therapeutic treatment of the primary lesion, approximately 50% to 60% of patients will later develop locoregional recurrence, and 20% of these patients will go on to develop distant metastasis. Despite surgical resection with negative histopathological margins, approximately 20% of patients will have local recurrence. There are various theories with respect to the cause of local cancer recurrence including field cancerization4 or that clinically relevant micrometastatic spread is not detectable by histopathological margins alone.5 In oral cavity cancer, between 23% and 27% of patients with SCCHN with a clinically negative neck on physical examination will have micrometastatic disease on elective neck dissection.6,7 The presence of lymphatic metastasis is currently the most significant prognostic factor affecting survival; the presence of this alone can reduce survival by 50%.8–10 These current statistics indicate that there is a significant need for a reliable blood marker to determine prognosis in patients with SCCHN, specifically those who may be at higher risk of locoregional or distant recurrence.
The concept of the development of metastasis involves a tumor cell or microembolus that dissociates from the primary tumor, circulates within lymphatic or vascular channels, and ultimately resides in a regional or distant site. The ability to detect such cells, known as circulating tumor cells (CTCs), may have an impact on the prognosis and treatment of patients with cancer by providing (1) definitive evidence for early occult spread of the primary cancer, (2) a risk factor for the development of future metastasis, and (3) a peripheral marker for treatment susceptibility and cancer surveillance. Furthermore, genetic analysis of CTCs may lead to targeted treatment strategies.11 Studies have linked circulating/ disseminated tumor cells to poor prognosis in breast,12 lung,13,14 prostate,15 and colorectal cancers.16 It should be noted, however, that the role of CTCs in patients with SCCHN is yet to be conclusively elucidated, and the mechanism between CTCs and cancer recurrence or metastasis is not yet known. Nevertheless, the presence of CTCs could be an independent marker of more aggressive cancers.
It is a diagnostic challenge to identify CTCs from a patient’s blood specimen when 1 mL of blood contains an average of 5 billion red blood cells (RBCs), 7 million white blood cells (WBCs), and 295 million platelets.17 A negative depletion method involves removal of normal, unwanted cells, such as RBCs through lysis, and normal lymphocytes through magnetic depletion of CD45+-labeled cells. In contrast, positive selection of tumor cells can be performed by magnetic selection of epithelial cells using antibodies binding to surface markers such as epithelial cell adhesion molecule (EpCAM). At present, there are 3 general types of CTC detection methods: (1) immunocytochemistry, which implies visual observations, (2) flow cytometry and/or image cytometry, and (3) reverse transcriptase–polymerase chain reaction. Prior to the use of 1 of these 3 detection methods, an enrichment method (using negative depletion or positive selection) can greatly increase both the sensitivity and specificity of the test.
A majority of the published studies on the detection of CTCs in the blood of patients with cancer uses a combination of a negative depletion step (removal of RBCs) and a positive selection of the CTCs using an antiepithelial antibody bound to a magnetic particle. The most well known of these methods available is the CellSearch System by Veridex LLC, Raritan, New Jersey.18,19 In addition, the “CTC Chip” technology uses a positive selection method to initially isolate cells expressing EpCAM and then allow staining with secondary antibody markers.20
However, the use of a positive selection technique for the CTCs introduces a potential, significant bias into the final detection analysis: the CTCs must express the surface marker to which the antibodies are specific. This bias has been recently experimentally demonstrated by a study from Sieuwerts et al.21 Specifically, these researchers demonstrated that the effectiveness of the CellSearch System varied significantly with respect to its ability to detect subtypes of human breast cancer cells spiked into healthy human blood. As expected, the CellSearch System was able to recover 85% of the breast cancer cells exhibiting the typical epithelial characteristics. However, as the cell types expressed a different phenotype characteristic of tumor-initiating stem cells (CD24 low and CD44 high), the recovery of the spiked cells drops to only 2%.21 EpCAM may be downregulated during epithelial-to-mesenchymal transition, in which cancer cells may acquire a more invasive, migratory phenotype.20 Therefore, using a positive selection technique may not identify CTCs in some patients. In contrast to the positive selection approaches, which introduce significant bias into the type of CTCs detected, we have been developing an enrichment method that is based only on negative depletion of normal cells. We recently demonstrated that this optimized method is able to obtain an average 5.66 log10 enrichment of CTCs in the blood of patients with head and neck cancer.22 In this initial study, we sought to determine the clinical significance of CTCs with regard to disease-free survival in patients with SCCHN and present our prospective clinical follow-up to this point.
