Abstract
Background
Schizophrenia is associated with abnormalities in emotional processing and social cognition, which may result from disruption of the underlying neural mechanism(s) governing emotional learning and memory. To investigate this possibility, we measured the acquisition and extinction of conditioned fear responses and delayed recall of extinction in schizophrenia and control subjects.
Methods
28 schizophrenia and 18 demographically-matched control subjects underwent a two-day fear conditioning, extinction learning and extinction recall procedure, in which skin conductance response (SCR) magnitude was used as the index of conditioned responses.
Results
During fear acquisition, 83% of the controls and 57% of the patients showed autonomic responsivity (‘responders’), and the patients showed larger SCRs to the stimulus that was not paired with the unconditioned stimulus (CS−) than the controls. Within the responder group, there was no difference between the patients and controls in levels of extinction learning; however, the schizophrenia patients showed significant impairment, relative to the controls, in context-dependent recall of the extinction memory. In addition, delusion severity in the patients correlated with baseline skin conductance levels.
Conclusions
These data are consistent with prior evidence for a heightened neural response to innocuous stimuli in schizophrenia and elevated arousal levels in psychosis. The finding of deficient extinction recall in schizophrenia patients who showed intact extinction learning suggests that schizophrenia is associated with a disturbance in the neural processes supporting emotional memory.
Keywords: schizophrenia, fear, conditioning, extinction, emotion, memory
INTRODUCTION
It has been recently recognized that impaired emotional function may play an important role in the symptoms and the overall functional disability associated with schizophrenia (1–5). Empirical studies have reported at least two types of emotional processing abnormalities in schizophrenia patients. First, patients with schizophrenia demonstrate deficits in emotion recognition across multiple sensory domains (6–12). Second, a bias to misassign emotional meaning to neutral, innocuous stimuli has been identified in schizophrenia patients using a wide range of experimental paradigms (13–16). In some studies, emotion recognition deficits in schizophrenia have been linked to impairments in cognition or overall functioning (5, 17). In contrast, mis-assignment of emotional meaning to neutral stimuli has been associated with psychotic symptoms (13, 15, 18, 19).
One possibility is that one or both of these abnormalities in emotional function arise from a disruption of the basic neuronal mechanism(s) that are responsible for encoding and retrieving the emotional value or meaning of a stimulus—emotional memory processes. A type of emotional memory, called extinction memory, has been studied extensively in rodents and humans using adaptations of Pavlovian fear conditioning paradigms (20–23). Pavlovian fear conditioning involves pairing an innocuous stimulus (the conditioned stimulus, CS) with an aversive sensation (the unconditioned stimulus, US), such as a shock, air puff or loud noise, which leads to the formation of a memory indicating that the CS signals danger (a ‘danger’ memory) (24–26). If the CS is subsequently presented several times without the US (extinction learning), a second, ‘safety’ memory is formed, linking the CS with the absence of the US. It has been shown that these two memories of a given CS can exist simultaneously, and can be retrieved independently at a later time (27–29). Importantly, the safety (extinction) memory is linked to the particular context in which it was formed; the extinction memory is retrieved only if the context in which it was encoded is present at retrieval (30, 31).
Studies of aversive conditioning in schizophrenia have produced mixed results, with findings of facilitated (32, 33), diminished (16, 34, 35) as well as normal (34, 36) aversive conditioning in schizophrenia patients relative to controls. These inconsistencies may be due in part to methodological differences among studies (for example, the absence of a non-aversive comparison condition (CS−) in some investigations), as well as to heterogeneity within patient samples. Heterogeneity was evident in several studies which found that one third to one half of the patients exhibited no learning at all, while the remaining patients showed normal acquisition of aversive conditioning (34, 36). These findings are consistent with evidence for the existence of a subpopulation of patients with schizophrenia with abnormally low or absent autonomic responses to salient stimuli (37).
To date, extinction memory has not been studied in schizophrenia. Although it has been established that patients with schizophrenia have difficulty using contextual information during cognitive (38, 39) and social cognitive (40, 41) processing, it is not known whether these deficits are related to abnormalities in context-dependent emotional memory processes.
In the current study, we sought to determine whether schizophrenia is associated with impairment in learning or memory of conditioned fear responses. We used a two-day, classical Pavlovian fear conditioning and extinction paradigm which had been previously adapted for use in humans (22, 23). On the first day of this paradigm, participants undergo fear conditioning during which one neutral stimulus is paired (CS+) and another is not paired (CS−) with an aversive electrical tactile stimulus (US). Following acquisition of conditioned fear responses, participants then undergo an extinction learning phase during which the CS+ is presented without the US. These two phases occur in distinct visual contexts (the acquisition context and the extinction context). Twenty-four hours later, participants are again exposed to the CS+ without the US, in the extinction context and in the acquisition context, and responses to the CS+ in the two contexts are measured within and across groups. Based on previous studies (22, 42, 43), we predicted that, on the second day of the study, healthy control participants would show minimal SCRs to the CS+ in the extinction context, indicating successful retrieval of the extinction memory (i.e., the memory that the CS+ no longer predicts the US). We also hypothesized that patients with schizophrenia would demonstrate impairment, relative to controls, in context-dependent retrieval of extinction memory.
METHODS AND MATERIALS
Participants
28 patients with DSM-IV-diagnosed schizophrenia and 18 control subjects, between 18 and 65 years old, were studied. Clinically stable, medicated patients with schizophrenia were recruited through the MGH Schizophrenia Clinical and Research Program (see Table 1). Healthy control subjects were recruited via advertisement. The healthy control subjects did not have any psychiatric, neurologic or severe medical disorders, or history of substance abuse during the previous three months, as determined by phone screening, questionnaires and the SCID (44). Capacity to provide informed consent was evaluated for each subject, and written informed consent was obtained from all subjects prior to enrollment in accordance with the guidelines of the Partners Healthcare Institutional Review Board. Each patient’s symptoms were evaluated by a trained rater using the Positive and Negative Symptom Scale (45).
Table 1.
