Abstract
Lenalidomide, an immunomodulatory agent, and flavopiridol, a broad cyclin-dependent kinase inhibitor, are both active therapies for clinical use in genomic high risk chronic lymphocytic leukemia (CLL). A high-performance liquid chromatographic assay with tandem mass spectrometric detection has been developed to simultaneously quantify lenalidomide and flavopiridol in human and mouse plasma to facilitate their combined clinical development. Samples were prepared by liquid-liquid extraction with acetonitrile- (ACN) containing internal standard (IS), genistein, followed by evaporation of solvent and reconstitution in 95/5 H2O/ACN. Lenalidomide and IS were separated by reverse phase liquid chromatography on a C-18 column using a gradient of H2O and ACN, each with 0.1% formic acid. Atmospheric pressure chemical ionization (APCI) in positive-ion mode with single reaction monitoring on a triple quadrupole mass spectrometer were applied to detect transitions of lenalidomide (260.06 > 149.10) and flavopiridol (402.09 > 341.02). Lower limits of quantification (LLOQ) of lenalidomide and flavopiridol were 1nM and 0.3nM respectively. Recoveries of lenalidomide and flavopiridol from human plasma ranged from 99% to 116% throughout their linear ranges. Within and between-run precision and accuracy of replicate samples were all less than 15%. This is the most sensitive analytical method reported to date for both lenalidomide and flavopiridol. This sensitivity will enable late terminal phase concentration measurements and accurate pharmacokinetic parameter estimation in a planned clinical trial with lenalidomide and flavopiridol in CLL patients.
Keywords: Lenalidomide, Pharmacokinetics, Flavopiridol, LCMS
Introduction
Lenalidomide (Revlimid®, CC-5013) is a potent immunomodulatory (IMiDs) agent derived from thalidomide. It has been shown to mediate direct toxicity and induce an immune response through marked activation of T and NK cells in multiple myeloma patients [1–4]. Lenalidomide has been evaluated in patients with multiple myeloma, myelodysplastic syndrome (MDS) and metastatic renal cell carcinoma [5–7], and recently was approved by the US Food and Drug Administration (FDA) for treatment of multiple myeloma and MDS. Two recent studies have demonstrated lenalidomide is also clinically active in high-risk genomic CLL patients including those with deletion(17p13.1) [8,9]. Our group has recently demonstrated that lenalidomide has unique B-cell activating features in a dose dependent fashion that may correlate with development of tumor flare (Andritsos et al, JCO in press). Pharmacokinetic variability of lenalidomide clearance may therefore contribute to this toxicity observed in CLL thereby justifying the need for a sensitive pharmacokinetic measurement to assess plasma levels.
Flavopiridol, initially established as a cyclin-dependent kinase (CDK) inhibitor, showed potent anticancer effects in preclinical studies [10]. In addition to CDK inhibition, flavopiridol has been shown to regulate the anti-apoptotic proteins, Mcl-1 and XIAP [11,12]. Although limited activity was observed in early clinical studies, recent trials with a modified dosing schedule based upon pharmacokinetic modeling to achieve and maintain a 1.5 μM plasma concentration of flavopiridol for 4–6 hours have produced a nearly 50% objective response rate in patients with refractory and high-risk genomic CLL [13].
Based on the different mechanisms of these two agents and their ability to produce responses in genomic high risk CLL, a clinical trial evaluating this combination is under development (National Cancer Institute trial, NCI 8046). To facilitate pharmacokinetic studies in this and future trials with these agents, the current method has been developed with particular focus on achieving superior sensitivity for accurate PK and protein binding characterization. Only two analytical methods have been reported for lenalidomide with lower limits of quantitation of 19 nM (5 ng/ml) [14] and 1.2 nM (0.3 ng/ml) in human plasma. Our group recently reported the most sensitive method for flavopiridol in human plasma with an LLOQ of 3 nM (manuscript submitted). High sensitivity is essential for full characterization of terminal elimination kinetics, which may be important in understanding inter-individual differences in response and toxicity. Additionally, high sensitivity enables improved measurement of protein binding for highly bound drugs, such as flavopiridol. We describe here the development and validation of a sensitive method for the simultaneous quantification of lenalidomide and flavopiridol in human plasma and we present PK data from an active clinical trial with lenalidomide.
