Abstract
Objective
Induced therapeutic hypothermia after pediatric cardiac arrest is an important intervention. We assessed the feasibility, effectiveness, side effects, and adverse events associated with a standardized surface cooling protocol.
Design
Prospective intervention trial
Setting
Urban, tertiary care children’s hospital
Patients
12 Pediatric cardiac arrest survivors
Interventions
Standardized surface cooling protocol
Measurements and Main Results
Patients (age: median 1.5 years, IQR[0.5, 6.25], CPR duration: median 18 min, IQR [10, 45]) were cooled by a standard surface cooling protocol for rapid induction and maintenance of goal rectal Temperature (T) 32–34°C for 24 hours, with prospectively defined rescue protocols. Side effects and clinical interventions were recorded. Median time to rectal T ≤34°C was 1.5 [1, 1.5] hours from cooling initiation and 6 [5, 6.5] hours from arrest. T was documented every 30 minutes. Maintenance target T 32–34°C was attained in 78% (414/531) of measurements, overshoot hypothermia <32°C in 15% (81/531), and overshoot hyperthermia >34°C in 7% (36/531). Mean bias between rectal vs esophageal T was −0.42 °C [95%CI, −0.49 to −0.35], and between rectal and bladder T was 0.16 °C [95%CI, 0.11 to 0.22]. Side effects observed included: hypokalemia <3.0mEq/L in 67% of patients and bradycardia < 2%ile for age in 58%. There were no episodes of bleeding or ventricular tachyarrhythmia that required treatment. Six of 12 (50%) patients survived to discharge.
Conclusions
A standard surface cooling protocol achieved rapid induction of hypothermia after pediatric cardiac arrest. During maintenance of hypothermia, 78% of measures were within target T 32–34°C. Commonly employed temperature sites (esophageal, rectal and bladder) were similar. Overshoot hypothermia and associated side effects were common, but there were no serious adverse events attributable to induced therapeutic hypothermia in this case series. Surface cooling protocols to induce and maintain therapeutic hypothermia after pediatric cardiac arrest are potentially feasible.
Keywords: induced hypothermia, heart arrest, children
Introduction
Impaired neurologic function and quality of life following pediatric cardiac arrest are common. Survival to hospital discharge ranges from 5–27%, with only half of survivors having a favorable neurologic outcome1–2, with associated long term physical, psychological, and quality of life burdens.
Induced therapeutic hypothermia after cardiac arrest can improve neurologic outcomes in selected circumstances. Following witnessed adult ventricular fibrillation (VF), therapeutic hypothermia to 32–34°C for 12–24 hours improves survival and neurologic outcomes.3–6 Induced therapeutic hypothermia for 72 hours after perinatal encephalopathy can improve outcome in selected neonates.7–10
In 2005, the International Liaison Committee on Resuscitation (ILCOR) recommended for children: “When subjects remain comatose after resuscitation, consider cooling them to 32 to 34°C.”11 The implementation of therapeutic hypothermia after pediatric cardiac arrest is sporadic with no prospective clinical trial proving efficacy. In a survey, less than 50% of providers self-report cooling patients following cardiac arrest, but 95% report willingness to randomize in an intervention trial.12 This was attributed to lack of efficacy evidence, difficulty with cooling techniques, and lack of explicit protocols. The authors called for prospective studies to assess the feasibility, safety, and effectiveness of therapeutic hypothermia protocols.
Our objectives were to prospectively assess the feasibility of rapid induction, maintenance, and controlled rewarming using a standard and practical therapeutic hypothermia surface cooling protocol following successful resuscitation from pediatric cardiac arrest. Our primary hypotheses were that this protocol would achieve rapid induction of cooling to 32–34°C within 6 hours of protocol initiation in at least 80% of cases, and that 80% of temperature measurements during maintenance would be between 32–34°C. Our secondary objectives were to describe the side effects and adverse events associated with this therapeutic hypothermia protocol during induction, maintenance and rewarming. We further sought to compare the agreement between core temperature measurements taken from commonly used core probe locations.
Materials and Methods
This study was approved by our hospital’s institutional review board and patient informed consent was obtained.
This was a prospective interventional study applying a standardized surface cooling protocol to children <21 years old following successful resuscitation from in- or out-of-hospital cardiac arrest. Patients were enrolled at a tertiary care pediatric institution from 9/2006 thru 7/2008. Cardiac arrest was defined as any event requiring >60 seconds of chest compressions. Patients were eligible for enrollment if they had return of spontaneous circulation (ROSC) from a cardiac arrest and the clinical team decided to implement induced therapeutic systemic hypothermia to a target range of 32 to 34°C for 24 hours. If > 8 hours had elapsed from the time of the cardiac arrest to protocol implementation, patients were not eligible.
Patients had continuous temperature monitoring in four commonly used locations when possible: rectum (90050, 90044, 9 and 12 Fr probes, accuracy: +0.2/−0.15 °C, Tyco Medical, Pleasanton, CA), axilla (skin) (Sure Temp Plus 692, accuracy: average error 0.055 °C, Welch Allyn, Skaneateles Falls, NY) , esophagus (90050, 90044, 9 and 12 Fr probes, accuracy: +0.2/−0.15 degrees °C, Tyco Medical, Pleasanton, CA), and bladder (foley temperature probes, 8–16 Fr, accuracy +/−0.2°C, Smith Medical, Rockland, MA). At the time of this study, rectal temperature was considered the primary temperature location for titration of therapy and was connected to the Gaymar cooling device for temperature servo-regulation. The esophageal and bladder temperatures were displayed on the bedside monitor (GE Solar). One patient had the esophageal temperature probe connected to the Gaymar cooling device due to anal atresia. We did not routinely connect to different devices to verify the measurement. If the rectal temperature differed from any other core measure (esophageal, bladder) by more than 1°C, the staff was instructed to re-check the location of the rectal probe, and the rectal probe in correct position was used to titrate the protocol interventions. Patients were cooled by a standard protocol (Figure 1 and 2) to a goal rectal temperature of 32–34°C (Induction Phase [I]), maintained at 32–34°C for 24 hours from induction initiation (Maintenance Phase [M]), and then rewarmed to 36.5°C (Rewarming Phase [R]) using a standardized protocol. Temperatures were documented from all sites every 30 minutes during I, M and R. Time (T) 0 was defined as the initiation of the standardized cooling protocol.