METHODS
BLOOD SAMPLES
After obtaining informed consent for participation in this institutional review board–approved study, blood samples of 10 to 18 mL were drawn from patients at the time of surgery for SCCHN at the James Cancer Hospital and Solove Research Institute at The Ohio State University, Columbus. Blood samples, collected in green-top BD Vacutainer tubes (BD Vacutainer Systems, Franklin Lakes, New Jersey), were stored at 4°C until processing, which occurred within 24 hours after procurement. Operators were blinded to all clinical and histopathological correlative information during the sampling and analysis process.
NEGATIVE DEPLETION METHOD
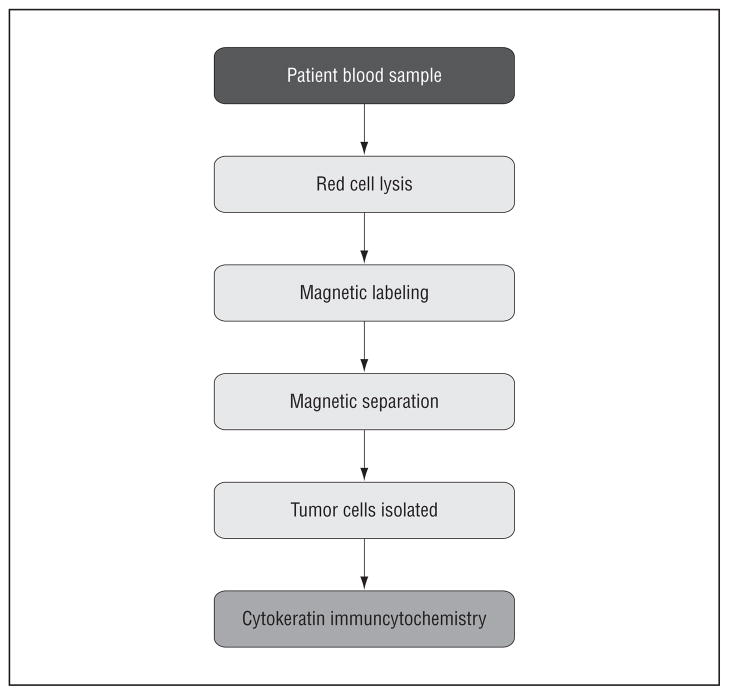
The optimized enrichment process has been previously presented (Yang et al22 and Balasubramanian et al23) and will only be summarized here. First, the blood sample was subjected to an RBC lysis step, followed with labeling using anti-CD45, tetrameric antibody complex (TAC; Stem Cell Technologies, Vancouver, British Columbia, Canada), and dextran-coated magnetic nanoparticles. The immunomagnetically labeled cells were then passed through our optimized magnetic deposition sorting system to deplete a majority of the peripheral blood leukocytes and obtain an enriched sample containing the CTCs. The enriched sample containing the suspended CTCs was then divided into multiple aliquots; one of these was subjected to a cytospin for immunocytochemistry staining, and a second aliquot was lysed to obtain RNA for further molecular analysis. An outline of the techniques performed is shown in Figure 1.
Figure 1.
Overall circulating tumor cell enrichment process.
