Abstract
Aims/hypothesis
Anti-zinc transporter (ZnT)8 autoantibodies are commonly detected in type 1 diabetic patients. We hypothesized that ZnT8 is also recognized by CD8+ T cells and aimed at identifying HLA-A2 (A*02:01)-restricted epitope targets.
Methods
Candidate epitopes were selected by ZnT8 plasmid DNA immunization of HLA-A2/DQ8-transgenic mice and tested for T-cell recognition in peripheral blood mononuclear cells of type 1 diabetic, type 2 diabetic and healthy subjects by IFN-γ enzyme-linked immunospot.
Results
Caucasian HLA-A2+ type 1 diabetic patients, both adults (83%) and children (60%),displayed ZnT8-reactive CD8+ T cells which recognized a single ZnT8186–194 (VAANIVLTV) epitope. This ZnT8186–194-reactive fraction accounted for 50–53% of total ZnT8-specific CD8+ T cells. Another ZnT8153–161 (VVTGVLVYL) sequence was recognized in 20–25% of type 1 diabetic adults and children, respectively. Both epitopes were type 1 diabetes-specific, being marginally recognized by type 2 diabetic and healthy subjects (7–12% for ZnT8186–194 and 0% for ZnT8153–161).
Conclusions/interpretation
ZnT8-reactive CD8+ T cells are predominantly directed against the ZnT8186–194 epitope and are detected in a majority of type 1 diabetic patients. The exceptional immunodominance of ZnT8186–194 may point to common environmental triggers precipitating beta-cell autoimmunity.
Keywords: autoimmunity, beta cell, environment, islet, SCL30A8, type 1 diabetes
Introduction
The autoimmune cascade leading to beta-cell destruction in type 1 diabetes is propagated by T-cell recognition of specific epitopes. Epitope mapping is therefore critical for staging autoimmunity during disease course and for developing and monitoring immunomodulatory therapies. Indeed, combination of multiple epitope markers is likely to track beta-cell autoimmunity more efficiently than any single epitope alone. Likewise, restoring immune tolerance to a broad antigen spectrum may be clinically more effective [1]. Zinc transporter 8 (ZnT8) was recently identified as a major type 1 diabetes antigen, with 60–80% of recent-onset type 1 diabetic subjects harboring antibodies (Abs) against its C-terminal domain [2]. As for other beta-cell antigens, such autoAbs are likely to be accompanied by autoreactive T cells targeting ZnT8 [1]. Indeed, ZnT8-reactive CD4+ and CD8+ T cells were recently described in Caucasian and Chinese type 1 diabetic patients, respectively [3, 4]. Further characterization of these CD8+ T cells is needed, given their central role in beta-cell autoimmunity [5, 6]. We here identify a highly immunodominant ZnT8186–194 epitope recognized by such cells.
Methods
ZnT8 peptide library
Seventy nonamer peptides selected as potential binders to HLA-A1, -A2, -A3, -A24, -B7, - B8 (ESM Table 1) were synthesized (>75% purity; ProImmune, Oxford, UK). Binding to recombinant HLA-A2 molecules was assessed using Class I REVEAL assays (ProImmune). Candidate epitopes were resynthesized at >90% purity.
DNA immunization
HLA-A2/DQ8-transgenic (A*02:01/DQB1*03:02) H-2Db−/−β2m−/−IAbβ−/−IAbα−/−IEbβ−/− mice were obtained by crossing HLA-A2- and HLA-DQ8-transgenic mice [7, 8].These mice were pretreated with cardiotoxin and vaccinated twice with non-coding plasmid or plasmid encoding full-length human ZnT8 (1:1 mixture of Arg/Trp325 isoforms) cloned into pcDNA3.1, as described [7]. The study was approved by the local ethics committee. Splenocytes were plated (5×105/well) in IFN-γ enzyme-linked immunospot (ELISpot) plates (Millipore, Molsheim, France) along with peptides (10 µM). After culturing for 20–24 h, plates were revealed with a biotinylated anti-IFN-γ Ab (U-CyTech, Utrecht, The Netherlands), alkaline phosphatase-conjugated streptavidin and NBT-BCIP. Triplicate wells were counted and averaged using a Bioreader 5000 Pro-SF (BioSys, Karben, Germany).
