Abstract
Functional connectivity measures based upon low-frequency blood-oxygenation-level-dependent functional magnetic resonance imaging (BOLD fMRI) signal fluctuations have become a widely used tool for investigating spontaneous brain activity in humans. Still unknown, however, is the precise relationship between neural activity, the hemodynamic response and fluctuations in the MRI signal. Recent work from several groups had shown that correlated low-frequency fluctuations in the BOLD signal can be detected in the anesthetized rat — a first step toward elucidating this relationship. Building on this preliminary work, through this study, we demonstrate that functional connectivity observed in the rat depends strongly on the type of anesthesia used. Power spectra of spontaneous fluctuations and the cross-correlation-based connectivity maps from rats anesthetized with α-chloralose, medetomidine or isoflurane are presented using a high-temporal-resolution imaging sequence that ensures minimal contamination from physiological noise. The results show less localized correlation in rats anesthetized with isoflurane as compared with rats anesthetized with α-chloralose or medetomidine. These experiments highlight the utility of using different types of anesthesia to explore the fundamental physiological relationships of the BOLD signal and suggest that the mechanisms contributing to functional connectivity involve a complicated relationship between changes in neural activity, neurovascular coupling and vascular reactivity.
Keywords: α-Chloralose, Medetomidine, Isoflurane, BOLD signal, fMRI
1. Introduction
Biswal et al. were the first to suggest using functional magnetic resonance imaging (fMRI) to obtain functional connectivity maps as a means of probing networks in the brain without the use of an explicit stimulus or task [1]. Further studies have confirmed that the principal source of contrast in these maps arises from fluctuations in the blood-oxygenation-level-dependent (BOLD) signal [2–4], suggesting that the fluctuations reflect coordinated variations of underlying regional brain activity. Functional connectivity MRI (fcMRI) is now an integral aspect of neuroimaging research in humans and has been applied to further our understanding of the brain at a systems level for normal function (e.g., language [5] and intelligence [6]) and for psychiatric and neurological disorders [7–9].
The image acquisition for fcMRI is largely identical with task-based fMRI and thus relies on the BOLD signal, which is known to be sensitive to changes in blood flow, oxygen utilization and blood volume. The relationship between the BOLD response to sensory stimulation and the change in neural activity has been examined in a series of important experiments [10–12]. However, little work has been done on the relationship between spontaneous neural activity and the low-frequency BOLD fluctuations that form the basis for functional connectivity measurements.
One of the reasons that the physiological basis of functional connectivity is not well characterized is that most studies have been performed in human subjects, limiting physiological measurements to noninvasive techniques. Recently, interest in animal studies of functional connectivity has grown rapidly and several groups have presented preliminary work in the rodent [13–18]. The translation of this technique to the rodent has made it possible to thoroughly explore the relationship between correlated MRI signals, local metabolic and hemodynamic changes and spontaneous neural activity. An important early study by Lu et al. showed that correlated MRI signals and coherent delta band oscillations are affected in the same way by increasing anesthesia depth, implying coupling between neural activity and functional connectivity [15].
One of the drawbacks of previous functional connectivity studies in the rodent is that they were performed with relatively low temporal resolution [repetition time (TR)=1.5–2 s] without monitoring and correcting for physiological cycles [15–18]. It is well established in the human functional connectivity literature that respiratory and cardiac processes can introduce correlation unrelated to brain function into the fMRI data [19,20]. The same processes could lead to undesirable contamination of the functional connectivity maps obtained in rodents. The differences in anatomy and the use of small-bore, high-field scanners for animal studies may cause the effects of physiological noise to be different from those observed in humans, and this noise has not been characterized in rodents. To minimize the effects of physiological noise, we employed high temporal resolution (10-Hz sampling) to resolve both primary cardiac and respiratory components, which are then removed by filtering, in our study.
For the rodent, functional imaging studies are typically performed in anesthetized animals, and the choice of anesthesia is critically important. The amplitude of the BOLD response to stimulation and the optimal stimulation frequency depend upon the choice of anesthetic [12,21–23]. α-Chloralose is the most widely used anesthesia for fMRI studies in the rat, and the earliest functional connectivity studies in the rodent were also performed in α-chloralose-anesthetized animals [13,24]. Medetomidine, another candidate anesthetic, has recently gained popularity for both fMRI and functional connectivity studies because it allows nonterminal experiments and longitudinal studies [16,22]. Isoflurane also shows potential for longitudinal exams, as the BOLD response to forepaw stimulation can be detected under low levels of the anesthesia [23] and Kannurpatti et al. have shown that correlated MRI signal fluctuations can also be mapped under these conditions [17].
The variety of anesthetic and imaging protocols in use makes it difficult to compare reports of functional connectivity in the rodent. The relative localization of signal correlation, average correlation value and presence of significant correlation in specific networks (e.g., bilateral somatosensory regions, bilateral basal ganglia) may depend on the type of anesthesia used. However, the range of physiological alterations induced by these anesthetics may prove advantageous in elucidating the basis of functional connectivity. Because each anesthetic has a characteristic effect on both neural activity and hemodynamics, alterations in functional connectivity attributable to anesthesia offer a glimpse into the physiological basis of the BOLD fluctuations.