Demographic characteristics of the full cohort of subjects (n = 46) and the responders (participants with ≥ 2 trials with SCRs ≥ .03 microseimens during the Acquisition phase) (n=31). Means are presented, with standard errors of the mean in parentheses. Antipsychotic medications taken by the patients, with the number of patients taking them in parentheses: clozapine (12), risperidone (5), olanzapine (5), aripiprazole (6), quetiapine (2), ziprazadone (1), perphenazine (1), haloperidol (1) and no antipsychotic medication (3). Only one patient was taking an anticholinergic medication (benztropine). Although the proportion of responders was 26% higher in the control than in the patient group, this difference did not reach statistical significance (Fischer’s Exact Test, p=.11). Within the patient group, there were no significant differences between the responders (n=15) and non-responders (n=12) on any clinical or outcome measure, although there was a trend towards a higher mean SCR to the CS+ trials during the Acquisition phase in the responders compared to the nonresponders (p=.09) and a trend towards a higher verbal I.Q. in the nonresponders compared to the responders (p=.09). Also, within the responder cohort, there were no significant differences between the controls (n=16) and patients (n=15) in demographic characteristics or selected level of electrical stimulation, with the exception that, as in the full cohort, mean verbal I.Q. was higher in the controls than in the patients (p=.004). a: Socioeconomic status, measured using the Hollingshead index; b: Verbal I.Q., measured using the North American Reading Test.
| Full cohort | Responders | |||
|---|---|---|---|---|
| Controls n=18: 7 female |
Patients n=28: 5 female |
Controls n=15: 5 female |
Patients n=16: 5 female |
|
|
|
||||
| Age (years) | 42.8 (3.4) | 43.6 (2.0) | 41.2 (3.8) | 42.8 (3.0) |
| Mean parental education (years) | 13.2 (0.7) | 13.4 (0.5) | 13.2 (0.9) | 13.1 (0.6) |
| Mean parental SESa | 2.7 (0.2) | 2.5 (0.2) | 2.8 (0.3) | 2.6 (0.3) |
| Verbal I.Q.b | 110.0 (1.6) | 104.2 (2.0) | 111.8 (1.2) | 101.6 (3.0 |
| Stimulation level | 2.6 (0.3) | 2.3 (0.2) | 2.7 (0.3) | 2.2 (0.3) |
| PANSS Total | 56.5 (2.1) | 58.9 (2.7) | ||
| PANSS Positive scale | 14.6 (1.0) | 15.1 (1.1) | ||
| PANSS Negative scale | 13.8 (0.7) | 13.7 (1.0) | ||
| PANSS General scale | 28.5 (1.2) | 30.1 (1.4) | ||
| PANSS delusion item | 2.9 (0.3) | 3.1 (0.4) | ||
| Duration of illness (years) | 18.7 (2.0) | 18.6 (2.8) | ||
| Chlorpromazine equivalents | 441.4 (70.6) | 347.8 (68.8) | ||
Psychophysiological Procedures
A Coulbourn Instruments Lablink V System (Allentown, PA) was used to record skin conductance level via a Coulbourn Isolated Skin Conductance Coupler (71–23). Electrodes were placed on the palm of the participant’s non-dominant hand. Electrodes were also attached to the second and third fingers of the dominant hand for the purpose of delivering the US (a 500 ms electrical stimulus). The US was generated by a Coulbourn Transcutaneous Aversive Finger Stimulator (E13–22). The intensity of the electrical US was set by each participant before the start of the procedure at a level that was “highly annoying but not painful.”
Fear Conditioning and Extinction Procedures
The fear conditioning and extinction procedures used in this study were similar to those previously described (22, 42). During both days of the procedure, participants sat in a comfortable chair in front of a computer monitor. After the electrodes were attached, participants were asked to passively view digital photographs of two rooms which contained lamps that appeared on the computer screen (see Figure 1).
Figure 1.
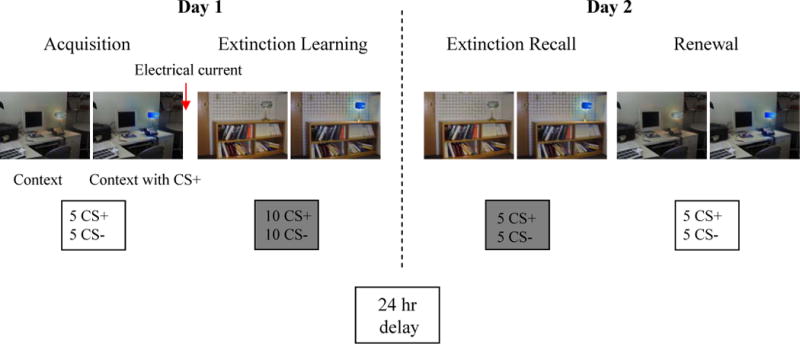
Schematic of experimental protocol. Photographs of the visual contexts (CX), within which conditioned stimuli (CS) were presented, are shown. In this example, a photograph of an office is the conditioning context (CX+) and a photograph of a conference room is the extinction context (CX−). The blue light is the conditioned stimulus that was paired with the electrical current (CS+) and later extinguished. The conditioned stimulus that was not paired with the electrical current, the CS− (which, in this example, would be a yellow light) is not shown here. Six seconds after the onset of the presentation of the room photograph, the lampshade in the room turns yellow or blue for 12 seconds (total stimulus duration for the room photograph: 18 seconds). The numbers of each stimulus type presented during each phase is indicated. The Extinction Learning phase was divided into an early and late phase that each included 10 trials (5 CS+ and 5 CS−), all presented within the CX−. Electrical current was delivered only during the Acquisition phase. Gray shading indicates the CX−. The Habituation phase is not shown for simplicity. Note that the order of the phases on Day 2, Extinction Recall and Renewal, were counterbalanced across subjects.
Photographs of the two rooms (a conference room and an office) constituted the two virtual contexts, CX. During the procedure, one context was associated (CX+) and one was not associated (CX−) with receiving the electrical stimulus. Each room CX contained a lamp. Two colors of the lit lampshade (blue or yellow) constituted the CSs. One CS was paired (CS+) and one was not paired (CS−) with presentations of the electrical stimulus. The selection of the CS+ and CS− colors, and the CX+ and CX− rooms, was counterbalanced across participants.
For each trial during the experiment, the CX was presented for 18 seconds: 6 seconds alone, followed by 12 seconds in combination with the CS+ or CS−. Skin conductance was recorded for 5 seconds prior to the presentation of the CX, during the 6-second presentation of the CX alone, and during the 12-second presentation of the CX plus the CS. The US occurred during the last 500 ms of the CS+. The average inter-trial interval was 16 seconds.
The experimental protocol was administered over two separate days. There were three phases on Day 1: Habituation, Acquisition, and Extinction Learning. The Habituation phase included trials in which the CS+ and CS− (4 trials of each) were presented in a counterbalanced manner within either the acquisition context (CX+) or the extinction context (CX−). The Acquisition phase included 5 CS+ and 5 CS− trials, all presented within the CX+. The Extinction Learning phase was divided into two sub-phases: early and late, which were separated by a 1-minute rest period. Each sub-phase included 5 CS+ and 5 CS− trials, all presented within the CX−. The electrical US was not delivered during the Extinction Learning phase.
