SUMMARY
BACE1 is the sole secretase for generating β–amyloid (Aβ) in vivo and is being actively pursued as a drug target for the treatment of Alzheimer’s disease. Transmembrane BACE1 exerts its biological activity by cleaving its membrane-bound cellular substrates. Here we reveal that BACE1 directly regulates the level of membrane-anchored full length Jagged1 (Jag1), a signaling molecule important for the control of neurogenesis and astrogenesis, via interaction with its cognate Notch receptor. We show that shedding of Jag1 is reduced in BACE1-null mice and up-regulated Jag1 enhances Notch signaling via cell-cell juxtacrine interactions. Further biochemical assays confirmed that overexpression of BACE1 enhanced cleavage of Jag1. Consequently, BACE1-null mice exhibit a significant increase in astrogenesis with a corresponding decrease in neurogenesis in their hippocampi during early development. Hence, BACE1 appears to function as a signaling protease that controls the balance of neurogenesis and astrogenesis via the Jag1-Notch pathway.
Keywords: BACE1, Alzheimer’s β-secretase, α-secretase, Jagged1, ADAM17, Notch, NICD, BLBP, GFAP, astrogenesis, neurogenesis
INTRODUCTION
BACE1 is widely recognized as the β–secretase that cleaves amyloid precursor protein (APP) at the N–terminal end of the β–amyloid peptide (Aβ) (Hussain et al., 1999; Lin et al., 2000; Sinha et al., 1999; Vassar et al., 1999; Yan et al., 1999). An excessive accumulation of Aβ results in aggregation and amyloid deposition, one of the two pathological hallmarks presentin brains of Alzheimer’s disease (AD)patients (Hardy and Selkoe, 2002). AD transgenic mouse models deficient in BACE1 abrogate the production of Aβ (Cai et al., 2001; Luo et al., 2001; Roberds et al., 2001). Hence, inhibition of BACE1 enzymatic activity is being actively investigated to treat or prevent AD. Because of this significant clinical application, knowledge concerning the broad physiological functions of BACE1 in the human brain is critical for understanding the full effects of BACE1 manipulations.
BACE1-null mice have been widely utilized for this purpose. Initial reports demonstrated that BACE1-null mice are mostly viable and healthy, but recent studies show that BACE1-null mice exhibit various abnormalities including hypomyelination (Hu et al., 2006; Hu et al., 2008; Willem et al., 2006), schizophrenia-like behaviors (Savonenko et al., 2008), epileptic seizures (Hitt et al., 2010; Hu et al., 2010), and retinal pathology associated with accumulation of age pigment (Cai et al., 2012). The abolished cleavage of BACE1 substrates, such as neuregulin-1 and voltage-gated sodium channel β-subunit, may account for the aforementioned phenotypes in BACE1-null mice (Hu et al., 2006; Hu et al., 2008; Hu et al., 2010; Kim et al., 2011; Willem et al., 2006).
In this report, we show that young BACE1-null mice exhibited increased numbers of astrocytes in their hippocampi during early development. Such an increase in astrocytes relates to enhanced astrogenesis with a concomitant reduction in neurogenesis. We further demonstrate that levels of full length Jagged1 (Jag1), a signaling molecule important for the control of astrogenesis via interaction with its cognate Notch receptor, are significantly increased in BACE1-null mice. This higher level of Jag1 in turn activates the Notch pathway by inducing the expression of genes that favor differentiation of neural precursor cells to astrocytes. Our biochemical assays show that Jag1 is shed by BACE1. Hence, we provide evidence that BACE1 regulates the Jag1-Notch pathway and that BACE1 deficiency alters the balance between astrogenesis and neurogenesis during early development in the mouse hippocampus.
RESULTS
Increased astrogenesis and decreased neurogenesis in BACE1-null dentate gyrus
BACE1 is richly expressed by neurons, especially during early development. In brain, BACE1 is more enriched in the hippocampus than in the cerebral cortex (Hu et al., 2006). To determine whether BACE1 affects brain development, we examined fixed young brain tissues using antibodies specific to different cell lineages. BACE1 deficiency caused a visible increase in GFAP-positive astrocytes (Figure 1A). This increase was seen in both male and female mice and was mostly restricted to the dentate gyrus of BACE1-null mice, while the difference in other brain regions was less evident (data not shown).
Figure 1. Increased astrocytes in BACE1–null mice.
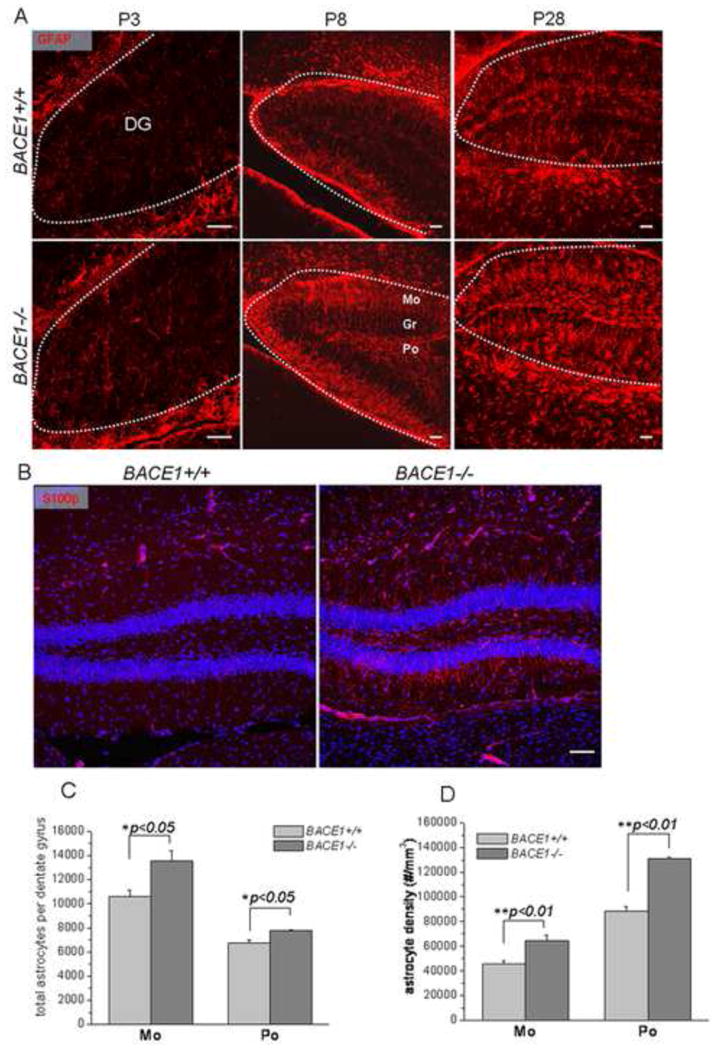
(A) Fixed brain slices were stained with antibodies specific to GFAP for radial glia and astrocytes (Smi22, A). The age of samples is specified in each panel. Apical radial processes were visibly greater in P8 BACE-null dentate gyrus (middle panel in A) while mature astrocytes were visibly greater in the polymorph (Po) and molecular (Mo) layers (middle and right panels in A). The contour of dentate gyrus is specified by the dashed line. Scale bar is 10 μm. (B) Fixed brain slices from P30 mouse brains were stained with antibodies specific to astrocytes by S100β for mature astrocytes. Astrocytic processes marked by S100β, extending from the granular layer (gr) to the molecular layer, were clearly greater in young adult BACE1-null dentate gyrus than in the wild-type controls. Nuclei were marked by TO-PRO-3. (C–D) Stereological quantification of astrocytes marked by GFAP was performed using mice at P28. The total astrocytes were calculated from the entire dentate gyrus in each mouse. The increases of astrocytes in both the molecular layer (Mo) and polymorph layer (Po) were significant (n=6 animals, * P<0.05, and ** P<0.01, two-way ANOVA).
