Abstract
Activation of the GABAA receptor results in inhibition of neuronal activity. One subunit of this multi-subunit receptor termed alpha 6 (Gabrα6) contributed to inflammatory temporomandibular joint (TMJ) nociception but TMJ disorders often include myofascial pain. To address Gabrα6 role in myofascial pain we hypothesized that Gabrα6 has an inhibitory role in myofascial nociceptive responses similar to inflammatory TMJ arthritis. To test this hypothesis a, myofascial nociceptive response was induced by placing a ligature bilaterally on the tendon attachment of the anterior superficial part of a male rat's masseter muscle. Four days after ligature placement Gabrα6 expression was reduced by infusing the trigeminal ganglia (TG) with small interfering RNA (siRNA) having homology to either the Gabrα6 gene (Gabra6 siRNA) or no known gene (control siRNA). After siRNA infusion nociceptive behavioral responses were measured, i.e., feeding behavior and head withdrawal after pressing upon the region above the ligature with von Frey filaments. Neuronal activity in the TG and trigeminal nucleus caudalis and upper cervical region (Vc–C1) was measured by quantitating the amount of phosphorylated extracellular signalregulated kinase (p-ERK). Total Gabrα6 and GABAA receptor contents in the TG and Vc–C1 were determined. Gabrα6 siRNA infusion reduced Gabrα6 and GABAA receptor expression and significantly increased the nociceptive response in both nociceptive assays. Gabra6 siRNA infusion also significantly increased TG p-ERK expression of the ligated rats. From these results we conclude GABAA receptors consisting of the Gabrα6 subunit inhibit TG nociceptive sensory afferents in the trigeminal pathway and have an important role in the regulation of myofascial nociception.
Keywords: pain, trigeminal ganglia, temporomandibular joint, GABA, myofascial
Introduction
The GABAA receptor can function to inhibit neuronal activity and is comprised of five separate subunits, many of which have several isoforms, including the GABAA receptor subunit alpha 6 (Gabrα6). Gabrα6 has recently been shown to be expressed in both neurons and satellite glia of the trigeminal ganglia (TG) (Puri et al., 2011, 2012). The Gabrα6 positive neuronal cell bodies in the TG project axons to the temporomandibular joint (TMJ) (Puri et al., 2011, 2012) and likely to the trigeminal nucleus caudalis and upper cervical region (Vc–C1) (Shigenaga et al., 1988). Orofacial pain, including TMJ nociception can be affected by changes in neuronal activity within the TG and Vc–C1. These changes are modulated, in part, by GABA receptor signaling (Ginestal and Matute, 1993; Kondo et al., 1995; Almond et al., 1996; Cairns et al., 1999; Takemura et al., 2000; Cai et al., 2001; Hayasaki et al., 2006; Anderson et al., 2009; Vit et al., 2009; Puri et al., 2012). Similarly, nociceptive studies on the tooth have shown that GABAergic neurons are activated in the Vc and trigeminal nucleus interpolaris (Vi) and that these neurons respond to GABAA receptor modulators muscimol and bicuculline (Takemura et al., 2000; Wu et al., 2010).
A recent study demonstrated there was an association between Gabrα6 expression and inflammatory TMJ nociception (Puri et al., 2012) but a large number of patients with TMD have masticatory muscle pain (Harness et al., 1990; Stohler, 1999). To date the role of Gabrα6 in myofascial nociception has not been studied. We hypothesized here that in addition to its role in inflammatory TMJ nociception that the GABAA receptor also has an inhibitory role in modulating myofascial nociception. To address this hypothesis we utilized a recent myofascial hypersensitivity model that induced nociception by placing a ligature around the tendon attachment of the anterior superficial part of a rat's masseter muscle (Guo et al., 2010). After ligature placement Gabrα6 expression was knocked down in the TG by small interfering RNA (siRNA) infusion and orofacial nociception was quantitated by measuring changes in feeding behavior and in response to the application of graded von Frey filaments to the region above the ligated muscle tendon (Guo et al., 2010; Puri et al., 2012). GABAA receptor levels in the TG and the Vc were determined by performing a Western blot for the β1, β2 and β3 subunits (Schofield et al., 1989; Ymer et al., 1989; Connolly et al., 1996). Western blot analysis was also utilized to measure expression of GABAA receptors containing the α6 subunit and to determine neuronal activity by measuring phosphorylated extracellular signal-regulated kinase (p-ERK) expression (Shimizu et al., 2006; Suzuki et al., 2007; Liverman et al., 2009).
Experimental Procedures
Animal care and welfare
All animal experiments were approved by the Texas A&M Health Science Center Baylor College of Dentistry Institutional Animal Care and Use Committee in accordance with the guidelines of the Office of Laboratory Animal Welfare, the USDA and National Research Council's “Guide for Care and Use of Laboratory Animals”. Male Sprague–Dawley rats (275– 300 g) were purchased from Harlan Industries, Houston, TX. Upon arrival the animals were housed individually in a temperature-controlled room (23 °C) under a 14/10-h light/dark cycle with lights on at 6:00 AM. The rats were given chow (Harlan Industries) and water ad libitum. The rats were acclimated to their surroundings for at least 3 days before cannulation surgery, see timeline in Table 1 for outline of experimentation.
Table 1.
Timeline of experiments.
| Days | Experiment |
| Preday -30 | Rats were purchased from Harlen Industries |
| Preday -27 | Initial filament testing was performed |
| Preday -26 | Cannulation of the trigeminal ganglia was completed |
| Preday -20 | Cannulated rats were placed in the feeder units |
| Preday -14 | Filament testing was performed |
| Preday -7 | Filament testing was performed |
| Preday -3 | Feeding data and body weight was analyzed |
| Preday -2 | Feeding data and body weight was analyzed |
| Preday -1 | Feeding data and body weight was analyzed |
| Lig +1 | Ligature placed on the tendon on the morning of day ligature 1 and feeding data and body weight was analyzed |
| Lig +2 | Feeding data and body weight was analyzed |
| Lig +3 | Feeding data and body weight was analyzed and filament testing was performed |
| Lig +4/siRNA +1 | siRNA infused into the trigeminal ganglia on the morning of day siRNA 1 and feeding data and body weight was analyzed |
| Lig +5/siRNA +2 | Feeding data and body weight was analyzed |
| Lig +6/siRNA +3 | Feeding data and body weight was analyzed and filament testing was performed |
| Sacrifice | 72 h after siRNA infusion the animals were sacrificed and the trigeminal ganglia and trigeminal nucleus caudalis tissue was isolated |
Guide cannula placement surgery
Rats were anesthetized with a mixture of ketamine (75 mg/kg) and xylazine (0.5 mg/kg) injected intramuscularly (Bellinger and Tillberg, 1997). Guide cannulas (22 GA, Plastics One Inc, Roanoke, VA, USA) were stereotaxically (David Kofp Instruments, Tujunga, CA, USA) placed in the TG bilaterally using coordinates 4.3 mm posterior of Bregma, 3.4 mm lateral of the midline at a depth of 9.5–10.3 mm from the dura (Paxinos and Watson, 2007). Stereotaxic coordinates were established in a preliminary trial by injecting India ink through the cannulas followed by a postmortem histological examination and in all animals cannula placement was confirmed by X-ray (Puri et al., 2012).