On Day 2, there were two phases: Extinction Recall and Renewal. The Extinction Recall phase included 5 CS+ and 5 CS− presented within the CX− without any US. During the Renewal phase, 5 CS+ and 5 CS− were presented within the CX+, and again no US was delivered. In half the participants, the Renewal phase preceded the Recall phase.
Instructions to Participants
Prior to the Habituation phase, participants were instructed that the purpose of this phase was to show them all the possible pictures that they would see throughout the study, and that no electrical current would be delivered. Prior to all subsequent phases of the study, participants were instructed that they “may or may not receive electrical stimulations.”
Following the Acquisition phase and at the start of Day 2, each participant was asked if they could recall the color of the light that was paired with the electrical stimulation, and to describe the room which contained the light that was paired with the stimulation.
Skin Conductance Level and Response Values and Data Analysis
Baseline SCL was calculated as the mean skin conductance values during the 5 seconds prior to the presentation of the context during the Habituation phase.
The SCR magnitude for each CS trial was calculated by subtracting the mean skin conductance level during the 2 seconds immediately prior to CS onset (during which the context alone was being presented) from the highest skin conductance level recorded during the 12-second CS duration. Thus, all SCRs to the CS+ and CS− reflect changes in skin conductance levels above any changes produced by the context. To minimize the impact of individual variation in fear acquisition on the extinction learning and extinction recall measures, the measures of extinction learning and extinction recall were normalized in each individual to their largest SCR during fear acquisition (22, 42). SCRs were square-root transformed prior to analysis. Since SCRs to the CS+ can only be extinguished after successful acquisition of those responses, extinction learning and recall were examined only in participants who showed SCRs during the Acquisition phase (with SCRs ≥ 0.03 microseimens in two or more of the 10 trials of the Acquisition phase), consistent with previous studies (22, 46).
Fear acquisition was calculated as the mean SCR for the last two trials of the CS+ minus the mean SCR for the last two trials of the CS− during the Acquisition phase. Extinction learning was calculated using an Extinction Learning Index, representing extinction learning success on Day 1: 100-((the average SCR for the last two trials of the Extinction Learning phase, divided by the largest SCR for the Acquisition phase) × 100). Extinction memory was calculated using an Extinction Retention Index, representing extinction recall success on Day 2, with the same formula as the Extinction Learning Index except that SCRs for the first two trials of the Extinction Recall phase were used.
Our a priori hypothesis regarding extinction recall was tested using Student’s t-test (α = 05, two-tailed). Differential fear acquisition (SCRs to the CS+ compared to SCRs to the CS− during the last two trials of the Acquisition phase) and differential context sensitivity (SCRs to the CS+ during the first two trials of the Extinction Recall phase compared to SCRs to the CS+ during the first two trials of the Renewal phase) were assessed within each group using paired t-tests (α = 05, one-tailed).
Fisher’s Exact Test was used to assess between-group differences in explicit recall of CS and CX identities (α = 05, two-tailed). Means are presented with standard errors of the mean.
RESULTS
Characteristics of the participants
There were no differences between the patients and controls in age, mean parental education, socioeconomic status, or in level of electrical stimulation chosen (see Table 1). Mean verbal IQ was lower in the patients than in the controls (t (43) = 2.07; p =.04).
Baseline SCL and Fear Acquisition
The mean SCL at baseline was non-significantly higher in the controls than the patients (controls: 3.55 ± .84; patients: 1.97 ± .37 microseimens; t (29) = 1.93, p=.06). Within-group analyses revealed that the control group demonstrated acquisition of differential fear conditioning to the CS+ versus CS− (t (17) = 2.65; p=.008), and the patients showed a trend towards acquisition of differential fear responses (t (27) = 1.61; p = .08). There were no statistically significant differences between the two groups in mean levels of fear acquisition (CS+ minus CS−) (controls: .29 ± .11; patients: .10 ± .07 microseimens; t (44) = 1.46, p=.15) or in magnitude of SCRs to the CS+ trials (controls: .09 ± .08; patients: .12 ± .06 microseimens; t (44) = .38, p=.71). However, the patients exhibited a significantly larger mean SCR to the CS− trials compared to the controls (controls: −.20 ± .09; patients: .02 ± .04 microseimens; t (44) = 2.62, p=.01).
Examination of the acquisition data of each subject revealed that 83% of the controls (15/18) and 57% of the patients (16/28) exhibited autonomic responsivity (≥ 2 trials with SCR ≥ .03 microseimens) during the Acquisition phase (‘responders’). Within this responder cohort, the controls again exhibited a non-significantly higher mean SCL than the patients (controls = 4.13 ± .94; patients = 2.49 ± .52 microseimens; t (29) = 1.55, p=.13). The responder patient and control groups did not differ in levels of fear acquisition (CS+ minus CS−) (controls: .33 ± .13; patients: .20 ± .12 microseimens; t (29) = .75, p=.46) or in magnitude of SCRs to the CS+ trials (controls: .10 ± .10; patients: .22 ± .10 microseimens; t (29) =.80, p=.43). However, within the responder group, the patients again showed a significantly larger mean SCR to the CS− trials compared to the controls (controls: −.22 ± .10; patients: .02 ± .06 microseimens; t (29) = 2.06, p=.048) (Figure 2A). Because we planned to measure fear extinction and extinction memory only in participants who displayed SCRs during acquisition (see Methods), further analyses were limited to the responders (15 controls, 16 patients).
Figure 2.
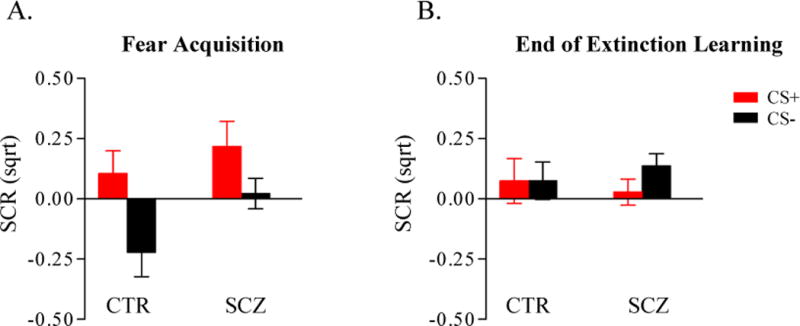
Plots of mean SCRs ± SEM during the last two trials of the CS+ (red bars) and CS− (black bars) during the Acquistion phase (A) and the Extinction Learning phase (B) in the controls (CTR, n = 15) and schizophrenia patients (SCZ, n = 16) within the responder cohort. For the responders, within-group analyses revealed that the controls demonstrated acquisition of differential fear conditioning to the CS+ versus CS− (t (14) = 2.57; p=.011), and the patients showed a trend towards acquisition of differential fear responses (t (15) = 1.61; p = .064), similar to the findings in the full cohort. There were no differences between the control and patient responder groups in overall fear acquisition and extinction learning (see text). Note that because measurements of SCRs to the conditioned stimuli are normalized to the SCRs to the context (see Methods), SCRs at the end of extinction can have negative values, which can lead to a value of the Extinction Learning Index that exceeds 100%. Sqrt: square-root transformed.