During early developmental stages, GFAP is expressed by both radial glia and mature astrocytes, and GFAP-positive radial glia in the subgranular zone of dentate gyrus are recognized as multi-potent neural precursor cells (NPCs) (Doetsch, 2003; Malatesta et al., 2008). Depending on the environmental and developmental cues, radial glia express either neurogenic marker proteins SRY-related HMG-box gene 2 (Sox2), doublecortin and nestin or a gliogenic marker such as brain lipid binding protein (BLBP) (Kempermann et al., 2004; von Bohlen Und, 2011). With reduced nestin expression, somata of multi-potent radial glia in the subgranular zone will differentiate into astrocytes and migrate across the granular layer toward the molecular layer (Brunne et al., 2010). When reaching the border between the granular and molecular layers, radial glia begin to express the astrocytic protein S100β. After reaching the molecular layer, GFAP-positive processes in radial glia ramify and become mature astrocytes. Astrogenesis usually begins after birth and rodent astrocytes mature by about two weeks of age (Bushong et al., 2004).
We noted that the increase in GFAP-labeled cells was relatively small at P3 (Figure 1A, left panels). The increase of radial glia in the granular layer of P8 BACE1-null dentate gyrus became evident and more radio-glial apical processes were extended across the granular layer (Figure 1A, middle panels). At P28, radial glial apical processes in wild-type (wt) mice were notably less abundant in the granular layer during normal growth. However, the intensity of GFAP-positive apical processes remained abundant in BACE1-null dentate gyrus, indicating higher numbers of radial glia are present. Moreover, the number of mature astrocytes with spongiform processes, marked by GFAP antibody in the polymorph and molecular layers, was significantly greater in BACE1-null dentate gyrus. This increase of mature astrocytes in BACE1-null mice was also evident if astrocytes were labeled with S100β antibody (Figure 1B). Further stereological quantification, based on the examination of P28 mouse brains, confirmed increases in total dentate astrocytes by 28.2% in the molecular layer and 15.7% in the polymorph layer (Figure 1C; 13567.3±795.2 astrocytes per BACE1−/− molecular layer vs. 10580.9±511.6 astrocytes per wt molecular layer; 7798.5±53.2 astrocytes per BACE1−/− polymorph layer vs. 6740.8±272.5 astrocytes per wt polymorph layer; n=6 animals, P<0.05, Student’s t-test). In addition, we also calculated the astrocyte density (defined as astrocyte numbers per mm3) in the molecular and polymorph layers and found increases by approximately 41.2% and 48.1% of astrocyte density in the BACE1-null molecular and polymorph layers, respectively (Figure 1D; 64857.8±4419.2 in BACE1−/− molecular layer vs. 45972.6±2330.8 in wt molecular layer; 130901.2±1287.4 in BACE1−/− polymorph layer vs. 88353.0±3533.8 in wt polymorph layer; n=6 animals, P<0.01, Student t-test).
In contrast, the number of mature neurons, labeled with the NeuN antibody, was visibly reduced in BACE1-null mice (Figure 2A). The reduction in mature neurons in BACE1-null mice was visible at the early ages of P3 and P8. A stereological quantification using samples from P28 mice showed reductions of neurons by 24.1% in the granule layer and 30.9% in the polymorph layer (Figure 2B; 106045.7±2722.8 neurons per BACE1−/− granular layer vs. 139648.2±5046.7 neurons per granular layer of wt mice; 5999.0±290.8 neurons per BACE1−/− polymorph layer vs. 8676.8±406.7 neurons per polymorph layer of wt, n=6 animals, P<0.01).
Figure 2. Decreased number of mature neurons in BACE1–null mice.
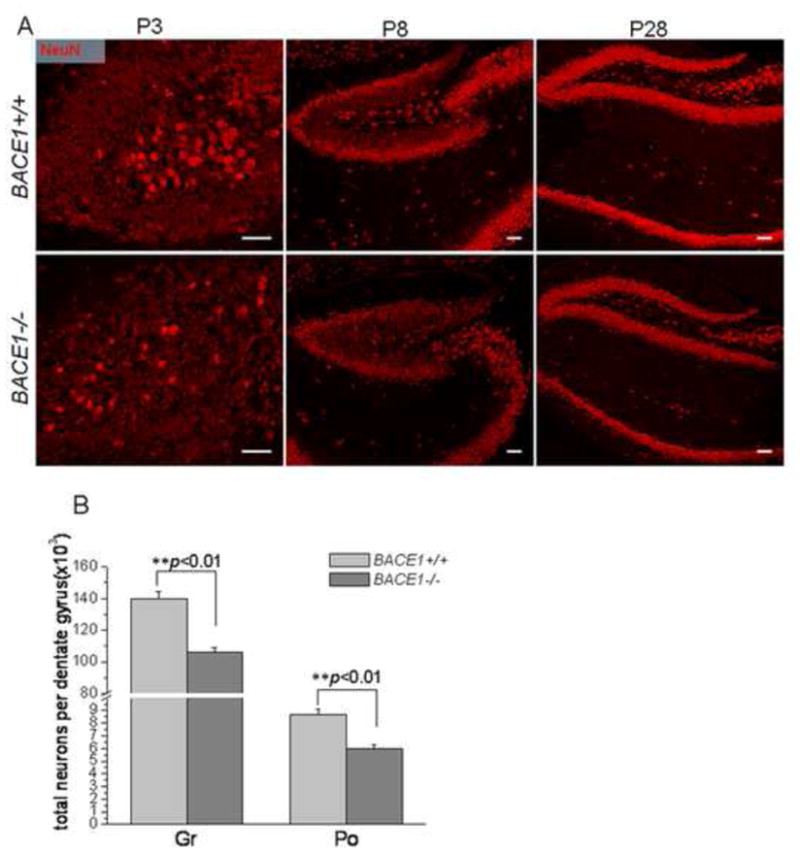
(A) Fixed brain slices from the indicated age of mouse brains were stained with antibodies specific to NeuN. The number of mature neurons in BACE1-null dentate gyrus was visibly smaller than in the wild-type controls. (B) Stereological quantification of total mature neurons marked by NeuN was performed using mice at P28. The reductions of neuronal number in the granular layer (Gr) and polymorph layer are displayed in separate bars (n=6 animals, ** P<0.01, two-way ANOVA).