Materials and Methods
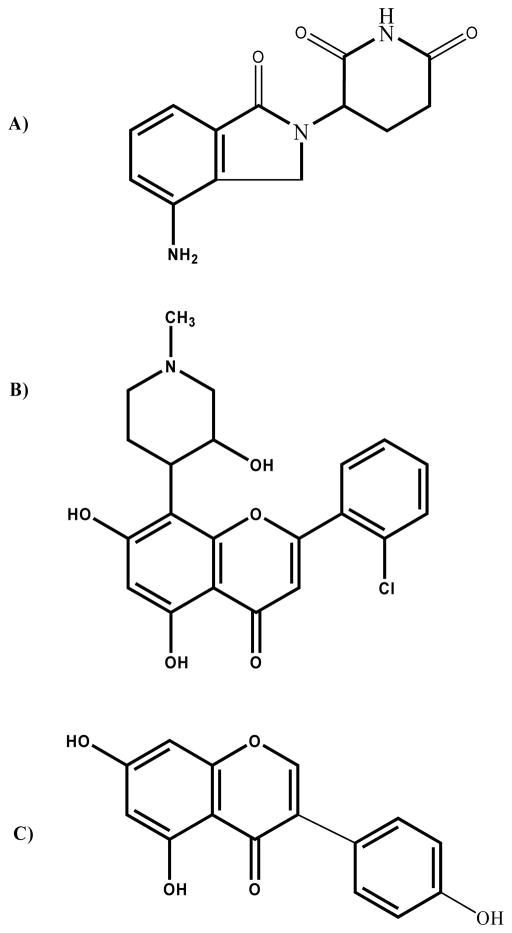
Human plasma was obtained from the American Red Cross (Columbus, OH). Lenalidomide (MW 260.3) and flavopiridol (HCl salt, MW 438.29) were obtained from the NCI. HPLC grade water and ACN were obtained from Thermo Fisher Scientific (Waltham, MA). The internal standard (IS), genistein (MW 270.24), and all other chemicals were purchased from Sigma (St. Louis, MO). Chemical structures of lenalidomide, flavopiridol and genistein are displayed in Figure 1.
Figure 1.
Chemical structures for a) lenalidomide (MW 260.3), b) flavopiridol (MW of HCl salt, 438.29), and c) genistein (MW 270.24).
Preparation of Stock Solutions and Calibration Samples
Individual stock solutions of lenalidomide, flavopiridol and genistein were prepared by dissolving compounds in DMSO at a concentration of 1 mM and stored in polypropylene centrifuge tubes (Life Science Products, Rochester, NY) at −20°C for up to 1 month. Concentrated (10x) working solutions containing equal concentrations of lenalodimide and flavopiridol between 3 nM and 10,000 nM were prepared in 5/95 ACN/H2O with 0.1% acetic acid and diluted into human plasma (35 μl into 315 μl plasma) to produce calibration samples ranging from 0.3 nM to 1 μM. Quality control (QC) samples were prepared at 3, 50 and 800 nM for both compounds. Blank samples were produced by adding 35μL of 5/95 ACN/H2O with 0.1% of acetic acid (without drug) to 315μL human plasma.
Sample Processing
Acetonitrile (1.0 ml) containing 200 nM IS was added to 350ul standards, QCs, blanks, zeros or unknown plasma samples to precipitate proteins. The mixtures were vortex mixed for 10 s and centrifuged at 16,000 x g for 10 min at 4°C. One mL of the supernatant was subsequently transferred to a clean centrifuge tube and dried in a refrigerated speed-vac system (Thermo Fisher). Samples were reconstituted in 150 μl of 5/95 ACN/H2O with 0.1% acetic acid, vortex mixed for 10s and centrifuged at 16,000 x g for 10 min at 4°C. 100 μl of the supernatant was transferred into autosampler vials with polypropylene inserts (Thermo Fisher) for subsequent analysis.