In this study, we examined the localization of and correlation between low-frequency BOLD fluctuations in the rat brain sequentially under two anesthetics, medetomidine and isoflurane. Results are compared with previously reported work with α-chloralose. High-temporal-resolution (100 ms) gradient-echo EPI data were collected to ensure adequate sampling of the primary components of the respiratory and cardiac cycles. Strong contributions from the low-frequency range were observed in the cortex, while respiratory and cardiac contributions were primarily confined to the surface and base of the brain. When the anesthesia was switched from medetomidine to isoflurane, the correlation between the filtered time courses grew less localized, suggesting that the correlations in this case may be due to generalized changes in cerebral hemodynamics. These results demonstrate that seed-based functional connectivity maps in the rodent are strongly influenced by the choice of anesthesia and indicate a need for caution in interpreting the low-frequency fluctuations as a direct reflection of neural activity.
2. Methods
2.1. Experimental methods
All experiments were conducted in compliance with guidelines set forth by the institutional animal care and use committees of Emory University. Seven adult male Sprague–Dawley rats were initially anesthetized with isoflurane anesthesia (5% for induction, 2%–3% through a nosecone for preparation). After placement in the scanner cradle, the rats were each given a bolus of medetomidine (0.05 mg/kg, sc) and isoflurane was discontinued. Fifteen minutes after bolus injection, anesthesia was continued with constant medetomidine infusion (0.1 mg/kg/h). Each rat was placed on a heated water pad to maintain rectal temperature at approximately 37°C while in the magnet. Each animal was secured in a head holder with ear bars and a bite bar to prevent head motion and strapped to a plastic cradle. Two needle electrodes were inserted beneath the skin of one forepaw to provide electrical stimulation as needed.
Rats were imaged on a 9.4-T/20-cm Bruker horizontal bore scanner using a single-shot gradient-echo EPI sequence to acquire T2*-weighted MR images. Setup included shimming, adjustments of echo spacing and symmetry and B0 compensation. The images were acquired with the following parameters: 64×64 matrix, effective echo time of 20 ms, TR of 100 ms and bandwidth of 200 kHz. A field of view of 1.92×1.92 cm2 was used, translating to in-plane resolutions of 300 µm. A 2-mm-thick coronal slice was positioned covering the bilateral primary somatosensory cortex (SI). A series of 60 images was acquired during a forepaw stimulation paradigm (9 Hz, 20 off–20 on–20 off, 4 mA) in order to identify the SI. Following the activation scan, a series of images was acquired without any stimulation. Each image series consisted of 3600 images. A second activation scan was completed to ensure a normal response, and then medetomidine was discontinued and the animal was placed on 2% isoflurane. Another scan was acquired without stimulation 15–30 min after the initiation of isoflurane.
In addition to the medetomidine and isoflurane data collected for this study, we also expanded upon our earlier work in this area [13] to include results obtained using α-chloralose anesthesia. The experimental details for the five α-chloralose-anesthetized animals were similar to those used here. The rats were intubated and artificially ventilated prior to scanning, and imaging was performed on an 11.7-T/30-cm Bruker scanner.
2.2. Analysis of stimulation data
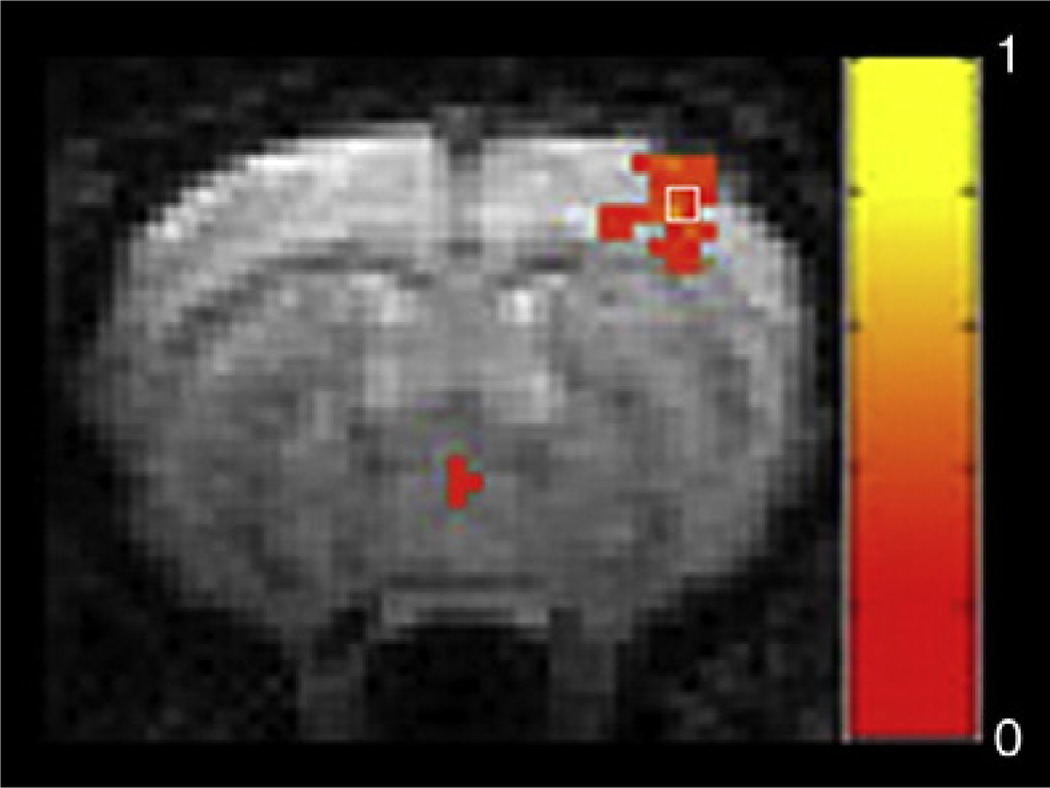
Image processing and analysis were performed in Matlab (MathWorks, Natick, MA), unless otherwise specified. For each rat, the SI was first identified from the scans in which the forepaw was stimulated. Using Matlab or STIMULATE [25], we generated a map from cross-correlation of the unfiltered image time course and a boxcar waveform representing the stimulation period. The correlation coefficient threshold was set at 0.2. Activation maps were then visually referenced in order to choose a 2×2 region of interest (ROI) within the SI that included the highest cross-correlation values. An example is shown in Fig. 1.