Study subjects
New-onset autoAb+ insulin-dependent type 1 diabetic adults (n=12; 8/4 males/females; median age 31 yr, range 24–60; diabetes duration 39 d, 0–140); children (n=10; 6/4 males/females; median age 12 yr, 1–16; diabetes duration 5 d, 0–80); type 2 diabetic patients (n=24; 15/9 males/females; median age 60 yr, 12–77; diabetes duration 12 yr, 0–42) and healthy controls (n=27; 12/15 males/females; median age 36 yr, 22–60) were recruited in Paris and Turin. Serum Abs were measured by RIA, using 35S-labeled GAD65, IA-2ic and ZnT8 (construct C-term CRCW JH6.2). All subjects were HLA-A2+ (HLA-A*02:01) by genotyping and gave written informed consent. Local ethics committees approved the study.
ELISpot assays
Peripheral blood mononuclear cells (PBMCs) prepared as described [5] were used either fresh or frozen-thawed with similar results [5, 9]. PBMCs (3×105/well) were seeded in anti-IFN-γ-coated ELISpot plates in AIM-V medium containing 0.5 ng/ml IL-7 and 10 µM peptide. A viral peptide mix (Flu MP58–66, Epstein-Barr virus BMLF280–288, cytomegalovirus pp65495–503) and phytohemagglutinin (1 µg/ml) were positive controls. Pyruvate dehydrogenase (PD)5–13 and DMSO diluent were negative controls. After 20–24 h, plates were developed and counted as above. For blocking experiments, anti-CD8 (OKT8; 25 µg/ml) or anti-HLA-A2 (BB7.2; 50 µg/ml) were added to wells. Readouts are expressed as spot-forming cells (SFC)/106 PBMCs after subtracting background responses against PD5–13 and no peptide (which were identical in all cases). The positive cutoff was set at 3SD above the average background, as determined by receiver-operator characteristics analysis [5]. Intra and inter-assay CVs are 14% and 9%, respectively [5].
Statistics
Values are expressed as mean±SD or median (range), according to distribution. Comparisons between proportions were made with the Fisher’s exact test.
Results
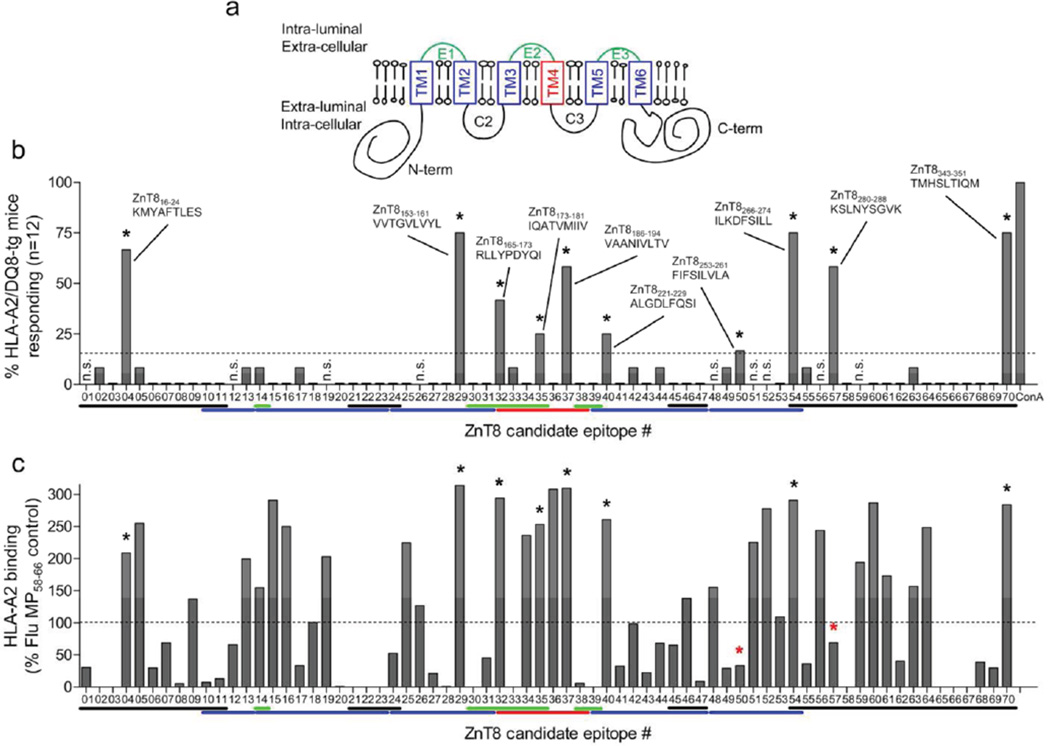