To determine whether the increase in astrocytes is due to a shift in the differentiation of multi-potent radial glia, we examined the expression of BLBP, which is spatially and temporally regulated during radial glial proliferation and differentiation (Feng et al., 1994). We found that BLBP-labeled radial glia in subgraular zones of BACE1-null mice were significantly increased (Figure S1). BLBP and GFAP double-positive radial glia, recognizable by triangularly-shaped somata (specified by circles in Figure 3A), were more readily identified in the BACE1-null granular layer of dentate gyrus than in the same region of wt mice. Quantification of BLBP and GFAP double-positive radial glia confirmed an increase by 54.4% in P8 and 91.2% in P20 BACE1-null mice, respectively (Figure 3B; P8: 4468.8±347.9 radial glia per BACE1−/− dentate gyrus vs. 2893.8±238.5 radial glia per wt dentate gyrus; P20: 3761.7±305.8 radial glia per BACE1−/− dentate gyrus vs. 1967.2±202.7 radial glia per wt dentate gyrus; n=5 animals, P<0.01). Moreover, the increased BLBP-positive cells in BACE1-null brain sections were also reflected by increased protein levels of BLBP in those mice (Figure 3C). Although BLBP protein levels in mouse hippocampi were developmentally reduced from P3 to P20 in parallel with the declined differentiation of BLBP-positive radial glia into astrocytes (Brunne et al., 2010), BLBP protein levels in BACE1-null hippocampi in all three age groups were higher than that in the corresponding age-matched control group (Figure 3D). Together, these results suggest that more radial glia differentiate into mature astrocytes in BACE1-null mice while neurogenesis is reduced during early developmental stages.
Figure 3. Increased radial glia in BACE1-null mice.
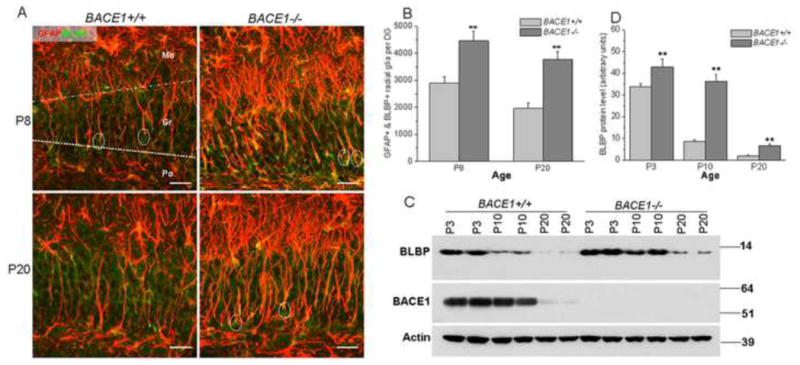
(A) Fixed brain slices were treated with antibodies to GFAP and BLBP, both being expressed by radial glia in the dentate gyrus. Somata of radial glia have a triangular shape (specified by circles) and the processes marked by both GFAP and BLBP usually projected toward the granular layer. Radial glia near the border of the granular and molecular layers usually differentiate into astrocytes, which have typical ramified processes in the molecular layers (single colored picture in Figure S1). Scale bar is 30 μm. (B) Comparison of GFAP and BLBP double positive radial glia in wt and BACE1−/− dentate gyrus was performed using a similar stereological quantification as described in Figure 1. Significantly greater numbers of total radial glia were present in BACE1-null dentate gyrus (n=5 animals, ** P<0.01, two-way ANOVA). (C) The protein level of BLBP was gradually reduced during postnatal growth. During early development, expression of BACE1 was also higher in P3 samples (compared to P20 samples). Increased protein levels of BLBP were evident in BACE1-null mice. Actin served as a loading control. (D) Bar graphs represent a relative quantification of western blots. A significant increase of BLBP was observed in all three ages of BACE1-null samples (n=3 independent experiments, ** P<0.01, two-way ANOVA).
Altered fates of NPCs in BACE1-null dentate gyrus
To determine whether increased GFAP and BLBP double positive radial glia in BACE1-null mice were due to an increase in dividing NPCs, we performed a pulse labeling experiment by intraperitoneally injecting BrdU in mice at the age of P18. After a 12 hr pulse, the total number of BrdU-positive cells was not noticeably different between the two groups in the basal region of the granular layer, and this was further confirmed by stereological quantification (Figure 4A; 7538.8±353.2 cells per wt dentate gyrus vs. 7011.3±324.0 per BACE1−/− dentate gyrus, n=5 animals). Despite similar numbers of NPCs in the subgranular zone, BrdU-positive cells in BACE1-null mice were also found in the polymorph layer and molecular layer while labeled NPCs in wt dentate gyrus were mostly restricted to the subgranular zone (Figure 4A). This observation appears unique to BACE1-null mice, which clearly indicates a differential fate in dividing NPCs.
Figure 4. Increased astrogenesis and reduced neurogenesis in BACE1-null mice.
