Abstract
A common in vitro model for studying acute mechanical damage in cartilage is to impact an isolated osteochondral or cartilage specimen with a metallic impactor. The mechanics of a cartilage-on-cartilage (COC) impact, as encountered in vivo, are likely different than those of a metal-on-cartilage (MOC) impact. The hypothesis of this study was that impacted in vitro COC and MOC specimens would differ in their impact behavior, mechanical properties, chondrocyte viability, cell metabolism, and histologic structural damage. Osteochondral specimens were impacted with either an osteochondral plug or a metallic cylinder at the same delivered impact energy per unit area, and processed after 14 days in culture. The COC impacts resulted in about half of the impact maximum stress and a quarter of the impact maximum stress rate of change, as compared to the MOC impacts. The impacted COC specimens had smaller changes in mechanical properties, smaller decreases in chondrocyte viability, higher total proteoglycan content, and less histologic structural damage, as compared to the impacted MOC specimens. If metal-on-cartilage impact conditions are to be used for modeling of articular injuries and post-traumatic osteoarthritis, the differences between COC and MOC impacts must be kept in mind.
Keywords: Articular cartilage, biochemical analysis, histology, impact testing, post-traumatic osteoarthritis
Introduction
Acute mechanical damage sustained by cartilage at the time of a joint impact injury is an important pathomechanical etiologic factor that leads from acute cartilage injury to post-traumatic osteoarthritis. Cartilage impact damage has been shown to cause acute and delayed chondrocyte death, metabolic dysfunction, and changes in cartilage mechanical properties. Because of its central role in injury-related cartilage degeneration, cartilage impact damage has been extensively studied using in vitro preparations. Most commonly, in vitro impaction of an osteochondral or cartilage specimen has been performed with a metallic impactor.
In clinical practice, articular fractures and cartilaginous injuries are the result of supra-physiologic forces transferred between two cartilaginous surfaces. Material and structural differences between two impacting osteochondral structures, compared to a metallic impactor striking cartilage, would be expected to affect stresses and stress rates of change during the impact. These differences in mechanical impact characteristics would in turn affect the deformation of the cartilage during impact, and therefore the pathologic strains encountered by the chondrocytes at the time of injury.
The objective of this study was to compare the cartilage injury characteristics of a cartilage-on-cartilage (COC) in vitro impact model to those of a conventional metal-on-cartilage (MOC) in vitro impact model. The first hypothesis was that with the same delivered impact energy per unit area, cartilage stresses and stress rates of change would be less for COC impacts compared to MOC impacts. The second hypothesis was that compared to MOC-impacted specimens, COC-impacted specimens would exhibit less structural damage, less mechanical property change, less chondrocyte death, and less chondrocyte metabolic dysfunction.
Methods
Osteochondral specimens measuring approximately 25 mm square and approximately 8 mm thick were excised from skeletally mature (12–24 month) bovine lateral tibial plateaus, from sites where meniscus did not cover the articular cartilage. Each specimen was attached to its own stainless steel plate using polycaprolactone as a mechanical bonding agent. Attachment of the plate through its reference corner holes automatically indexed the specimen to a reproducible position within all testing fixtures and microscopes, and allowed load applications, measurements, and harvesting cuts to be made in known, reproducible locations (Figure 1). The osteochondral specimens were impacted with the same delivered energy per area, using either a metallic (n = 18) or cartilage (n = 16) impactor. The impact behavior, mechanical properties, chondrocyte viability, cell metabolism, and structural damage of the specimens were determined.
Figure 1.
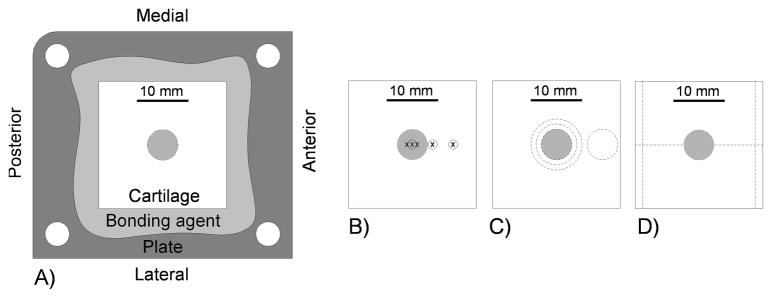
A) Schematic of osteochondral specimen on base plate, and locations of the B) mechanical tests (circles) and imaging (x’s), C) cuts for biochemistry tests, and D) cuts for histological slides on the osteochondral specimens. The impact area in the center of the specimen is indicated by shading.
Impact behavior
The metal impactor (Figure 2A) was a brass cylinder having a flat end with a rounded edge and an impact diameter of 5.5 mm. The cartilage impactors (Figure 2B) were obtained from the femoral condyle apposing each test specimen, using a mosaicplasty harvesting system (Mosaicplasty DP-Disposable Harvesting System, Smith & Nephew Endoscopy, Andover, MA). Four or five osteochondral plugs were punched, and the plug that was the flattest and that had a cartilage surface most perpendicular to the plug sides was chosen as the cartilage impactor for that specimen. The plugs were 6.5 mm in diameter and approximately 15 mm in length. Each plug was press-fit into a brass sleeve, leaving approximately 3 mm of cartilage and subchondral bone outside the sleeve. The opposite end of the plug was braced by filling the remainder of the brass sleeve with polycaprolactone. This sleeve was in turn press-fit into a brass sphere, which was then secured within the drop tower prior to impact.