Extinction learning
The patient and control groups demonstrated equivalent levels of extinction learning, as reflected by their responses to the CS+ at the end of the Extinction Learning phase (controls: .07 ± .09; patients: .03 ± .05 microseimens; t (29) = .66, p=.51) (Figure 2B) and their mean Extinction Learning Index (controls: 89.5 % ± 11.9; patients: 92.5 % ± 16.1; t (29) = .15; p=.88) (Figure 3A).
Figure 3.
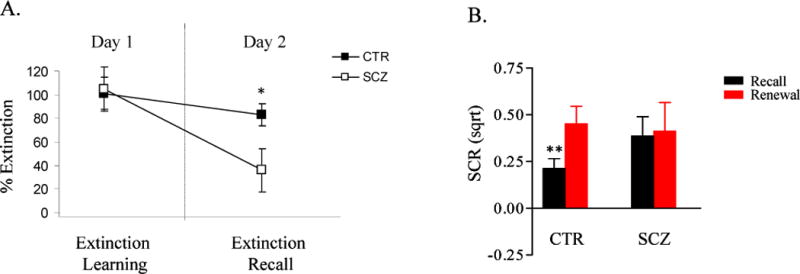
A. A plot of mean percent extinction ± SEM of the two groups during the Extinction Learning (Day 1) and Extinction Recall (Day 2) phases. Extinction learning and extinction recall are each normalized to the highest levels of fear (SCR) attained during the Acquisition phase and are expressed as a percentage of that maximal fear response that is extinguished (the mean Extinction Learning Index and Extinction Retention Index are shown on the left and right of this plot, respectively; see Methods). The two groups do not differ with respect to extinction learning, but extinction recall is significantly lower in the patients than in the controls. CTR: control group, n=15; SCZ: schizophrenia group, n=16; *p = .034. Sqrt: square-root transformed.
B. A plot of mean SCRs ± SEM to the CS+ of the two groups during the Extinction Retention (black bars) and Renewal (red bars) phases on Day 2, showing that SCRs to the CS+ are modulated by context in the controls (n=15) but not in the patients (n=16). **p = .008.
Extinction recall after 24 hours
Despite the comparable levels of extinction learning shown by the schizophrenia and control groups, the patients with schizophrenia demonstrated impaired recall of the extinction memory, with a significantly lower Extinction Retention Index than the control subjects (controls: 74.2 % ± 8.0; patients: 33.7 % ± 16; t (29) = 2.23; p=.034) (Figure 3A). Further, the controls showed normal context-sensitivity during retrieval, with significantly lower SCRs to the CS+ in the extinction, compared to the acquisition, context (t (14) =2.77; p=.008); in contrast, the schizophrenia patients did not demonstrate a significant effect of context on SCRs to the CS+ on Day 2 (t (15) =.22; p=.41) (Figure 3B).
Explicit learning
There was no difference between the two groups in explicit learning on Day 1 (see Table 2). However, debriefing at the beginning of Day 2 revealed that while the schizophrenia patients and controls were equally likely to correctly recall the identities of the CS+ and CS−, the patients were less likely to recall the identities of the CX+ and CX− than the controls (Fisher’s Exact Test, p=.02).
Table 2.
Percentage of subjects within each group (15 controls, 16 patients with schizophrenia) demonstrating successful recall of the identities of the CS+ (vs. CS−) and CX+ (vs. CX−) with the p values of the corresponding statistical comparisons (Fisher’s Exact Test).
| Control | Schizophrenia | P value | |
|---|---|---|---|
|
|
|||
| % recalled CS+ on Day 1 | 86.7 | 75 | .65 |
| % recalled CX+ on Day 1 | 100 | 92.9 | .48 |
| % recalled CS+ on Day 2 | 86.7 | 68.8 | .39 |
| % recalled CX+ on Day 2 | 100 | 62.5 | .02 |
Correlations
There were no correlations among the four primary outcome measures within either group, with the exception of an inverse correlation between mean SCL and the extinction learning index within the control group only (Rho=−.69; p=.004; n=15). Also, there were no significant correlations between magnitudes of fear acquisition, extinction learning or extinction memory recall and the PANSS total and subscale scores within the patient group. However, a significant correlation was found between SCL and the PANSS Positive subscale scores (Rho = .69; p= .0028; n=16) (Figure 4A). Additional analyses revealed that the correlation between SCL and positive symptom severity was due to an association between baseline SCL and severity of delusional thinking (Rho = .80; p=.0002; n=16) (Figure 4B) and also levels of suspiciousness and persecution (Rho=.55; p=.027; n=16). No other items within the PANSS Positive subscale were significantly correlated with skin conductance levels. Also, there were no significant correlations between the reported findings and age, I.Q., duration of illness or dose of antipsychotic medication.
Figure 4.
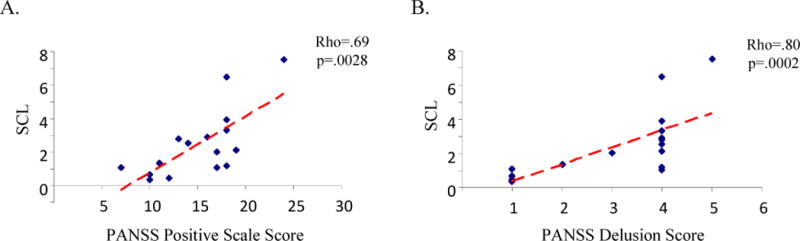
Scatter plots of the correlations between baseline skin conductance level (SCL) and the PANSS Positive Scale score (A) and the PANSS delusion score (B) within the schizophrenia group (n =16).
DISCUSSION
Summary of findings
In this study, a substantial proportion (43%) of the patients with schizophrenia displayed minimal autonomic responsivity, consistent with previous findings (37). The patients who had intact autonomic responses (responders) demonstrated levels of fear acquisition and extinction learning that were comparable to the controls; however, these patients showed impairment, and loss of context sensitivity, in delayed recall of extinction. Moreover, the reduction in extinction recall manifested by abnormal SCRs in the patients was accompanied by a parallel reduction in explicit recall of the identities of the safe and dangerous contexts.