Liquid chromatography and mass spectrometry
Samples contained in autosampler vials were loaded into an Agilent (Santa Clara, CA) 1100 HPLC system connected to a ThermoFisher TSQ Quantum Discovery Max mass spectrometer controlled by LCQuan software. The HPLC system comprised a dual pump with static mixer, vacuum degasser, heated column compartment and well-plate autosampler. A reversed phase Zorbax (Agilent) C-18 column (3.5 μm, 2.1 × 50 mm) coupled with a Metaguard (Varian, Walnut Creek, CA) C-18 guard column (5 μm, 2 × 10 mm) were utilized for separation of lenalidomide and flavopiridol. Mobile phases were de-ionized water (A) and ACN (B), each with 0.1% formic acid. Initial conditions were 100% A for 3 min followed by a 0.3 min linear gradient to 90% B. This was held for 3 min before a 0.3 min linear gradient return to 100% A for equilibration for the remainder of the 10 min run. A constant flow rate of 0.4ml/min was maintained throughout the run. Sample injection volumes were 20 μl.
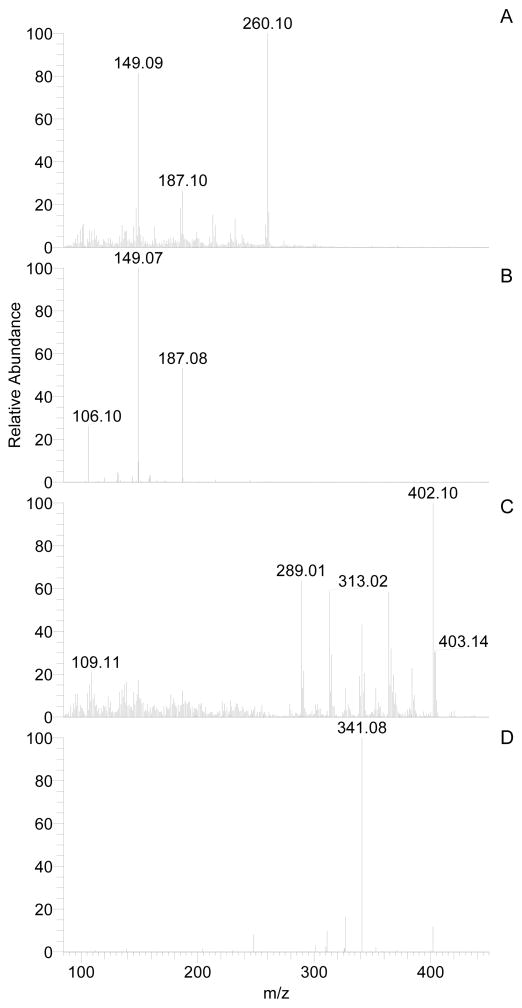
Lenalidomide, flavopiridol, and IS were ionized by atmospheric pressure chemical ionization (APCI) in positive ion mode ([M+H]+) and fragmented with argon for single reaction monitoring (SRM) on a ThermoFisher TSQ Quantum Discovery Max mass spectrometer. Final TSQ parameter settings were as follows: collision energy, 26 V; scan time, .03 s; scan width, .002 m/z; Q1 and Q3 peak widths, 0.2 full width at half maximum m/z; chrom filter, 8 s; collision gas pressure, 1.5 mTorr. Mass transitions monitored were 260.06 > 149.10 (lenalidomide), 402.09 > 341.02 (flavopiridol) and 271.09 > 152.90 (IS), [M + H]+ (see Figure 2). Peaks were integrated using the Interactive Chemical Integration System (ICIS) algorithm, and least squares regression was utilized with 1/x weighting to fit a straight line for the peak area ratios (lenalidomide/IS and flavopiridol/IS) vs. analyte concentration.
Figure 2.
Mass spectra of parent (a, c) or product (b, d) ions from lenalidomide (a, b) and flavopiridol (c, d). Mobile phase samples containing 100 nM individual compounds were infused via syringe at a flow rate of 5 μl/min without LC flow. Source collision induced dissociation energy was set to −10 V causing fragmentation of parent ions in the full scans (a and c). Collision gas and collision energy were set to 1.5 mTorr and 28 V, respectively.