Figure 2.
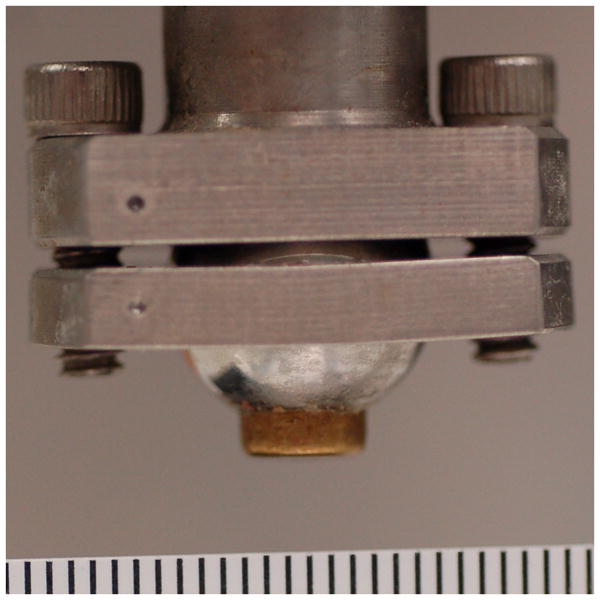
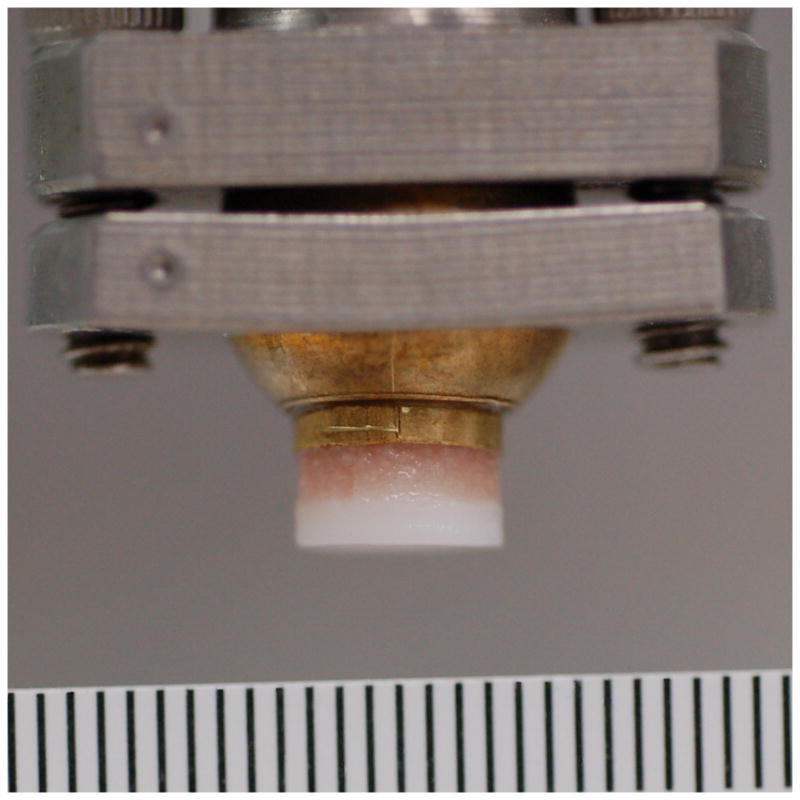
A) Metal and B) cartilage impactors. Ruler markings are 1 mm.
A drop tower, previously described,1 was used to impact each specimen with an energy of 3.09 J/cm2 (Figure 3). Since the diameter of the cartilage impactor (6.5 mm, limited by available mosaicplasty system sizes) was greater than the diameter of the metal impactor (5.5 mm), the impact mass was increased accordingly (0.592 vs. 0.824 kg for MOC vs. COC, respectively, each from a height of 12.7 cm) so that the two impactors delivered the same impact energy per unit area. The cartilage surface was kept moist with frequent application of culture medium, and the impactions were done within a laminar airflow hood. Impact accelerometer data were recorded at 100 kHz. Maximum impact stress, maximum impact stress rate of change, and the amount of higher-frequency content (percent of frequency spectrum greater than 1 kHz) in the impact signal were calculated. This high-frequency content measure was demonstrated in a previous study to be useful as an estimation of the amount of acute structural cartilage injury.1
Figure 3.