IMMUNOCYTOCHEMISTRY
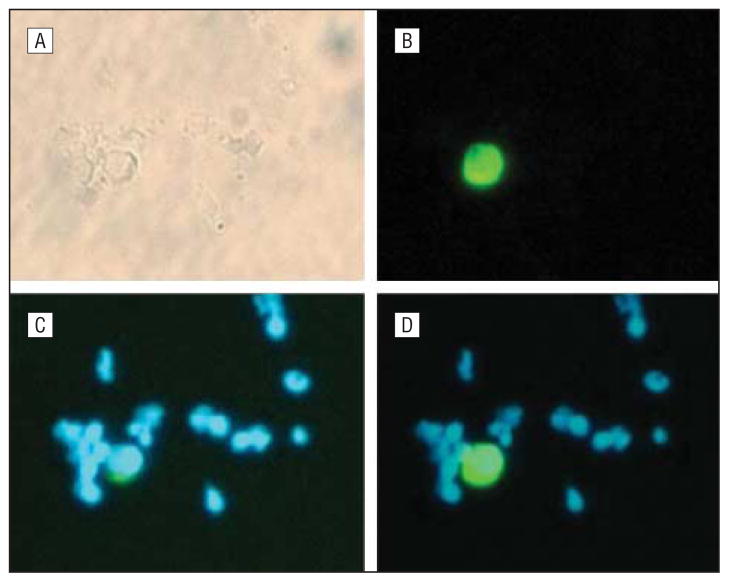
The cytospin was obtained using a Shannon cytospin instrument (Thermo Scientific, Pittsburgh, Pennsylvania) and fixed in 1% formaldehyde for 10 minutes. For the immunostaining, a 1:10 dilution of the anticytokeratin fluorescein isothiocyanate conjugated (FITC) antibody CK3-6H5 (MiltenyiBiotec, Auburn, California), which recognizes cytokeratin 8, 18, and 19, was applied to the cytospin and incubated at 37°C for 30 minutes in a humidity chamber. Next, the slide was washed in phosphate-buffered saline 3 times for 5 minutes each time and air dried. The air-dried slides were mounted in Vectashield mounting medium with 4,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, California). Slides were observed on a Nikon fluorescent microscope (Nikon Corp, Tokyo, Japan), equipped with filters for DAPI and FITC emissions. Following the published criteria used by Partridge et al24 and Riethdorf et al19 to identify a CTC, the cell must (1) be double positive for FITC and DAPI staining; (2) have an intact membrane; and (3) have a high nuclear to cytoplasmic ratio and be as large, or larger when compared with surrounding peripheral blood leukocytes; and (4) be negative for CD45-PE. In our current system, criteria 4 is indirect, since we are magnetically labeling the CD45-positive lymphocytes with the TAC complex and magnetically removing them; therefore, it is assumed that the cells remaining are not CD45 positive. Figure 2 shows a representative photograph of this technique described in our previously published work: (A) bright field microscopy, (B) filters appropriate for FITC (anti–pan-cytokeratin, green cytoplasm), (C) filters appropriate for DAPI stain (nuclei blue), and (D) combined image of (B) and (C).22 The final concentration of CTCs per milliliter of peripheral blood was calculated using the number of CTCs on the cytospin using appropriate dilution factors during the negative depletion process.
Figure 2.
Circulating tumor cell (CTC) immunocytochemistry technique. A, Light microscopy; B, cytokeratin (cytoplasm stains green); C, DAPI (4,6-diamidino-2-phenylindole) (nucleus stains blue); and D, combined cytokeratin and DAPI with high nuclear to cytoplasmic ratio (defined a CTC) (original magnification ×200 for all images). Reprinted with permission from Yang et al.22
INCLUSION/EXCLUSION CRITERIA AND DATA ANALYSIS
The inclusion criteria for patients in this study and prospective clinical follow-up were the following: (1) final pathological diagnosis of SCCHN, (2) successful enrichment (after RBC lysis, depleting nucleated cells by at least 1 log10 enrichment), and (3) clinical follow-up data exist. Without a high level of enrichment, the ability to detect CTCs can be questionable. The use of this criteria resulted in exclusion of the following: 2 samples for less than 1 log10 enrichment, 2 samples for non-cancer-related mortality (ie, perioperative myocardial infarction), 2 samples with non-SCCHN on final pathological analysis, and 5 samples owing to failure of patient to follow-up after the surgery. Statistical analysis was performed with the log-rank test for Kaplan-Meier survival plots and the χ2 and t tests for calculation of P values as given in the Table.
Table.