Fig. 1.
Map of activation acquired from one rat during forepaw stimulation after the medetomidine scan and prior to the isoflurane scan. Cross-correlation values were thresholded at 0.2 and overlaid on the first EPI image of the series. Normal activation is seen in the SI contralateral to the stimulation. The box indicates the 2×2 ROI, including the highest cross-correlation value obtained during activation, which was used as the seed for functional connectivity analysis.
2.3. Power spectral analysis
Prior to further analysis, motion correction was performed on each series of functional connectivity images with AFNI [26], using the center image of the time series as base. Each time series was inspected using STIMULATE to detect any transient effects in the imaging sequence due to nonequilibrium magnetization, and the data sets were truncated by approximately 100 images to eliminate these effects.
Power spectral density analysis was performed on the user-selected 2×2 ROIs in the SI. The four pixel time courses in each ROI were de-trended, averaged and normalized. The power spectral density estimate was calculated and plotted via Welch’s method.
Spectra from individual time series and from different locations in the brain were also examined for increased power at cardiac and respiratory frequencies. Maps were created in order to assess spatial localization of physiological noise and the low-frequency contributions. We visually examined the spectra of time courses obtained from different locations in the brain for cardiac and respiratory peaks (at approximately 4.5 and 1 Hz, respectively) and noted the frequencies of the corresponding peaks for each data set. Bandpass filtering (third-order Butterworth filter) was used to isolate the time courses corresponding to the physiological peaks. A low-pass (0–0.2 Hz) filter was used to isolate low-frequency contribution. The filtered time courses were normalized, and the temporal variance was calculated and mapped to reflect spatial localization of the contribution due to different peaks.
2.4. Functional connectivity
For functional connectivity analysis using seed-based cross-correlation, time courses were low-pass filtered. A 0.15-Hz cutoff frequency was used in order to isolate the low-frequency region of the signal in which spontaneous fluctuations have previously been observed [13]. Low-pass filtering removes signal contributions from higher-frequency physiological effects, such as respiration and heart rate. The time courses from each of the four chosen pixels in the ROI were averaged together after removal of linear trends and normalization through subtraction of the time course mean and division by the standard deviation of the time course. The resulting reference time course from the seed region was then cross-correlated with each remaining time course from the entire de-trended, normalized data set. A map of correlation coefficients was obtained and used for statistical analysis. From the seed-based correlation map, relative to the seed pixels, user-selected clusters of 4×4 pixels within the ipsilateral and contralateral SI and secondary somatosensory cortex (SII), ipsilateral and contralateral caudate putamen (CP) and contralateral SI regions of the rat brain were averaged. Histograms of all the seed-based cross-correlation coefficients in the brain were created, normalized and plotted for each anesthetic condition. The number of pixels in the bilateral 4×4 user-defined regions in the SI with cross-correlation coefficients crossing a threshold of 0.2 was recorded and compared with the number of cross-correlation coefficients exceeding the threshold in the rest of the brain to provide a measure of the specificity of the technique. The time courses from the ROIs in ipsilateral SII and CP were also used as seeds to create cross-correlation maps, and the measurements were repeated.
3. Results
All rats exhibited normal activation in the SI during stimulation of the contralateral forepaw (Fig. 1) in both the fMRI data set acquired prior to the medetomidine functional connectivity scan and the fMRI data set acquired between the medetomidine scan and the isoflurane scan.