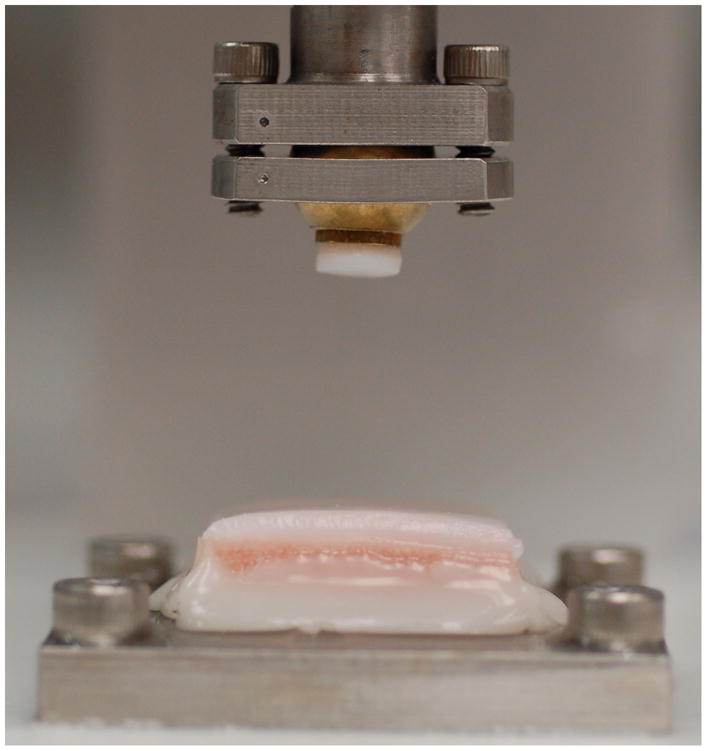
Cartilage impactor in drop tower, and osteochondral specimen to be impacted.
Mechanical properties
Cartilage mechanical properties were measured by stress relaxation using a custom-built indentor. The indentor, run by LabVIEW, incorporated a 500 g load cell (060-0911-14, Honeywell Sensing and Control, Columbus, OH), an impermeable 3 mm spherical diameter indentor tip, a vertical stepper motor for the indentation, and two horizontal stepper motors for motion between indentation positions. The indentor was housed in an incubator with settings of 37°C, 98% humidity, and 5% CO2. Cartilage thickness was first measured with a handheld ultrasonic probe (Model 35DL Thickness Gage, Olympus NDT Inc., Waltham, MA). A laser alignment device indicated the desired locations on the cartilage for making the thickness measurements. The stress relaxation test consisted of ramping the indentor tip to 10% strain over 0.1 second, then holding it there for 500 seconds. Force data were recorded at 400 Hz during the subsequent first five seconds, then at 40 Hz for the remainder of the test. Indentation was measured at the center of the impact area, adjacent to the impact area, and remotely from the impact area (0 mm, 4 mm, and 8 mm anterior to the center of the impact area, respectively; Figure 1B) at three time points – just before impact, two hours after the impact, and then 14 days after the impact (just before specimens went to biochemical or histological processing).
The indentation force-versus-time data were used to calculate poro-viscoelastic mechanical properties of the cartilage (based on the model of DiSilvestro et al.2), employing an axisymmetric Abaqus finite element (FE) model and a Matlab-driven optimization procedure. A specimen-specific FE model was generated for each cartilage thickness. The Matlab optimization procedure ran an FE simulation with an initial set of material properties, compared the FE-model force-versus-time curve to that from the mechanical test, and then iteratively adjusted the properties until the error between the two curves was minimized. Poisson’s ratio was set to 0.05. The poro-viscoelastic mechanical properties calculated were equilibrium modulus, permeability, and relaxation coefficient. The equilibrium modulus was determinate of the final stress at the end of the indentation test. The relaxation coefficient primarily governed the peak force, and it dominated the initial relaxation behavior. The permeability also influenced the relaxation process, becoming the dominant term after the solid-phase viscoelastic effect had dissipated. Mechanical property data were normalized to the pre-impact values of each specimen.
Chondrocyte viability
Chondrocyte viability was measured using confocal imaging (Bio-Rad 1024, Zeiss Microscopy, LLC, Thornwood, NY), with Calcein-AM being the marker for viable cells. Specimens were imaged within the impact area (at the center of the impact area, 1 mm anterior to the center and 1 mm posterior to the center), at one point adjacent to the impact area (4 mm anterior to the center of the impact area), and at one point remote from the impact site area (8 mm anterior to the center of the impact area) (Figure 1B). Imaging was done before the impact (and before the mechanical property measurements), and then at either 1, 2, or 7 days after impact. The pre-impact imaging was also used to qualitatively screen the specimens for an acceptably high density of viable cells. Pre-impact and post-impact imaging was done at the same sites by positioning each specimen using a custom x-y stage driver having a precision of 15 μm. The confocal microscope imaged live cells over an approximate 0.54 by 0.54 mm square area, from the articular surface to a depth of 80–100 μm below the surface, in 20 μm intervals. Custom image analysis software determined the number of viable cells at each depth interval, and the number of viable cells per mm3 for each point. The values for the three points within the impact area were averaged. Post-impact and pre-impact live cell density numbers were compared at each region for each specimen, with data presented as a change from the pre-impact value.