Validation
Method validation was performed according to the Food and Drug Administration guidelines [15] and included specificity, recovery, accuracy, precision and stability. Validation runs included blank (no analytes or IS), zero (IS only) and calibration samples (analytes plus IS), although blanks and zeros were not used for standard curve regression.
Specificity and recovery
Assay specificity was evaluated at the LLOQ of lenalidomide (1 nM) and flavopiridol (0.3 nM) in blank plasma from six individuals to identify interfering background signals. Plasma samples were extracted as described above and analyzed for lenalidomide, flavopiridol, and IS to ensure adequate signal-to-noise ratios and lack of significant variability. Ion suppression via matrix effect was evaluated using the common method of comparing peak areas from 3, 50, and 800 nM drug (lenalidomide and flavopiridol at equal concentrations) in neat solution (95% water/5% ACN with 0.1% acetic acid) before and after it was used to reconstitute dried plasma extract (e.g. matrix, as produced according to the described plasma preparation methods). Replicate plasma samples (n = 5) produced with 3 nM of one analyte (ie, either lenalidomide or flavopiridol) and either 800 nM or none of the second analyte were prepared and compared to plasma QC samples to evaluate ion suppression effects of each analyte on the other. A set of replicate (n = 5) plasma QC samples in this study enabled recovery determinations, which were estimated by comparison with the mobile phase samples.
Linearity
Plasma calibration curves were established with 0.3, 1, 3, 10, 30, 100, 300, and 1000 nM of both lenalidomide and flavopiridol. Blanks were not used in the regression, and the calibration curve line was not forced through the origin.
Accuracy and precision
Accuracy and precision were assessed by analysis of quintuplicate QC samples at 3, 50 and 800 nM. Within-run (n = 5) and between-run (n = 5 × 3 runs) accuracy and precision were calculated for each of the three concentrations using calculated concentrations as determined by individual standard curves for each run.
Stability
Stock solution stability was evaluated by producing replicate QC samples with freshly-prepared and 28-day-old stock solutions of lenalidomide and flavopiridol. Calculated concentrations were based on standard curves from freshly-made stock. For freeze-thaw stability, replicate QC samples were produced and stored at −70°C. For long-term temperature stability, QC samples at 800 nM remained frozen for 42 days before processing and analysis. Freeze-thaw samples were removed from the freezer and placed on the bench top at room temperature. Within 30 minutes of thawing, samples were placed back in the −70°C freezer for up to 72 hours. After three complete freeze-thaw cycles, samples were processed and analyzed. Post-preparative stability was evaluated by re-analyzing samples after they sat in the autosampler for 12 hours at room temperature.
Lenalidomide PK analysis
An IRB approved clinical trial of lenalidomide and temsirolimus in patients with relapsed multiple myeloma is actively accruing patients at the James Cancer Hospital in Columbus, Ohio (NCI 7314). Plasma samples were collected on the first day of treatment after patients received their first oral dose of lenalidomide. Whole blood (5 mL) was collected into heparinized tubes pre-dose and 0.5, 1, 2, 3, 4, 6, 8, and 24 hours after dosing, and plasma was separated and stored within 30 minutes of blood collection. Samples were immediately frozen at −70°C and stored for up to 4 weeks prior to analysis.
Results and Discussion
Assay Conditions
Initial attempts to include lenalidomide in our previously-reported LC-ESI/MS flavopiridol quantitation method [16] resulted in low lenalidomide signal. Therefore various mass spec and chromatography conditions were evaluated to optimize both lenalidomide and flavopiridol signals. Electrospray (ESI) and APCI sources were evaluated both in negative and positive ion modes. Positive mode APCI provided superior signal intensity for lenalidomide compared to negative mode APCI and either positive or negative mode ESI. Positive mode APCI was approximately equivalent to flavopiridol sensitivity observed with positive mode ESI, and it was therefore adopted for further development. Genistein was selected as the preferred internal standard based on our previous experience with its use as a commercially available, structurally similar internal standard for flavopiridol quantitation (in press), and since it also yielded sufficient signal in positive mode APCI.