In addition, during fear acquisition, the patients showed higher SCRs to the CS− than the controls, and baseline skin conductance levels in the responder patient group correlated with delusion severity.
Reduced autonomic responsivity and abnormal responses to the CS− during fear acquisition
The finding of a population of schizophrenia patients with minimal or absent autonomic responses to sensory stimuli has been repeatedly replicated (37, 47, 48). The average proportion of patients with schizophrenia classified as nonresponders in previous studies is approximately 40%, compared to the 5–10% of nonresponders found in healthy and non-schizophrenia psychiatric samples (37), consistent with the rates found in the current study. It has been demonstrated in studies of unmedicated schizophrenia patients that some degree of autonomic hyporesponsivity is intrinsic to the disorder (49–54).
The discrepancies among the results of studies of aversive conditioning in schizophrenia (16, 32–36) may be due in part to this heterogeneity in autonomic responsivity. In the current study, we found some evidence for an impairment in the acquisition of conditioned fear responses in schizophrenia; although the difference between the mean levels of fear acquisition between the schizophrenia and control groups did not reach significance, the effect size was 0.41 (Cohen’s d) (in the full cohort of subjects), suggesting that the failure to find a significant difference between the groups in fear acquisition may have reflected a Type II error.
The trend towards a reduction in differential fear acquisition in the schizophrenia group was at least in part due to an abnormally elevated response to the CS−, replicating a previous, similar finding (16). These results are consistent with the increasing evidence for the presence of a behavioral and neural bias towards misattributing emotional meaning to innocuous, emotionally neutral stimuli in individuals with schizophrenia (13, 15, 18, 55). Functional neuroimaging studies have detected abnormally elevated activity in response to neutral, nonaversive stimuli in patients with schizophrenia in the amygdala (18, 56, 57), parahippocampal gyrus (18), ventral striatum (16), cingulate gyrus (55) and lateral prefrontal cortex (15). Taken together, the consistent replication of this overall finding, and its relationship to abnormal fear conditioning and dysfunction of emotional learning and memory circuitry, suggest that aberrant neural responses to neutral stimuli in schizophrenia arise from disruption of a basic emotional memory mechanism.
Extinction recall deficit
The finding of abnormally reduced recall of extinction memory in patients with intact autonomic responsivity suggests that some patients with schizophrenia are impaired in a specific type of emotional memory, namely the retrieval of a ‘safety signal’ (58). Although our finding is limited to a subgroup of schizophrenia patients with intact autonomic responsivity, results of an exploratory analysis of the data from the full cohort of subjects (28 patients, 18 controls) (data not shown) were the same as the findings for the responders, with impaired extinction recall, following intact extinction learning, in the patients in comparison to the controls. Thus extinction recall may be impaired in a substantial proportion of patients with schizophrenia.
The retrieval of extinction memory relies on a network of limbic brain regions which includes the medial prefrontal cortex, hippocampus and amygdala (20, 21, 23, 26, 43, 58–60), areas which show abnormal levels of activity during emotional processing in schizophrenia (19, 55, 57, 61–64). An fMRI study of extinction recall in healthy humans found that the success of extinction recall was predicted by the magnitude of both medial prefrontal and hippocampal activation (43). The hippocampus is thought to mediate the effects of context on Pavlovian fear conditioning and extinction (58); the loss of context-sensitivity in the schizophrenia patients during the retrieval phases of this study suggests that impaired extinction recall in schizophrenia could reflect dysfunction of the hippocampus. This hypothesis is supported by a large body of neuroimaging and post-mortem evidence for structural and functional abnormalities of the hippocampus in schizophrenia (65–68).
Because schizophrenia is characterized by deficits in episodic memory and in other cognitive processes (69), it is possible that general cognitive abnormalities in the patients may have contributed to the impairment in explicit recall of the identities of the contexts on Day 2, the deficit in extinction recall, or both. We could not address this question directly in this study, because we did not measure explicit memory capacity or other aspects of cognitive function except I.Q. (which did not predict extinction recall success in either group). Because the relationship between explicit episodic memory processes and implicit, automatic ones such as extinction recall is not fully understood, studies which formally measure both types of memory in the same subjects will shed further light on this question.
These results have some implications for studies of the treatment of schizophrenia. Extinction recall in rodents depends upon N-methyl-D-aspartate (NMDA) glutamate receptor activity (70, 71). In basic investigations of the pathophysiology of schizophrenia, antagonism of the NMDA receptor has been used as a pharmacological model of schizophrenia symptoms, primarily because of the striking similarities between the psychological effects of NMDA receptor antagonists and both the negative and positive symptom clusters of schizophrenia (72, 73). Clinical trials have found mixed evidence for efficacy of agonists at the glycine modulatory site of the NMDA receptor in the treatment of cognitive deficits and negative symptoms in schizophrenia patients (74–77). Because an extinction recall impairment in schizophrenia may have greater biological ‘proximity’ to neuronal dysfunction than conventional measures of psychopathology, future trials of such agents may benefit from targeting quantifiable NMDA-dependent processes such as extinction memory recall.
Elevated skin conductance levels in delusions
The correlation between severity of delusional ideation and baseline skin conductance levels is in line with previous studies which have found elevated levels of spontaneous skin conductance responding in patients with high levels of positive symptoms (50, 78) and with findings of elevated skin conductance levels in schizotypal individuals (79). Elevated arousal during delusional states could account for the presence of attentional and memory biases towards threatening (81, 82), generally emotional (83) or affectively ambiguous (13, 84) information in delusional patients.
Limitations
The interpretation of our results is limited by the fact that we did not examine our data for the potential confounding effects of gender, subclasses of antipsychotic medication, caffeine and nicotine, due to power constraints imposed by the modest number of subjects. Another important limitation of this study is that we cannot exclude the possibility that antipsychotic treatment influenced our results, although we found no evidence for such an effect. Studies conducted in animals have found that D2 dopamine receptor blockade diminishes the acquisition of Pavlovian (86, 87) and operant (88–90) aversive conditioning. However, in the current study, normalizing the primary outcome measures to the maximal acquired fear response within individuals likely eliminated or minimized this potential confound, and we found no evidence for differences between the patients and controls within the responder group in responses to the CS+ or in overall levels of fear acquisition. Interestingly, D2 dopamine receptor antagonists have been shown to facilitate extinction learning (88, 91), and recall of extinction (91) in animals. Thus, if antipsychotic treatment improves extinction recall, the intrinsic impairment in schizophrenia may be underestimated by the present study.
Acknowledgments
This work was funded by NIMH (K23 MH076054 (DJH) and K24 MH002025 (DCG)) and the National Alliance for Research in Schizophrenia and Depression (NARSAD) with the Sidney J. Baer Trust. We are grateful to Dr. Eric Maclin for valuable assistance with the statistical analyses.