Biochemical analyses
Cell metabolism was evaluated by use of [3H]proline incorporation (collagen synthesis) and by measurement of total proteoglycan content (via total glycosaminoglycan content). At 14 days, eight MOC and eight COC specimens were processed for biochemical analysis. The specimens were first incubated in 0.01 mCi [3H]proline (PerkinElmer, Waltham, MA) for 18.5 hours in culture medium, then washed in fresh culture medium for 4 hours to allow for final incorporation. Full-thickness cartilage specimens were then extracted using dermal punches to cut a 6 mm diameter central disc; concentric, annular rings between 6–8 mm and between 8–10 mm diameter; and a 6 mm diameter remote disc (Figure 1C). The discs and rings were further rinsed for 20 hours in PBS at 4 degrees Celsius to remove any un-incorporated [3H]proline. The four sections from each specimen were analyzed separately. [3H]proline incorporation and total glycosaminoglycan (GAG) content were measured using a papain digestion and DMMB assay method adapted from Hoemann et al.3 The cartilage plugs referenced above were measured for wet tissue mass, and digested in a phosphate-buffered EDTA solution with Papain (500 μg/ml Papain type III, Sigma, St. Louis, MO) for 4 hours at 65 degrees Celsius and centrifuged at 12,000g for 10 minutes at room temperature. [3H]proline incorporation was assessed by first pipetting 100 μl of papain-digested sample into 1 ml of liquid scintillation cocktail (OptiPhase SuperMix, Wallac/PerkinElmer, Waltham, MA), then mixing the solution carefully. Each sample was analyzed twice using a liquid scintillation counter (Trilux 1450 Microbeta Liquid Scintillation & Luminescence Counter, Wallac/PerkinElmer, Waltham, MA) to find average counts per minute per wet tissue mass. Total GAG content was measured in papain digests of the cartilage plugs referenced above. Using 1,9-dimethylmethylene blue (44.9 μM, Sigma, St. Louis, MO) and shark chondroitin sulfate (Sigma, St. Louis, MO) as a standard, absorbance measurements were taken at 530 nm using an endpoint method (Vmax Kinetic Microplate Reader, Molecular Devices, Sunnyvale, CA). Each sample was assayed three times, calculated for a dilution factor, and those values were normalized to wet tissue mass and averaged.
Histological analyses
After 14 days, five MOC and five COC specimens were processed for histological analysis. To maintain spatial information about each histological section relative to the impact zone, two parallel M-L reference lines were cut to center the impact area in the center of the section, and the specimen was then cut through the center of the impact zone in the A-P direction (Figure 1D). Specimens thus registered were prepared using OARSI guidelines for histological preparation4 and embedded in paraffin wax for sectioning parallel to the A-P direction. Five-micron-thick sections were selected and stained with Safranin-O. Sections were digitized at a resolution of 322.25 nm/pixel using an Olympus VS110 Virtual Microscopy System (Olympus Corporation of the Americas, Center Valley, PA) and exported in a TIF format at a resolution of 1.61 μm/pixel for automated and manual histological evaluation.
Digitized sections were evaluated using a bovine cartilage-specific automated version of the Mankin (HHGS) Scale5 implemented within a custom Matlab routine, and by manual application of the Histologic Structural Damage Score (HSDS).1 The HSDS was developed to describe acute cartilage mechanical damage, independently of any biologic response. Application of the HSDS involved three different observers grading each digitized section on the sum of articular surface cracking (scored as 0, 1, or 2) and cartilage crushing (scored as 0, 3, 4, or 5), and averaging the observers’ scores.
Statistical analysis
The data were statistically analyzed using Minitab and SAS. The two-tailed Wilcoxon rank-sum test was used to compare MOC and COC data for the mechanical property, biochemical, and histological tests. For the chondrocyte viability tests, the Mantel-Haenzel test was used to compare MOC and COC data, adjusted for time, and Friedman’s test was used to compare chondrocyte viability outcomes between time points separately for MOC and COC data. Statistical significance was set at α = 0.05.
Results
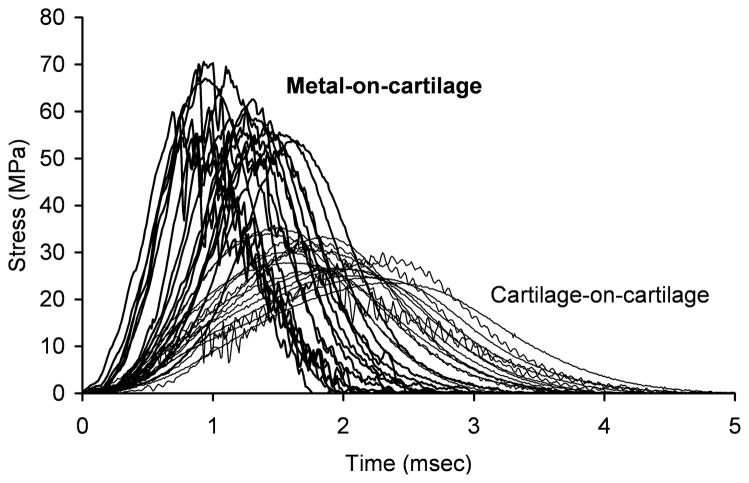
The COC impacts resulted in about half the maximum stress (29.3 ± 3.6 vs. 60.2 ± 6.0 MPa, p < 0.0001) and a quarter of the maximum stress rate of change (30.9 ± 8.0 vs. 123.5 ± 26.9 GPa/sec, p < 0.0001) of the MOC impacts (Figure 4). The COC impacts also resulted in less high-frequency content in the impact signal than did the MOC impacts (12.5% ± 8.7% vs. 22.1% ± 11.7%, p = 0.006). Impact data were not collected for one MOC specimen because of operator error. The osteochondral impactor plugs displayed damage from the impaction. This damage typically consisted of visible fissuring of the cartilage surface, buckling of the cartilage, or crushing of the bone. In an extreme case, the impact caused a piece of the osteochondral plug to break off; the corresponding impacted specimen was kept in the study.