Nociception testing using meal duration assay
Six days after cannulation (Table 1) the rats were housed individually in sound-attenuated chambers equipped with photobeam computer-activated pellet feeders (Med Assoc. Inc., East Fairfield, VT, USA) and given 45-mg rodent chow pellets (Product No. FO 165, Bioserv, Frenchtown, NJ, USA). When a rat removed a pellet from the feeder trough, a photobeam placed at the bottom of the trough was no longer blocked, signaling the computer to drop another pellet. The computer recorded the date, recorded the time and kept a running tally of the total daily food consumption. In these analyses, a meal was defined using a 10-min end of meal criterion (i.e., a meal was bracketed before and after by a 10-min period of no pellets being taken) and the minimum meal size was set at 135 mg [i.e., three pellets] (Castonguay et al., 1986). Meal duration was then calculated using proprietary and Med Associate computer programs. We have previously shown in male and female rats that meal duration can be used as a non-invasive biological marker for TMJ nociception for up to 19 days (Kerins et al., 2003, 2004; Bellinger et al., 2007; Kramer et al., 2010) and for pulp pain (Kramer et al., 2012). Changes in meal duration are the result of deep nociceptive responses, most likely from muscles that function in the mastication process. Patients experiencing TMJ pain also have longer chewing cycles and the cycle length is attenuated when TMJ pain is diminished (Hansdottir and Bakke, 2004; Bakke and Hansdottir, 2008; Pereira et al., 2009). The lengthening of meal duration during TMJ pain is a “guarding behavior”, which can be argued is an operationally defined nociceptive behavior (Sternberg and Wachterman, 2000). Ten animals were included per treatment group in this assay and these same animals were tested with the filament assay shown below.
Nociception testing using filaments
A series of calibrated von Frey filaments were applied to the skin of sham and ligated rats above the tendon attachment of the anterior superficial portion of the masseter muscle on specific days (Table 1). A withdrawal of the head after contact with the probing filament was defined as a response. Each von Frey filament was applied five times at intervals of a few sec. The response frequencies (EF50) were calculated as described previously (Guo et al., 2010). Briefly, the response frequencies [(number of responses/number of stimuli) × 100%] to a range of von Frey filament forces were determined and a stimulus–response frequency curve was plotted. After a non-linear regression analysis, the half maximal response [i.e., EC50 values calculated by Prism 5.0 software (GraphPad, Inc.), here termed EF50] value was derived from the stimulus response curve. We used the EF50 values as a measure of mechanical sensitivity. A smaller EF50 value indicates greater sensitivity and a larger value less sensitivity. Filament testing reflects deep hyperalgesia, because previous work has shown that lidocaine injections into the deep tissue surrounding the ligature attenuate hyperalgesia, whereas lidocaine injection into the overlying skin had no effect (Shimizu et al., 2009; Guo et al., 2010). Ten animals were included per treatment group. These 10 animals were in the feeders (to measure meal duration) and were briefly removed for filament testing, thus filament tests were performed on the same animals in which we obtained the meal duration data.
Ligature surgery
Rats were anesthetized with ketamine and xylazine as stated above. Two 4.0 chromic gut ligatures, spaced >3.0mm apart, were placed around the tendon of the anterior superficial portion of the masseter muscle (Guo et al., 2010). Surgical access to the tendon was from the interior of the mouth. The incision in the mouth was closed with a single 5.0 polyglycolic acid suture using a 13-mm 3/8 needle. Sham-operated rats received the same surgery, but the tendon was not ligated.
Trigeminal ganglion infusions
Rats were anaesthetized with 5% isoflurane and infused bilaterally with 5 μl of control or siRNA solution over a 5-min period using an infusion pump (KDS Model 310 Plus, Holliston, MA). Gabrα6 siRNA (2.5 μg) or control siRNA (2.5 μg) (Invitrogen, Carlsbad, CA) was mixed with linear polyethyleneimine (PEI) (in vivo JetPEI, Cat# 201–10, PolyPlus Transfection, Illkirch, France) to increase the transfection efficiency (Boussif, 1995). The ratio of cationic PEI amines (N) to nucleic acid phosphates (P) was N/P = 6. This amount of siRNA and PEI was based on a previous study where siRNA knockdown was performed in vivo (Kramer et al., 2010).
The control siRNA had no homology to any known gene and was identified as Silencer Negative Control #1 siRNA (5′-AGUACUGCUUACGAUACGGtt-3′, 5′- CCGUAUCGUAAGCAGUACUtt-3′) and the Gabrα6 siRNA sequence was sense (5′-GGAACGAUCCUGUACACCAtt-3′), anti-sense (3′-UGGUGUACAGGAUCGUUCCat-5′). Animals were alert and mobile less than 5 min after removal from isoflurane.
Western blot analysis
Three days following infusion (Table 1) the rats were anesthetized with carbon dioxide gas and quickly decapitated. The TG from each rat just rostral of V1 and 2 mm caudal of V3 was dissected after removal of the brain. A slice of caudal brainstem was also collected from a tissue block that included a 2-mm segment beginning 5 mm caudal to the obex. This tissue block included the caudal laminated (Vc) and upper cervical spinal cord (C1). The tissue block was turned coronally and the upper Vc–C1 was harvested using a 15-gauge needle as a punch. The tissue was then homogenized in 300 μl of T-Per tissue protein extraction reagent containing Halt Protease Inhibitor (Thermo Scientific, Rockford, IL, USA). The homogenates were centrifuged at 20,200g for 10 min at 4 °C. The supernatant was removed. Total protein was determined in each sample using a BCA protein assay (Thermo Scientific) and 15 μg of total protein was loaded into each well of a 4– 12% Bis-Tris acrylamide gel (Invitrogen). The gel was electrophoresed at 200 V for 35 min and the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (Bio Rad, Hercules, CA, USA). The membrane was rinsed in Tris-buffered saline containing 0.1% Tween-20 and then blocked for 1 h in this buffer containing 5% milk. After three more rinses the membranes were placed in the block solution with either an antibody against (1) Gabrα6 (1:2000) or (2) GABAA receptor subunit beta - Gabrβ1 (1:2000) or (3) Gabrβ2 (1:1000) or (4) Gabrβ3 (1:2000) or (5) against p-ERK (1:2000) or (6) against β-actin (1:2000). These rabbit polyclonal antibodies were obtained from either Millipore (Billerica, MA, USA) or Cell Signaling Technology (Danvers, MA, USA). The membranes were probed with: (1) with anti-Gabrα6, stripped (Re-blot Plus Mild, Millipore) (2) probed with anti-Gabrβ1, stripped; (3) probed with anti-Gabrβ2, stripped; (4) probed with anti-Gabrβ3, stripped; (5) probed with anti-p-ERK; and (6) stripped again and probed with anti-β-actin. For each antibody an overnight incubation at 4 °C was completed, the membranes were rinsed three times and then incubated with a horse radish peroxidase (HRP)-conjugated goat anti-rabbit antibody (1:500, Cell Signaling) at room temperature for 90 min. After incubation in this second antibody the membranes were rinsed three times and reacted with the ECL Plus Western Blotting Detection System (GE Healthcare). After exposure of the membrane to the film and development of the film, the bands were quantitated with Image J software. An area and mean value were multiplied to obtain the optical density of the band. Values were reported as a ratio of the optical density of the protein band of interest (i.e., Gabrα6, Gabrβ or p-ERK) divided by the optical density of the β-actin band. Controls in which the primary antibody was not included showed no signal (data not shown). The TG was isolated from 10 animals per treatment group. The C1 region was not isolated from all animals as a result of technical difficulties thus the Vc–C1 was isolated from three animals per treatment group. Previous studies showed pre-absorption of the Gabrα6 antibody with a Gabrα6 peptide resulted in elimination of the Gabrα6 specific band (Puri et al., 2012).
TG collection, tissue processing and immunofluorescence
After removal of the TG, one ganglion was used for Western blot analysis (see above), three ganglia were randomly chosen from each treatment group for sectioning and staining and were placed in 5% paraformaldehyde fixative and later stored in a 25% sucrose solution. Fixed TG were sagittally cut at a thickness of 24 μm on a cryostat. The sections were stored in 25% sucrose until used. Immunohistochemistry was completed on floating sections by first quenching 30 min with 0.3% hydrogen peroxide followed by three 20-min phosphate-buffered saline (PBS) rinses and a 1-h blocking step (PBS, 5% normal goat serum, 0.3% Triton X-100). Following three rinses in PBS, the sections were incubated overnight at 4 °C with the primary antibodies in a solution of PBS, 1% bovine serum albumin (BSA) and 0.3% Triton X-100. The primary monoclonal antibodies included a mixture antineuronal marker NeuN (1:1000, Millipore) and a rabbit polyclonal antibody against p-ERK (1:500, Cell Signaling Technology). After the overnight incubation with primary antibodies the slides were rinsed three times in PBS followed by a 2-h incubation step with fluorescently tagged secondary antibodies (PBS, 1% BSA, 0.3% Triton X-100). The secondary antibodies included a mixture of goat anti-rabbit 488 and a goat anti-mouse 633 from Invitrogen. After incubation with the secondary antibodies the sections were rinsed again three times in PBS, placed on slides and the sections were mounted with fluoromount-G medium (Southern Biotech, Birmingham, AL, USA). Images were captured using a Nikon epifluorescent microscope and a Photometrics CoolSnap K4 CCD camera (Roper Scientific, Inc, Duluth, GA, USA). Nikon Imaging Software-Elements (Melville, NY) controlled the camera. Controls in which the primary antibody was not included showed no signal (data not shown).