We used DNA immunization [7] to pinpoint naturally processed and presented HLA-A2- restricted epitopes derived from ZnT8 (Fig. 1a). Ten candidates were selected based on positive responses (i.e. >3SD above background) in >15% (≥2/12) of immunized mice (Fig. 1b; ESM Table 1). None was positive in control-immunized mice. Similar results were obtained using HLA-A2-transgenic mice lacking the DQ8 transgene [7] (data not shown). Most candidate epitopes were located either in the fourth transmembrane region (3/10) or in the C-terminal domain (3/10). None overlapped with the Arg/Trp325 polymorphic region. Binding assays to recombinant HLA-A2 molecules were performed in parallel (Fig. 1c). While most (8/10; 80%) candidate epitopes identified by DNA immunization were strong HLA-A2 binders, 2 epitopes (#50, ZnT8253–261; and #57, ZnT8280–288) were weak binders. In addition, several epitopes (22/30; 73%) that were strong HLA-A2 binders did not elicit significant responses in DNA-immunized mice. Thus, compared to an epitope selection strategy based on HLA-binding affinity, DNA immunization allowed us to focus on fewer candidates (10/70 vs. 30/70; 14% vs. 43%), including 2 weak-binding peptides. Moreover, DNA immunization selects candidates on the additional criterion of natural processing and presentation [7].
Figure 1. HLA-A2-restricted ZnT8 candidate epitopes.
a ZnT8 protein structure and topology. The 3 intra-luminal loops are shown in green, the 4 extra-luminal domains in black, the 6 transmembrane (TM) domains (TM1–TM6) in blue, except for TM4 (red). b ZnT8 candidate epitopes selected by DNA immunization on HLA-A2/DQ8-transgenic mice. Splenocytes were recalled with the 70 peptides listed in ESM Table 1. Each bar represents the percent of mice responding to the designated peptide or to concanavalin A (ConA) positive control. The dotted line represents the positive cut-off (>15% of mice responding) and selected epitopes are indicated by asterisks. Color lines below X-axis show the position of each epitope within the ZnT8 structure, following the color code of panel a. c Binding of ZnT8 peptides to recombinant HLA-A2.1 in vitro. The same peptide library was tested using the Class I REVEAL binding assay (Proimmune). Results are expressed as percent binding and the dotted line shows the positive cut-off (>100% binding compared to the reference Flu MP58–66 peptide). Asterisks indicate peptides selected by DNA immunization, two of which (red asterisks) are weak binders.