Figure 2 displays parent and product spectra for lenalidomide and flavopiridol. With source collision-induced dissociation set to −10 V (this setting provided maximum parent intensity), some fragmentation occurred in the absence of collision gas pressure, as indicated (see Figure 2). Flavopiridol mass transitions were similar to those reported by our group previously (in press).
Various mobile phase modifiers were evaluated before selecting water and ACN, each with 0.1% formic acid, for separation through a C-18 column. Previous reports of lenalidomide quantitative assays suggested acidic mobile phases for isocratic separation through a C-18 matrix [14,17]. Due to tailing issues with flavopiridol [18,19], we evaluated rapid gradient conditions and ammonium formate modifiers. Good flavopiridol peak shape was attainable with formic acid (0.1%) and a rapid gradient through a 50 mm Zorbax extended C-18 column. Lenalidomide retention necessitated column equilibration and initial conditions of 100% water. Inclusion of any organic component in the initial condition prevented lenalidomide retention on the column. Therefore, the 100% water initial condition was employed for this method.
Validation
Recovery
Acetonitrile plasma protein precipitation and extraction was previously shown to yield greater than 90% recovery for flavopiridol [16]. We therefore sought to determine if lenalidomide recovery would be sufficient using this simple procedure. Comparison of peak areas and area ratios (lenalidomide/IS) in neat solution and after sample processing with ACN protein precipitation indicated excellent recovery for both lenalidomide and flavopiridol (Table 1).
Table 1.
Recovery of lenalidomide and flavopiridol from human plasma following protein precipitation with acetonitrile (n = 5).
| Nominal Concentration (nM) | Lenalidomide Recovery (mean ± S.D., %) | Flavopiridol Recovery (mean ± S.D., %) |
|---|---|---|
| 3 | 108 ± 12 | 110 ± 19 |
| 50 | 114 ± 7 | 100 ± 15 |
| 800 | 116 ± 12 | 99 ± 11 |
Specificity and Sensitivity
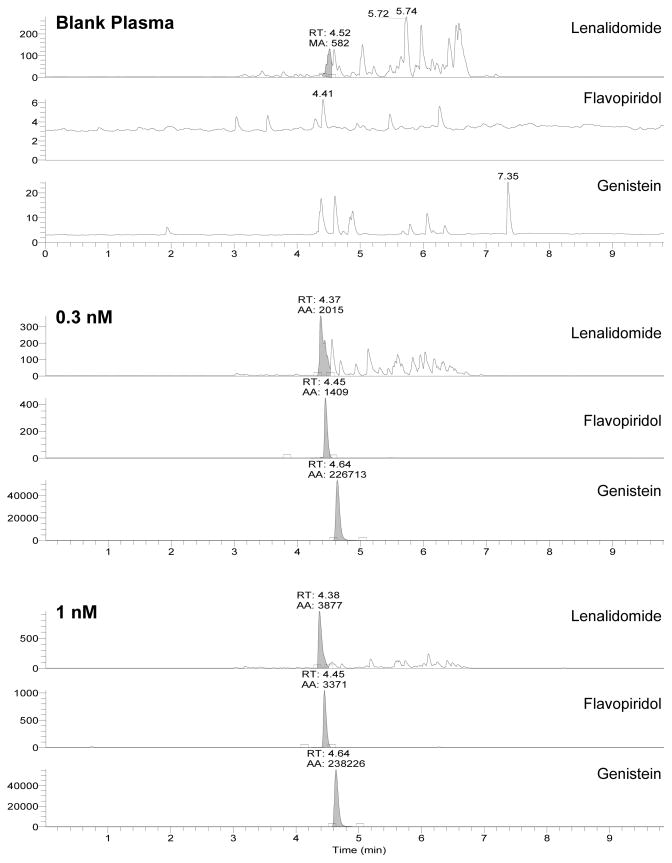
Blank plasma was evaluated for interfering signals as shown in Figure 3. Background signal with absolute intensity up to 3 × 102 (arbitrary units) was typically evident in the lenalidomide channel, although a majority of peaks occur at retention times (RT) later than lenalidomide (4.38 min.). Signal-to-noise ratios always exceeded the required 5/1 ratio for lenalidomide at its LLOQ of 1 nM compared to integrated background peaks eluting near 4.38 min. in blank plasma injections. A few interfering peaks also occurred in the IS channel, however these were always less than 102 intensity. Background in the flavopiridol channel was very low with peak intensities near 10. No signal overlap is expected in the various analyte channels with SRM detection. This is illustrated in Figure 3, where IS is present with equal concentrations of lenalidomide and flavopiridol at their respective LLOQs of 0.3 nM and 1 nM.