Statistical analysis
Meal duration and body weight data were analyzed (ABstat software, V1.94 or Graph Pad Prism version 5.0, Graph Pad, San Diego, CA, USA) by a three-way analysis of variance (ANOVA) with the independent variables being the control siRNA or Gabrα6 siRNA infusion, sham or ligature surgery and the day of treatment. The dependent variable was meal duration or body weight. Further analysis of the dark meal duration was completed by a three-way ANOVA using the dependent variable of meal duration and the independent variable treatment (i.e., Ligature/control siRNA group, Ligature/Gabra6 siRNA) and days (i.e., Lig +5/siRNA +2 and Lig +6/siRNA +3). Statistical significance was further analyzed with a Duncan or Bonferroni post hoc test. Filament data were analyzed using the Mann–Whitney U test. Western data were analyzed by a two-way ANOVA. The independent variables included the control siRNA or Gabrα6 siRNA infusion and sham or ligature surgery. The dependent variable was optical density ratio. Data with p < 0.05 were considered significant. All values were presented as mean ± SEM (standard error of the mean).
Results
Gabrα6 knock-down in the TG increased the nociceptive response
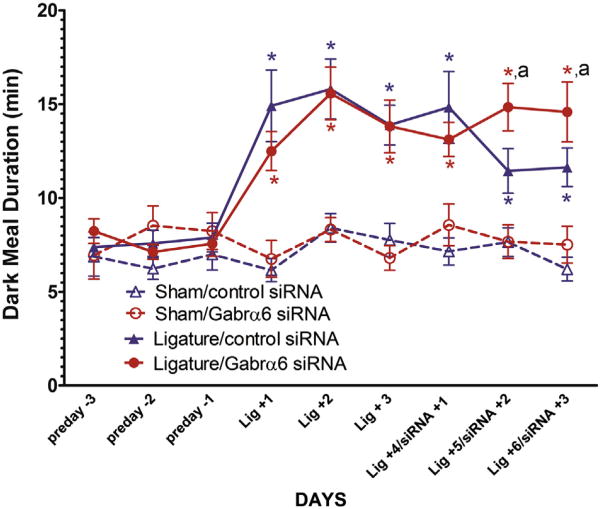
The dark phase meal duration (Fig. 1) significantly increased on days Lig +1, Lig +2, Lig +3, Lig +4 Lig +5 and Lig +6 in both the Ligature/control siRNA group and the Ligature/Gabrα6 siRNA-infused group when compared to their respective non-ligatured groups (see Table 1 for days). At 24 h following TG infusion (i.e., Lig +4/siRNA +1) there was no difference in dark meal duration between the Ligatured/Gabrα6 siRNA-infused group and the Ligatured/control siRNA-infused group (Fig. 1). However, at both 48 (Lig +5/siRNA +2) and 72 h (Lig +6/siRNA +3) the dark meal duration of the Ligature/control siRNA-infused group was significantly reduced compared to the Ligature/Gabrα6 siRNA-infused group (Fig. 1).
Fig. 1.
Dark meal duration was lengthened after Gabrα6 knock-down in the trigeminal ganglia suggesting an increase in the nociceptive response. Male rats were cannulated, placed in the feeders (preday -3, preday -2 preday -1), the tendons were ligatured (Lig +1, Lig +2, Lig +3) and 72 h later the trigeminal ganglion was infused with control siRNA or Gabrα6 siRNA (Lig +4/siRNA +1, Lig +5/siRNA +2, Lig +6/siRNA +3). Dark meal duration was measured for each treatment group. ANOVA and post hoc testing was completed with data from 10 animals per treatment group using the dependent variable of meal duration and the independent variables of Sham, Ligature and control siRNA, Gabar6 siRNA and days, *p < 0.05. The letter “a” indicates that there was a significant main effect (p < 0.05) for treatment (Ligature/control siRNA and Ligature/Gabra6 siRNA) and days (Lig +5/siRNA +2 and Lig +6/siRNA +3). Values are the mean ± SEM.
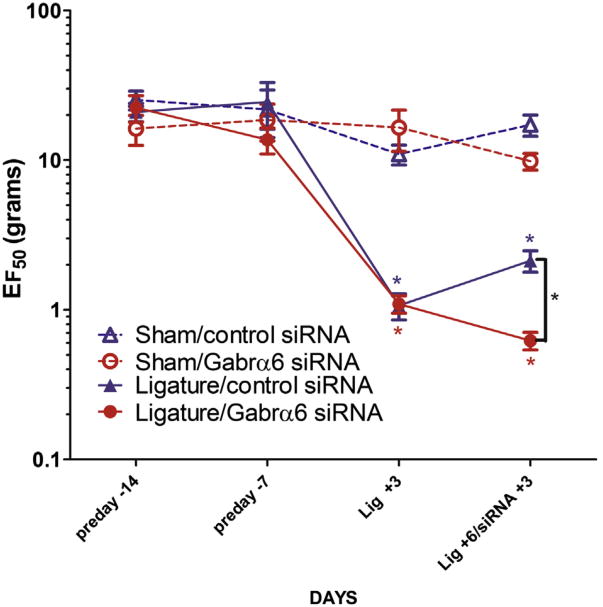
In filament tests the ligated groups displayed significantly greater sensitivity (a decrease EF50) than the sham-operated rats (Fig. 2). TG Gabrα6 siRNA infusion significantly enhanced the sensitivity of the ligatured rats compared to the control siRNA-infused ligatured animals. The data are comparable to the meal duration finding.
Fig. 2.
Filament testing of the ligatured region indicated nociceptive responses increased after Gabrα6 knock-down in the trigeminal ganglia. Cannulated male rats were tested with von Frey filaments over the tendon attachment of the anterior superficial portion of the masseter muscle 14 and 7 days before ligature (preday -14, preday -7). Then a ligature was place on the tendon of the masseter muscle and 72 h after surgery filament testing was performed (Lig +3). After ligature testing siRNA was infused into the trigeminal ganglia and 72 h after infusion filament testing was performed (Lig +6/siRNA +3). Data were analyzed using a Mann–Whitney U test, *p < 0.05. Values are the mean ± SEM. Ten animals were in each treatment group.
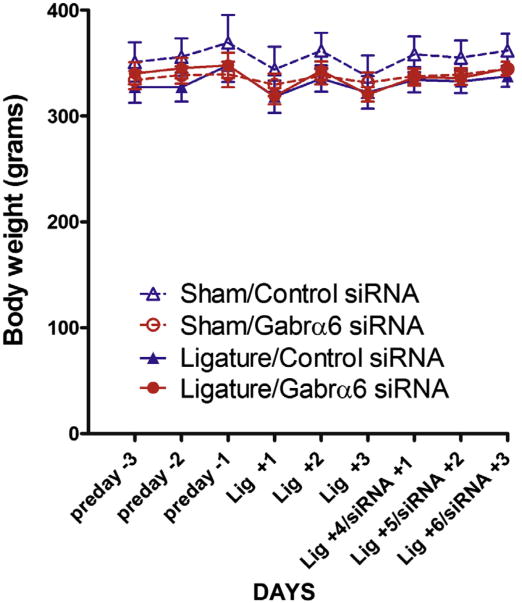
Body weight after treatment
Body weight of the animals was not significantly affected by either ligature surgery or siRNA infusion (Fig. 3).