Figure 1.
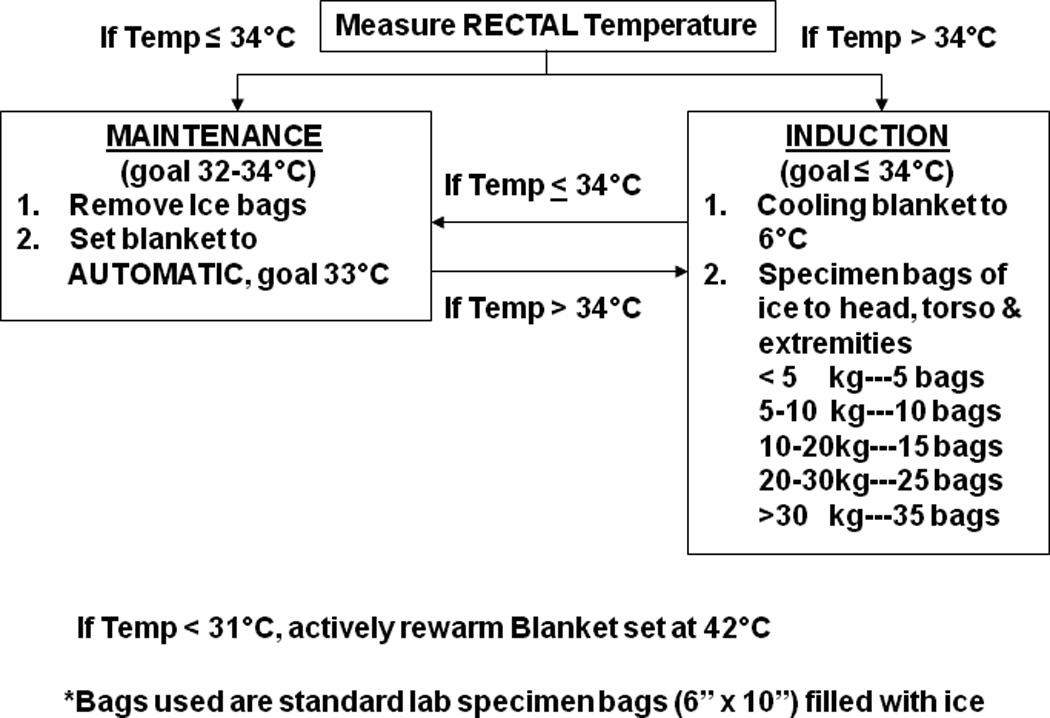
Induction and Maintenance Cooling Protocol from T= 0 to T=24 C: Celsius, kg: kilograms
Figure 2.
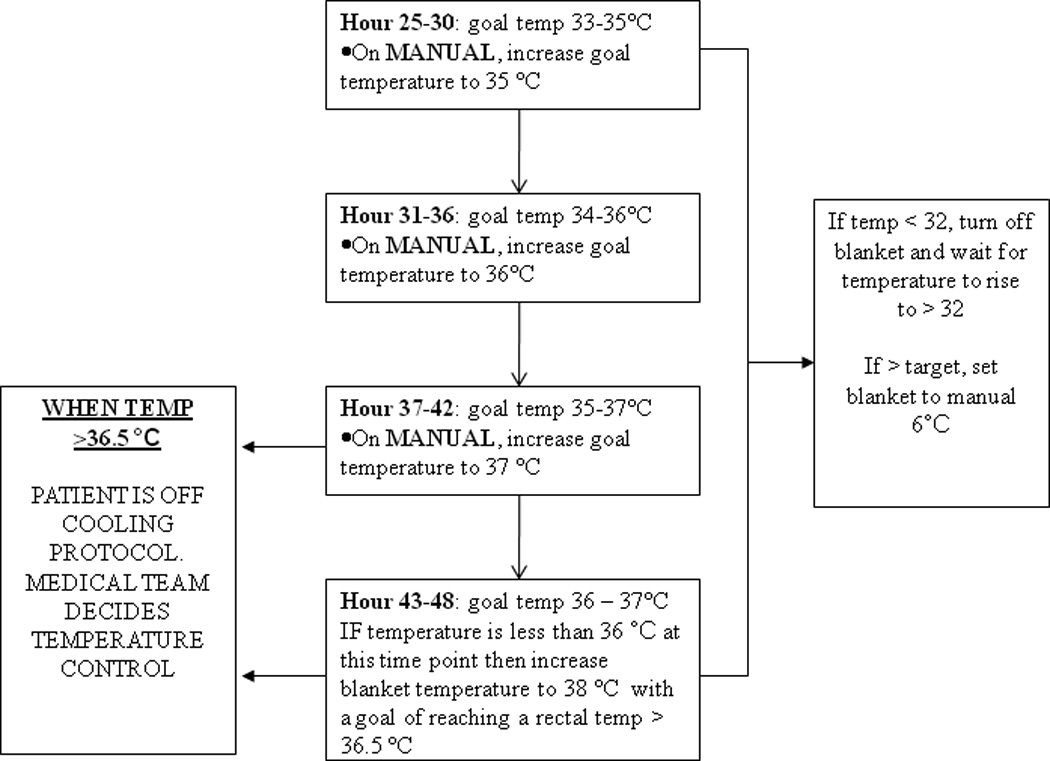
Standardized rewarming protocol from T= 24 up to T= 48. C: Celsius, kg: kilograms
Induction was initiated using surface cooling with 6 inch × 10 inch plastic bags filled with ice placed around the head, axilla, groin and extremities separated from the patient by a single layer of a light cloth blanket and a servo-controlled cooling blanket placed beneath the patient and directly on the skin (Gaymar Medi-therm II Model MTA-5900.) The number of ice bags used was calculated based on patient body weight as shown in Figure 1. Pediatric ( Gaymar # 9177) and adult size blankets (Gaymar# 9176) were utilized and blanket size was chosen so that as much of the patient as possible was on the blanket. Ice cold fluid boluses were not employed. During induction, the cooling blanket was set to the manual mode 6°C until the rectal temperature was 34°C, then to the automatic mode 33°C. If patients were ≤34°C on admission, the blanket was set to the automatic mode at 33°C (i.e., treated as maintenance phase). A prospectively designated rescue and titration protocol was utilized (Figure 1).