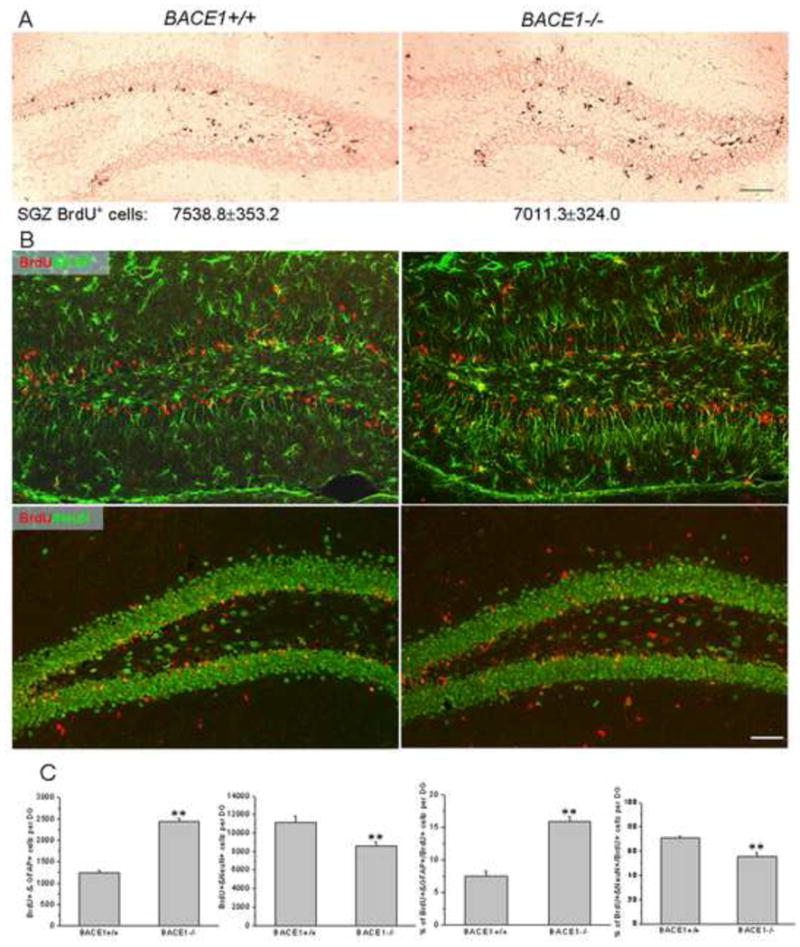
(A) A single injection of BrdU was administered to P18 mice and BrdU-incorporated dividing cells were detected by an antibody specific to BrdU by immunohistochemical staining. BrdU-positive dividing cells were mainly seen in the subgranular zone while some BrdU-positive cells were also seen in the molecular layer in BACE1-null mice. Scale bars=40μm. BrdU-positive dividing cells only in the subgranular zone were quantified from each mouse by using the stereological quantification. The number represents the total BrdU-positive cells in the basal subgranular region per dentate. The difference in total BrdU-positive cells in this region did not differ significantly (n=5 animals, Student’s t-test). (B) P11 mice were injected with BrdU once per day for five consecutive days and brains were examined at P30. The brain sections were treated with monoclonal antibodies to BrdU (red) and GFAP or NeuN. BrdU+/GFAP+-positive astrocytes were mainly in the molecular and polymorphic layers, and significantly more of these astrocytes were observed in BACE1-null dentate gyrus. BrdU+/NeuN+ -positive cells were mainly localized in the granular layer, including the subgranular zone. Scale bar = 30μm. (C) The quantified total double-positive astrocytes or neurons from each dentate gyrus are quantified. The percentages of double-positive astrocytes or neurons over the total BrdU single-positive cells are also shown in bar graphs. N=5 animals, P<0.01, Student’s t-test.
For fate determination of BrdU-positive cells, P11 mice were intraperitoneally injected with BrdU daily for five consecutive days and sacrificed at P30 for analysis. In treated wt mice, BrdU and GFAP double-positive dividing radial glia were restricted to the subgranular zone of dentate gyrus (Figure 4B, upper panels). During early developmental stages, these radial glia migrate in both directions, i.e., across the granular layer to form astrocytes with a typical morphology of mature astrocytes in the molecular layer (Brunne et al., 2010). We noted that the numbers of BrdU and GFAP double positive astrocytes were visibly greater in the BACE1-null molecular layer compared to that in the wt molecular layer (Figure 4B). Quantitative analysis further revealed that BrdU and GFAP double positive astrocytes were almost double (increased by 97.4%) in BACE1-null dentate molecular and polymorph layers compared to wt controls (Figure 4C; 2444.2±78.0 in BACE1−/− vs. 1238.3±64.4 in wt, n=5 animals, P<0.01). Moreover, BACE1 deficiency resulted in a higher percentage of dividing NPCs to differentiate into astrocytes in the dentate gyrus (Figure 4C; 15.9±0.8% in BACE1−/− vs. 7.5±0.8% in wt, n=5 animals, P<0.01).
On the other hand, significantly less numbers of multi-potent radial glia adopted the fate of neurogenesis in forming BrdU and NeuN double positive cells, which are primarily located in the granular layer in BACE1-null mice (Figure 4B, lower panels). This reduced neurogenesis was confirmed by the 23% decrease of BrdU and NeuN double –positive neurons in the BACE1-null dentate granular layer (Figure 4C; 8575.7±492.4 granule cells in BACE1−/− vs. 11134.5±749.2 granule cells in wt, n=5, P<0.01). A reduction in neurogenesis in BACE1-null brain was further evidenced by a smaller percentage of BrdU-positive NPCs differentiating into neurons compared to wt animals (Figure 4C; 55.7±3.3% in BACE1−/− vs. 70.5±1.8% in wt, n=5, P<0.01, Student’s t-test). Together, our data indicate that BACE1 deficiency favors astrogenesis by increasing the percentage of multi-potent radial glia to differentiate into astrocytes.
Increased Jagged1-Notch signaling in BACE1-null mice
To explore the molecular mechanism underlying enhanced astrogenesis in BACE1-null mice, we examined a panel of proteins important for astrogenesis. We found that Notch activation was significantly increased, as increased NICD production was evident in BACE1-null mice (Figure 5A). We also observed that the activity of Notch was greater in the wt P3 hippocampus and gradually weakened during early mouse growth, as NICD levels were lower from P3 to P10 to P20 (Figure 5A). In BACE1-null mice, NICD levels were increased in each of these three age groups compared to wt mice (Figure 5B), indicating a higher Notch signaling activity with BACE1 deficiency.
Figure 5. Altered Jag1-Notch signaling in BACE1-null mice.