Figure 3.
Mass chromatograms of blank plasma, 0.3 nM lenalidomide and flavopiridol (LLOQ), or 1 nM lenalidomide (LLOQ) and flavopiridol. Single reaction monitoring channels include lenalidomide (260.06 > 149.10), flavopiridol (402.09 > 341.02), and genistein (271.09 > 152.90).
Lenalidomide, flavopiridol, and IS eluted with RTs of 4.38, 4.46, and 4.63 min. While elution of these compounds does not necessarily require separation for SRM detection, ion suppression was a concern and therefore evaluated in conjunction with plasma matrix effects. No concentration or signal intensity differences were detected (all comparisons were within 15% of nominal values) when lenalidomide and flavopiridol were added to QC samples at drastically different concentrations. In addition, no signal intensity differences were evident with either compound when evaluated in the absence of IS. These results indicate a lack of significant interference between the compounds. Comparison of lenalidomide and flavopiridol peak areas in neat solution and in neat solution with reconstituted plasma extract also demonstrated no measurable attenuation of signal.
Linearity
Calibration samples (0.3, 1, 3, 10, 30, 100, 300 and 1000 nM) and quintuplicate QC samples (3, 50 and 800 nM) containing equivalent concentrations of lenalidomide and flavopiridol were prepared daily and used to determine method accuracy and precision. All standard curves exhibited linearity with R2 values of 0.998 or better (the 0.3 nM level was not included for lenalidomide). Table 2 lists mean concentrations and percent coefficients of variation (CV%) for calibration standards from 5 individual analytical runs.
Table 2.
Mean calculated concentrations, accuracy, and coefficients of variation (CV) for calibration standards (n = 5 separate analytical runs).
| Nominal Conc. (nM) | Lenalidomide | Flavopiridol | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean Calc. Conc. (nM) | Accuracy (%) | CV (%) | Mean Calc. Conc. (nM) | Accuracy (%) | CV % | |
| 0.3 | - | - | - | 0.30 | 99.4 | 10.3 |
| 1 | 0.9 | 87.8 | 11.8 | 0.96 | 95.8 | 11.3 |
| 3 | 3.0 | 101.5 | 4.3 | 3.0 | 98.7 | 12.8 |
| 10 | 11.7 | 116.9 | 9.6 | 10.7 | 106.9 | 8.3 |
| 30 | 31.9 | 106.2 | 8.1 | 31.9 | 106.8 | 8.5 |
| 100 | 108.2 | 108.2 | 3.6 | 104.9 | 104.9 | 3.5 |
| 300 | 304.4 | 101.5 | 10.8 | 286.3 | 95.4 | 5.4 |
| 1000 | 971.9 | 97.2 | 2.9 | 1014.7 | 101.5 | 8.0 |
Accuracy and Precision
Method accuracy and precision were determined from replicate analysis of QC samples. Within-run accuracy and precision were determined from quintuplicate specimens prepared at each of the 3 QC concentrations. Between-batch accuracy and precision was determined with grand means and standard deviations from runs on 3 separate days (n = 15). Accuracy (% deviation) ranged from 2% to 14% of expected values, and precision (CV%) ranged from 5% to 14% at the low, medium, and high QC concentrations (Table 3).
Table 3.