Standardized controlled rewarming began at T=24 hours (24 hours after cooling initiation) with a target to rewarm to 36.5°C rectal temperature up to 24 hours (Figure 2). The blanket, in the manual mode, was increased by 1°C every 6 hours. We switched from automatic mode to manual mode during rewarming so patients would passively rewarm. In our previous experience when patients were rewarmed in the automatic mode, the blanket temperature would often increase to 42°C with increased risk of overshoot hyperthermia.
During maintenance, overshoot hypothermia was defined as a T <32°C and overshoot hyperthermia was defined as a T >34°C. “Excellent” protocol performance was prospectively defined as <10% of rectal T measurements outside the 32–34°C range, good as <20%, and fair as 30% of rectal T.
Post-resuscitation care decisions regarding sedation, paralysis, ventilator and hemodynamic management, and medication administration were made by the clinical team and recorded. On-call support for the cooling protocol was available 24 hours per day, 7 days per week by the therapeutic hypothermia study team, a group of pediatric intensivists. In addition to ad hoc access by the clinical team, a member of the study team contacted the bedside nurse daily to answer questions and offer support.
Neurologic outcome was measured according to Pediatric Utstein Guidelines 13 using the Pediatric Cerebral Performance Category (PCPC). A non-blinded, trained critical care physician assigned the PCPC retrospectively based upon documentation of patient condition on admission prior to arrest and at discharge. The PCPC is a standard validated six-point scale categorizing degrees of functional impairment with 1 representing normal cerebral performance and 6 representing brain death.14 Favorable neurologic outcome was defined a priori as a discharge PCPC of 1 or 2, or a change in the pre-arrest PCPC from the post-arrest discharge PCPC of < 2.
Known side effects of mild to moderate hypothermia, such as cold diuresis, arrhythmia, bradycardia, electrolyte abnormalities (hypokalemia), shivering, bleeding, leukopenia, thrombocytopenia, and infection were documented.15 Patients were monitored for ventricular arrhythmias. Ventricular tachycardia was defined as >3 consecutive premature ventricular contractions (PVCs). Bradycardia was defined as a heart rate < 2%ile for age.16 An “episode of hypotension” was defined as a documented systolic blood pressure < 5%ile for age.17 “Sustained hypotension” was defined as a documented systolic blood pressure < 5%ile for age for > 1 hour. “Vasopressor dependent shock” was defined as documented hypotension requiring the initiation of inotropic or vasopressor therapy. Hypokalemia was defined as a potassium <3 mEq/L; leukopenia as white blood cells <5, 000 /µL, and thrombocytopenia as platelets <100,000 / µL. Clinically significant bleeding was prospectively defined as bleeding requiring red blood cell transfusion, as determined by the treating clinical team. Infections were documented as positive cultures from trachea, urine or blood. The protocol did not require routine surveillance cultures.
Usual clinical care practices by patient and phase of cooling were documented hourly including: inotropic and vasopressor support, dextrose containing fluids, insulin infusion, ventilation modality, surfactant use, nitric oxide administration, central venous catheter and arterial catheter placement, antipyretic administration, and steroid administration. Descriptive statistics and analysis were performed using Stata 10 (College Station, Tx) and Microsoft Excel 2003. Data were not normally distributed and are presented as medians [inter-quartile ranges]. Agreement between temperature monitoring sites was determined by Bland-Altman analysis.18
Results
During the study period, 38 patients survived cardiac arrests and were admitted to the PICU, 20 patients met inclusion criteria and 100% were approached for consent. Two families declined and 5 parent/legal guardians were unavailable for consent within the eight hour time window for enrollment. Thirteen patients were enrolled. One patient was excluded from analysis secondary to clinical team decision to use an alternative cooling protocol. Twelve patients (60% of eligible cardiac arrest patients, 92 % of patients who started cooling by protocol) met criteria for analysis.
Patient Pre-arrest Characteristics and Outcomes
Patient and event characteristics are summarized in Table 1. Six patients were neurologically normal prior to their cardiac arrest. Six of 12 (50%) patients survived to discharge: 3 (25%) with a favorable neurologic outcome and 3 (25%) with an unfavorable neurologic outcome.
Table 1.
Patient and Event Characteristics.
| Study Number |
Arrest Age (years) |
Gender | Weight (kg) |
Arrest Cause |
Duration of CPR |
Arrest Location |
First Documented rhythm |
Shock | Epinephrine doses |
Discharge PCPC |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | F | 57 | asphyxia | 11 | Out-of-hospital | asystole | n/a | 1 | Dead |
| 2 | 14 | M | 70 | drug overdose | 14 | Out-of-hospital | asystole | n/a | 2 | Dead |
| 3 | 16 | M | 68 | unknown | 6 | In-hospital | asystole | n/a | 0 | Dead |
| 4 | 3.7 | F | 14 | asphyxia | 16 | Out-of-hospital | asystole | n/a | 3 | Dead |
| 5 | 0.8 | M | 8 | asphyxia | 20 | In-hospital | PEA | n/a | 7 | 3 |
| 6 | 0.2 | M | 5 | near SIDS | 47 | Out-of-hospital | asystole | n/a | 5 | 5 |
| 7 | 3.5 | M | 12 | asphyxia | 50 | Out-of-hospital | asystole to VT | 2 | 2 | Dead |
| 8 | 2.2 | F | 13 | near drowning | 5 | Out-of-hospital | asystole | n/a | 0 | 1 |
| 9 | 0.2 | M | 5 | Near SIDS | 75 | Out-of-hospital | asystole to VT | 6 | 7 | Dead |
| 10 | 0.6 | M | 10 | VT | 45 | Out-of-hospital | VT to PEA | 1 | 3 | 1 |
| 11 | 0.8 | F | 10 | asphyxia | 25 | Out-of-hospital | asystole | n/a | 2 | 5 |
| 12 | 0.4 | M | 8 | near SIDS | 2 | Out-of-hospital | unknown | n/a | 0 | 1 |
Kg: kilograms, CPR: cardiopulmonary resuscitation, VT, pulseless ventricular tachycardia, PEA, pulseless electrical activity SIDS, Sudden Infant Death Syndrome, PCPC, pediatric performance category. PCPC =1: normal, 3: moderate abnormal, 5: vegetative.