Fig. 3.
Body Weight was not significantly affected by ligature or siRNA infusion. Body weight measurements were taken before and after ligature surgery and siRNA infusion. See Fig. 1 for treatment groups. Values are the mean ± SEM. Ten animals were in each treatment group.
Knock-down of Gabrα6 decreased GABAA receptor levels in the TG and Vc–C1
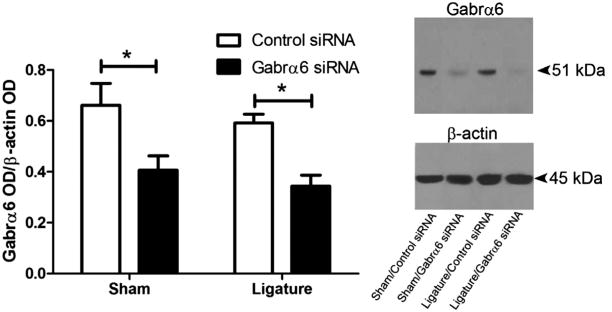
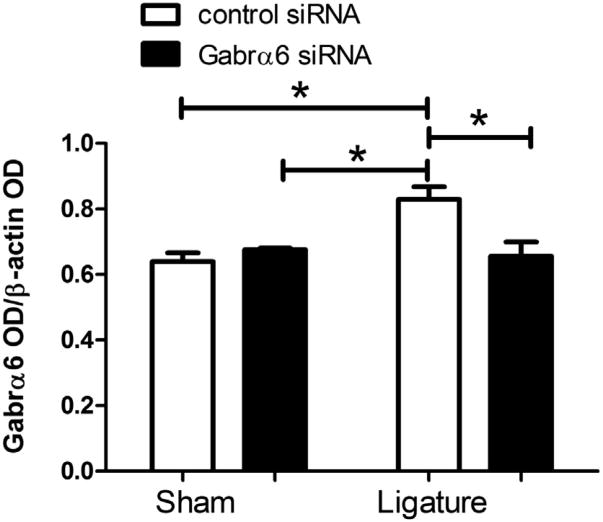
Gabrα6 expression was significantly decreased in the TG 72 h after infusion of the Gabra6 siRNA in both sham and ligatured rats (Fig. 4). We also quantitated expression of Gabra6 in the Vc–C1 because primary afferents projecting from the TG can terminate in the Vc–C1 region (Marfurt, 1981; Jacquin et al., 1983; Pfaller and Arvidsson, 1988) and we aimed to determine if any changes were occurring on the terminals of these primary afferents. In the non-ligatured groups Gabrα6 levels in the Vc–C1 were not affected by Gabrα6 siRNA infusions. On the other hand, ligation increased Gabrα6 levels, whereas infusion with Gabrα6 siRNA returned Gabrα6 to a baseline level (Fig. 5).
Fig. 4.
Gabrα6 expression was significantly reduced 72 h after infusion of Gabrα6 siRNA into the TG. A Western blot was completed after isolating protein from the trigeminal ganglia. Trigeminal ganglia were isolated 6 days after ligature surgery and 72 h after siRNA infusion. Surgery included a sham operation or an operation where a ligature was placed on the tendon attachment of the anterior superficial portion of the masseter muscle. Antibodies used in the Western blot were against the Gabrα6 and β-actin protein, 15 μg of total protein was loaded per lane. Histogram values were reported as a ratio of the optical density of the Gabrα6 band divided by the optical density of the β-actin band. A two-way ANOVA and post hoc testing was completed with data from 10 animals per treatment group, *p < 0.05. Values are the mean ± SEM. The top right image is a Western blot probed with a Gabrα6 antibody and this same blot was stripped and probed with a β-actin antibody.
Fig. 5.
Knock-down of Gabrα6 reduced Gabrα6 expression in trigeminal nucleus caudalis. Western blots were completed using 15 μg of total protein per lane. Antibodies used in the Western were against Gabrα6 and β-actin. Histogram values were reported as a ratio of the optical density of the Gabrα6 band divided by the optical density of the β-actin band. A two-way ANOVA was performed (independent variables were control siRNA/Gabrα6 siRNA and sham/ligature) (dependent variable was the ratio of the OD), post hoc tests were completed comparing each group, *p < 0.05. The caudalis tissue was isolated from only a subset of rats thus, there were three animals included in each treatment group. Values are the mean ± SEM.
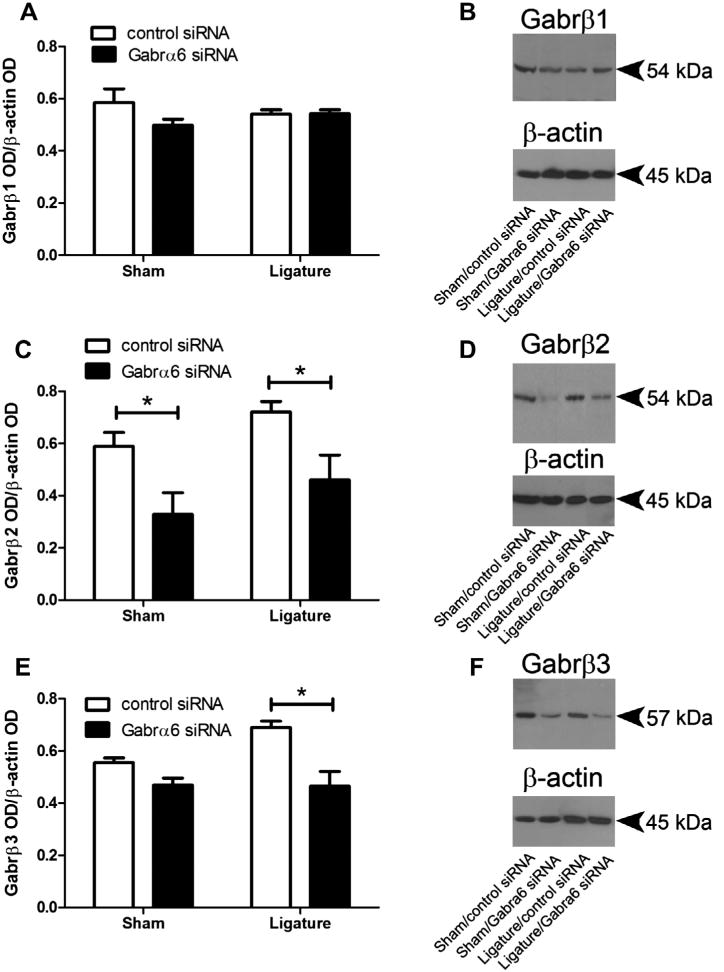
α and β subunits are necessary for a functional GABAA receptor and the β subunit consists of three isoforms Gabrβ1, β2 and β3 (Schofield et al., 1989; Ymer et al., 1989; Connolly et al., 1996). By quantitating the amount of the three β subunits (Fig. 6) we expected to determine changes in the total amount of GABAA receptor (Nusser et al., 1999). Thus, GABAA receptor levels in the TG and Vc–C1 were determined by performing a Western blot for the Gabrβ1, β2 and β3 subunits for each treatment group. While Gabra6 siRNA infusion did not change TG Gabrβ1 (Fig. 6A) it did significantly decrease both Gabrβ2 and Gabrβ3; the latter only in the ligatured group (Fig. 6C, E). In the Vc– C1 there was no significant change in the levels of either Gabrβ1 F(1,9) = 0.10, n.s., p ≥ 0.75 or β2 F(1,9) = 0.07, p ≥ 0.79 or β3 F(1,9) = 1.70, p ≥ 0.22 to ligation or siRNA infusion.
Fig. 6.