Figure 4.
Impact stress-versus-time signals from metal-on-cartilage and cartilage-on-cartilage specimens.
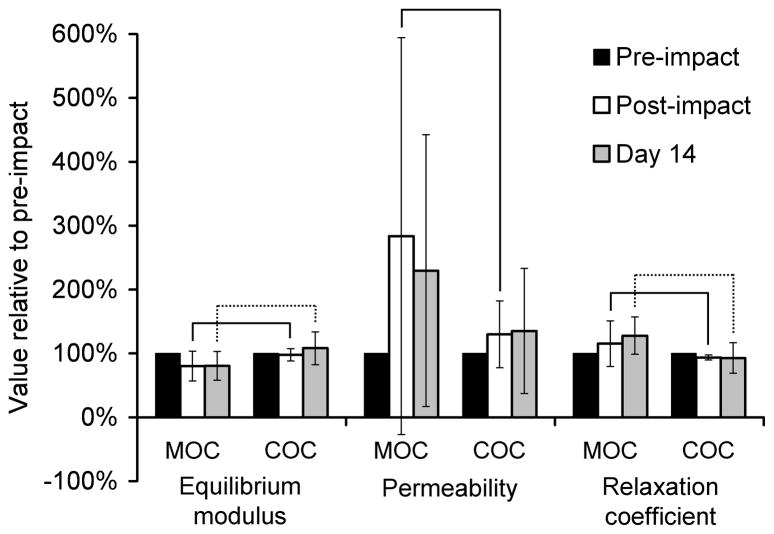
At the impact center, the MOC specimens showed more change in the poro-viscoelastic mechanical properties than did the COC specimens (Figure 5). The MOC and COC specimens were significantly different in normalized equilibrium modulus, permeability, and relaxation coefficient at two hours post-impact, and significantly different in normalized equilibrium modulus and relaxation coefficient at Day 14. At the adjacent and remote sites, none of the normalized mechanical properties were significantly different between the MOC and COC specimens, and the changes from the pre-impact values were minimal (data not shown). One MOC and one COC specimen were excluded because they became infected before Day 14.
Figure 5.
Poro-viscoelastic mechanical properties of cartilage impacted with a metal (MOC) or cartilage (COC) impactor, at the impact center. Error bars indicate standard deviations. Data were normalized to the pre-impact value for each specimen. Solid brackets indicate groups that were significantly different at post-impact, and dotted brackets indicate groups that were significantly different at Day 14.
Within the impact area, the MOC specimens experienced a greater percentage of chondrocyte death than the COC specimens across the three time points (Figure 6), with the impactor type having an effect overall (p = 0.0048). Because of positioning discrepancies between the pre-impact and post-impact imaging sessions (see Discussion section for more detail), some imaging data had to be eliminated; the subsequent number of specimens per time point was 3–5 for within the impact area and for the remote point, and 2–5 for the adjacent point. Follow-up Wilcoxon rank-sum comparisons at each time point were not performed because of the small number of specimens available at each time point. No significant difference in the viability changes within the impact area was detected between any of the days for either the MOC (p = 0.44) or COC (p = 0.25) groups. At the adjacent and remote sites, the impactor type did not have a significant effect on the change in chondrocyte viability across the three time points (p = 0.32 and 0.92, respectively) (data not shown).
Figure 6.
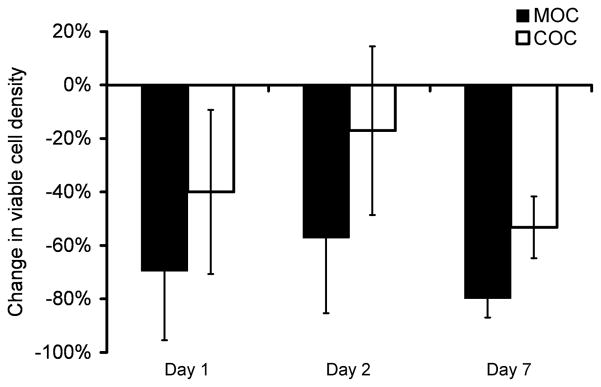
Chondrocyte viability of cartilage impacted with a metal (MOC) or cartilage (COC) impactor, within the impact area. Data are relative to the pre-impact value for each specimen. The impactor type had an overall effect on the percentage of chondrocyte death (p = 0.0048). No significant difference in the viability changes was detected between any of the days for either the MOC (p = 0.44) or COC (p = 0.25) groups.