Temperature Data
Temperatures were simultaneously documented from 4 probe locations: rectal probe in all 12, axillary (skin) probe in 11, esophageal probe in 11 and bladder probe in 11.
A total of 3583 measurements were documented at 30 minute time intervals during induction, maintenance and rewarming: 999 rectal, 844 axillary, 872 esophageal and 868 bladder measurements.
Hypothermia Induction
All 12 (100%) patients were cooled to ≤34°C within 4 hours of initiation of cooling, and within 8 hours of cardiac arrest onset. Three (25%) patients had rectal T ≤34°C on admission to the pediatric intensive care unit. After excluding those 3 patients, the median time from initiation of hypothermia induction to ≤34°C was 1.5 hours [1, 1.5]. The median time from cardiac arrest onset to ≤34°C rectal was 6 hours [5, 6.5].
Hypothermia Maintenance
Table 2 summarizes individual patients’ T measurements. For all patients, 1949 measurements were obtained at 30 minute intervals from probes during this period: 531 rectal, 450 axillary, 482 esophageal and 486 bladder. During maintenance, 78% (414/531) of rectal temperatures were within goal range. Figure 3. Overshoot hypothermia (<32°C) occurred in 15% (81/531) of rectal T measurements and severe overshoot hypothermia (< 31°C) occurred in only 2% (11/531) of rectal T measurements. Overshoot hyperthermia (>34°C) occurred in 7 % (36/531) of rectal T measurements. Only 4/12 (33%) patients achieved ≥ 80% of maintenance rectal T measurements within the goal range 32–34°C. The percentage of measurements between 32–34°C per patient was 78% [68%–94%]. Figure 3
Table 2.
Individual patients time to 34°C, maintenance temperature compliance (% measurements within each temperature parameter) and time to rewarm.
| Patient number |
Initial Rectal Temperature (°C) At T=0 |
Time to 34°C from ROSC (hours) |
Time to 34°C from cooling initiation (hours) |
Overshoot hypothermia (% <32°C) |
Goal temperature (% 32 to 34°C) |
Overshoot hyperthermia (% >34°C) |
Time to rewarm- (36.5°C) (hours) |
|---|---|---|---|---|---|---|---|
| 1 | 37.8 | 6 | 2 | 22 | 67 | 11 | 10.5 |
| 2 | 34.5 | 5 | 1 | 28 | 53 | 19 | 16.5 |
| 3 | 37.9 | 8 | 4 | 32 | 78 | 0 | 16.5 |
| 4 | 34.1 | 6.5 | 1 | 0 | 100 | 0 | 15.5 |
| 5 | 31.7 | n/a | 0 | 27 | 73 | 0 | 18 |
| 6 | 33 | n/a | 0 | 0 | 96 | 4 | 21 |
| 7 | 35.9 | 4.5 | 1.5 | 13 | 72 | 15 | 13.5 |
| 8 | 37.3 | 5 | 1 | 21 | 73 | 6 | 24 |
| 9 | 33.6 | n/a | 0 | 25 | 75 | 0 | 0 |
| 10 | 34.7 | 6 | 1 | 0 | 96 | 4 | 10 |
| 11 | 36.5 | 8 | 1.5 | 7 | 93 | 0 | 20.5 |
| 12 | 34.9 | 5.5 | 1.5 | 4 | 79 | 17 | 24 |
ROSC: return of spontaneous circulation, T=time
Figure 3.
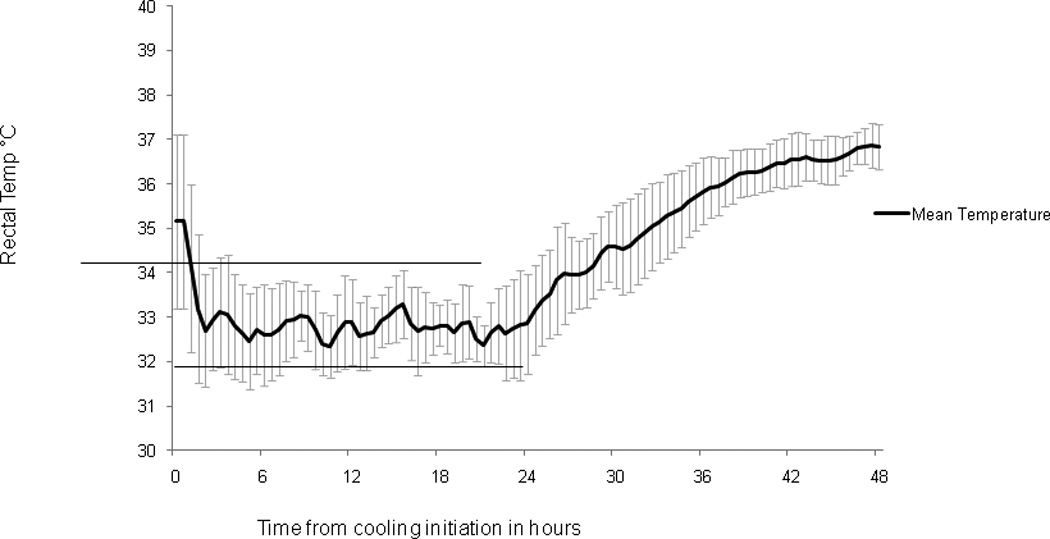
Mean (+/− standard deviation) rectal temperature during therapeutic hypothermia induction, maintenance and rewarming for all study patients. Dark line denotes mean temperatures over time. Lines at 32 and 34 degrees denote goal temperature range during maintenance of hypothermia.