The high temporal resolution of the functional connectivity scans obtained in this study allowed clear separation of low-frequency respiratory and cardiac peaks in the power spectra from individual voxels. The power spectra from seed regions chosen in the cortex showed structure in the low frequencies (<0.2 Hz) that was not present in the spectra of seeds chosen outside the brain. Peaks corresponding to respiration (~1 Hz) and cardiac pulsation (~4–5 Hz) could be seen in spectra from some seed regions, particularly near the sagittal sinus. Fig. 2 shows the spatial distribution of power in the respiratory range and the cardiac range. The respiratory component localizes to the surface of the brain near the sagittal sinus and to the base of the brain. The primary contribution from the cardiac cycle was observed near large vessels at the base of the brain. These results are similar to the distribution of physiological noise observed in mechanically ventilated rats anesthetized with α-chloralose [14].
Fig. 2.
Spatial distribution of the temporal variance in the respiratory (left) and cardiac (right) bands in one rat. Respiratory and cardiac components exhibit localized areas of high contribution on the surface and at the base of the brain.
Power spectra from the SI and the resulting seed-based cross-correlation maps for each anesthetic condition in individual rats are shown in Fig. 3. High power in the low-frequency range was observed in most rats under both medetomidine (row 1, bottom) and isoflurane (row 2, bottom), with the occasional appearance of a clear peak at 0.1–0.15 Hz. No consistent peak was observed above 0.2 Hz for either anesthesia. These results are in agreement with previous work performed in animals under α-chloralose, an example of which is also shown in Fig. 3 (far right).
Fig. 3.
Cross-correlation maps for a seed region in the SI thresholded at a minimum of 0.2 and overlaid on the first EPI image of the series (top figures in both rows) and power spectral density for the time course from the seed region for frequencies between 0 and 0.5 Hz (bottom figures). The top row was acquired with medetomidine anesthesia; the bottom, with isoflurane. Each column contains data from a single rat. It is clear that the extent of correlation with the seed region increases when the anesthesia is switched to isoflurane. Power spectra under both anesthetics exhibit high power in the very low frequencies, sometimes with a clear peak at 0.1 Hz. For comparison, a cross-correlation map and power spectral density plot from a rat anesthetized with α-chloralose are shown at the far right.
Most of the seed-based cross-correlation maps for the medetomidine scans show high correlation in both ipsilateral SI and contralateral SI (row 1, top). These maps qualitatively resemble the seed-based cross-correlation maps previously reported in rats anesthetized with α-chloralose. Correlation values ranged from 0.3 to 0.68 (average=0.47±0.13) in ipsilateral SI and from 0.1 to 0.53 (average=0.25±0.48) in contralateral SI. The ratio of pixels crossing the correlation threshold (0.2) in anatomically defined SI to pixels crossing the threshold in the rest of the brain was 0.15±0.06. In the isoflurane scans from the same rat using the same seed region, high correlation values are much more widespread and encompass the entire cortex, as well as much of the subcortical area (row 2, top). Correlation values ranged from 0.26 to 0.84 (average=0.56±0.19) in ipsilateral SI and from 0.19 to 0.75 (average=0.48±0.18) in contralateral SI. The ratio of pixels crossing the correlation threshold in the SI to pixels crossing the threshold in the rest of the brain was 0.05±0.008. The average correlation values for isoflurane and medetomidine data were not significantly different in ipsilateral SI but were significantly higher for isoflurane in contralateral SI (paired t test, P<.05). There was no significant difference between the numbers of pixels crossing the threshold in the SI for the two anesthetics, but the ratio of pixels inside the SI to those from the rest of the brain was significantly lower for the isoflurane scans (P<.01).
Fig. 4 shows the normalized histograms for seed-based cross-correlation values for α-chloralose, medetomidine and isoflurane scans from all rats. The α-chloralose and medetomidine distributions are similar in shape, with peak values near zero. For isoflurane, however, the distribution is broader and the mean of the distribution is shifted to toward positive values, reflecting the increased cross-correlation observed in the maps from individual rats.
Fig. 4.
Normalized histograms of the distribution of cross-correlation coefficients for rats anesthetized with α-chloralose (blue), medetomidine (red) or isoflurane (green). The distribution for α-chloralose is similar in shape to that for medetomidine but shifted slightly. The peak for both distributions is near zero. In contrast, the distribution for isoflurane is broader and the peak is shifted toward positive values.
Seed regions were chosen in the SI, SII and CP, and cross-correlation maps were created (Fig. 5). For medetomidine, high correlation values are localized to symmetrical bilateral areas and each seed region exhibits a distinctive functional connectivity map. For isoflurane, however, high correlation values are obtained throughout the cortex, and little difference in the functional connectivity map is observed for seed regions in the SI or SII. Maps from rats anesthetized with α-chloralose exhibit less localization than the medetomidine maps but more than the isoflurane maps.
Fig. 5.