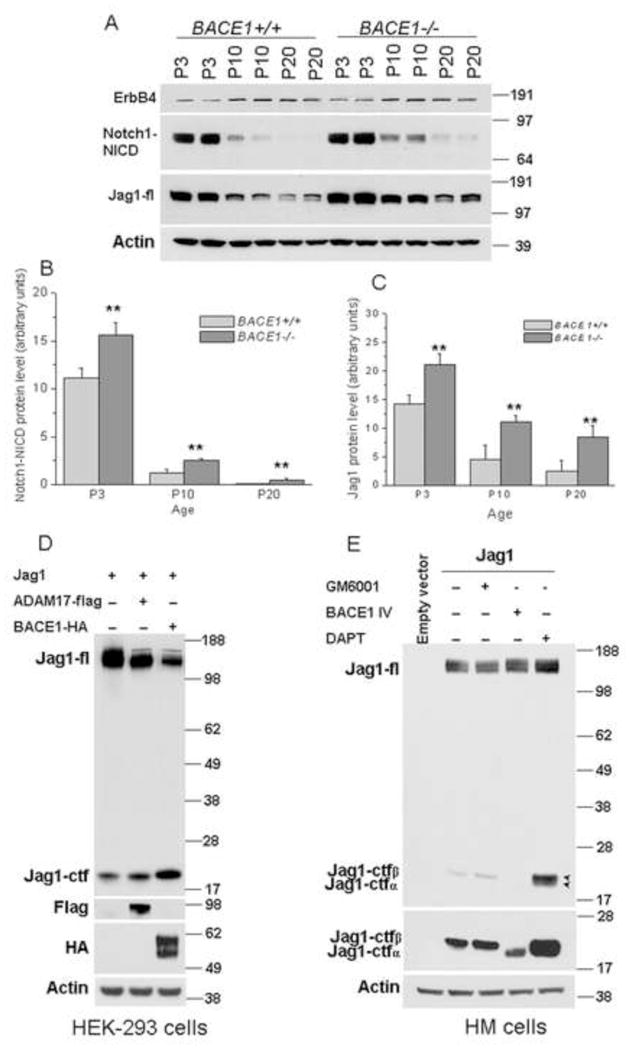
(A) Protein lysates from hippocampi were used for comparing levels of the indicated proteins. The level of NICD is developmentally regulated and increased NICD was seen in three age groups of BACE1-null mice compared to wild-type controls. Full-length Jag1 was elevated in BACE1-null mice while ErbB4 levels were not changed. (B–C) The relative protein levels of NICD and Jag1 are presented as bar graphs. Three separate experiments were performed and the results were used for quantification (** P<0.01, two-way ANOVA). (D) The Rat Jag1 expression construct was transfected with empty vector or together with BACE1 or ADAM17 in HEK-293 cells and protein lysates were examined by western blot analysis. A significant increase in the processed Jag1 C-terminal fragment (Jag1-ctf) was visible in both BACE- and ADAM17-expressing cells. (E) Jag1 was transiently transfected in HM cell line, which was generated by stably overexpressing BACE1 in HEK-293 cells. The transfected cells were then treated with BACE1 inhibitor IV, α-secretase inhibitor GM6001 or γ-secretase inhibitor DAPT. Jag1-ctfα and Jag1-ctfβ stands for α-secretase- and BACE1-cleaved fragments, respectively.
Notch activation is normally mediated by cell-cell interactions with Notch on one cell and its membrane-anchored ligand, belonging to the DSL (Delta/Serrate/Lag-2) family, on neighboring cells (Kopan and Ilagan, 2009). Interactions between the extracellular domains of DSL ligands and the Notch receptor trigger subsequent proteolytic cleavage of Notch and the release of NICD. To determine how Notch becomes more active in BACE1-null mice, we examined the levels of Serrate/Jagged 1 (Jag1) and Jagged 2 (Jag2), two main ligands expressed by hippocampal neurons during early mouse development (Stump et al., 2002). While changes in Jag2 were not evident (data not shown), protein levels of full length Jag1 were significantly elevated in BACE1-null hippocampi (Figure 5A), and this reduced processing of Jag1 was statistically significant (Figure 5C). It has been previously demonstrated that only membrane-anchored Jag1 has the capacity to mediate Notch activation via cell-cell juxtacrine signaling, and specific ectodomain shedding of Jag1 appears to down-regulate Jag1 signaling activity (Kopan and Ilagan, 2009). Hence, our data suggest an enhanced Jag1-Notch signaling activity in BACE1-null mice via reduced proteolytic processing of Jag1.
BACE1 regulates Jag1 signaling activity via proteolysis
BACE1, a type I transmembrane aspartyl protease, is predicted to shed its substrates residing on the membrane (Yan et al., 2001). We therefore examined whether Jag1 is directly cleaved by BACE1 by performing Western blot analysis on HEK-293 cells co-transfected with BACE1 and Jag1. Previously, Jag1 was shown to be shed by α-secretase in cultured cells (Ikeuchi and Sisodia, 2003; LaVoie and Selkoe, 2003), and ADAM17 appears to be active in processing Jag1 (Boyer-Di et al., 2007; Parr-Sturgess et al., 2010). We noted that overexpressed BACE1 cleaved Jag1, as production of Jag1 C-terminal fragment (Jag1-ctf) was increased in cells co-expressing BACE1 and Jag1 (Figure 5D). Similar increases in Jag1-ctf levels were also observed in cells co-expressing ADAM17 and Jag1. When Jag1 was expressed in HM cells, which stably overexpress HA-tagged BACE1, only one Jag1-ctf band was visible because of high BACE1 activity (Figure 5E). Treatment of the transfected cells with metalloprotease inhibitor GM6001 slightly increased the level of Jag1-ctf. If BACE1 activity was inhibited by BACE1 inhibitor IV, full length Jag1 was increased. Noticeably, Jag1-ctf migrated faster, suggesting increased α-secretase cleavage under this condition (shown in the long exposed condition, Figure 6E middle panel). Blocking γ-secretase activity using DAPT resulted in accumulation of two Jag1-ctf fragments in the low exposure condition (specified in arrowheads in Figure 5E). These results indicated that the slower migrating Jag1-ctf is a BACE1-cleaved product. We thus designated these two bands as Jag1-ctfα and Jag1-ctfβ.
Discussion
BACE1 was the first discovered membrane-anchored aspartyl protease in the vertebrate system; its rich expression in the neuron and in early developmental stages implies the importance of this protein in various brain functions. In this study, we showed that BACE1 effectively cleaves Jag1, a member of the DSL protein family. In BACE1-null mice, levels of full length Jag1 were significantly increased. Our functional study shows that the increased full length Jag1 shifts the balance of astrogenesis and neurogenesis. Since a proper balance between neurogenesis and gliogenesis is important for brain function, BACE1 is undoubtedly an important molecule in the control of normal brain functions.
In the developing mammalian brain, differentiation into neurons from NPCs begins at the embryonic stage while differentiation into astroglia from NPCs begins postnatally. During neonatal mouse development, the Notch signaling pathway has been implicated to control the switch of neural precursor cell (NPCs) differentiation to gliogenesis by irreversibly inhibiting neurogenesis (Ever and Gaiano, 2005; Morrison et al., 2000). Notch can be activated by interaction with a membrane-anchored ligand such as Jag1 through cell-cell juxtacrine interactions (Kopan and Ilagan, 2009). Although several transmembrane-anchored Notch ligands such as Delta, Jag1 and Jag2 can potentially activate Notch, Jag1 appears to be the predominant ligand expressed in the early developmental dentate gyrus (Stump et al., 2002). Jag1 was indeed predominantly expressed by neurons (Figure S2) and we do not yet know the contribution of other Notch ligands. Noticeably, NICD was detected in P0 and P3 nestin-positive hippocampal neurons (Figure S3), consistent with a prior report that Notch is expressed by NPCs in the early developmental hippocampus (Stump et al., 2002).