Rewarming
Only 11 patients underwent rewarming as 1 patient met criteria for irreversible cessation of neurologic function during the cooling phase and was removed from the cooling protocol. Following maintenance, patients were rewarmed to 36.5°C over a median 16.5 [12.8, 20.6] hours. Table 2
Temperature Probe Comparison
During maintenance (32°C −34°C), the mean bias between rectal and esophageal probes was −0.42 °C [95%CI, −0.49 to −0.35], the upper limit of agreement was 1.13, and the lower limit of agreement was −1.97. The mean bias between rectal and bladder was 0.16 °C [95%CI, 0.11 to 0.22], the upper limit of agreement was 1.27, and the lower limit of agreement was −0.94. The mean bias between rectal and skin was 0.57 °C [95%CI, 0.47 to 0.67], the upper limit of agreement was 2.74, and the lower limit of agreement was −1.6. The mean bias between esophageal and bladder temperature probes was −0.14 [95%CI, −.48 to – 0.2]. The upper limit of agreement was 0.8 and the lower limit of agreements was −1.1. Simultaneous temperature measurements over time are visually depicted in Figure 4.
Figure 4.
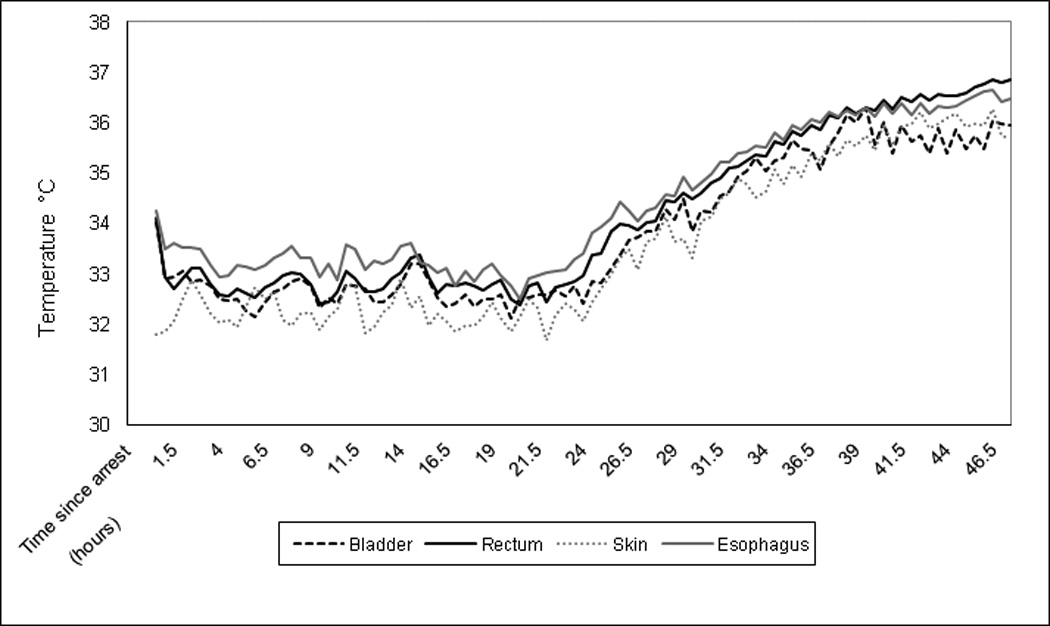
Rectal, bladder, skin and esophageal temperatures (°C) measured at each time point are graphed over the 48 hours of induction, maintenance and rewarmin
Clinician Interventions
All patients had central venous access placed before or during cooling (I or M). Ten patients had arterial lines placed: 4 preinduction, 2 during I, and 4 during M. Two patients were unable to have arterial lines placed.
All patients were mechanically ventilated for the duration of cooling and rewarming. One required high frequency oscillation for ARDS and one received surfactant. Three patients were treated with inhaled nitric oxide for hypoxemia.
Ten patients received antibiotics empirically on admission for potential infections. Four received hydrocortisone for vasopressor refractory shock and one received methylprednisolone for bronchospasm. Five patients received acetaminophen during rewarming, but no patient received ibuprofen. Three patients received furosemide: 1 during maintenance and two during rewarming.
Sedation & paralysis
Seven patients received sedating infusions (infusion for at least two hours) of fentanyl and midazolam during cooling and rewarming. Four patients received intermittent (at least one bolus dose) doses of fentanyl and/or midazolam. One patient received no sedating medications. Intermittent paralysis was administered in 75% (8/12) of patients (I: 5, M: 6, and R: 3) and continuous infusions were administered in 42% (5/12) of patients (I: 2, M; 4, and R: 5). Shivering was noted in 10 of 12 patients. One patient did not shiver and one patient received continuous paralysis for clinical illness (i.e., shivering could not be assessed). One patient received dexmedetomidine during maintenance and rewarming for sedation.
Neurologic monitoring
Eleven (92%) patients underwent prolonged EEG monitoring (>30 minutes), 1 intermittently and 10 for a total of 72 continuous hours during maintenance, rewarming and 24 hours of normothermia.19 Five patients received anticonvulsant medications for electrographic seizures. Nine patients had brain computerized tomography scans (CT) within 48 hours of their cardiac arrest. Seven had CTs prior to initiation of cooling, 1 during maintenance and 1 during rewarming. No CT had evidence of acute intracranial hemorrhage. Patients were transported to CT with temperature probes in place. No patient had an MRI during the 48 hours of the cooling and rewarming protocols.
Fluid, electrolytes & nutrition
Fluid
Net fluid balance by phase of therapeutic hypothermia was: I: −0.3 ml/kg [−1.3, 1.3], M: 67.2 ml/kg [29.4, 111], and R: 82 ml/kg [60, 88]. Urine output by phase was: I: 3.3 ml/kg/hr [2, 5.9], M: 2.6 ml/kg/hr [3.3, 4.4], and R: 2.4 ml/kg/hr [1.2, 3.9].
During cooling (I and M), 9 patients were hyperglycemic (glucose > 150 mg/dL). All nine received insulin infusions and 7 dextrose containing fluids. Four patients were hypoglycemic (glucose < 60mg/ dL), all while receiving insulin infusions and all received dextrose bolus therapy. 2 patients had a glucose < 40mg/dL. During rewarming, 8 patients received dextrose containing fluids. Seven were hyperglycemic and three were treated with insulin infusions. Four were hypoglycemic: two were being treated with insulin which was discontinued and two had increases in their IV fluids dextrose concentrations. All received bolus dose dextrose.