Patient Characteristics Including Age, Sex, Tumor Site, Overall Stage, Nodal Status, Adjuvant Therapy, Smoking, and Alcohol Use
| Characteristic | Patients (N = 48)
|
P Value | |
|---|---|---|---|
| CTCs Present (n = 34 [71%]) | No CTCs Present (n = 14 [29%]) | ||
| Age, range, y | 62.1 | 59.5 | .57 |
| Men/women, % | 62/38 | 79/21 | .26 |
| Smoking ≥ 15 P-Y, No. (%) | 26 (77) | 9 (64) | .39 |
| EtOH, moderate, No. (%) | 17 (50) | 7 (50) | >.99 |
| Follow-up, mean, mo | 19.1 | 18.9 | .17 |
| Tumor site, No. (%) | .50 | ||
| Oral cavity | 19 (56) | 6 (40) | |
| Oropharynx | 6 (18) | 4 (27) | |
| Larynx | 8 (24) | 4 (27) | |
| Hypopharynx | 1 (3) | 1 (7) | |
| Overall stage, No. (%) | .98 | ||
| 1 | 3 (9) | 1 (7) | |
| 2 | 9 (27) | 4 (29) | |
| 3 | 6 (18) | 3 (21) | |
| 4 | 16 (47) | 6 (43) | |
| Pathological nodal involvement, No. (%) | 13 (41) | 6 (60) | .42 |
| Adjuvant therapy, No. (%) | 21 (62) | 9 (64) | .87 |
| Nucleated cell log depletion, mean (SD), log10 | 2.53 (0.70) | 2.57 (0.63) | .85 |
| Total cell log depletion, mean (SD), log10 | 5.32 (0.61) | 5.31 (0.64) | .98 |
Abbreviations: CTCs, circulating tumor cells; EtOH, ethanol; P-Y, pack-years.
RESULTS
Following the inclusion and exclusion criteria previously described, we are reporting the follow-up data of 48 patients. The mean (SD) prospective time of follow-up for patients not presenting with a recurrence or cancer-related mortality was 19.0 (10.1) months (range, 2–38 months); clinically detectable recurrences were seen as early as 2 months after the blood sample was drawn. The mean (SD) total cell enrichment was 5.32 (0.61) log10 (range, 4.15–7.11 log10), and the mean (SD) nucleated cell enrichment was 2.53 (0.70) log10 (range, 1.41–4.35 log10). A summary of patient characteristics including age, sex, tumor site, overall stage, nodal status, adjuvant therapy, smoking, and alcohol use is provided in the Table; there were no significant differences between these 2 groups. In addition, 9 of 46 patients (20%) had microscopically positive surgical margins on final pathologic analysis of the primary tumor, and therefore adjuvant therapy with chemoradiation was recommended. Only 1 patient was not compliant with completing chemoradiation therapy, and this patient developed progression of locoregional disease. Of the remaining 8 patients, 4 (50%) with positive microscopic margins had no evidence of disease during follow-up.
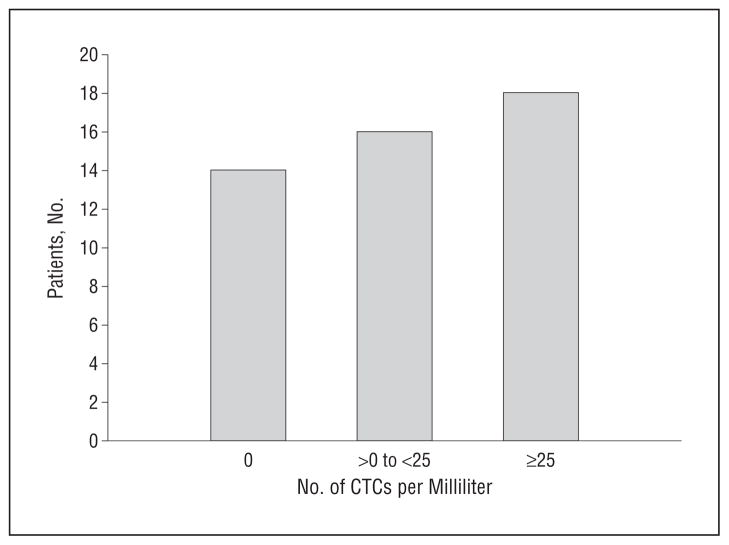
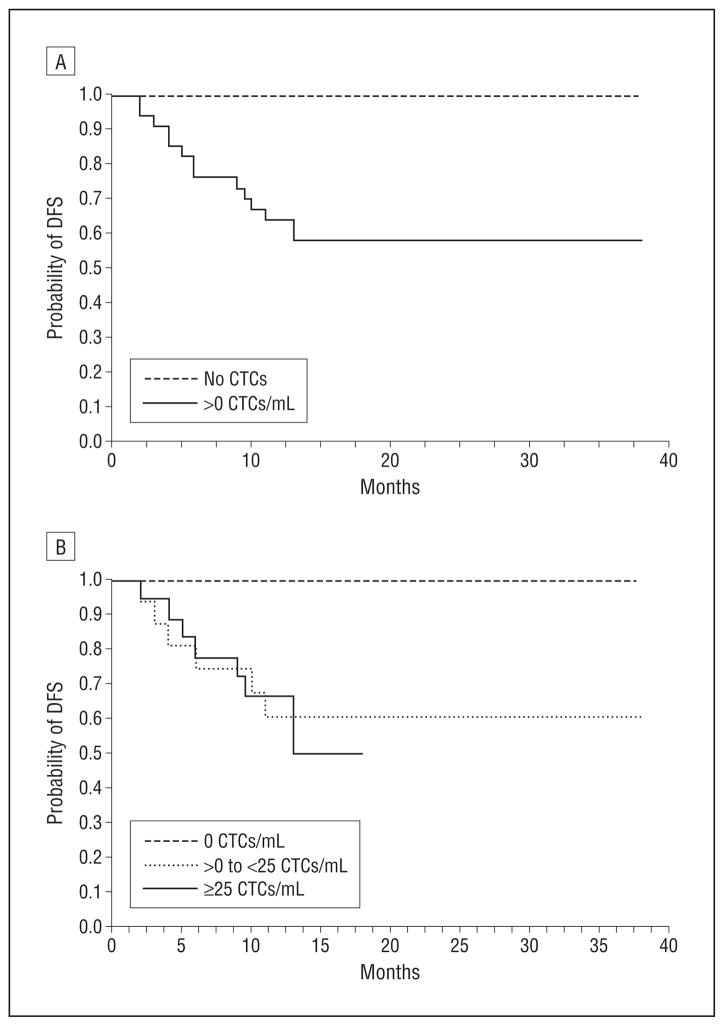