Enhanced astrogenesis in BACE1-null mice was observed in the P3-BACE1-null mouse hippocampal dentate gyrus, and this became gradually more evident, as increased GFAP-positive astrocytes in the P28 mouse dentate gyrus were greater in the molecular and polymorph layers of BACE1-null dentate gyrus compared to wt (Figure 1A). Numbers of mature astrocytes, marked by S100β antibody, were also greater in BACE1-null dentate gyrus than in wt (Figure 1B). Such an increase in astrogenesis appeared to be due to enhanced differentiation of multi-potent radial glia to adopt an astrogenesis fate. We observed that a significant portion of radial glia migrated across the granular layer of BACE1-null mice and that this migration was significantly greater than in wt mice (Figure 2 and Figure S1). We also quantified diving radial glia neural stem cells (one section from every ten sections, a total of six sections from each animal). Notably, BrdU-positive dividing cells in wt dentate gyrus were mostly localized in the subgranular zone. We found that the number of BrdU-positive cells after a 12-hr pulse was slightly greater in BACE1-null mice than in their wt littermates. Quantification showed that this increase was mainly in the molecular layer and polymorphic layer because the total radial glia neural stem cells in subgranular zone were not changed significantly based on our stereological quantification shown in Figure 4A–B. In the molecular or polymorph layer, our BrdU-labeling results showed that these astrocytes express GFAP (Figure 4C), S100β or Adlh1 (data not shown), confirming that mature astrocytes are differentiated from radial glia. On the other hand, BrdU and NeuN-double positive mature neurons were mainly localized in the granular layer (Figure 4E), indicating that these BrdU-positive radial glia in the subgranular zone would predominantly form granule cells. Hence, enhanced astrogenesis apparently reduces neurogenesis in BACE1-null mice, presumably due to lateral inhibition of neurogenesis due to enhanced NICD levels.
In the P3 BACE1-null dentate gyrus, the numbers of mature neurons marked by NeuN antibody in the entire dentate gyrus were clearly smaller than in wt controls (Figure 2A). Although the gap was narrowed by P28, the reduction of neurogenesis never recovered to normal levels. Hence, we provide evidence that BACE1 controls the balance of hippocampal astrogenesis and neurogenesis and that increased astrogenesis can impair normal neurogenesis.
Our biochemical assays indicate that Jag1 is a cellular substrate of BACE1 as cleavage of Jag1 by BACE1 is reduced when BACE1 is inhibited (Figure 5). Correspondingly, enhancing cleavage of Jag1 is seen upon BACE1 overexpression. On the Western blot, it appeared that BACE1- and ADAM17-cleaved Jag1 C-terminal fragments migrate almost identically. Our recent in vitro assays have further confirmed that the cleavage sites of Jag1 by ADAM17 and BACE1 are indeed in close proximity (data not shown). Similarly, BACE1- and ADAM10- cleaved Neuregulin-1 products, separated by only eight amino acids, are also not readily separable (Luo et al., 2011). Additional mutagenesis studies and in vitro mapping experiments are being conducted for further validation. During early development, both BACE1 (various prior publications) and Jag1 (Figure S2) are expressed by neurons. BACE1 deficiency clearly causes reduced shedding of Jag1 by enhancing full length Jag1 levels (Figure 5). In turn, the up-regulated Jag1 signaling will activate Notch, which is expressed by radial glial cells to release NICD; NICD was found to co-exist with nestin in neural stem cells during early development (Figure S3). Hence, our data demonstrate that BACE1 functions as a signaling protease that controls the Jag1-Notch signaling pathway.
In summary, BACE1 exerts its physiological functions via the regulated processing of its cellular substrates. In this study we show that the Jag1-Notch signaling pathway is regulated by BACE1 activity. Since BACE1 is one of the most promising drug targets for Alzheimer’s therapy, it is logical to ask whether BACE1 inhibition will affect neurogenesis. Despite the importance of BACE1 in balancing astrogenesis and neurogenesis, the Jag1-Notch pathway is more active during early developmental stages and less active in the adult; BACE1 activity is significantly higher in early development than in adult (Figure 3C). Increased astrocytes during early developmental stages are expected to have long-lasting effects on brain functions. It is known that astrocytes, the most abundant cell type in the brain, regulate synaptic function and plasticity by close association with synapses (Clarke and Barres, 2013). While it is unclear whether an increased number of astrocytes in the dentate gyrus will significantly affect synaptic function, reduced neurogenesis potentially affects neuronal functions. BACE1-null mice display both impaired long term depression and a specific deficit in mossy fiber long term potentiation (Wang et al., 2008) as well as epileptic seizures (Hu et al., 2010). We postulate that these deficits are related to the altered neurogenesis and astrogenesis in BACE1-null mice. Looking ahead, it will be important to determine whether the inhibition of BACE1 will have a significant effect in the adult on the hippocampal functions that are associated with the altered astrogenesis and neurogenesis observed in this study.
EXPERIMENTAL PROCEDURES
Animals
BACE1-null mice
Generation of BACE1–null mice was described by Cai et al. (Cai et al., 2001), and the colonies have gone through at least 10 congenic breedings with C57Bl6 mice. The primers (HC69: AGGCAGCTTTGTGGAGATGGTG; HC70: CGGAAATCGGAAAGGCTACTCC; and HC77: TGGATGTGGAATGTGTGCGAG) were used to genotype BACE1–null mice (Cai et al., 2001). The primer pair (TGAAAAGCTGCACCTCATTG and CTGGTTGACCTGAGCTGTGA) was used to amplify a DIG-labeled 544 bp DNA fragment for Southern blotting (Roche Applied Science), which was used to ensure complete deletion of BACE1 in BACE1–null mice. All experimental protocols were approved by the Animal Care and Use Committee at the Cleveland Clinic in compliance with the guidelines established by the public Health Service Guide for the Care.
Cell proliferation and differentiation analyses
To analyze neural differentiation, 15mg/kg of BrdU was injected once daily for 5 d beginning at P11 (4 wt vs. 4 BACE1-null mice), and the animals were sacrificed after an additional 14 d and brain sections were examined with double-labeling by using primary antibodies against BrdU and SMI22 (clones: Mab1B4, Mab2E1 and Mab4A11; Sternberger) or BrdU and NeuN (clone: MAB377; Chemicon). Labeled cells were quantified by stereology.
Immunofluorescent confocal microscopy
Confocal experiments were performed according to standard methods as previously described (He et al., 2004). After transcardial perfusion with 4% paraformaldehyde, the mouse brain was surgically removed and immersed in 20% sucrose overnight at 4°C. Brains were sagittally cut into 16μm–thick sections on a freezing microtome (Microm GmbH, Walldorf, Germany). Sections were permeabilized with 0.3% Triton X–100 for 30 min. After being rinsed in PBS three times to remove the detergent, the sections were heated by microwave in 0.05 M citrate–buffered saline (pH 6.0) for 5 min, blocked with 5% normal goat serum, and incubated with individual primary antibodies at the following dilutions: SMI22 (1:1000), S100β (1:500; Sigma), Aldh1L1 (1:3; clone: N103/39; NeuroMab), BLBP (1;1000; Millipore), and NeuN (1:1000). After washing with PBS three times, sections were incubated with secondary antibodies conjugated with Alexa fluor 488 or Alexa fluor 568 (Molecular Probes).