Electrolytes
Hypokalemia < 3 mEq/L was present in 8 patients during at least one phase: I: 3, M: 8, and R: 2. Five were treated with potassium containing IV fluids and bolus dose potassium, two with only potassium containing fluids and one with only potassium boluses. Only two patients were hypokalemic during rewarming. One was treated with potassium containing intravenous fluids and potassium bolus supplementation. None suffered life threatening arrhythmias. Hyperkalemia > 5.5 mEq/L was present in 1 patient immediately on admission and improved during induction and maintenance. There was no hyperkalemia during rewarming.
Hypomagnesemia (magnesium < 1.5 mg/dL) was observed in maintenance for 3 patients and rewarming for 2 patients. Hypocalcemia (ionized calcium < 1 mmol/L) was observed in maintenance for 3 patients and rewarming for 1 patient. Hypophosphatemia (phosphorous < 2.5 ) was observed in maintenance for 3 patients and rewarming for 1 patient mg/dL).
Nutrition
One patient received trophic (<5ml/ hour) feeds during cooling and four patients received trophic nasogastric feeds during rewarming. Eight were NPO throughout cooling and rewarming. One patient received parenteral nutrition.
Associated Events and Complications
Bradycardia defined as at least 1 documented heart rate < 2%ile for age16 was observed in 7 patients, none requiring intervention. Two patients had frequent PVCs and bigeminy; one of whom had suffered a VT arrest, was diagnosed with an underlying prolonged QT syndrome, and was treated with a lidocaine infusion. No other arrhythmias were reported. During induction, there were no documented episodes of hypotension or sustained hypotension, but 2 patients had vasopressor dependent shock. During maintenance, 1 patient had a documented episode of hypotension, 1 had sustained hypotension, and 7 had vasopressor dependent shock. During rewarming, 2 two patients had episodes of hypotension, 2 had sustained hypotension and 6 had vasopressor dependent shock.
There was no documented skin injury attributable to cooling therapy.
Leukopenia (<5, 000/µL) was documented in 6 patients, one due to leukemia prior to initiation of the standardized hypothermia protocol. Thrombocytopenia (<100, 000/µL) was noted in 4 patients, one due to leukemia prior to the initiation of the hypothermia protocol Three subjects received red blood cell transfusions during the cooling and rewarming protocol, none for acute bleeding. One patient received a platelet transfusion, 4 patients FFP transfusions, 3 patients albumin transfusions and one Hetastarch transfusion during the protocol period. There were no new documented positive blood, tracheal or urine cultures in the five days following the cooling and rewarming protocol.
There were no repeat cardiac arrests during the study protocol or escalation to extra-corporeal membrane oxygenation.
Discussion
This prospective study establishes that a standardized pediatric surface cooling protocol can successfully cool patients after cardiac arrest to goal rectal temperatures ≤34°C within 2 hours of initiation of cooling, and within 8 hours of cardiac arrest. During maintenance, 78% of rectal temperatures were within target therapeutic hypothermia range. However, only one-third of patients had ≥80% of temperature measurements in the goal range of 32°C −34°C during maintenance. Overshoot hypothermia (rectal T <32°C) was common occurring at least once in 9/12 (75%) of patients, but only 15% of measured values. Overshoot hyperthermia (rectal T >34°C) was also common occurring at least once in 7/12 (58%) patients, but only 7% of measured values. Further, the mean bias between rectal and esophageal probes was −0.42 °C [95%CI, −0.49 to −0.35], and core temperature probe sites (esophageal, rectal, and bladder) were comparable during maintenance. In this series, no severe adverse events attributable to the standardized induced therapeutic hypothermia surface cooling protocol were identified.
Induction
We rapidly induced hypothermia in the 9 patients whose temperatures were >34°C on admission. From the time of protocol initiation, patient’s core temperatures were ≤ 34°C within 2 hours. However, time to hypothermia from ROSC was limited by common barriers of transfer from outside hospitals, cranial imaging and central venous and arterial access. As a result, the median time to a rectal temperatures ≤ 34°C from ROSC was 6 hours.
To minimize side effects associated with cooling, it is important to monitor for known side effects. Surface cooling can rapidly drop patient temperature, especially when paralyzed and packed in ice. In neonatal trials, overshoot hypothermia to T< 32C occurred with induction of surface cooling.7, 10, 20 Even with intense surveillance and support, overshoot hypothermia <32C occurred in 15% of T measurements during maintenance.
Electrolyte disturbance, specifically hypokalemia in association with hyperglycemia and insulin administration, is common during induction, but can be aggressively managed to minimize the risk of arrhythmia. Both adult and neonatal randomized controlled trials showed no significant difference between treatment groups for known side effects of hypokalemia, hypoglycemia, bleeding, or ventricular fibrillation.7–8, 10, 20 Notably, bradycardia was significantly higher in the hypothermia treated populations.3, 7–8, 20 Explicit fluid and electrolyte management was not reported, and may have varied between treatment groups thus minimizing the difference in side effects. Despite this, hypokalemia is common and should be anticipated. Diuresis is associated with induction and early maintenance of therapeutic hypothermia. In this study, net fluid balance was positive 67.2 ml/kg [29.4, 111] ml/kg during induction and maintenance. This is comparable to the adult findings of net fluid balance of 3280 ml/hr [2160, 4390] during the first 24 hours of therapy.21
Preclinical data indicate that the rapid achievement of hypothermia after global cerebral ischemia improves both histologic tissue and clinical behavioral outcomes.22 Furthermore, delay in cooling may negate the benefit of hypothermia, although the exact time window for benefit is not yet known.22 A protocol that rapidly induces hypothermia is important in light of these data. 25% of patients were hypothermic on arrival, and of the other 9 patients, all but one of had a rectal T ≤34° C in <2 hours. The adult therapeutic hypothermia after cardiac arrest literature does not report a similar speed of hypothermia induction using surface cooling techniques.23 The rapidity of cooling in our study may be due to our aggressive use of bags of ice or children’s increased surface area to mass ratio facilitating conductive cooling.