Footnotes
FINANCIAL DISCLOSURES
Scott Rauch received funds for research through MGH for Brain Stimulation Therapy from Medtronics, Inc.; through MGH for VNS from Cyberonics; and through MGH on anxiolytic action from Cephalon. Dr. Rauch also received honoraria from Novartis for consultation on emerging treatments; Neurogen for his participation as a consultant on emerging trends in anxiety associated with insomnia; Sepracor for his consultation on fear/conditioning/extinction and from Primedia for his participation in developing a CE activity and Medtronics, Inc for his attendance of the Advisory Board meeting on the Anatomy and Neuroscience of anxiety and depression. Dr. Rauch is also a trustee at McLean Hospital and also serves on the Board at Massachusetts Society for Medical Research (MSMR) as well as on the National Foundation of Mental Health (NFMH) Board.
Donald Goff received funds for research from Pfizer, Cephalon, and Janssen; speaking honoraria from Pfizer, Lilly, BristolMyerSquib, VerusMed, Letters and Science, Primedia, SG Cowen, Vista Research, Organon, Xytis, UCLA, Wyeth, Forest Laboratories, Loma Linda University, Psychiatric Times, Ohio State University, Genactics, Van Andel Research Institute; consulting honoraria from Xenoport, Dainippon Sumitomo Pharma America, Inc, and Genactics; honoraria for serving on an advisory board from Solvay/Wyeth, BristolMyerSquib, Proteus, Vanda Pharmaceuticals, Organon, and Xytis; and an honorarium for serving on a DSMB from Solvay/Wyeth.
Note that no direct conflict is anticipated. All relationships are listed for full disclosure.
The remaining authors have no financial disclosures.
References
- 1.Sergi MJ, Rassovsky Y, Widmark C, Reist C, Erhart S, Braff DL, Marder SR, Green MF. Social cognition in schizophrenia: relationships with neurocognition and negative symptoms. Schizophr Res. 2007;90(1–3):316–24. doi: 10.1016/j.schres.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 2.Smith B, Fowler DG, Freeman D, Bebbington P, Bashforth H, Garety P, Dunn G, Kuipers E. Emotion and psychosis: links between depression, self-esteem, negative schematic beliefs and delusions and hallucinations. Schizophr Res. 2006;86(1–3):181–8. doi: 10.1016/j.schres.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Penn DL, Corrigan PW, Bentall RP, Racenstein JM, Newman L. Social cognition in schizophrenia. Psychol Bull. 1997;121(1):114–132. doi: 10.1037/0033-2909.121.1.114. [DOI] [PubMed] [Google Scholar]
- 4.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 5.Hooker C, Park S. Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Res. 2002;112:41–50. doi: 10.1016/s0165-1781(02)00177-4. [DOI] [PubMed] [Google Scholar]
- 6.Moberg PJ, Arnold SE, Doty RL, Kohler C, Kanes S, Seigel S, Gur RE, Turetsky BI. Impairment of odor hedonics in men with schizophrenia. Am J Psychiatry. 2003;160(10):1784–9. doi: 10.1176/appi.ajp.160.10.1784. [DOI] [PubMed] [Google Scholar]
- 7.Addington J, Saeedi H, Addington D. Facial affect recognition: a mediator between cognitive and social functioning in psychosis? Schizophr Res. 2006;85(1–3):142–50. doi: 10.1016/j.schres.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Crespo-Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Watkins GL, Ponto LL, Hichwa RD. Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. Journal of the American Medical Association. 2001;286:427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- 9.Leitman DI, Hoptman MJ, Foxe JJ, Saccente E, Wylie GR, Nierenberg J, Jalbrzikowski M, Lim KO, Javitt DC. The neural substrates of impaired prosodic detection in schizophrenia and its sensorial antecedents. Am J Psychiatry. 2007;164(3):474–82. doi: 10.1176/ajp.2007.164.3.474. [DOI] [PubMed] [Google Scholar]
- 10.Malaspina D, Coleman E. Olfaction and social drive in schizophrenia. Arch Gen Psychiatry. 2003;60(6):578–84. doi: 10.1001/archpsyc.60.6.578. [DOI] [PubMed] [Google Scholar]
- 11.Hoekert M, Kahn RS, Pijnenborg M, Aleman A. Impaired recognition and expression of emotional prosody in schizophrenia: review and meta-analysis. Schizophr Res. 2007;96(1–3):135–45. doi: 10.1016/j.schres.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin Psychol Rev. 2002;22:789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- 13.Holt DJ, Titone D, Long LS, Goff DC, Cather C, Rauch SL, Judge A, Kuperberg GR. The misattribution of salience in delusional patients with schizophrenia. Schizophr Res. 2006;83:247–256. doi: 10.1016/j.schres.2005.12.858. [DOI] [PubMed] [Google Scholar]
- 14.Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ, Kanes SJ, Gur RE, Gur RC. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry. 2003;160:1768–1774. doi: 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]
- 15.Corlett PR, Murray GK, Honey GD, Aitken MR, Shanks DR, Robbins TW, Bullmore ET, Dickinson A, Fletcher PC. Disrupted prediction-error signal in psychosis: evidence for an associative account of delusions. Brain. 2007;130(Pt 9):2387–400. doi: 10.1093/brain/awm173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen J, Willeit M, Zipursky RB, Savina I, Smith AJ, Menon M, Crawley AP, Kapur S. The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology. 2008;33(3):473–9. doi: 10.1038/sj.npp.1301437. [DOI] [PubMed] [Google Scholar]
- 17.Kerr SL, Neale JM. Emotion perception in schizophrenia: specific deficit or further evidence of generalized poor performance? J Abnorm Psychol. 1993;102:312–318. doi: 10.1037//0021-843x.102.2.312. [DOI] [PubMed] [Google Scholar]
- 18.Surguladze S, Russell T, Kucharska-Pietura K, Travis MJ, Giampietro V, David AS, Phillips ML. A reversal of the normal pattern of parahippocampal response to neutral and fearful faces is associated with reality distortion in schizophrenia. Biol Psychiatry. 2006;60(5):423–31. doi: 10.1016/j.biopsych.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Holt DJ, Phillips ML. In: The human amygdala in schizophrenia, in The Human Amygdala. Phelps EA, Whalen PJ, editors. New York: Guilford; In press. [Google Scholar]