Lenalidomide and flavopiridol intra-batch and inter-batch precision and accuracy (n = 5 for intra-batch and n = 15 for inter-batch calculations).
| Compound | (nM) | Within-batch | Between-batch | ||
|---|---|---|---|---|---|
| Precision (% CV) | Accuracy (%) | Precision (% CV) | Accuracy (%) | ||
| Lenalidomide | 3 | 14 | 102 | 14 | 114 |
| 50 | 11 | 108 | 5 | 105 | |
| 800 | 8 | 91 | 12 | 100 | |
|
| |||||
| Flavopiridol | 3 | 10 | 92 | 13 | 96 |
| 50 | 5 | 108 | 5 | 107 | |
| 800 | 6 | 91 | 8 | 100 | |
Stability
Stock solution stability was evaluated by producing QC samples with freshly-prepared and 28-day-old stock solutions of lenalidomide and flavopiridol. Observed differences between the QC samples were within the accuracy and precision limits of 15% deviation from nominal concentration and 15% CV. Therefore the stock solution was deemed to be stable for up to 28 days. Results for freeze-thaw and post-preparative stability were similar, as indicated in Table 4. Long-term stability samples indicated lack of significant degradation, as samples measured within 16% of nominal values. Stability data indicates no significant degradation of lenalidomide or flavopiridol under the storage or handling conditions evaluated. Both flavopiridol and genistein stability had been evaluated previously and were found to be stable under conditions identical or similar to those in this method (manuscript submitted).
Table 4.
Stability of lenalidomide and flavopirodol in human plasma and in mobile phase post-processing.
| Lenalidomide | Flavopirodol | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Accuracy (mean ±S.D., %) | Accuracy (mean ±S.D. %) | |||||
|
| ||||||
| 3nM | 50nM | 800nM | 3nM | 50nM | 800nM | |
| Freeze-thaw stability | 103 ± 12 | 93 ± 5 | 89 ± 8 | 96 ± 19 | 103 ± 20 | 90 ± 11 |
| Post-preparative stability | 91.7 ± 4.7 | 95.0 ± 5.2 | 95.8 ± 9.0 | 91.9 ± 6.3 | 96.0 ± 3.9 | 92.0 ± 5.0 |
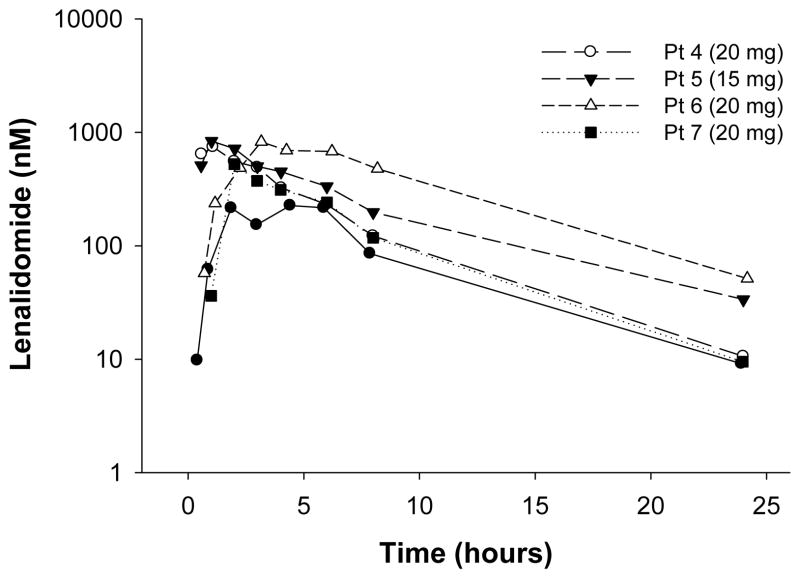
Pharmacokinetics
The method described above is being used to quantify lenalidomide concentrations in multiple myeloma patients being treated with the combination of lenalidomide and temsirolimus in an ongoing trial. The concentration versus time profiles of lenalidomide in four patients receiving lenalidomide on day 1 of treatment are shown in Figure 4. After dosing with 15 or 20 mg lenalidomide, 24-hour concentrations ranged between 3 nM and 20 nM. Given our recent finding that B-cell activation may occur with low doses of lenalidomide in CLL patients (Andritsos et al, JCO in press), high sensitivity will be critical for accurate estimation of lenalidomide PK parameters. The 1 nM LLOQ achieved with this method provides the best opportunity for accurate terminal phase measurements and PK parameter estimations for very low lenalidomide doses administered in the current and upcoming clinical trials.
Figure 4.
Lenalidomide plasma concentration vs. time profiles for four patients following a 15- or 20-mg oral dose of lenalidomide. Lenalidomide concentrations were determined with the validated method described herein.