Intravenous cooling has been evaluated in adults. Rapid cooling with ice cold intravenous fluids is feasible achieving rates of 1.7° C/hr. 24–27 Endovascular cooling catheters rapidly induce therapeutic hypothermia following adult cardiac arrest.28 While these methods may be rapid, use of these methods in children are concerning because of the concerns of large volume infusions leading to pulmonary edema, potential for arrhythmia when administered centrally and risk for thrombosis with large endovascular catheters in small pediatric central veins. To date, alternative intravenous methods to induce hypothermia have not been systematically evaluated in children following cardiac arrest.
Maintenance
While we achieved hypothermia quickly in this protocol, compliance of 78% during maintenance was below our a priori definition of “excellence” (>90% of rectal T values in target range), and only met our a priori definition of “fair” (>70% of rectal T values in target range) cooling compliance. Even with systematic implementation of this protocol, overshoot hypothermia was common.
Overshoot cooling to temperatures <32°C has been noted in children29–30 and adults utilizing external cooling protocols.31 Despite our prospective implementation training and available support team, we noted similar findings. To induce hypothermia we packed our subjects in ice while monitoring and controlling temperature from a rectal temperature probe. The observed overshoot hypothermia may be explained by the delay in rectal temperature change when compared to other core temperature probe sites during active cooling, 32 the cooling device servo-control algorithm, or the use of ice bags for rapid induction.
Rewarming
This protocol attempted to control the rewarming of patients by approximately 1 degree every 6 hours with the goal of minimizing rebound hyperthermia, hyperkalemia, and vasodilatation associated hypotension. Rewarming was achieved in patients over 16.5 [12.8, 20.6] hours. While many of our patients were on potassium containing fluids during this time, there was no evidence of hyperkalemia. In contrast, in one adult study a significant difference in potassium levels between normothermia and hypothermic groups was noted during rewarming with evidence of hyperkalemia in the hypothermic group.3
Temperature Comparison
We compared our unit's common core temperature probe sites. At the time of our study, usual care did not include tympanic T monitoring. While rectal temperature probes have traditionally been our standard or care, we sought to determine whether rectal, esophageal and bladder temperatures could be used interchangeably during hypothermia. For the 9 patients who underwent induction of hypothermia, there were +/− 2 degrees of difference between the rectal, bladder and esophageal probes. During induction of hypothermia, rectal and bladder temperatures can lag behind other core temperatures such as the esophageal temperature.15, 32 During maintenance, esophageal and bladder and rectal and bladder temperature probes had low mean biases indicating agreement. The rectum, although easily monitored, may not be reflective of core temperature during induction and rewarming.
Each of these monitoring locations has potential benefits and limitations. Rectal temperature probes are often displaced with care and defecation. Esophageal probes are easy to place, have minimal displacement, and are more easily observed. Finally, bladder temperature probes are easily placed, but only provide effective temperature data with adequate urine production. Notably, these probes may contain metal and may not be MRI compatible. Although tympanic membrane temperature has been shown to correlate well with brain temperature33, continuous 72 hour tympanic membrane temperature monitoring was not practical nor usual practice in our unit during the time of this study.
Retrospective Descriptive Studies of Pediatric Therapeutic Hypothermia after Cardiac Arrest
Recently, two retrospective pediatric studies of induced therapeutic hypothermia after cardiac arrest compared outcomes between normothermic and hypothermic controls. Both evaluations showed no association of outcome with induced therapeutic hypothermia after multivariable logistic regression analysis.29–30 Doherty et al. performed a multicenter retrospective cohort study across 5 centers, of which only three utilized hypothermia. 88% of the study population was children with underlying heart disease and 94% of the arrests were in-hospital. 29 In our current study, no patients had underlying heart disease and only 17% suffered in-hospital arrests.
In contrast, Fink et al. evaluated a single center’s retrospective experience with induced therapeutic hypothermia for primarily asphyxia-associated cardiac arrests. Only 8% of patients had underlying heart disease and only 9% suffered in-hospital arrests. There was no standard protocol for induction, maintenance or rewarming during this time period. While they observed no significant difference in outcome between the normothermic and hypothermic groups, it was evident that patients treated with therapeutic hypothermia had suffered more severe injury reflected in their requirement of more epinephrine doses to obtain ROSC and longer cardiac arrest duration prior to achieving ROSC.30 Both of these studies provide important assessments of the historical landscape of therapeutic hypothermia use following pediatric cardiac arrest, but are limited by their retrospective approach and lack of an explicit protocol for induction, maintenance and rewarming.
Neither retrospective descriptions, nor our current prospective single center pilot trial account for individual hospital, patient or provider variability, and thus may not be generalizable to the pediatric cardiac arrest population at large.
An effective and feasible protocol for induction, maintenance and rewarming is critical to the efficacy and safety of cooling after cardiac arrest. Induced therapeutic hypothermia to 32–34°C has been systematically evaluated in neonates with hypoxic ischemic encephalopathy and adults following cardiac arrest.3, 8, 10, 34–35 While adult studies evaluating the efficacy of therapeutic hypothermia after witnessed ventricular fibrillation cardiac arrest showed improved survival and neurologic outcomes in patients cooled to 32 to 34°C, not all patients in the hypothermic arm reached goal hypothermic temperature during the course of the study.3, 34 In addition, the “usual care” arms were often hyperthermic and not maintained in a “therapeutic normothermia” range. Effective implementation of an intervention is necessary to accurately assess the efficacy of a therapy. Our current pilot study demonstrates that therapeutic hypothermia after pediatric cardiac arrest can be feasible, relatively effective and safe for selected patients with a protocol. This data is one more preliminary study that sets the stage for a randomized, controlled trial of therapeutic hypothermia after cardiac arrest (clinicaltrials.gov identifier NCT00880087).
This study had several limitations. Our current prospective study of a standard surface cooling protocol was performed in a small, consecutive, convenience sample of patients at a single institution. Thus, small numbers prevented the ability to perform a formal safety analysis. Only 60% of eligible cardiac arrest patients (92% of eligible patients who started the cooling protocol) were enrolled which may reflect physician's indecision to cool specific patients. Intensive protocol education and monitoring were not objectively quantified, nor was the learning curve that occurs when new protocols are disseminated. The availability of an expert team to support and advise the clinical team on hypothermia therapy and answer questions 24 hours per day may limit the generalizablity of this protocol. Finally, actual brain temperature was not measured. All core temperatures monitored were surrogates for actual brain temperature.