- 20.Sotres-Bayon F, Bush DE, Ledoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- 21.Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60(4):337–43. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42:456–464. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 23.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73(1):61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ledoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 26.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12(2):120–50. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 27.Bouton ME, Garcia-Gutierrez A, Zilski J, Moody EW. Extinction in multiple contexts does not necessarily make extinction less vulnerable to relapse. Behav Res Ther. 2006;44(7):983–94. doi: 10.1016/j.brat.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 29.Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem. 2002;9(6):402–7. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27(4):840–4. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouton ME, Frohardt RJ, Sunsay C, Waddell J, Morris RW. Contextual control of inhibition with reinforcement: Adaptation and timing mechanisms. J Exp Psychol Anim Behav Process. 2008;34(2):223–36. doi: 10.1037/0097-7403.34.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sears LL, Andreasen NC, O’Leary DS. Cerebellar functional abnormalities in schizophrenia are suggested by classical eyeblink conditioning. Biol Psychiatry. 2000;48(3):204–9. doi: 10.1016/s0006-3223(00)00247-x. [DOI] [PubMed] [Google Scholar]
- 33.Spain B. Eyelid conditioning and arousal in schizophrenic and normal subjects. J Abnorm Psychol. 1966;71:260–266. doi: 10.1037/h0023596. [DOI] [PubMed] [Google Scholar]
- 34.Kosmidis MH, Breier A, Fantie BD. Avoidance learning in schizophrenia: a dissociation between the effects of aversive and non-aversive stimuli. Schizophr Res. 1999;38:51–59. doi: 10.1016/s0920-9964(98)00181-9. [DOI] [PubMed] [Google Scholar]
- 35.Hofer E, Doby D, Anderer P, Dantendorfer K. Impaired conditional discrimination learning in schizophrenia. Schizophr Res. 2001;51:127–136. doi: 10.1016/s0920-9964(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 36.Howe ES. GSR conditioning in anxiety states, normals, and chronic functional schizophrenic subjects. J Abnorm Psychol. 1958;56:183–189. doi: 10.1037/h0047365. [DOI] [PubMed] [Google Scholar]
- 37.Ohman A. Electrodermal activity and vulnerability to schizophrenia: a review. Biol Psychol. 1981;12(2–3):87–145. doi: 10.1016/0301-0511(81)90008-9. [DOI] [PubMed] [Google Scholar]
- 38.Barch DM, Carter CS, MacDonald AW, III, Braver TS, Cohen JD. Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J Abnorm Psychol. 2003;112:132–143. [PubMed] [Google Scholar]
- 39.Hemsley DR. The development of a cognitive model of schizophrenia: placing it in context. Neurosci Biobehav Rev. 2005;29:977–988. doi: 10.1016/j.neubiorev.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Green MJ, Waldron JH, Simpson I, Coltheart M. Visual processing of social context during mental state perception in schizophrenia. J Psychiatry Neurosci. 2008;33(1):34–42. [PMC free article] [PubMed] [Google Scholar]
- 41.Green MJ, Waldron JH, Coltheart M. Emotional context processing is impaired in schizophrenia. Cognit Neuropsychiatry. 2007;12(3):259–80. doi: 10.1080/13546800601051847. [DOI] [PubMed] [Google Scholar]
- 42.Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, Rauch SL. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behav Neurosci. 2006;120(6):1196–203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- 43.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62(5):446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 44.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders. New York: The New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- 45.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 46.Phelps EA, Delgado MR, Nearing KI, Ledoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 47.Gruzelier JH, Venables PH. Skin conductance orienting activity in a heterogeneous sample of schizophrenics. J Nerv Ment Dis. 1972;155(4):277–87. doi: 10.1097/00005053-197210000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Bernstein AS, Frith CD, Gruzelier JH, Patterson T, Straube E, Venables PH, Zahn TP. An analysis of the skin conductance orienting response in samples of American, British, and German schizophrenics. Biol Psychol. 1982;14:155–211. doi: 10.1016/0301-0511(82)90001-1. [DOI] [PubMed] [Google Scholar]
- 49.Zahn TP, Carpenter WT, Jr, McGlashan TH. Autonomic nervous system activity in acute schizophrenia: I. method and comparison with normal controls. Arch Gen Psychiatry. 1981;38(3):251–8. doi: 10.1001/archpsyc.1981.01780280019001. [DOI] [PubMed] [Google Scholar]
- 50.Zahn TP, Jacobsen LK, Gordon CT, McKenna K, Frazier JA, Rapoport JL. Autonomic nervous system markers of psychopathology in childhood-onset schizophrenia. Arch Gen Psychiatry. 1997;54(10):904–912. doi: 10.1001/archpsyc.1997.01830220020003. [DOI] [PubMed] [Google Scholar]
- 51.Gruzelier J, Connolly J, Eves F, Hirsch S, Zaki S, Weller M, Yorkston N. Effect of propranolol and phenothiazines on electrodermal orienting and habituation in schizophrenia. Psychol Med. 1981;11(1):93–108. doi: 10.1017/s0033291700053319. [DOI] [PubMed] [Google Scholar]
- 52.Gruzelier JH, Hammond NV. The effect of chlorpromazine upon psychophysiological, endocrine and information processing measures in schizophrenia. J Psychiatr Res. 1978;14(1–4):167–82. doi: 10.1016/0022-3956(78)90019-5. [DOI] [PubMed] [Google Scholar]
- 53.Zahn TP. On the bimodality of the distribution of electrodermal orienting responses in schizophrenic patients. J Nerv Ment Dis. 1976;162(3):195–9. doi: 10.1097/00005053-197603000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Straube ER. On the meaning of electrodermal nonresponding in schizophrenia. J Nerv Ment Dis. 1979;167(10):601–11. doi: 10.1097/00005053-197910000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Holt DJ, Lakshmanan B, Freudenreich O, Goff DC, Rauch SL, Kuperberg GR. Increased activity to ambiguous social information within a cortical midline network in schizophrenia, under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hall J, Whalley HC, McKirdy JW, Romaniuk L, McGonigle D, McIntosh AM, Baig BJ, Gountouna VE, Job DE, Donaldson DI, Sprengelmeyer R, Young AW, Johnstone EC, Lawrie SM. Overactivation of fear systems to neutral faces in schizophrenia. Biol Psychiatry. 2008;64(1):70–3. doi: 10.1016/j.biopsych.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 57.Holt DJ, Kunkel L, Weiss AP, Goff DC, Wright CI, Shin LM, Rauch SL, Hootnick J, Heckers S. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr Res. 2006;82:153–162. doi: 10.1016/j.schres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 58.Corcoran KA, Quirk GJ. Recalling safety: cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectr. 2007;12(3):200–6. doi: 10.1017/s1092852900020915. [DOI] [PubMed] [Google Scholar]