Knock-down of Gabrα6 resulted in a decrease in GABAA receptor β subunit expression in the trigeminal ganglia. Histogram values for (A) Gabrβ1, (C) Gabrβ2 and (E) Gabrβ3 were reported as a ratio of the optical density of the β subunit band divided by the optical density of the β-actin band. The Western blots were completed using antibodies for the three β subunits comprising the GABAA receptor, (B) Gabrβ1, (D) Gabrβ2 and (F) Gabrβ3. A two-way ANOVA was performed (independent variables were control siRNA/Gabrα6 siRNA and sham/ligature) (dependent variable was the ratio of the OD), post hoc tests were completed comparing each group, *p < 0.05, 10 animals per group. Values are the mean ± SEM.
Increased neuronal activity after knock-down of the Gabrα6 subunit
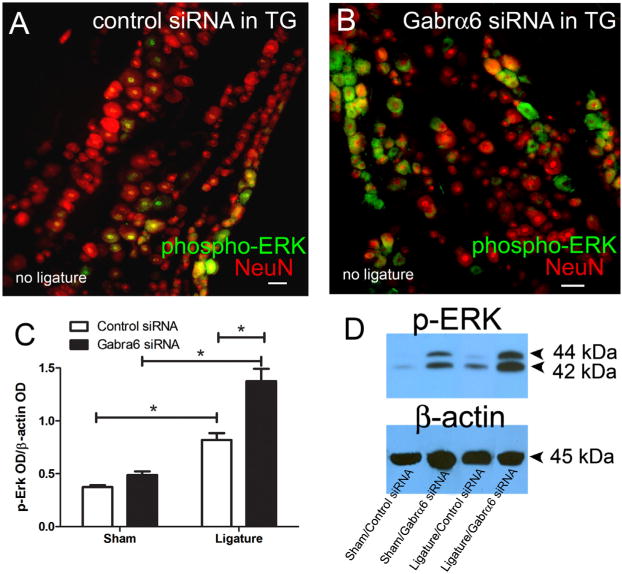
p-ERK is widely used as a marker for neuronal activation in both the TMJ and tooth pulp models (Shimizu et al., 2006; Suzuki et al., 2007; Liverman et al., 2009). p-ERK was observed in NeuN positive neurons of the TG (Fig. 7A, B). p-ERK expression significantly increased in the TG of ligated rats as compared to the non-ligated group (Fig. 7C, D). p-ERK expression was further significantly increased with a combination of ligature and infusion with Gabrα6 siRNA (Fig. 7C, D).
Fig. 7.
Neuronal activity, as measured by p-ERK, increased after knock-down of Gabrα6. Male rats' trigeminal ganglia were infused with control siRNA or Gabrα6 siRNA 6 days after ligature surgery and 72 h after siRNA infusion the rats were sacrificed and the trigeminal ganglia were isolated. In panels A and B the trigeminal ganglia were stained for p-ERK (green) and NeuN (red). Images are representative of rats in the no ligature groups. Scale bar = 50 μm. (C, D) Western blots were completed using 15 μg of total protein per lane from sham and ligatured rats that were infused with siRNA. Antibodies used in the Western blot were p-ERK and β-actin. (C) Histogram values were reported as a ratio of the optical density of the p-ERK band divided by the optical density of the β-actin band. A two-way ANOVA was performed; independent variables were control siRNA/Gabrα6 siRNA and sham/ligature. The dependent variable was the ratio of the OD. Post-hoc tests were completed comparing each group, *p < 0.05, 10 animals per group. Values are the mean ± SEM. (D) The top right image is a Western blot probed with a p-ERK antibody and this same blot was stripped and probed with a β-actin antibody.
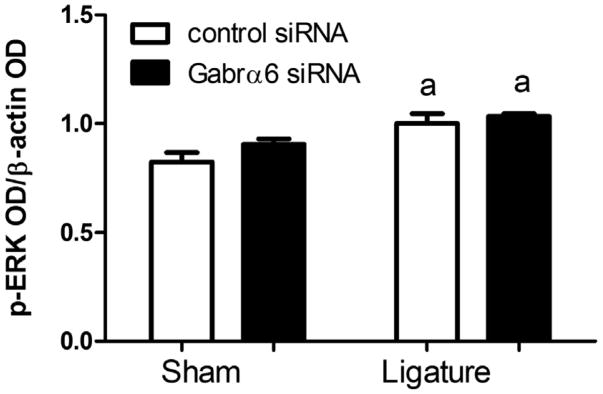
In the Vc–C1 ligation significantly enhanced p-ERK expression but Gabrα6 siRNA infusion had no further effect (Fig. 8).
Fig. 8.
Knock-down of Gabrα6 effect on trigeminal nucleus caudalis p-ERK expression. Western blots were completed using 15 μg of total protein per lane isolated from the trigeminal nucleus caudalis. Antibodies used in the Western were against p-ERK and β-actin. Histogram values were reported as a ratio of the optical density of the p-ERK band divided by the optical density of the β-actin band. The letter “a” indicates that there was a significant main effect for ligature F(1,7) = 16.7, p < 0.01 as determined by a two-way ANOVA when using the dependent variable of the p-ERK band divided by the optical density of the β-actin band and the independent variables of ligature and siRNA treatment. The caudalis tissue was isolated from only a subset of rats thus, there were three animals included in each treatment group. Values are the mean ± SEM.
Discussion
siRNA with Gabrα6 sequence was taken up by cells in the TG and reduced Gabrα6 expression within the TG (Puri et al., 2012). This Gabrα6 siRNA treatment also reduced Gabrα6 expression in the Vc–C1 region after ligature. Note: primary afferents projecting from the TG cells terminate in the Vc–C1 region (Marfurt, 1981; Jacquin et al., 1983; Pfaller and Arvidsson, 1988). A decrease in Gabrα6 expression within the cell bodies of the TG should result in a decrease in Gabrα6 expression in the primary afferent terminals located in the Vc–C1 region since GABAA receptor protein from the TG transports to the afferent presynaptic terminals in the Vc–C1, as demonstrated by the fact that presynaptic terminals contain functional GABAA receptors (Grudt and Henderson, 1998).
Ligation of the tendon alone did not alter Gabrα6 expression in the TG, similarly TMJ inflammation does not significantly alter Gabrα6 expression in the TG (Puri et al., 2011, 2012). Ligation did increase Gabrα6 expression in the Vc–C1 region. This contrasts work involving nerve ligation which reduced GABAA receptor expression in the dorsal root ganglia (Fukuoka et al., 1998). On the other hand peripheral inflammation was shown to increase expression of GABAA and GABAB receptors in the dorsal horn (Castro-Lopes et al., 1992, 1994; McCarson and Enna, 1999). GABA binding in the dorsal horn also increases in the presence of inflammation (Castro-Lopes et al., 1995), suggesting that changes in GABAA receptors expression are different in TG and spinal cord and technique specific, i.e., nerve ligation versus inflammation. Thus the different methods for inducing the nociceptive response could modulate Gabra6 expression differently and one must carefully evaluate the method for inducing pain in determining the role of the GABAA receptor.
Gabrα6 knock-down increased nociceptive responses
Meal duration and von Frey measurements indicated greater sensitivity following Gabrα6 siRNA infusion of ligatured rats, reflecting involvement of Gabrα6 in nociception. Since the meal duration of the non-ligatured Gabrα6 siRNA-infused group did not differ from the non-ligatured control siRNA group it shows infused Gabrα6 siRNA by itself does not affect meal duration, i.e., nociception. These data show TG Gabrα6 siRNA infusion increased the nociceptive response of the ligatured animals. We recently demonstrated that there was an association between Gabrα6 and inflammatory TMJ nociception (Puri et al., 2011, 2012) but the current study expands the role of Gabrα6 as an important regulator of orofacial nociception. The fact that body weight was not altered after ligation and siRNA infusion supports the idea that the changes in feeding behavior (i.e., meal duration) were not the result of loss of appetite due to illness after treatment (i.e., ligation or infusion). The results here also show that ligature increased neuronal activity (i.e. p-ERK) in both the TG and Vc–C1 region and a reduction in Gabrα6 expression further increased neuronal activity in the TG. Since orofacial nociceptive responses are inhibited by GABAergic signaling (Cairns et al., 1999; Viggiano et al., 2004) a decrease in GABAA receptors should result in a greater nociceptive response (Almond et al., 1996; Viggiano et al., 2004). Consistent with this idea is that neuronal activity in the medullary region increased after reducing Gabrα6 expression in the TG of a rat with TMJ inflammation (Puri et al., 2012).