Conclusion
Following return of a spontaneous circulation after pediatric cardiac arrest, rapid induction and maintenance of hypothermia with a standard surface cooling protocol is feasible. Core temperature sites (esophageal, rectal and bladder) were similar during maintenance. This feasibility study sets the stage for future evaluations of surface cooling management protocols to induce and maintain therapeutic hypothermia after cardiac arrest in children.
Acknowledgements
Support: Endowed Chair of Pediatric Critical Care Medicine. CTRC grant UL1-RR-024134
The nurses and staff of the Children’s Hospital of Philadelphia Pediatric Intensive Care Unit who tirelessly strove for excellence to implement and document for the purposes of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Alexis Topjian, Rebecca Ichord and Vinay Nadkarni each receive NHLBI funding as investigators for the Therapeutic Hypothermia After Cardiac Arrest study.
References
- 1.Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. Jama. 2006;295:50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 2.Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Circulation. 2009;119:1484–1491. doi: 10.1161/CIRCULATIONAHA.108.802678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 4.Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 5.2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;112:IV1–IV203. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen N, Hovdenes J, Nilsson F, et al. Outcome, timing and adverse events in therapeutic hypothermia after out-of-hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53:926–934. doi: 10.1111/j.1399-6576.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 7.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 8.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD003311.pub2. CD003311. [DOI] [PubMed] [Google Scholar]
- 10.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 11.2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: pediatric advanced life support. Pediatrics. 2006;117:e1005–e1028. doi: 10.1542/peds.2006-0346. [DOI] [PubMed] [Google Scholar]
- 12.Haque IU, Latour MC, Zaritsky AL. Pediatric critical care community survey of knowledge and attitudes toward therapeutic hypothermia in comatose children after cardiac arrest. Pediatr Crit Care Med. 2006;7:7–14. doi: 10.1097/01.pcc.0000192322.45123.80. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa) Circulation. 2004;110:3385–3397. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 14.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 15.Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med. 2009;37:1101–1120. doi: 10.1097/CCM.0b013e3181962ad5. [DOI] [PubMed] [Google Scholar]
- 16.The Harriet Lane Handbook. 13th ed. Mosby: 1993. [Google Scholar]
- 17.Part 10: pediatric advanced life support. Resuscitation. 2000;46:343–399. doi: 10.1016/s0300-9572(00)00296-3. [DOI] [PubMed] [Google Scholar]
- 18.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 19.Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009;72:1931–1940. doi: 10.1212/WNL.0b013e3181a82687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Zeiner A, Holzer M, Sterz F, et al. Mild resuscitative hypothermia to improve neurological outcome after cardiac arrest. A clinical feasibility trial Hypothermia After Cardiac Arrest (HACA) Study Group. Stroke. 2000;31:86–94. doi: 10.1161/01.str.31.1.86. [DOI] [PubMed] [Google Scholar]
- 22.Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993;21:1348–1358. doi: 10.1097/00003246-199309000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Holzer M. Devices for rapid induction of hypothermia. Eur J Anaesthesiol Suppl. 2008;42:31–38. doi: 10.1017/S0265021507003274. [DOI] [PubMed] [Google Scholar]
- 24.Bernard S, Buist M, Monteiro O, Smith K. Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation. 2003;56:9–13. doi: 10.1016/s0300-9572(02)00276-9. [DOI] [PubMed] [Google Scholar]
- 25.Virkkunen I, Yli-Hankala A, Silfvast T. Induction of therapeutic hypothermia after cardiac arrest in prehospital patients using ice-cold Ringer's solution: a pilot study. Resuscitation. 2004;62:299–302. doi: 10.1016/j.resuscitation.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Polderman KH, Rijnsburger ER, Peerdeman SM, Girbes AR. Induction of hypothermia in patients with various types of neurologic injury with use of large volumes of ice-cold intravenous fluid. Crit Care Med. 2005;33:2744–2751. doi: 10.1097/01.ccm.0000190427.88735.19. [DOI] [PubMed] [Google Scholar]
- 27.Kim F, Olsufka M, Longstreth WT, Jr, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation. 2007;115:3064–3070. doi: 10.1161/CIRCULATIONAHA.106.655480. [DOI] [PubMed] [Google Scholar]
- 28.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37:S186–S202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 29.Doherty DR, Parshuram CS, Gaboury I, et al. Hypothermia therapy after pediatric cardiac arrest. Circulation. 2009;119:1492–1500. doi: 10.1161/CIRCULATIONAHA.108.791384. [DOI] [PubMed] [Google Scholar]
- 30.Fink EL, Clark RS, Kochanek PM, Bell MJ, Watson RS. A tertiary care center's experience with therapeutic hypothermia after pediatric cardiac arrest. Pediatr Crit Care Med. 2009 doi: 10.1097/PCC.0b013e3181c58237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merchant RM, Abella BS, Peberdy MA, et al. Therapeutic hypothermia after cardiac arrest: unintentional overcooling is common using ice packs and conventional cooling blankets. Crit Care Med. 2006;34:S490–S494. doi: 10.1097/01.CCM.0000246016.28679.36. [DOI] [PubMed] [Google Scholar]
- 32.Weingart S, Mayer S, Polderman K. Rectal probe temperature lag during rapid saline induction of hypothermia after resuscitation from cardiac arrest. Resuscitation. 2009 doi: 10.1016/j.resuscitation.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Zweifler RM, Voorhees ME, Mahmood MA, Parnell M. Rectal temperature reflects tympanic temperature during mild induced hypothermia in nonintubated subjects. J Neurosurg Anesthesiol. 2004;16:232–235. doi: 10.1097/00008506-200407000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 35.Shankaran S, Laptook A, Wright LL, et al. Whole-body hypothermia for neonatal encephalopathy: animal observations as a basis for a randomized, controlled pilot study in term infants. Pediatrics. 2002;110:377–385. doi: 10.1542/peds.110.2.377. [DOI] [PubMed] [Google Scholar]