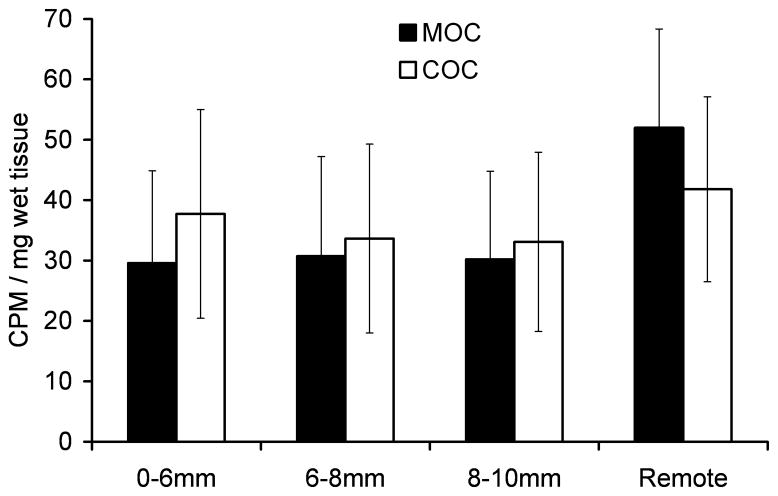
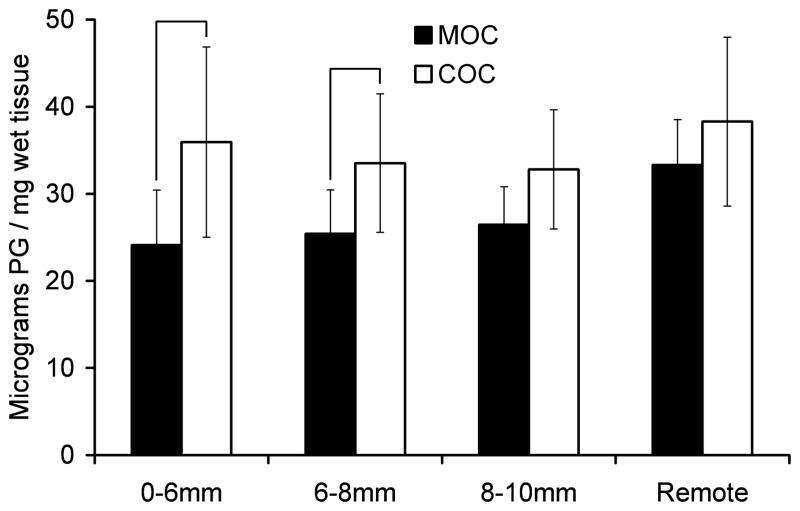
No significant difference was detected in [3H]proline incorporation between the MOC and COC specimens at any of the sections studied (Figure 7A). The COC specimens had higher total proteoglycan content than did the MOC specimens, with the difference being significant for the central disc (0–6 mm) and adjacent ring (6–8 mm) sections (Figure 7B).
Figure 7.
A) [3H]proline incorporation and B) total proteoglycan (PG) content for cartilage impacted with a metal (MOC) or cartilage (COC) impactor. Cartilage was processed at 14 days post-impact. Error bars indicate standard deviations. Brackets indicate groups that were significantly different at each section.
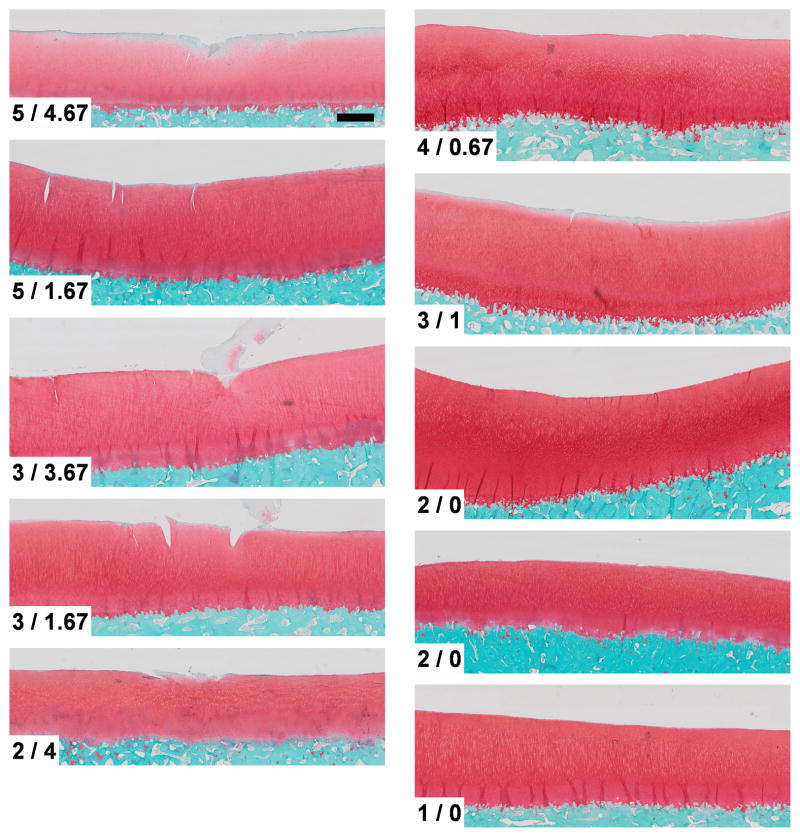
The COC specimens overall had less structural damage than the MOC specimens (Figure 8). The COC specimens had at most very minor surface cracking, while the MOC specimens usually had a greater degree of cracking and some crushing. The MOC and COC specimens scored 3.60 ± 1.34 and 2.40 ± 1.14, respectively, on the Mankin Scale (p = 0.197), and scored 3.14 ± 1.39 and 0.33 ± 0.47, respectively, on the HSDS (p = 0.011).