Cross-correlation maps created using seeds in the SI (left column), SII (center) and CP (right) in rats anesthetized with α-chloralose (top row), medetomidine (center) or isoflurane (bottom). Lower correlation but greater localization is present in the medetomidine scans than in the isoflurane or α-chloralose scans. Cortical localization is greater in α-chloralose scans than in isoflurane scans.
Cross-correlation values between time courses from each seed region and the time courses from ipsilateral and contralateral areas were measured from 4×4 pixel regions (Fig. 6). High correlation between all pairs of regions is observed under isoflurane. Under medetomidine, correlation between bilateral areas is high but correlation between networks is reduced. A paired t test on values obtained from isoflurane and medetomidine scans in the same rat indicates significantly lower correlation between ipsilateral SI and SII and ipsilateral SII and CP during medetomidine anesthesia (P<.05). Because the α-chloralose data were obtained in a different experimental setting, the results cannot be directly compared, but the correlation values indicate relatively high connectivity between cortical areas (SI and SII) but low correlation between cortical areas and the CP.
Fig. 6.
Summary of average correlation values for seed areas in ipsilateral SI, SII or CP and ROIs in contralateral SI, ipsilateral SII, contralateral SII, ipsilateral CP and contralateral CP measured for animals under isoflurane, medetomidine or α-chloralose. Lower internetwork correlation is observed in the medetomidine scans, with paired t tests between medetomidine and isoflurane conditions obtained from the same animal indicating significant differences (P<.05) for correlation values between iSI and iSII and between iSII and iCP.
4. Discussion
In recent years, several groups have reported observations of functional connectivity in the anesthetized rat [13–18]. The rat is one of the most widely studied animals in neuroscience and has been the subject of many fMRI experiments [10,27–29], making it an excellent candidate in which to explore the physiological basis of spontaneous BOLD fluctuations. One important difference between typical human studies and rodent studies of functional connectivity is that it is usually necessary to utilize anesthesia in the rodent, both to reduce motion and to prevent stress. This study examined the effect of anesthesia on functional connectivity using sequential acquisition of data under medetomidine and isoflurane in the same rat.
4.1. Comparison with human studies
In all rats imaged for this study, bilateral patterns of connectivity were observed, particularly in the somatosensory cortex, similar to results obtained using seed-based correlation in human studies [30]. The patterns of connectivity in the anesthetized rats were also in accordance with previous rodent studies. Seed-based correlation maps for medetomidine and α-chloralose exhibited the more localized correlation seen in typical human studies, while maps created using isoflurane data showed a widespread pattern of high correlation that encompassed large regions of the cortex. This finding suggests that functional connectivity studies performed using medetomidine or α-chloralose are likely to be more comparable with functional connectivity studies performed in awake humans.
4.2. Separation of respiratory and cardiac contributions
In human studies, the TR (often on the order of 1–2 s) is typically too long to resolve the cardiac frequency, and consequently cardiac pulsation can introduce unwanted correlation. Previous studies in rodents have also employed long TRs [15–18], ignoring the possible effects of physiological cycles such as respiration and cardiac pulsation. These effects have not been studied in freely breathing rodents and could be significant due to the high magnetic field strength and large changes in susceptibility. In this study, data acquisition parameters were optimized to acquire a single slice centered over the SI while maintaining a rapid sampling rate of 100 ms. Imaging with such high temporal resolution prevents aliasing of the primary components of respiratory and cardiac contributions into the low frequencies upon which the study is focused. This is illustrated through the power spectrum estimation of the BOLD signal, in which distinct peaks occur in the low frequencies and in the cardiac and respiratory rate ranges. The primary respiratory and cardiac components are essentially eliminated by the application of the low-pass filter. Even for the high-temporal-resolution data obtained in this study, it is possible that low-frequency contributions attributable to respiratory fluctuations or the cardiovascular system are present [19,20]. These contributions are not well characterized and are difficult to pinpoint and remove. For more complete correction for physiology or for studies requiring longer TRs, synchronized recording of cardiac and respiratory cycles would enable retrospective correction techniques [31,32].
Heart rate and blood oxygenation level were measured using a pulse oximeter, and the values just prior to each functional connectivity scan were recorded. The average heart rate increased significantly when the anesthesia was changed from medetomidine to isoflurane (272.5±24.3 vs. 346.9±23.7, P<.01). After the heart rate reaches 300 bpm, the Nyquist criterion is no longer met and the primary cardiac component will be aliased. However, even at a heart rate of 7 Hz (higher than any recorded in this study), the primary aliased peak should not appear below 3 Hz and thus should not affect the low-frequency signals. Again, this statement is not applicable to other contributions from the cardiovascular system and does not include the effects of variable heart rate. Based upon the spatial distribution of the primary cardiac peak (Fig. 2), however, we expect minimal contamination from cardiac components in the SI.
The oxygenation level of the blood also changed over the course of the study (97.8±1.5 for medetomidine vs. 93.5±3.3 for isoflurane, P<.01). All animals were freely breathing, and the drop in oxygenation may be an effect of the isoflurane anesthesia. A lower oxygenation level may amplify the MRI signal changes related to spontaneous fluctuations in blood flow and metabolism, which could account for some of the high signal correlation observed in the isoflurane data.