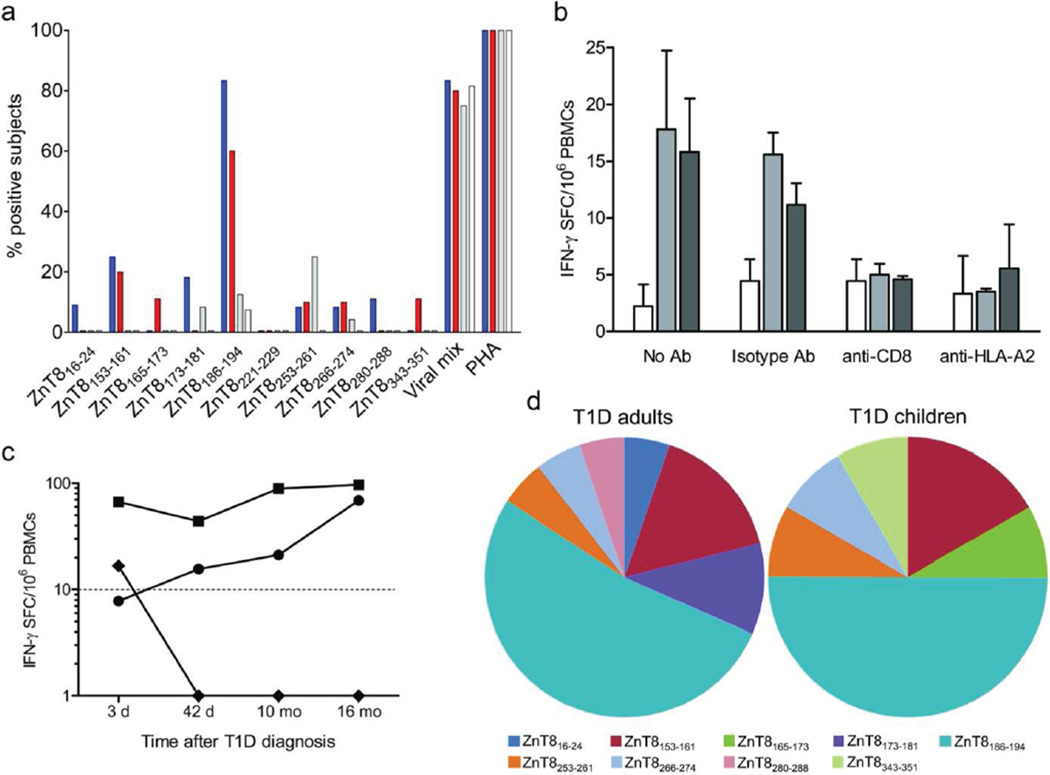
The identified peptides were subsequently tested for CD8+ T-cell recognition using PBMCs from type 1 diabetic, type 2 diabetic and healthy subjects. Results are summarized in Fig. 2a and raw data in ESM Figures 1–2. All but one peptide (ZnT8221–229) were recognized by PBMCs of at least one type 1 diabetic patient. ZnT8153–161 (#29; VVTGVLVYL) and ZnT8186–194 (#37; VAANIVLTV) emerged as immunodominant. ZnT8153–161 was recognized in 25% of type 1 diabetic adults and 20% of type 1 diabetic children, but not by type 2 diabetic and healthy subjects. ZnT8186–194 was targeted in 73% of type 1 diabetic patients, without significant differences between adults (83%) and children (60%). PBMCs from type 2 diabetic patients and healthy controls rarely recognized this epitope (12% and 7%, respectively; p≤0.009 for both compared to type 1 diabetic adults or children), while ZnT8253–261 (#50; FIFSILVLA) was more recognized in type 2 (25%) than in type 1 diabetic (8% adults, 10% children) and healthy subjects (0%; p=0.007 for comparison between type 2 diabetic and healthy). Both ZnT8153–161 and ZnT8186–194 were strong HLA-A2 binders, were located in transmembrane regions and positive in 75% and 58% of immunized mice, respectively. Fourteen IFN-γ ELISpot assays were performed using two different ZnT8186–194 peptide batches, giving identical results in 13 (93%). Moreover, reactivity to ZnT8 epitopes was neutralized both by anti-CD8 and anti-HLA-A2 Abs (Fig. 2b), demonstrating that IFN-γ responses originated from CD8+ T cells recognizing HLA-A2-restricted epitopes. As previously observed for GAD- and IA-2-reactive CD8+ T cells [5, 7] and for ZnT8-reactive CD4+ T cells [3], there was no correlation between anti-ZnT8 T-cell and autoAb responses (data not shown).