Conclusion
We describe the development and validation of a simple analytical assay for simultaneous measurement of lenalidomide and flavopiridol in human plasma. This assay is the most sensitive reported to date for both compounds with lower quantification limits of 1nM for lenalidomide and 0.3 nM for flavopiridol. This superior sensitivity will enable accurate PK parameter estimates in current and future trials with these agents.
Acknowledgments
This work was supported by the Specialized Center of Research from the Leukemia and Lymphoma Society, National Cancer Institute grants, P01 CA95426, P01 CA101956, and U01CA76576-10, the CALGB Foundation, and the D. Warren Brown Foundation.
References
- 1.Gupta D, Treon SP, Shima Y, et al. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001;15:1950–1961. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 2.Hideshima T, Chauhan D, Shima Y, et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–2950. [PubMed] [Google Scholar]
- 3.Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163:380–386. [PubMed] [Google Scholar]
- 4.Davies FE, Raje N, Hideshima T, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- 5.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 6.Patel PH, Kondagunta GV, Schwartz L, et al. Phase II trial of lenalidomide in patients with metastatic renal cell carcinoma. Invest New Drugs. 2007 doi: 10.1007/s10637-007-9107-y. [DOI] [PubMed] [Google Scholar]
- 7.Richardson PG, Schlossman RL, Weller E, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100:3063–3067. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- 8.Chanan-Khan A, Miller KC, Musial L, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24:5343–5349. doi: 10.1200/JCO.2005.05.0401. [DOI] [PubMed] [Google Scholar]
- 9.Ferrajoli A, Faderl S, Ravandi F, et al. The JAK-STAT pathway: a therapeutic target in hematological malignancies. Curr Cancer Drug Targets. 2006;6:671–679. doi: 10.2174/156800906779010227. [DOI] [PubMed] [Google Scholar]
- 10.Pepper C, Thomas A, Fegan C, et al. Flavopiridol induces apoptosis in B-cell chronic lymphocytic leukaemia cells through a p38 and ERK MAP kinase-dependent mechanism. Leuk Lymphoma. 2003;44:337–342. doi: 10.1080/1042819021000029984. [DOI] [PubMed] [Google Scholar]
- 11.Gojo I, Zhang B, Fenton RG. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin Cancer Res. 2002;8:3527–3538. [PubMed] [Google Scholar]
- 12.Rosato RR, Almenara JA, Kolla SS, et al. Mechanism and functional role of XIAP and Mcl-1 down-regulation in flavopiridol/vorinostat antileukemic interactions. Mol Cancer Ther. 2007;6:692–702. doi: 10.1158/1535-7163.MCT-06-0562. [DOI] [PubMed] [Google Scholar]
- 13.Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tohnya TM, Hwang K, Lepper ER, et al. Determination of CC-5013, an analogue of thalidomide, in human plasma by liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;811:135–141. doi: 10.1016/j.jchromb.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Guidance for Industry on Bioanalytical Method Validation. 2001
- 16.Phelps MA, Rozewski DM, Johnston JS, et al. Development and validation of a sensitive liquid chromatography/mass spectrometry method for quantitation of flavopiridol in plasma enables accurate estimation of pharmacokinetic parameters with a clinically active dosing schedule. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;868:110–115. doi: 10.1016/j.jchromb.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saravanan G, Rao BM, Ravikumar M, et al. Development of an HPLC assay method for lenalidomide. Chromatographia. 2007;66:287–290. [Google Scholar]
- 18.Stinson SF, Hill K, Siford TJ, et al. Determination of flavopiridol (L86 8275; NSC 649890) in human plasma by reversed-phase liquid chromatography with electrochemical detection. Cancer Chemother Pharmacol. 1998;42:261–265. doi: 10.1007/s002800050815. [DOI] [PubMed] [Google Scholar]
- 19.Zhai SP, Sausville E, Figg WD. A high-performance liquid chromatography method using ultraviolet detection for the quantitation of flavopiridol from human plasma. Biomedical Chromatography. 2002;16:379–382. doi: 10.1002/bmc.166. [DOI] [PubMed] [Google Scholar]