4.3. Anesthesia-dependent changes
Anesthetics cause physiological changes that can affect the BOLD signal, including alterations in neural activity, vascular reactivity and neurovascular coupling. The anesthesia of choice for fMRI studies in the rodent has typically been α-chloralose, but because it is not suitable for longitudinal experiments, several groups have explored possible alternatives. Isoflurane is widely used for anatomical imaging in rodents and can be used for fMRI studies at very low concentrations, obtaining an average BOLD change during forepaw stimulation of around 2%–3% [23]. Isoflurane suppresses neural activity, reduces cerebral metabolism and is a strong vasodilator for the cerebrovascular system. Possibly due to the high baseline CBF induced, higher doses of isoflurane (>1 MAC) tend to suppress the BOLD response. Medetomidine is gaining in popularity as an anesthetic for rodent fMRI because it is also suitable for longitudinal studies [22]. Signal changes during forepaw stimulation are similar to those observed under isoflurane, on the order of 2%–4%, and there appears to be a wider range of acceptable anesthetic depths. Medetomidine is a selective α2-adrenergic agonist that induces bradycardia and increases blood pressure.
In the current experiments, we chose to focus on these two anesthetics because they can be used for longitudinal studies. The order of administration was not randomized. Each rat was initially anesthetized with isoflurane to facilitate handling, and then isoflurane was discontinued and the medetomidine infusion was started. After data were acquired under medetomidine, the infusion was discontinued and switched to isoflurane once more. Most of the image setup (positioning, shimming) was performed while the animal was anesthetized with medetomidine, so that by the time functional connectivity data were acquired, at least 45 min had passed since the short initial dose of isoflurane. Rats typically awoke from isoflurane anesthesia within a few minutes of its discontinuation, so it is likely that any lingering effects of the initial dose were negligible. In addition, the use of isoflurane as an inducing agent prior to further anesthetization with medetomidine or α-chloralose is a common practice for functional studies in the rodent [27–29]. We chose to acquire the medetomidine data first because it allows us to ensure that normal activation during forepaw stimulation is obtained prior to acquiring functional connectivity data. It is possible but more difficult to detect activation in response to a stimulation using isoflurane anesthesia, and results are similar to those observed with medetomidine [23].
When medetomidine is discontinued in favor of isoflurane during the second transition, there may be a lingering effect of the medetomidine, which does not clear from the animal as rapidly as isoflurane. To minimize this effect, we did not collect data under isoflurane until at least 20–30 min after the switch. A fairly high dose of isoflurane (2%) was administered, and its effects are likely to dominate any lingering effects of medetomidine.
Ideally, data would be obtained from a single rat for all three anesthetics described in this study (isoflurane, medetomidine and α-chloralose). In practice, this is difficult because the possibility of interactions from previous anesthetics complicates the interpretation of the data even more with additional experiments or requires unfeasibly long experiments to allow the complete washout of the anesthetics between data collection. In addition, α-chloralose requires the animals to be intubated and mechanically ventilated — conditions that are not necessarily ideal for the other two anesthetics. The work presented here, with the α-chloralose data acquired in a similar but not identical experimental setting, allows a preliminary examination of how these anesthetics affect the detection and measurement of functional connectivity in the rodent. It should be noted that while the α-chloralose data were acquired on an 11.7-T MRI system rather than the 9.4-T system used for the medetomidine/isoflurane studies, the higher field strength is not expected to significantly affect the power spectrum of the low-frequency BOLD signal or the correlation between time courses from different areas, and indeed, the α-chloralose data measured at 11.7 T were qualitatively similar to those obtained by Lu et al. at 9.4 T [15].
4.4. Anesthesia dependence of functional connectivity
The detection of a BOLD response to forepaw stimulation under all of the anesthetic agents used for this study indicates that some form of neurovascular coupling is preserved, even though it may differ from that of the awake animal [21–23]. While the BOLD signal is also the basis for the fluctuations used to map functional connectivity, the effects of anesthesia could be more complicated for fcMRI than for traditional fMRI studies. For example, a reduction in connectivity between two brain areas could result from a loss of synchrony in the neural activity or a reduction in the amplitude of the BOLD fluctuations due to an alteration in the neurovascular coupling. Peltier et al. have shown that the administration of sevoflurane in humans can reduce or eliminate functional connectivity [33]. However, a study by Kiviniemi et al. found that sedatives such as midazolam can increase the amplitude and synchrony of the low-frequency BOLD fluctuations [34]. The conflicting findings may be due to different mechanisms of action with dissimilar effects on neural activity or to the effect of the anesthetic on the cerebrovascular system. The relationship between spontaneous neural activity, hemodynamic variations and BOLD fluctuations is not well understood.