Figure 2. Recognition of HLA-A2-restricted ZnT8 epitopes in different study subjects.
a Percent of new-onset type 1 diabetes (T1D) adults (blue bars; n=12), new-onset T1D children (red bars; n=10), type 2 diabetes (T2D; grey bars; n=24) and healthy subjects (white bars; n=27) responding to each individual epitope by IFN-γ ELISpot. b IFN-γ ELISpot responses were measured in a T1D adult using PBMCs stimulated in the presence of no Ab, isotype control Ab, anti-CD8 or anti-HLA-A2 Ab. Mean ± SD counts of triplicate wells are given without background subtraction for each of the indicated peptide stimuli: no peptide (white bars), ZnT8186–194 (light grey bars) and ZnT8253–261 (dark grey bars). c Patient A03 was tested at 4 subsequent time points for PBMC reactivities against the indicated peptides: ZnT8186–194 (circles), ZnT8153–161 (diamonds) and viral mix (squares). Results are expressed as mean IFN-γ SFC/106 PBMCs and are background-subtracted. d Relative distribution of epitope specificities. The percent prevalence of each epitope out of all epitopes recognized among new-onset T1D adults (left; n=19) and children (right; n=12) is shown.
The median frequency of ZnT8 epitope-reactive CD8+ T cells in new-onset type 1 diabetic patients (34.5 SFC/106 PBMCs, i.e. 0.003%, range 0.0007–0.08%) was similar to that previously observed for other beta-cell epitopes [5, 7]. Moreover, one type 1 diabetic adult patient (A03) was studied at 4 different time points (Fig. 2c). While IFN-γ responses to a viral mix remained fairly stable over time, frequencies of ZnT8186–194-reactive T cells secreting IFN-γ gradually increased over time, while the corresponding ZnT8153–161-reactive T cells rapidly disappeared.
The immunodominance of ZnT8186–194 was also evident when considering its relative representation among all epitopes recognized (Fig. 2d). Indeed, ZnT8186–194 was the most frequently targeted peptide both in type 1 diabetic adults and children (53% and 50% of total ZnT8-reactive CD8+ T-cell responses, respectively) compared to other epitopes.
Discussion
ZnT8 is clearly a major target of autoreactive CD8+ T cells in Caucasian new-onset type 1 diabetic patients. Strikingly, the overwhelming majority of ZnT8-reactive T cells (50–53%) and of type 1 diabetic adults (83%) and children (60%) recognized a single HLA-A2-restricted ZnT8186–194 epitope. Another ZnT8153–161 epitope ranked second, being recognized in 20–25% of type 1 diabetic patients. Such a high degree of epitope focusing is surprising, as autoreactivity is usually more widely distributed against different sequences [5]. Indeed, the two most immunodominant epitopes described to date are GAD114–123 [5] and PPI15–24 [6], which are recognized by ~50% of type 1 diabetic adults compared to the 83% scored by ZnT8186–194. An increase in ZnT8186–194-reactive IFN-γ responses along disease course was also observed in one patient, contrary to what observed for most epitope reactivities, including ZnT8153–161, which rapidly wane after diagnosis [9]. ZnT8253–261 was instead recognized by 25% of type 2 diabetic patients (25%), a population seldom included in previous T-cell studies. The phenotype of these patients was not distinctive; they were all Ab-negative and all but one were not insulin-treated. It is possible however that larger ad hoc studies focusing on patients of uncertain classification may reveal a discrete subset.
The epitopes here identified do not overlap with those targeted by CD4+ T cells [3]. The subdominant ZnT8153–161 epitope was also recognized by PBMCs of Chinese HLA-A2+ recent-onset type 1 diabetic patients [4]. On the contrary, the novel ZnT8186–194 emerges here as the major epitope recognized. Intriguingly, we recently described the same ZnT8186–194 epitope and its contiguous ZnT8178–186 sequence as targets of autoAb responses in ~60% of Sardinian type 1 diabetic patients [10]. These autoAbs were cross-reactive with homologous sequences of the Mycobacterium avium paratuberculosis (MAP) 3865c protein, prompting the hypothesis that MAP could be an environmental trigger for type 1 diabetes via a molecular mimicry mechanism [10]. It will be relevant to investigate whether also ZnT8186–194-reactive CD8+ T cells cross-recognize MAP3865c133–141, which may explain their high prevalence.