- 59.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60(4):376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26(37):9503–11. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gur RE, Loughead J, Kohler CG, Elliott MA, Lesko K, Ruparel K, Wolf DH, Bilker WB, Gur RC. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch Gen Psychiatry. 2007;64(12):1356–66. doi: 10.1001/archpsyc.64.12.1356. [DOI] [PubMed] [Google Scholar]
- 62.Holt DJ, Weiss AP, Rauch SL, Wright CI, Zalesak M, Goff DC, Ditman T, Welsh RC, Heckers S. Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biological Psychiatry. 2005;57:1011–1019. doi: 10.1016/j.biopsych.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 63.Gur RE, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI, Kohler C, Alsop D, Maldjian J, Ragland JD, Gur RC. An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry. 2002;159:1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- 64.Taylor SF, Liberzon I, Decker LR, Koeppe RA. A functional anatomic study of emotion in schizophrenia. Schizophr Res. 2002;58:159–172. doi: 10.1016/s0920-9964(01)00403-0. [DOI] [PubMed] [Google Scholar]
- 65.Heckers S, Konradi C. Hippocampal neurons in schizophrenia. J Neural Transm. 2002;109:891–905. doi: 10.1007/s007020200073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 67.Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998;55(5):433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- 68.Weiss AP, Heckers S. Neuroimaging of declarative memory in schizophrenia. Scand J Psychol. 2001;42(3):239–250. doi: 10.1111/1467-9450.00234. [DOI] [PubMed] [Google Scholar]
- 69.Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev. 2003;13:43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- 70.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53(6):871–80. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 71.Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 2001;21(22):9009–17. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158(9):1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- 73.Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003;169(3–4):215–33. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 74.Duncan EJ, Szilagyi S, Schwartz MP, Bugarski-Kirola D, Kunzova A, Negi S, Stephanides M, Efferen TR, Angrist B, Peselow E, Corwin J, Gonzenbach S, Rotrosen JP. Effects of D-cycloserine on negative symptoms in schizophrenia. Schizophr Res. 2004;71(2–3):239–48. doi: 10.1016/j.schres.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 75.Goff DC, Tsai G, Levitt J, Amico E, Manoach D, Schoenfeld DA, Hayden DL, McCarley R, Coyle JT. A placebo-controlled trial of D-cycloserine added to conventional neuroleptics in patients with schizophrenia. Arch Gen Psychiatry. 1999;56(1):21–27. doi: 10.1001/archpsyc.56.1.21. [DOI] [PubMed] [Google Scholar]
- 76.Goff DC, Tsai G, Manoach DS, Flood J, Darby DG, Coyle JT. D-cycloserine added to clozapine for patients with schizophrenia. Am J Psychiatry. 1996;153(12):1628–1630. doi: 10.1176/ajp.153.12.1628. [DOI] [PubMed] [Google Scholar]
- 77.Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, Carpenter WT. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164(10):1593–602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- 78.Maina G, Barzega G, Bellino S, Bogetto F, Ravizza L. Type I and type II schizophrenia: relations between tonic electrodermal activity and clinical ratings before and after haloperidol treatment. Psychiatry Res. 1995;57(1):49–56. doi: 10.1016/0165-1781(95)02354-y. [DOI] [PubMed] [Google Scholar]
- 79.Raine A, Venables PH, Mednick S, Mellingen K. Increased psychophysiological arousal and orienting at ages 3 and 11 years in persistently schizotypal adults. Schizophr Res. 2002;54(1–2):77–85. doi: 10.1016/s0920-9964(01)00354-1. [DOI] [PubMed] [Google Scholar]
- 80.Williams LM, Das P, Liddell BJ, Olivieri G, Peduto AS, David AS, Gordon E, Harris AW. Fronto-limbic and autonomic disjunctions to negative emotion distinguish schizophrenia subtypes. Psychiatry Res. 2007;155(1):29–44. doi: 10.1016/j.pscychresns.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 81.Bentall RP, Kaney S. Content specific information processing and persecutory delusions: an investigation using the emotional Stroop test. Br J Med Psychol. 1989;62(Pt 4):355–364. doi: 10.1111/j.2044-8341.1989.tb02845.x. [DOI] [PubMed] [Google Scholar]
- 82.Fear C, Sharp H, Healy D. Cognitive processes in delusional disorders. Br J Psychiatry. 1996;168:61–67. doi: 10.1192/bjp.168.1.61. [DOI] [PubMed] [Google Scholar]
- 83.Kinderman P. Attentional bias, persecutory delusions and the self-concept. Br J Med Psychol. 1994;67(Pt 1):53–66. doi: 10.1111/j.2044-8341.1994.tb01770.x. [DOI] [PubMed] [Google Scholar]
- 84.Phillips ML, Senior C, David AS. Perception of threat in schizophrenics with persecutory delusions: an investigation using visual scan paths. Psychol Med. 2000;30(1):157–167. doi: 10.1017/s0033291799001397. [DOI] [PubMed] [Google Scholar]
- 85.Allen P, Freeman D, McGuire P. Slow habituation of arousal associated with psychosis proneness. Psychol Med. 2007;37(4):577–82. doi: 10.1017/S0033291706009615. [DOI] [PubMed] [Google Scholar]
- 86.Greba Q, Gifkins A, Kokkinidis L. Inhibition of amygdaloid dopamine D2 receptors impairs emotional learning measured with fear-potentiated startle. Brain Res. 2001;899(1–2):218–26. doi: 10.1016/s0006-8993(01)02243-0. [DOI] [PubMed] [Google Scholar]
- 87.Inoue T, Tsuchiya K, Koyama T. Effects of typical and atypical antipsychotic drugs on freezing behavior induced by conditioned fear. Pharmacol Biochem Behav. 1996;55(2):195–201. doi: 10.1016/s0091-3057(96)00064-0. [DOI] [PubMed] [Google Scholar]
- 88.Li M, Parkes J, Fletcher PJ, Kapur S. Evaluation of the motor initiation hypothesis of APD-induced conditioned avoidance decreases. Pharmacol Biochem Behav. 2004;78(4):811–9. doi: 10.1016/j.pbb.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 89.Blackburn JR, Pfaus JG, Phillips AG. Dopamine functions in appetitive and defensive behaviours. Prog Neurobiol. 1992;39(3):247–79. doi: 10.1016/0301-0082(92)90018-a. [DOI] [PubMed] [Google Scholar]
- 90.Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure Behav Brain Res. 1994;65(2):221–9. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 91.Ponnusamy R, Nissim HA, Barad M. Systemic blockade of D2-like dopamine receptors facilitates extinction of conditioned fear in mice. Learn Mem. 2005;12(4):399–406. doi: 10.1101/lm.96605. [DOI] [PMC free article] [PubMed] [Google Scholar]