Anesthetics can potentially alter functional connectivity by modifying the characteristics of spontaneous neural activity (amplitude, frequency, synchrony) or by changing properties of the vasculature. While the effects of medetomidine and isoflurane on neural activity, metabolism and cerebral blood flow have not been completely explored, general trends may be extrapolated from previous studies. Neural activity is typically suppressed in a monotonic fashion with increasing doses of anesthesia. Luckl et al. reported that the doses of α-chloralose commonly used for fMRI result in higher frequencies and baseline power in the EEG as compared with 1%isoflurane [35]. A similar study found that the primary effect of medetomidine is to increase power in the lower frequencies, particularly delta and theta, without suppressing higher-frequency activity [36]. In cats, Ferron et al. showed that anesthetization with 1.5% isoflurane produces EEG patterns similar to slow wave sleep, while anesthetization with 2.5% results in periods of flat EEG punctuated by bursts of activity [37]. Based on these results, we expect that high-frequency electrical activity would be greater under medetomidine and α-chloralose than the 2% level of isoflurane used in the study. The relative contribution of higher-frequency neural activity determines the baseline energy consumption of the brain [38]. Thus, we would expect the medetomidine-anesthetized rat to be in a higher-energy state and the isoflurane-anesthetized rat to be in a lower-energy state. Previous work has shown that in lower-energy states, activity was more limited to local cortical areas, while in higher states, activity in response to a stimulus was much more widespread [38]. In this study, however, while low-frequency BOLD signal fluctuations were present under all anesthetics, the spatial distribution of correlation with time courses from seeds based in somatosensory cortex was much broader for isoflurane (presumably the lower-energy state) than for medetomidine or α-chloralose. These findings highlight both the importance of understanding anesthesia-related differences in functional connectivity studies in rodents and the potential for using anesthetic administration to probe the physiological basis of functional connectivity in future experiments.
We expected that the dose of isoflurane used in these experiments would greatly suppress or eliminate the correlation of the BOLD signal. High levels of isoflurane suppress neural activity and so should provide greater contrast between the isoflurane and the hypnotic agents, α-chloralose and medetomidine, which cause less reduction in neural activity. Despite the suppression of neural activity expected in rats under 2% isoflurane, widespread correlation was observed in the MRI signal. One possible explanation for this phenomenon is that cerebrovascular fluctuations are induced or amplified by the anesthesia. A general increase in the amplitude of cerebrovascular fluctuations could result in correlations that are less localized than in the medetomidinezr α-chloralose-anesthetized rats. A similar change in the amplitude of the fluctuations and the power spectra was previously observed in rats after exsanguination [39], which indicates that the purely vascular processes could account for the widespread correlation. Another possibility is that the vasodilation induced by the isoflurane allows the hemodynamic fluctuations linked to local neural activity to effectively spread through a larger cortical area than under medetomidine, where vasoconstriction may contribute to localization.
Low-frequency fluctuations in cerebral blood flow, cerebral blood volume and metabolic markers such as cytochrome aa3 redox have been observed with other modalities [40–42]. Because multimodality studies have yet to be performed, it is still unclear to what extent fluctuations in metabolism and blood flow are linked in the anesthetized rodent. It is plausible, for instance, that fluctuations in metabolism are tied tightly to fluctuations in local firing rates, while variations in blood flow include contributions from both neural activity and purely vascular oscillations. Because the BOLD mechanism combines cerebral blood flow, blood volume and oxygen metabolism, alterations in any of these parameters will impact the lowfrequency signal fluctuations and the relative contribution of the processes (dependent on imaging parameters, such as TR and echo time) may alter the localization of the signal. For example, the short TR used in this study to improve temporal resolution also increases sensitivity to changes in blood flow due to inflow of unsaturated spins.
The highly localized correlation patterns observed in rats under medetomidine anesthesia argue for a neural origin of the low-frequency fluctuations. It should be noted that the large standard deviation in the average contralateral value for medetomidine as compared with isoflurane may be a result of this specificity. The ROI for contralateral cortex was defined anatomically and may not completely coincide with the area of peak correlation for medetomidine. Because the location of correlation was highly specific to known bilateral networks in the medetomidine-anesthetized rats, it may be advantageous to utilize medetomidine rather than isoflurane for longitudinal functional connectivity studies. The widespread correlation observed in isoflurane data made it difficult to distinguish maps based on a seed in the SI from maps based on a seed in the SII, and patterns of connectivity for subcortical regions were in particular poorly confined to anatomical networks.
Given the growing interest in using functional connectivity in clinical studies as a noninvasive diagnostic and predictive tool, it is important to rigorously investigate the physiological basis of this phenomenon. The work described in this article shows that the spatial extent of correlation used to map functional connectivity is strongly influenced by the choice of anesthesia and suggests that anesthesia-related modulations of both cerebrovascular dynamics and neural activity influence the measurement of functional connectivity in the rodent. Further studies in the rodent will be needed to fully understand the relationship between spontaneous fluctuations in electrical activity, metabolism and hemodynamics. In particular, multimodality studies of spontaneous electrical activity and blood flow fluctuations under each anesthetic would be valuable in separating the effect of each agent on the cerebrovasculature from its effect on neural activity.