Stereological quantification
Volume of the Dentate Gyrus
Serial 20-μm frozen coronal sections were cut through the rostrocaudal extent of the hippocampus. The volume of the dentate gyrus was evaluated on every tenth section through the rostrocaudal extent of the dentate gyrus. After Nissl staining, the whole hippocampal image was captured by a Leica DMR microscope with a QImaging Retiga-2000R CCD camera, using a 2.5x objective to take P28 mice hippocampal images. The volumes of the molecular layer, granular layer and polymorph layer of the dentate gyrus were measured using Cavalieri’s direct estimator (Gundersen et al., 1988b). Briefly, the area of each layer in the dentate gyrus was measured (μm2) using Image J software, according to the boundary criteria of Franklin & Paxinos (1997) for the mouse. The total volume of each layer in the dentate gyrus was calculated from V= ΣA×T×10, where ΣA is the sum of the layer area, T is the section thickness and 10 is the periodicity of the section sample.
Cell Number in the Dentate Gyrus
Serial sections through the rostrocaudal extent of the dentate gyrus were selected at 10-section intervals for immunoflorescent staining with the primary antibody anti-NeuN or -GFAP and counter-staining with TO-PRO-3 to mark nuclei. For neural differentiation experiments, the selected sections were double immunoflorescently stained with NeuN/BrdU and GFAP/BrdU. Cells in different layers of the dentate gyrus were captured by a Leica SP5 Confocal Microscope using a 40x oil objective. Images were collected by z-series through a distance of 10 μm in 2 μm steps beginning at 2 μm from the section surface. Cells with labeled nuclei were counted using Image J software. The number (NV) of specific labeled cells (NeuN-, GFAP-, BrdU-, NeuN/BrdU-, and GFAP/BrdU-positive cells) was determined in each layer of the dentate gyrus using the optical disector method (Gundersen et al., 1988a). Nv= ΣQ−/ΣVDis, where ΣQ− is the sum of the cells counted and ΣVDis is the sum of the disector volumes. The disector volume was calculated from VDis=ADis×h, where ADis is the area of the counting layer, and h is the disector height (10 μm, the z-axis length of focus). The total cell number (N) in each layer of the dentate gyrus was calculated from N= NV×V.
Proteolytic cleavage of Jag1
For cultured cells, rat Jag1 expression construct (generous gift from the lab of Dr. Raphael Kopan) was transfected together with an empty vector, BACE1 or ADAM17 expression construct in HEK-293 cells for 24–48 hrs. ADAM17 expression construct (pRK5F-TACE; #31713) was obtained from Addgene (Liu et al., 2009). Protein lysates were prepared according to standard procedures and equal amounts of protein were used for western blotting analysis with primary antibodies (BLBP, 1:1000; BACE1, 1:1000; Cleaved Notch1, 1:500, Cell Signaling; ErbB4, 1:1000, Upstate; Jagged1, 1:200, Santa Cruz; HA antibody, 1:1000, Roche; both anti-actin in 1:5000 and anit-Flag in 1:1000, Sigma).
Supplementary Material
Figure S1. Increased BLBP-labeled radial glia in BACE1-null mice. Fixed brain slices dissected from the indicated age mouse were stained with antibodies to BLBP, a protein largely expressed by radial glia in the dentate gyrus. Significantly more radial glia are seen in BACE1-null subgranular layer. Mo stands for the molecular layer; Po stands for polymorph layer; Gr stands for granular layer. Scale bars=30μm.
Figure S2: Expression of Jag1 by hippocampal neurons. To determine the expression of Jag1 in developmental mouse brains, we incubated fixed brain sections from postnatal day 8 wild type mice with antibody specific to Jag1. It was clear that Jag1 is expressed by neurons, which were marked by the NeuN antibody.
Figure S3: Notch is activated by neural stem cells. To examine cell types that showed activation of Notch signaling pathway, we stained fixed brain sections from new born (P0) and postnatal day three (p3) mice (BACE1 +/+ and BACE1 −/−) with antibodies specific to NICD for activated Notch and nestin for neural stem cells. NICD appears mainly in cells that are also nestin-positive (higher magnification shown in insets). Consistent with the declined NICD levels during mouse growth (see western in Figure 2), NICD-positive neural stem cells were visibly less in P3 than in P0 brain sections, consistent with the developmental neurogenesis.
Highlights.
BACE1 control the balance of neurogenesis and astrogenesis;
Reduced neurogenesis is seen in BACE1-null dentate gyrus;
Increased Notch signaling correlates with increased astrogenesis;
BACE1 directly cleaves Jag1and regulates its signaling activity.
Acknowledgments
We would like to thank Drs. Hoonkyo Suh and Jeremy Rich (Cleveland Clinic Stem Cell Department) for discussions on neurogenesis during this study and Dr. Chris Nelson for critical reading of the manuscript. We are very grateful to Drs. Raphael Kopan and Mary Blandford (Washington University) for providing us with rat Jag1 expression construct. Plasmid pRK5F-TACE, deposited by Dr. Rik Derynck, was purchased from Addgene. This work is partially supported by NIH grants to RY (NS074256 and AG025493).