Figure 8.
Histologic sections (stained with Safranin-O) for cartilage impacted with a (left) metal (MOC) or (right) cartilage (COC) impactor and processed at 14 days post-impact. Scale bar is 1 mm. Mankin Scale/Histologic Structural Damage Scale (HSDS)1 are indicated. The MOC and COC specimens had a statistically significant difference (p = 0.011) in HSDS.
Discussion
Impacting an osteochondral specimen with an osteochondral plug resulted in about half of the impact maximum stress and a quarter of the impact maximum stress rate of change as did impacting an osteochondral specimen with a metallic impactor at the same delivered impact energy per unit area (Figure 4). In addition, the amount of higher-frequency content in the impact signal, indicative of the amount of acute structural cartilage injury,1 was considerably less for the COC impacts than for the MOC impacts. An osteochondral impactor plug would dissipate more of the delivered impact energy than would a metallic impactor (as indicated in part by the overt damage of the osteochondral impactor plugs upon impaction), thus reducing the amount of energy ultimately imparted to the impacted specimen and affecting the subsequent results. These findings likely have biologic significance in that many previous in vitro preparations of cartilage damage have demonstrated that cartilage metabolic response, impact-related changes in chondrocyte viability, and cartilage structural damage are affected by loading magnitudes6–11 and loading rates.7,8,10,11 These findings also have clinical significance when considering hemiarthroplasty and metallic osteochondral defect fillers, as an illustration of nonphysiologic cartilage stresses associated with a metal-on-cartilage situation.12
The differences between the MOC and COC impacts resulted in differences in the mechanical, biochemical, and histological properties of the impacted specimens. The MOC-impacted specimens clearly had greater structural damage (Figure 8), which was likely contributory to the marked increase in permeability and decrease in equilibrium modulus compared to the COC specimens (Figure 5). The greater structural damage in the MOC specimens was also reflected in an overall greater quantity of chondrocyte death in the MOC specimens (Figure 6). The lesser amount of proteoglycans in the MOC-impacted tissue compared with the COC-impacted tissue after 14 days may be the result of increased catabolic activity in response to increased mechanical damage. However, the higher permeability of the MOC specimens at 2 hours post-impact suggests that mechanical damage to the collagen fiber network that helps to retain proteoglycans may also be at least partly responsible for this lesser amount. [3H]proline uptake at 14 days was lower within and near the impact area than in the remote site. Although uptake was slightly higher within the COC impact area than within the MOC impact area, the difference was not statistically significant. While these results indicated that the COC and MOC protein synthesis rates were similar after 14 days, significant differences might have occurred at earlier time points.
Limitations concerning the chondrocyte viability measurements were the high variability and small sample sizes per time point, which resulted from positioning discrepancies between the two imaging sessions. The precision of the custom x-y stage driver was 15 μm, thus minimizing horizontal positioning errors. Changes in z-axis focal plane positioning likely resulted in much greater error, as impact-associated surface irregularities made accurate repeated positioning at an identical initial z-plane difficult. Differences in this starting position could therefore have affected the focal plane position of the subsequent imaging intervals, thus introducing inaccuracy into the viable cell counts. The imaging data from several locations consequently needed to be eliminated when the overall appearance of the remaining chondrocyte landmark locations within the image was judged to be too different between the two imaging sessions.
Milentijevic et al.13 impacted a cartilage explant with another cartilage explant. An explant pair was placed in a confining chamber, with their articular surfaces contacting each other. The explant pair was then impacted at 350 MPa/sec to 50 MPa. In contrast to when a cartilage explant was directly impacted with a metal impactor at 350 MPa/sec to 15, 30, or 60 MPa, neither of the paired impacted explants had any detectable chondrocyte death. To the authors’ knowledge, this is the only previous study involving impaction of isolated cartilage-on-cartilage explants.