Gabrα6 knock-down reduced GABAA expression
GABAA receptor content was assayed by measuring the amount of Gabrβ1, Gabrβ2 and Gabrβ3. Nusser et. al., demonstrated that by reducing Gabrα6 in the cerebellum GABAA β2, β3 and γ2 subunits decreased by 50%, 20% and 40%, respectively resulting in half the number of GABAA receptors (Nusser et al., 1999). It is possible that other α subunits, e.g., α1 and α5 subunits, found in all the TG neurons might compensate for depletion of the α6 subunits (Hayasaki et al., 2006), but previous work has shown that alternate subunits did not rescue a Gabrα6 deletion (Jones et al., 1997). Thus, our observed reduction in Gabrβ2 and Gabrβ3 after Gabrα6 siRNA infusion was interpreted as a decrease in GABAA receptor content in the TG (Nusser et al., 1999). Our results also show a decrease in Gabrα6 expression in the Vc–C1 region, although we did not measure a change in Gabrβ1, Gabrβ2 or Gabrβ3 content within the Vc–C1. It is possible that Gabrβ expression did decrease in the Vc–C1 but Western blots were simply not sensitive enough to detect a change since a majority of GABAA receptors in the sensory trigeminal nuclei are interneurons (Ginestal and Matute, 1993; Avendano et al., 2005). Thus, the signal might have been obscured by the unchanging levels of Gabrβ of these cells. More sensitive techniques for quantitation of Gabrβ receptor subunits, such as receptor binding assays, could reveal potential reductions in the amount of GABAA receptor after Gabrα6 knock-down.
Changes in Gabrα6 expression reduced GABAA receptor content and increased the nociceptive response, but neurotransmitters such as serotonin and nitric oxide can alter GABAA receptor expression and change the nociceptive response. Serotonin-norepinephrine reuptake inhibitors have been shown to alter the nociceptive response in the ligatured rat model used in this study (Guo et al., 2010). By altering orofacial responses through changes in trigeminal activity (Oshima et al., 2006; Nakai K et al., 2010; Loyd et al., 2011; Choi et al., 2012) and presynaptic GABA release (Schmitz et al., 1995; Koyama et al., 2002; Turner et al., 2004), serotonin has the potential to alter GABA transmission after ligature. Reactive oxygen species mediate pain in different animal models (Kim et al., 2004; Wang et al., 2004; Schwartz et al., 2008) and can affect orofacial pain by altering neuronal activity in the Vc (Viggiano et al., 2005, 2010). In a recent study reactive oxygen species (e.g. nitric oxide) reduced GABA-mediated inhibition potentially through a reduction in presynaptic GABA release (Yowtak et al., 2011) consistent with the idea that reactive oxygen species can alter GABA transmission after ligature.
Role of TG GABAA on nociception
Primary afferents projecting from the TG potentially have a role in the increased nociceptive response. Remember the TG afferents terminate in the Vc–C1 region (Marfurt, 1981; Jacquin et al., 1983; Pfaller and Arvidsson, 1988) and have functional presynaptic GABAA receptors (Grudt and Henderson, 1998). A decrease in GABAA content within these afferents (i.e., at the Vc–C1) would reduce the inhibitory pathway dependent on GABAA. GABAA-dependent tonic inhibition occurs within the substantia gelatinosa region of the Vc (Han and Youn, 2008) a region where small-diameter unmyelinated C and thinly myelinated Aδ primary afferent fibers conveying orofacial nociceptive information terminate (Lopez de Armentia et al., 2000; Sessle, 2000). Tonic inhibitory currents are generated by GABAA receptors in the Vc that include α4, α5 and α6 subunits (Neelands and Macdonald, 1999; Farrant and Nusser, 2005; Mody, 2005). GABA binding to its receptor can induce chloride channel opening and presynaptic inhibition (varez-Leefmans et al., 1988; Sung et al., 2000). This presynaptic inhibition resulting from the chloride is dependent on active inward pumping of Cl− ions potentially by the presence of the NKCC1 cotransporter or a lack of the KCC2 cotransporter in the primary afferents (Toyoda et al., 2005; Price et al., 2006).
Conclusion
In conclusion, a reduction in the Gabrα6 subunit resulted in a greater nociceptive response created by ligation of the tendon attachment of the anterior superficial part of male Sprague–Dawley rat's masseter muscle. The increased nociceptive response as measured by meal duration and von Frey filaments was accompanied by an increase in neural activity in the TG and Vc–C1. We suggest that by inducing a reduction in Gabrα6 there were lower levels of the inhibitory GABAA receptor in the TG cells as well as in the afferent terminals projecting to the Vc–C1. The results suggest that the GABAA receptor comprised of the Gabrα6 subunit was an important regulator of myofascial nociception.
Acknowledgments
This study was supported by NIDCR grants DE016059 (LLB) and DE022129 (PRK). We would like to thank Adam Hauser and Meilin Howard for their technical help in these studies.
Abbreviations
- ANOVA
analysis of variance
- BSA
bovine serum albumin
- Gabrα6
GABAA receptor subunit alpha 6
- Gabrβ
GABAA receptor subunit beta
- PBS
phosphate-buffered saline
- PEI
polyethyleneimine
- p-ERK
phosphorylated extracellular signalregulated kinase
- siRNA
small interfering RNA
- TG
trigeminal ganglia
- TMJ
temporomandibular joint
- Vc–C1
trigeminal nucleus caudalis and upper cervical region
Footnotes
Disclosures: There are no known conflicts of interest.