The distribution of CTCs per milliliter of blood in our patients with SCCHN is shown in Figure 3. From these data, a Kaplan-Meier survival plot was made with respect to the probability of disease-free survival as a function of months after the initial sample was taken for 2 groups: patients with no CTCs detected (group A) and patients with greater than 0 CTCs/mL of blood (group B). This plot is presented in Figure 4A, which shows improved disease-free survival in patients with no CTCs present (P= .01). Of particular note, we observed no instances of cancer recurrence or disease-related mortality in patients with no detectable CTCs. Refining the data into 3 groups (A, no CTCs; B, >0 to <25 CTCs/mL peripheral blood; and C, ≥25 CTCs/mL peripheral blood), we observed that those patients with a higher number of CTCs were more likely to have a worse clinical outcome (P= .04) (Figure 4B).
Figure 3.
Distribution of circulating tumor cells (CTCs) per milliliter in peripheral blood of patients with squamous cell carcinoma of the head and neck.
Figure 4.
Kaplan-Meier disease-free survival plot with regard to the presence or absence (A) and number (B) of circulating tumor cells (CTCs) in peripheral blood. DFS indicates disease-free survival.
COMMENT
This study has shown that the visually confirmed presence of CTCs in the peripheral blood of patients with SCCHN, using a high-performance negative depletion technique, was predictive of disease recurrence and/or cancer-related mortality (P= .01). The absence of CTCs may be promising as an indicator for disease-free survival and continued prospective follow-up on these patient outcomes is needed. Furthermore, the concentration of CTCs (number of CTCs per milliliter of blood) may be suggestive of outcome as shown in Figure 4B. A study by Cristofanilli et al18 on 177 patients with meta-static breast cancer concluded that the number of CTCs was an independent predictor of progression-free survival as well as overall survival. Similarly, Partridge et al,24 in a study on samples of bone marrow and central venous blood, collected preoperatively or postoperatively from patients with head and neck cancer, concluded that detection of disseminated tumor cells before or during surgery indicated a high risk of local and distant recurrence and reduced survival.
Partridge et al,24 as with our study, used a negative enrichment approach prior to immunocytochemistry or reverse transcriptase–polymerase chain reaction analysis of the blood samples. Unfortunately, they did not report the level of enrichment (in our case, log10 depletion). In our experience, establishing a minimum level of log10 depletion as a threshold prior to analysis is critical. If insufficient enrichment is achieved, CTCs may not be identified by visual inspection of cytospins. Tong et al25 experimentally demonstrated specifically that the final purity needed for a positive detection of a spiked cancer cell into human blood ranged from 1 cancer cell in 103 total nucleated cells to 1 cancer cell in 105 total nucleated cells. Our nucleated cell enrichment had a mean (SD) of 2.56 (0.64) (range, 1.41–4.35).