Ideally, an osteochondral impactor would be used for impacting an osteochondral or cartilage specimen in vitro, for closest equivalence to in vivo impacts. However, the desired amount of damage to the impacted surface might not be achievable with an osteochondral impactor plug, because of impactor plug damage resulting from the impact. Use of osteochondral impactors also introduces an additional source of variability in the experimental setup. One workaround might be to determine an impact mass and drop height, with a metal impactor, that would provide an impact response that is similar to that achieved with an osteochondral impactor, using finite element analysis.14 A metal impactor could then be used in vitro with those experimental parameters. Another method might be to select an impact mass and drop height that with a metal impactor causes a desired amount of injury to the specimen, such as an amount of damage similar to that observed with in vivo impacted specimens. Alternatively, substituting the impacting cartilage with a surrogate material that is less susceptible to impact damage, but still provides an equivalent impact response, would provide another option to improve the biofidelity of in vitro experimentation.
In conclusion, impact characteristics, cartilage mechanical property changes, cartilage structural damage, cartilage biochemical response, and chondrocyte viability changes were significantly different in bovine tibial plateau specimens that were impacted with a typical metallic impactor compared to an osteochondral impactor. If metal-on-cartilage impact conditions are to be used for modeling of articular injuries and post-traumatic osteoarthritis, the differences between COC and MOC impacts must be kept in mind.
Acknowledgments
This research was funded by NIH P50 AR055533. The authors also thank Smith and Nephew for donating the mosaicplasty systems, and thank Mr. Thomas Baer, Dr. Steve Hillis, Mr. Brice Journot, Ms. Gail Kurriger, Ms. Erin Main, and Dr. M. James Rudert for their assistance. Dr. McKinley is a committee chairman for the Orthopaedic Trauma Association.
References
- 1.Heiner AD, Martin JA, McKinley TO, et al. Frequency content of cartilage impact force signal reflects acute histologic structural damage. Cartilage. 2012;3:314–322. doi: 10.1177/1947603511430706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiSilvestro MR, Zhu Q, Wong M, et al. Biphasic poroviscoelastic simulation of the unconfined compression of articular cartilage: I--Simultaneous prediction of reaction force and lateral displacement. J Biomech Eng. 2001;123:191–197. doi: 10.1115/1.1351890. [DOI] [PubMed] [Google Scholar]
- 3.Hoemann CD, Sun J, Chrzanowski V, Buschmann MD. A multivalent assay to detect glycosaminoglycan, protein, collagen, RNA, and DNA content in milligram samples of cartilage or hydrogel-based repair cartilage. Anal Biochem. 2002;300:1–10. doi: 10.1006/abio.2001.5436. [DOI] [PubMed] [Google Scholar]
- 4.Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. [PubMed] [Google Scholar]
- 6.Milentijevic D, Torzilli PA. Influence of stress rate on water loss, matrix deformation and chondrocyte viability in impacted articular cartilage. J Biomech. 2005;38:493–502. doi: 10.1016/j.jbiomech.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Newberry WN, Garcia JJ, Mackenzie CD, et al. Analysis of acute mechanical insult in an animal model of post-traumatic osteoarthrosis. J Biomech Eng. 1998;120:704–709. doi: 10.1115/1.2834882. [DOI] [PubMed] [Google Scholar]
- 8.Scott CC, Athanasiou KA. Design, validation, and utilization of an articular cartilage impact instrument. Proc Inst Mech Eng [H] 2006;220:845–855. doi: 10.1243/09544119JEIM97. [DOI] [PubMed] [Google Scholar]
- 9.Verteramo A, Seedhom BB. Effect of a single impact loading on the structure and mechanical properties of articular cartilage. J Biomech. 2007;40:3580–3589. doi: 10.1016/j.jbiomech.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson PJ, Ewers BJ, Haut RC. Blunt injuries to the patellofemoral joint resulting from transarticular loading are influenced by impactor energy and mass. J Biomech Eng. 2001;123:293–295. doi: 10.1115/1.1378576. [DOI] [PubMed] [Google Scholar]
- 11.Natoli RM, Scott CC, Athanasiou KA. Temporal effects of impact on articular cartilage cell death, gene expression, matrix biochemistry, and biomechanics. Ann Biomed Eng. 2008;36:780–792. doi: 10.1007/s10439-008-9472-5. [DOI] [PubMed] [Google Scholar]
- 12.Anderson DD, Tochigi Y, Rudert MJ, et al. Effect of implantation accuracy on ankle contact mechanics with a metallic focal resurfacing implant. J Bone Joint Surg Am. 2010;92:1490–1500. doi: 10.2106/JBJS.I.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milentijevic D, Helfet DL, Torzilli PA. Influence of stress magnitude on water loss and chondrocyte viability in impacted articular cartilage. J Biomech Eng. 2003;125:594–601. doi: 10.1115/1.1610021. [DOI] [PubMed] [Google Scholar]
- 14.Judd KT, Heiner AD, Goreham-Voss CM, McKinley TO. Cartilage-on-cartilage vs. metal-on-cartilage impact characteristics. Trans 57th ORS. 2011;36:2142. doi: 10.1002/jor.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]