Supplementary Material
Acknowledgements
This study was supported by grants from the Juvenile Diabetes Research Foundation (JDRF grant 1-2008-106), the European Foundation for the Study of Diabetes (EFSD/JDRF/Novo Nordisk European Programme in Type 1 Diabetes Research 2007), the INSERM Programme National de Recherche sur le Diabète 2007, the Fondation Recherche Médicale (grant Installation Nouvelle Equipe), the Ile-de-France CODDIM (grant Soutien aux Jeunes Equipes) and the Societé Francophone du Diabète, to RM. RM is an INSERM Avenir Investigator and recipient of an APHP-Inserm Contrat Hospitalier de Recherche Translationelle. JCH and HWD acknowledge the JDRF Grant 4-2007-1056, University of Colorado Diabetes Research Center NIH grants P30-DK-57516 and R01-DK- 052068. We wish to thank all the patients that contributed to this research with their blood donations and Anna Falaschi-Jones, Stephanie Marchand and Caroline Dumange for their help with patient databases.
Abbreviations
- Ab
antibody
- ELISpot
enzyme-linked immunospot
- MAP
mycobacterium avium paratuberculosis
- PBMC
peripheral blood mononuclear cell
- SFC
spot-forming cells
- ZnT8
zinc transporter 8.
Footnotes
The original publication is available at springerlink.com
Contribution statement. MS and GA designed and performed experiments, participated in data analysis and interpretation and edited the manuscript; EL and CB participated in study design and data interpretation, provided blood samples and clinical data and edited the manuscript; CR, JCC, DDL, BB, DL, JFG, OL, GB participated in research discussion, provided blood samples and clinical data and reviewed the manuscript; FAL participated in research discussion, provided mice and reviewed the manuscript; LAS participated in study design and data interpretation and reviewed the manuscript; JCH and HWD participated in study design, data analysis and interpretation, performed experiments, provided reagents, and edited the manuscript; RM coordinated the study, designed experiments, participated in data analysis and interpretation and wrote the manuscript.
Duality of interest. The authors declare that there is no duality of interest associated with this manuscript.
References
- 1.Brezar V, Carel JC, Boitard C, Mallone R. Beyond the hormone: insulin as an autoimmune target in type 1 diabetes. Endocr Rev. 2011;32:623–669. doi: 10.1210/er.2011-0010. [DOI] [PubMed] [Google Scholar]
- 2.Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA. 2007;104:17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dang M, Rockell J, Wagner R, et al. Human type 1 diabetes is associated with T cell autoimmunity to zinc transporter 8. J Immunol. 2011;186:6056–6063. doi: 10.4049/jimmunol.1003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu X, Xu X, Gu R, et al. Prediction of HLA class I-restricted T-cell epitopes of islet autoantigen combined with binding and dissociation assays. Autoimmunity. 2012;45:176–185. doi: 10.3109/08916934.2011.622014. [DOI] [PubMed] [Google Scholar]
- 5.Mallone R, Martinuzzi E, Blancou P, et al. CD8+ T-cell responses identify beta cell autoimmunity in human type 1 diabetes. Diabetes. 2007;56:613–621. doi: 10.2337/db06-1419. [DOI] [PubMed] [Google Scholar]
- 6.Skowera A, Ellis RJ, Varela-Calvino R, et al. CTLs are targeted to kill beta cells in patients with type-1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest. 2008;118:3390–3402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blancou P, Mallone R, Martinuzzi E, et al. Immunization of HLA class I transgenic mice identifies autoantigenic epitopes eliciting dominant responses in type 1 diabetes patients. J Immunol. 2007;178:7458–7466. doi: 10.4049/jimmunol.178.11.7458. [DOI] [PubMed] [Google Scholar]
- 8.Nabozny GH, Baisch JM, Cheng S, et al. HLA-DQ8 transgenic mice are highly susceptible to collagen-induced arthritis: a novel model for human polyarthritis. J Exp Med. 1996;183:27–37. doi: 10.1084/jem.183.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinuzzi E, Novelli G, Scotto M, et al. The frequency and immunodominance of islet-specific CD8+ T-cell responses change after type 1 diabetes diagnosis and treatment. Diabetes. 2008;57:1312–1320. doi: 10.2337/db07-1594. [DOI] [PubMed] [Google Scholar]
- 10.Masala S, Paccagnini D, Cossu D, et al. Antibodies recognizing Mycobacterium avium paratuberculosis epitopes cross-react with the beta-cell antigen ZnT8 in Sardinian type 1 diabetic patients. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026931. e26931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.