In our study, it is interesting to note the significantly higher probability of disease-free survival of patients with no detectable CTCs. As indicated in the Table, there were patients in overall stages 1 to 4 in both groups. It was interesting to note that the presence of CTCs did not correlate with other prognostic indicators such as the presence of nodal metastasis (including specific N staging) or overall stage, suggesting that CTCs are a potentially independent poor prognostic risk factor for SCCHN. Certainly, further prospective data collection and additional patient enrollment are necessary to determine the independent prognostic role of CTCs in SCCHN.
A legitimate criticism of this work is whether false-positive results can occur from non-CTCs staining positive for the anti–pan-cytokeratin antibody used in this study. It has been suggested that illegitimate expression of cytokeratin genes could lead to such a situation, so the morphological assessment of the cell is of critical importance to differentiate a CTC from a hematopoietic cell.24 Interestingly, it has been demonstrated in a microarray study of 866 leukemia and lymphoma cases, that 1.5% of B- or T-cell lymphomas had aberrant cytokeratin expression; cytokeratin expression was not found in acute leukemias.26 All of the patients in this study have been independently diagnosed as having SCCHN (none of the 48 patients had lymphoma); yet only 70.8% of the patient samples were positive for CTCs based on visual inspection and positive for the binding of anticytokeratin antibody. Therefore, not all patients with cancer have detectable cytokeratin-positive cells in their blood. If cytokeratin-positive cells (based on the binding of the anti–pan-cytokeratin antibody) are present in normal blood, one would expect a much higher percentage of patients with these positively stained cells.
Our overall enrichment method and staining protocol is based on a previously published study, which used 2 squamous cell carcinoma cell lines spiked into buffy coats purchased from the American Red Cross. Specifically, more than 38 enrichments were conducted in which the final samples were visually counted for final number of total cells and spiked cancer cells using the staining protocol in this study. Because these spiked cancer cells were larger than typical peripheral blood leukocytes with noticeably larger nuclei and bright cytokeratin staining, there was no ambiguity in visual observations between leukocytes and cancer cells. In all of those visually identified samples, the only positively stained cytokeratin cells were the larger cells with large nuclei. We never observed a peripheral blood leukocyte or any other cell type that stained with the anticytokeratin antibody.25 We have also conducted our enrichment and staining protocol on blood samples from 10 healthy donors, which demonstrated no cytokeratin-positive cells.
An important advantage to the negative depletion technique can be demonstrated by 2 specific patients in this study. A 58-year-old woman with a notable smoking and alcohol consumption history, who had 2 simultaneous cancers, lung and supraglottic laryngeal, was found to have 29.4 CTCs/mL of blood. This patient had only a diagnostic biopsy performed, and she had evidence of distant metastasis at the time of blood sampling. This was the only patient included in this study who did not have an open surgical procedure because there was evidence of meta-static disease. Another patient, a 63-year-old man, with recurrent laryngeal cancer, who was found to have a lung cancer several months after total laryngectomy, had 529.7 CTCs/mL of blood. When such cancers share risk factors (ie, smoking), while rare, this situation can occur.
In our study, 35 patients (73%) had at least a 15 pack-year smoking history, and 24 patients (50%) had at least a moderate-heavy alcohol consumption (10–20 drinks per week). Given such clinical scenarios, the characterization of CTCs is confounded because there could be the possibility of different cancers producing their own populations of CTCs. In this situation, only a negative depletion technique, like the one we present, does not instill bias for a specific type of cancer (ie, cancer that expresses EpCAM) as does a positive selection technique, and it allows for multimarker analysis on the same CTCs. We are currently advancing such techniques to investigate the use of multimarker confocal microscopy and further delineate multiple populations of CTCs, including markers that are not typically associated with epithelial cells.22
The patients in this study are being followed up prospectively, and there are still many unanswered questions regarding the biological basis and role of CTCs in SCCHN. This article highlights our initial clinical results, but we do not yet know what the long-term follow-up of these patients will yield. Unfortunately, compliance and follow-up in this patient population can be a limitation; of this initial group of patients, 5 have failed to return to follow-up examination since their surgery. We continue prospective long-term follow-up of all of the patients in this study, with the ultimate goal being to incorporate CTC identification into the management of patients with SCCHN.