References
- Almond JR, Westrum LE, Henry MA. Post-embedding immunogold labeling of gamma-aminobutyric acid in lamina II of the spinal trigeminal subnucleus pars caudalis: I: A qualitative study. Synapse. 1996;24:39–47. doi: 10.1002/(SICI)1098-2396(199609)24:1<39::AID-SYN5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Anderson WB, Graham BA, Beveridge NJ, Tooney PA, Brichta AM, Callister RJ. Different forms of glycine- and GABA(A)-receptor mediated inhibitory synaptic transmission in mouse superficial and deep dorsal horn neurons. Mol Pain. 2009;5:65. doi: 10.1186/1744-8069-5-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avendano C, Machin R, Bermejo PE, Lagares A. Neuron numbers in the sensory trigeminal nuclei of the rat: A GABA- and glycine-immunocytochemical and stereological analysis. J Comp Neurol. 2005;493:538–553. doi: 10.1002/cne.20778. [DOI] [PubMed] [Google Scholar]
- Bakke M, Hansdottir R. Mandibular function in patients with temporomandibular joint pain: a 3-year follow-up. Oral Surg Oral Med Oral PatholOral RadiolEndod. 2008;106:227–234. doi: 10.1016/j.tripleo.2007.12.034. [DOI] [PubMed] [Google Scholar]
- Bellinger L, Tillberg L. Third ventricle cannulations, injection, and constant infusions using alzet pumps in the rat. In: Wellman PJ, Hoebel BG, editors. Ingestive behavior protocols. New York, NY: Society for the Study of Ingestive Behavior, Inc; 1997. pp. 135–147. [Google Scholar]
- Bellinger LL, Spears R, King CM, Dahm F, Hutchins B, Kerins CA, Kramer PR. Capsaicin sensitive neurons role in the inflamed TMJ acute nociceptive response of female and male rats. Physiol Behav. 2007;90:782–789. doi: 10.1016/j.physbeh.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Boussif O. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai BB, Cairns BE, Sessle BJ, Hu JW. Sex-related suppression of reflex jaw muscle activity by peripheral morphine but not GABA. Neuroreport. 2001;12:3457–3460. doi: 10.1097/00001756-200111160-00016. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Sessle BJ, Hu JW. Activation of peripheral GABAA receptors inhibits temporomandibular joint-evoked jaw muscle activity. J Neurophysiol. 1999;81:1966–1969. doi: 10.1152/jn.1999.81.4.1966. [DOI] [PubMed] [Google Scholar]
- Castonguay TW, Kaiser LL, Stern JS. Meal pattern analysis: artifacts, assumptions and implications. Brain Res Bull. 1986;17:439–443. doi: 10.1016/0361-9230(86)90252-2. [DOI] [PubMed] [Google Scholar]
- Castro-Lopes JM, Tavares I, Tolle TR, Coito A, Coimbra A. Increase in GABAergic cells and GABA levels in the spinal cord in unilateral inflammation of the hindlimb in the rat. Eur J Neurosci. 1992;4:296–301. doi: 10.1111/j.1460-9568.1992.tb00877.x. [DOI] [PubMed] [Google Scholar]
- Castro-Lopes JM, Tavares I, Tolle TR, Coimbra A. Carrageenan-induced inflammation of the hind foot provokes a rise of GABA-immunoreactive cells in the rat spinal cord that is prevented by peripheral neurectomy or neonatal capsaicin treatment. Pain. 1994;56:193–201. doi: 10.1016/0304-3959(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Castro-Lopes JM, Malcangio M, Pan BH, Bowery NG. Complex changes of GABAA and GABAB receptor binding in the spinal cord dorsal horn following peripheral inflammation or neurectomy. Brain Res. 1995;679:289–297. doi: 10.1016/0006-8993(95)00262-o. [DOI] [PubMed] [Google Scholar]
- Choi IS, Cho JH, An CH, Jung JK, Hur YK, Choi JK, Jang IS. 5-HT(1B) receptors inhibit glutamate release from primary afferent terminals in rat medullary dorsal horn neurons. Br J Pharmacol. 2012;167:356–367. doi: 10.1111/j.1476-5381.2012.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem. 1996;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Tokunaga A, Kondo E, Miki K, Tachibana T, Noguchi K. Change in mRNAs for neuropeptides and the GABA(A) receptor in dorsal root ganglion neurons in a rat experimental neuropathic pain model. Pain. 1998;78:13–26. doi: 10.1016/S0304-3959(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Ginestal E, Matute C. Gamma-aminobutyric acidimmunoreactive neurons in the rat trigeminal nuclei. Histochemistry. 1993;99:49–55. doi: 10.1007/BF00268020. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Henderson G. Glycine and GABAA receptor-mediated synaptic transmission in rat substantia gelatinosa: inhibition by mu-opioid and GABAB agonists. J Physiol. 1998;507(Pt 2):473–483. doi: 10.1111/j.1469-7793.1998.473bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wang H, Zou S, Wei F, Dubner R, Ren K. Long lasting pain hypersensitivity following ligation of the tendon of the masseter muscle in rats: a model of myogenic orofacial pain. Mol Pain. 2010;6:40. doi: 10.1186/1744-8069-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SM, Youn DH. GABAA receptor-mediated tonic currents in substantia gelatinosa neurons of rat spinal trigeminal nucleus pars caudalis. Neurosci Lett. 2008;441:296–301. doi: 10.1016/j.neulet.2008.06.048. [DOI] [PubMed] [Google Scholar]
- Hansdottir R, Bakke M. Joint tenderness, jaw opening, chewing velocity, and bite force in patients with temporomandibular joint pain and matched healthy control subjects. J Orofac Pain. 2004;18:108–113. [PubMed] [Google Scholar]
- Harness DM, Donlon WC, Eversole LR. Comparison of clinical characteristics in myogenic, TMJ internal derangement and atypical facial pain patients. Clin J Pain. 1990;6:4–17. doi: 10.1097/00002508-199003000-00003. [DOI] [PubMed] [Google Scholar]
- Hayasaki H, Sohma Y, Kanbara K, Maemura K, Kubota T, Watanabe M. A local GABAergic system within rat trigeminal ganglion cells. Eur J Neurosci. 2006;23:745–757. doi: 10.1111/j.1460-9568.2006.04602.x. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Semba K, Egger MD, Rhoades RW. Organization of HRP-labeled trigeminal mandibular primary afferent neurons in the rat. J Comp Neurol. 1983;215:397–420. doi: 10.1002/cne.902150405. [DOI] [PubMed] [Google Scholar]
- Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Makela R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AD, Sieghart W, Somogyi P, Smith AJ, Wisden W. Ligandgated ion channel subunit partnerships: GABAA receptor alpha6 subunit gene inactivation inhibits delta subunit expression. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerins CA, Spears R, Bellinger LL, Hutchins B. The prospective use of COX-2 inhibitors for the treatment of temporomandibular joint inflammatory disorders. Int J Immunopathol Pharmacol. 2003;16:1–9. [PubMed] [Google Scholar]
- Kerins C, Carlson D, McIntosh J, Bellinger L. A role for cyclooxygenase II inhibitors in modulating temporomandibular joint inflammation from a meal pattern analysis perspective. J Oral Maxillofac Surg. 2004;62:989–995. doi: 10.1016/j.joms.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Kondo E, Kiyama H, Yamano M, Shida T, Ueda Y, Tohyama M. Expression of glutamate (AMPA type) and gammaaminobutyric acid (GABA)A receptors in the rat caudal trigeminal spinal nucleus. NeurosciLett. 1995;186:169–172. doi: 10.1016/0304-3940(95)11316-o. [DOI] [PubMed] [Google Scholar]
- Koyama S, Matsumoto N, Murakami N, Kubo C, Nabekura J, Akaike N. Role of presynaptic 5-HT1A and 5-HT3 receptors in modulation of synaptic GABA transmission in dissociated rat basolateral amygdala neurons. Life Sci. 2002;72:375–387. doi: 10.1016/s0024-3205(02)02280-4. [DOI] [PubMed] [Google Scholar]
- Kramer PR, Kerins CA, Schneiderman E, Bellinger LL. Measuring persistent temporomandibular joint nociception in rats and two mice strains. Physiol Behav. 2010;99:669–678. doi: 10.1016/j.physbeh.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PR, He J, Puri J, Bellinger LL. A non-invasive model for measuring nociception after tooth pulp exposure. J Dent Res. 2012;91:883–887. doi: 10.1177/0022034512454297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liverman CS, Brown JW, Sandhir R, Klein RM, McCarson K, Berman NE. Oestrogen increases nociception through ERK activation in the trigeminal ganglion: evidence for a peripheral mechanism of allodynia. Cephalalgia. 2009;29:520–531. doi: 10.1111/j.1468-2982.2008.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Armentia M, Cabanes C, Belmonte C. Electrophysiological properties of identified trigeminal ganglion neurons innervating the cornea of the mouse. Neuroscience. 2000;101:1109–1115. doi: 10.1016/s0306-4522(00)00440-1. [DOI] [PubMed] [Google Scholar]