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Boyer-Di PJ, Wright-Crosnier C, Groyer-Picard MT, Driancourt C, Beau I, Hadchouel M, Meunier-Rotival M. Biological function of mutant forms of JAGGED1 proteins in Alagille syndrome: inhibitory effect on Notch signaling. Hum Mol Genet. 2007;16:2683–2692. doi: 10.1093/hmg/ddm222. [DOI] [PubMed] [Google Scholar]
- Brunne B, Zhao S, Derouiche A, Herz J, May P, Frotscher M, Bock HH. Origin, maturation, and astroglial transformation of secondary radial glial cells in the developing dentate gyrus. Glia. 2010;58:1553–1569. doi: 10.1002/glia.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Ellisman MH. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int J Dev Neurosci. 2004;22:73–86. doi: 10.1016/j.ijdevneu.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- Cai J, Qi X, Kociok N, Skosyrski S, Emilio A, Ruan Q, Han S, Liu L, Chen Z, Bowes RC, Golde T, Grant MB, Saftig P, Serneels L, De SB, Joussen AM, Boulton ME. beta-Secretase (BACE1) inhibition causes retinal pathology by vascular dysregulation and accumulation of age pigment. EMBO Mol Med. 2012;4:980–991. doi: 10.1002/emmm.201101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Ever L, Gaiano N. Radial ‘glial’ progenitors: neurogenesis and signaling. Curr Opin Neurobiol. 2005;15:29–33. doi: 10.1016/j.conb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Feng L, Hatten ME, Heintz N. Brain lipid-binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron. 1994;12:895–908. doi: 10.1016/0896-6273(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988a;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988b;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- He W, Lu Y, Qahwash I, Hu XY, Chang A, Yan R. Reticulon family members modulate BACE1 activity and amyloid-beta peptide generation. Nat Med. 2004;10:959–965. doi: 10.1038/nm1088. [DOI] [PubMed] [Google Scholar]
- Hitt BD, Jaramillo TC, Chetkovich DM, Vassar R. BACE1−/− mice exhibit seizure activity that does not correlate with sodium channel level or axonal localization. Mol Neurodegener. 2010;5:31. doi: 10.1186/1750-1326-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, He W, Diaconu C, Tang X, Kidd GJ, Macklin WB, Trapp BD, Yan R. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 2008;22:2970–2980. doi: 10.1096/fj.08-106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- Hu X, Zhou X, He W, Yang J, Xiong W, Wong P, Wilson CG, Yan R. BACE1 deficiency causes altered neuronal activity and neurodegeneration. J Neurosci. 2010;30:8819–8829. doi: 10.1523/JNEUROSCI.1334-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain I, Powell D, Howlett DR, Tew DG, Meek TD, Chapman C, Gloger IS, Murphy KE, Southan CD, Ryan DM, Smith TS, Simmons DL, Walsh FS, Dingwall C, Christie G. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol Cell Neurosci. 1999;14:419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- Ikeuchi T, Sisodia SS. The Notch ligands, Delta1 and Jagged2, are substrates for presenilin-dependent “gamma-secretase” cleavage. J Biol Chem. 2003;278:7751–7754. doi: 10.1074/jbc.C200711200. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kim DY, Gersbacher MT, Inquimbert P, Kovacs DM. Reduced sodium channel Na(v)1.1 levels in BACE1-null mice. J Biol Chem. 2011;286:8106–8116. doi: 10.1074/jbc.M110.134692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie MJ, Selkoe DJ. The Notch ligands, Jagged and Delta, are sequentially processed by alpha-secretase and presenilin/gamma-secretase and release signaling fragments. J Biol Chem. 2003;278:34427–34437. doi: 10.1074/jbc.M302659200. [DOI] [PubMed] [Google Scholar]
- Lin X, Koelsch G, Wu S, Downs D, Dashti A, Tang J. Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc Natl Acad Sci U S A. 2000;97:1456–1460. doi: 10.1073/pnas.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Xu P, Lamouille S, Xu J, Derynck R. TACE-mediated ectodomain shedding of the type I TGF-beta receptor downregulates TGF-beta signaling. Mol Cell. 2009;35:26–36. doi: 10.1016/j.molcel.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Prior M, He W, Hu X, Tang X, Sheng W, Yadav S, Kiryu-Seo S, Miller R, Trapp BD, Yan R. Cleavage of neuregulin-1 by BACE1 or ADAM10 produces differential effects on myelination. J Biol Chem. 2011 doi: 10.1074/jbc.M111.251538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, Martin L, Louis JC, Yan Q, Richards WG, Citron M, Vassar R. Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Appolloni I, Calzolari F. Radial glia and neural stem cells. Cell Tissue Res. 2008;331:165–178. doi: 10.1007/s00441-007-0481-8. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, Anderson DJ. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000;101:499–510. doi: 10.1016/s0092-8674(00)80860-0. [DOI] [PubMed] [Google Scholar]
- Parr-Sturgess CA, Rushton DJ, Parkin ET. Ectodomain shedding of the Notch ligand Jagged1 is mediated by ADAM17, but is not a lipid-raft-associated event. Biochem J. 2010;432:283–294. doi: 10.1042/BJ20100321. [DOI] [PubMed] [Google Scholar]
- Roberds SL, Anderson J, Basi G, Bienkowski MJ, Branstetter DG, Chen KS, Freedman SB, Frigon NL, Games D, Hu K, Johnson-Wood K, Kappenman KE, Kawabe TT, Kola I, Kuehn R, Lee M, Liu W, Motter R, Nichols NF, Power M, Robertson DW, Schenk D, Schoor M, Shopp GM, Shuck ME, Sinha S, Svensson KA, Tatsuno G, Tintrup H, Wijsman J, Wright S, McConlogue L. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer’s disease therapeutics. Hum Mol Genet. 2001;10:1317–1324. doi: 10.1093/hmg/10.12.1317. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci U S A. 2008;105:5585–5590. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, Zhao J, McConlogue L, John V. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- Stump G, Durrer A, Klein AL, Lutolf S, Suter U, Taylor V. Notch1 and its ligands Delta-like and Jagged are expressed and active in distinct cell populations in the postnatal mouse brain. Mech Dev. 2002;114:153–159. doi: 10.1016/s0925-4773(02)00043-6. [DOI] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- von Bohlen Und HO. Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell Tissue Res. 2011;345:1–19. doi: 10.1007/s00441-011-1196-4. [DOI] [PubMed] [Google Scholar]
- Wang H, Song L, Laird F, Wong PC, Lee HK. BACE1 knock-outs display deficits in activity-dependent potentiation of synaptic transmission at mossy fiber to CA3 synapses in the hippocampus. J Neurosci. 2008;28:8677–8681. doi: 10.1523/JNEUROSCI.2440-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratman NC, Mathews WR, Buhl AE, Carter DB, Tomasselli AG, Parodi LA, Heinrikson RL, Gurney ME. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- Yan R, Han P, Miao H, Greengard P, Xu H. The transmembrane domain of the Alzheimer’s beta-secretase (BACE1) determines its late Golgi localization and access to beta -amyloid precursor protein (APP) substrate. J Biol Chem. 2001;276:36788–36796. doi: 10.1074/jbc.M104350200. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G, editors. The Mouse Brain in Stereotaxic Coordinates. Academic Press; New York: 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Increased BLBP-labeled radial glia in BACE1-null mice. Fixed brain slices dissected from the indicated age mouse were stained with antibodies to BLBP, a protein largely expressed by radial glia in the dentate gyrus. Significantly more radial glia are seen in BACE1-null subgranular layer. Mo stands for the molecular layer; Po stands for polymorph layer; Gr stands for granular layer. Scale bars=30μm.
Figure S2: Expression of Jag1 by hippocampal neurons. To determine the expression of Jag1 in developmental mouse brains, we incubated fixed brain sections from postnatal day 8 wild type mice with antibody specific to Jag1. It was clear that Jag1 is expressed by neurons, which were marked by the NeuN antibody.
Figure S3: Notch is activated by neural stem cells. To examine cell types that showed activation of Notch signaling pathway, we stained fixed brain sections from new born (P0) and postnatal day three (p3) mice (BACE1 +/+ and BACE1 −/−) with antibodies specific to NICD for activated Notch and nestin for neural stem cells. NICD appears mainly in cells that are also nestin-positive (higher magnification shown in insets). Consistent with the declined NICD levels during mouse growth (see western in Figure 2), NICD-positive neural stem cells were visibly less in P3 than in P0 brain sections, consistent with the developmental neurogenesis.