CONCLUSIONS
We have previously published a technique for the identification of CTCs in the blood of patients with SCCHN,22 and this is our first clinical report of patient outcomes. From a clinical standpoint, to date, with a mean overall follow-up of 19.0 months, improved disease-free survival was seen in patients with no detectable CTCs (P=.01); increasing numbers of CTCs also correlated with worse prognosis (P= .04). Interestingly, there was no association between stage or nodal status and presence of CTCs.
This is an ongoing study, and we plan to continue to accrue patients to expand our knowledge of CTCs in SCCHN. Further prospective follow-up on these patients will ultimately determine the long-term significance of CTCs and their potential role. Further genetic analysis of CTCs, including real-time polymerase chain reaction, may identify molecular changes to characterize CTCs relative to the primary tumor. The development of a prognostic blood test is of critical importance to improve clinical outcome in patients with SCCHN.
Acknowledgments
Funding/Support: This work is supported by National Science Foundation (grant BES-0124897); the National Cancer Institute (grant R01 CA97391-01A1); the State of Ohio Third Frontier Program (grant ODOD 26140000: TECH 07-001); and the National Cancer Institute CCC Core Grant (P30 CA16058).
Footnotes
Financial Disclosure: None reported.
Previous Presentation: This study was presented at the annual meeting of the American Head and Neck Society; April 28-29, 2010; Las Vegas, Nevada.
Additional Contributions: Agnes Hurtuk assisted with patient consents, and Ronda Huston helped obtain patient medical charts.
Author Contributions: Drs K. R. Jatana and Balasubramanian shared first authorship of this article. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: K. R. Jatana, Balasubramanian, Lang, Ozer, Schuller, and Chalmers. Acquisition of data: K. R. Jatana, Balasubramanian, Lang, Yang, C. A. Jatana, White, Agrawal, and Teknos. Analysis and interpretation of data: K. R. Jatana, Balasubramanian, Lang, Yang, C. A. Jatana, and Chalmers. Drafting of the manuscript: K. R. Jatana, Balasubramanian, C. A. Jatana, Ozer, and Chalmers. Critical revision of the manuscript for important intellectual content: K. R. Jatana, Balasubramanian, Lang, Yang, White, Agrawal, Schuller, Teknos, and Chalmers. Statistical analysis: K. R. Jatana, Balasubramanian, and Chalmers. Obtained funding: Teknos and Chalmers. Administrative, technical, and material support: K. R. Jatana, Balasubramanian, Lang, Yang, C. A. Jatana, White, and Chalmers. Study supervision: K. R. Jatana, Ozer, Schuller, Teknos, and Chalmers.
References
- 1.Franceschi D, Gupta R, Spiro RH, Shah JP. Improved survival in the treatment of squamous carcinoma of the oral tongue. Am J Surg. 1993;166(4):360–365. doi: 10.1016/s0002-9610(05)80333-2. [DOI] [PubMed] [Google Scholar]
- 2.Zarbo RJ, Crissman JD. The surgical pathology of head and neck cancer. Semin Oncol. 1988;15(1):10–19. [PubMed] [Google Scholar]
- 3.Gath HJ, Brakenhoff RH. Minimal residual disease in head and neck cancer. Cancer Metastasis Rev. 1999;18(1):109–126. doi: 10.1023/a:1006268621730. [DOI] [PubMed] [Google Scholar]
- 4.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6(5):963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Loree TR, Strong EW. Significance of positive margins in oral cavity squamous carcinoma. Am J Surg. 1990;160(4):410–414. doi: 10.1016/s0002-9610(05)80555-0. [DOI] [PubMed] [Google Scholar]
- 6.McGuirt WF, Jr, Johnson JT, Myers EN, Rothfield R, Wagner R. Floor of mouth carcinoma: the management of the clinically negative neck. Arch Otolaryngol Head Neck Surg. 1995;121(3):278–282. doi: 10.1001/archotol.1995.01890030020004. [DOI] [PubMed] [Google Scholar]
- 7.Yuen AP, Wei WI, Wong YM, Tang KC. Elective neck dissection versus observation in the treatment of early oral tongue carcinoma. Head Neck. 1997;19 (7):583–588. doi: 10.1002/(sici)1097-0347(199710)19:7<583::aid-hed4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Jones AS, Roland NJ, Field JK, Phillips DE. The level of cervical lymph node metastases: their prognostic relevance and relationship with head and neck squamous carcinoma primary sites. Clin Otolaryngol Allied Sci. 1994;19(1):63–69. doi: 10.1111/j.1365-2273.1994.tb01150.x. [DOI] [PubMed] [Google Scholar]