- Loyd DR, Weiss G, Henry MA, Hargreaves KM. Serotonin increases the functional activity of capsaicin-sensitive rat trigeminal nociceptors via peripheral serotonin receptors. Pain. 2011;152:2267–2276. doi: 10.1016/j.pain.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfurt CF. The central projections of trigeminal primary afferent neurons in the cat as determined by the tranganglionic transport of horseradish peroxidase. J Comp Neurol. 1981;203:785–798. doi: 10.1002/cne.902030414. [DOI] [PubMed] [Google Scholar]
- McCarson KE, Enna SJ. Nociceptive regulation of GABA(B) receptor gene expression in rat spinal cord. Neuropharmacology. 1999;38:1767–1773. doi: 10.1016/s0028-3908(99)00121-5. [DOI] [PubMed] [Google Scholar]
- Mody I. Aspects of the homeostaic plasticity of GABAA receptor-mediated inhibition. J Physiol. 2005;562:37–46. doi: 10.1113/jphysiol.2004.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai K, Nakae A, Oba S, Mashimo T, Ueda K. 5-HT2C receptor agonists attenuate pain-related behaviour in a rat model of trigeminal neuropathic pain. Eur J pain. 2010;14:999–1006. doi: 10.1016/j.ejpain.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Neelands TR, Macdonald RL. Incorporation of the pi subunit into functional gamma-aminobutyric acid(A) receptors. Mol Pharmacol. 1999;56:598–610. doi: 10.1124/mol.56.3.598. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Ahmad Z, Tretter V, Fuchs K, Wisden W, Sieghart W, Somogyi P. Alterations in the expression of GABAA receptor subunits in cerebellar granule cells after the disruption of the alpha6 subunit gene. EurJ Neurosci. 1999;11:1685–1697. doi: 10.1046/j.1460-9568.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- Oshima K, Takeda M, Tanimoto T, Katsuumi I, Matsumoto S. Tooth-pulp-evoked rostral spinal trigeminal neuronal excitation is attenuated by the activation of 5-HT3 receptors via GABAergic interneurons in the rat. Brain Res. 2006;1109:70–73. doi: 10.1016/j.brainres.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Amsterdam, The Netherlands: Elsevier Inc; 2007. [Google Scholar]
- Pereira LJ, Steenks MH, de WA, Speksnijder CM, an der BA. Masticatory function in subacute TMD patients before and after treatment. J Oral Rehabil. 2009;36:391–402. doi: 10.1111/j.1365-2842.2008.01920.x. [DOI] [PubMed] [Google Scholar]
- Pfaller K, Arvidsson J. Central distribution of trigeminal and upper cervical primary afferents in the rat studied by anterograde transport of horseradish peroxidase conjugated to wheat germ agglutinin. J Comp Neurol. 1988;268:91–108. doi: 10.1002/cne.902680110. [DOI] [PubMed] [Google Scholar]
- Price TJ, Hargreaves KM, Cervero F. Protein expression and mRNA cellular distribution of the NKCC1 cotransporter in the dorsal root and trigeminal ganglia of the rat. Brain Res. 2006;1112:146–158. doi: 10.1016/j.brainres.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri J, Bellinger LL, Kramer PR. Estrogen in cycling rats alters gene expression in the temporomandibular joint, trigeminal ganglia and trigeminal subnucleus caudalis/upper cervical cord junction. J Cell Physiol. 2011;226:3169–3180. doi: 10.1002/jcp.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri J, Vinothini P, Reuben J, Bellinger LL, Ailing L, Peng YB, Kramer PR. Reduced GABA(A) receptor alpha6 expression in the trigeminal ganglion alters inflammatory TMJ hypersensitivity. Neuroscience. 2012;213:179–190. doi: 10.1016/j.neuroscience.2012.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Empson RM, Heinemann U. Serotonin reduces inhibition via 5-HT1A receptors in area CA1 of rat hippocampal slices in vitro. J Neurosci. 1995;15:7217–7225. doi: 10.1523/JNEUROSCI.15-11-07217.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield PR, Pritchett DB, Sontheimer H, Kettenmann H, Seeburg PH. Sequence and expression of human GABAA receptor alpha 1 and beta 1 subunits. FEBS Lett. 1989;244:361–364. doi: 10.1016/0014-5793(89)80563-0. [DOI] [PubMed] [Google Scholar]
- Schwartz ES, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain. 2008;138:514–524. doi: 10.1016/j.pain.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000;11:57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Sera M, Nishimori T, Suemune S, Nishimura M, Yoshida A, Tsuru K. The central projection of masticatory afferent fibers to the trigeminal sensory nuclear complex and upper cervical spinal cord. J Comp Neurol. 1988;268:489–507. doi: 10.1002/cne.902680403. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Asano M, Kitagawa J, Ogiso B, Ren K, Oki H, Matsumoto M, Iwata K. Phosphorylation of extracellular signalregulated kinase in medullary and upper cervical cord neurons following noxious tooth pulp stimulation. Brain Res. 2006;1072:99–109. doi: 10.1016/j.brainres.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Guo W, Wang H, Zou S, LaGraize SC, Iwata K, Wei F, Dubner R, Ren K. Differential involvement of trigeminal transition zone and laminated subnucleus caudalis in orofacial deep and cutaneous hyperalgesia: the effects of interleukin-10 and glial inhibitors. Mol Pain. 2009;5:75. doi: 10.1186/1744-8069-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg WF, Wachterman MW. Experimental studies of sex-related factors influencing nociceptive responses: nonhuman animal research. In: Fillingim RB, editor. Sex, gender and pain. Vol. 17. Seattle: IASP Press; 2000. pp. 71–88. [Google Scholar]
- Stohler CS. Muscle-related temporomandibular disorders. J Orofac Pain. 1999;13:273–284. [PubMed] [Google Scholar]
- Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci. 2000;20:7531–7538. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I, Harada T, Asano M, Tsuboi Y, Kondo M, Gionhaku N, Kitagawa J, Kusama T, Iwata K. Phosphorylation of ERK in trigeminal spinal nucleus neurons following passive jaw movement in rats with chronic temporomandibular joint inflammation. J OrofacPain. 2007;21:225–231. [PubMed] [Google Scholar]
- Takemura M, Shimada T, Shigenaga Y. GABA(A) receptor-mediated effects on expression of c-Fos in rat trigeminal nucleus following high- and low-intensity afferent stimulation. Neuroscience. 2000;98:325–332. doi: 10.1016/s0306-4522(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Toyoda H, Yamada J, Ueno S, Okabe A, Kato H, Sato K, Hashimoto K, Fukuda A. Differential functional expression of cation-Cl-cotransporter mRNAs (KCC1, KCC2, and NKCC1) in rat trigeminal nervous system. Brain Res Mol Brain Res. 2005;133:12–18. doi: 10.1016/j.molbrainres.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Turner TJ, Mokler DJ, Luebke JI. Calcium influx through presynaptic 5-HT3 receptors facilitates GABA release in the hippocampus: in vitro slice and synaptosome studies. Neuroscience. 2004;129:703–718. doi: 10.1016/j.neuroscience.2004.08.020. [DOI] [PubMed] [Google Scholar]
- varez-Leefmans FJ, Gamino SM, Giraldez F, Norgueron I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J Physiol. 1988;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggiano A, Monda M, Viggiano A, Chiefari M, Aurilio C, De Luca B. Evidence that GABAergic neurons in the spinal trigeminal nucleus are involved in the transmission of inflammatory pain in the rat: a microdialysis and pharmacological study. Eur J Pharmacol. 2004;496:87–92. doi: 10.1016/j.ejphar.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Viggiano A, Monda M, Viggiano A, Viggiano D, Viggiano E, Chiefari M, Aurilio C, De Luca B. Trigeminal pain transmission requires reactive oxygen species production. Brain Res. 2005;1050:72–78. doi: 10.1016/j.brainres.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Viggiano E, Monda M, Viggiano A, Viggiano A, Aurilio C, De Luca B. Persistent facial pain increases superoxide anion production in the spinal trigeminal nucleus. Mol Cell Biochem. 2010;339:149–154. doi: 10.1007/s11010-009-0378-9. [DOI] [PubMed] [Google Scholar]
- Vit JP, Ohara PT, Sundberg C, Rubi B, Maechler P, Liu C, Puntel M, Lowenstein P, Castro M, Jasmin L. Adenovector GAD65 gene delivery into the rat trigeminal ganglion produces orofacial analgesia. Mol Pain. 2009;5:42. doi: 10.1186/1744-8069-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- Wu LA, Huang J, Wang W, Wang W, Li YQ, Wang XJ, Wu SX. Activation of GABAergic neurons following tooth pulp stimulation. J Dent Res. 2010;89:532–536. doi: 10.1177/0022034510363231. [DOI] [PubMed] [Google Scholar]
- Ymer S, Schofield PR, Draguhn A, Werner P, Kohler M, Seeburg PH. GABAA receptor beta subunit heterogeneity: functional expression of cloned cDNAs. EMBO J. 1989;8:1665–1670. doi: 10.1002/j.1460-2075.1989.tb03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yowtak J, Lee KY, Kim HY, Wang J, Kim HK, Chung K, Chung JM. Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain. 2011;152:844–852. doi: 10.1016/j.pain.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]