- 9.Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer. 1993;71(2):452–456. doi: 10.1002/1097-0142(19930115)71:2<452::aid-cncr2820710228>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 10.Snow GB, Annyas AA, van Slooten EA, Bartelink H, Hart AA. Prognostic factors of neck node metastasis. Clin Otolaryngol Allied Sci. 1982;7(3):185–192. doi: 10.1111/j.1365-2273.1982.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 11.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4(6):448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 12.Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micro-metastasis in breast cancer. NEJM. 2005;353(8):793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 13.Pantel K, Izbicki J, Passlick B, et al. Frequency and prognostic significance of isolated tumour cells in bone marrow of patients with non-small-cell lung cancer without overt metastases. Lancet. 1996;347(9002):649–653. doi: 10.1016/s0140-6736(96)91203-9. [DOI] [PubMed] [Google Scholar]
- 14.Kasimir-Bauer S, Schleucher N, Weber R, Neumann R, Seeber S. Evaluation of different markers in non-small cell lung cancer: prognostic value of clinical staging, tumour cell detection and tumour marker analysis for tumour progression and overall survival. Oncol Rep. 2003;10(2):475–482. [PubMed] [Google Scholar]
- 15.Köllermann J, Weikert S, Schostak M, et al. Prognostic significance of disseminated tumor cells in the bone marrow of prostate cancer patients treated with neoadjuvant hormone treatment. J Clin Oncol. 2008;26(30):4928–4933. doi: 10.1200/JCO.2007.15.0441. [DOI] [PubMed] [Google Scholar]
- 16.Leinung S, Würl P, Schönfelder A, Weiss CL, Röder I, Schönfelder M. Detection of cytokeratin-positive cells in bone marrow in breast cancer and colorectal carcinoma in comparison with other factors of prognosis. J Hematother Stem Cell Res. 2000;9(6):905–911. doi: 10.1089/152581600750062354. [DOI] [PubMed] [Google Scholar]
- 17.McKenzie . Textbook of Hematology. Baltimore, MD: Williams & Wilkins Inc; 1996. [Google Scholar]
- 18.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 19.Riethdorf S, Fritsche H, Müller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13(3):920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 20.Maheswaran S, Haber DA. Circulating tumor cells: a window into cancer biology and metastasis. Curr Opin Genet Dev. 2010;20(1):96–99. doi: 10.1016/j.gde.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sieuwerts AM, Kraan J, Bolt J, et al. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst. 2009;101(1):61–66. doi: 10.1093/jnci/djn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, Lang JC, Balasubramanian P, et al. Optimization of an enrichment process for circulating tumor cells from the blood of head and neck cancer patients through depletion of normal cells. Biotechnol Bioeng. 2009;102(2):521–534. doi: 10.1002/bit.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balasubramanian P, Yang L, Lang JC, et al. Confocal images of circulating tumor cells obtained using a methodology and technology that removes normal cells. Mol Pharm. 2009;6(5):1402–1408. doi: 10.1021/mp9000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Partridge M, Brakenhoff R, Phillips E, et al. Detection of rare disseminated tumor cells identifies head and neck cancer patients at risk of treatment failure. Clin Cancer Res. 2003;9(14):5287–5294. [PubMed] [Google Scholar]
- 25.Tong X, Yang L, Lang JC, Zborowski M, Chalmers JJ. Application of immuno-magnetic cell enrichment in combination with RT-PCR for the detection of rare circulating head and neck tumor cells in human peripheral blood. Cytometry B Clin Cytom. 2007;72(5):310–323. doi: 10.1002/cyto.b.20177. [DOI] [PubMed] [Google Scholar]
- 26.Adams H, Schmid P, Dirnhofer S, Tzankov A. Cytokeratin expression in hematological neoplasms: a tissue microarray study on 866 lymphoma and leukemia cases. Pathol Res Pract. 2008;204(8):569–573. doi: 10.1016/j.prp.2008.02.008. [DOI] [PubMed] [Google Scholar]