Acknowledgments
We thank the Laboratory of Functional and Molecular Imaging, National Institutes of Health, for allowing use of their 11.7-T MRI system.
References
- 1.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 2.Biswal B, Hudetz AG, Yetkin FZ, Haughton VM, Hyde JS. Hypercapnia reversibly suppresses low-frequency fluctuations in the human motor cortex during rest using echo-planar MRI. J Cereb Blood Flow Metab. 1997;17(3):301–308. doi: 10.1097/00004647-199703000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 1997;10(4–5):165–170. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Peltier SJ, Noll DC. T(2)(*) dependence of low frequency functional connectivity. Neuroimage. 2002;16(4):985–992. doi: 10.1006/nimg.2002.1141. [DOI] [PubMed] [Google Scholar]
- 5.Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, et al. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol. 2000;21(9):1636–1644. [PMC free article] [PubMed] [Google Scholar]
- 6.Song M, Zhou Y, Li J, Liu Y, Tian L, Yu C, et al. Brain spontaneous functional connectivity and intelligence. Neuroimage. 2008;41(3):1168–1176. doi: 10.1016/j.neuroimage.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 7.Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA. Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum Brain Mapp. 2008;29(11):1265–1275. doi: 10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2007;104(47):18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang K, Liang M, Wang L, Tian L, Zhang X, Li K, et al. Altered functional connectivity in early Alzheimer’s disease: a resting-state fMRI study. Hum Brain Mapp. 2007;28(10):967–978. doi: 10.1002/hbm.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinker G, Bock C, Busch E, Krep H, Hossmann KA, Hoehn-Berlage M. Simultaneous recording of evoked potentials and T2*-weighted MR images during somatosensory stimulation of rat. Magn Reson Med. 1999;41(3):469–473. doi: 10.1002/(sici)1522-2594(199903)41:3<469::aid-mrm7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 12.Huttunen JK, Grohn O, Penttonen M. Coupling between simultaneously recorded BOLD response and neuronal activity in the rat somatosensory cortex. Neuroimage. 2008;39(2):775–785. doi: 10.1016/j.neuroimage.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 13.Williams KA, Peltier S, LaConte S, Keilholz SD. MRI evidence of resting state connectivity in rodent brain. Proc Int Soc Magn Reson Med. 2006:2119. [Google Scholar]
- 14.Majeed WM, Magnuson M, Keilholz SD. Spatiotemporal dynamics of low frequency fluctuations in BOLD fMRI of the rat. J Magn Reson Imag. 2009;30:384–393. doi: 10.1002/jmri.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA, et al. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci USA. 2007;104(46):18265–18269. doi: 10.1073/pnas.0705791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao F, Zhao T, Zhou L, Wu Q, Hu X. BOLD study of stimulation-induced neural activity and resting-state connectivity in medetomidine-sedated rat. Neuroimage. 2008;39(1):248–260. doi: 10.1016/j.neuroimage.2007.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kannurpatti SS, Biswal BB, Kim YR, Rosen BR. Spatio-temporal characteristics of low-frequency BOLD signal fluctuations in isoflurane-anesthetized rat brain. Neuroimage. 2008;40(4):1738–1747. doi: 10.1016/j.neuroimage.2007.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pawela CP, Biswal BB, Cho YR, Kao DS, Li R, Jones SR, et al. Resting-state functional connectivity of the rat brain. Magn Reson Med. 2008;59(5):1021–1029. doi: 10.1002/mrm.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31(4):1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 20.Katura T, Tanaka N, Obata A, Sato H, Maki A. Quantitative evaluation of interrelations between spontaneous low-frequency oscillations in cerebral hemodynamics and systemic cardiovascular dynamics. Neuroimage. 2006;31(4):1592–1600. doi: 10.1016/j.neuroimage.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Austin VC, Blamire AM, Allers KA, Sharp T, Styles P, Matthews PM, et al. Confounding effects of anesthesia on functional activation in rodent brain: a study of halothane and alpha-chloralose anesthesia. Neuroimage. 2005;24(1):92–100. doi: 10.1016/j.neuroimage.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Weber R, Ramos-Cabrer P, Wiedermann D, van Camp N, Hoehn M. A fully noninvasive and robust experimental protocol for longitudinal fMRI studies in the rat. Neuroimage. 2006;29(4):1303–1310. doi: 10.1016/j.neuroimage.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex. 2007;17(4):942–950. doi: 10.1093/cercor/bhl005. [DOI] [PubMed] [Google Scholar]
- 24.Lu HGL, Rea W, Stein EA, Yang Y. Resting-state functional connectivity in rat brain. Proc Int Soc Magn Reson Med. 2006:532. [Google Scholar]
- 25.Strupp JP. Stimulate: a GUI based fMRI analysis software package. Neuroimage. 1996;3(3):S607. [Google Scholar]
- 26.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 27.Keilholz SD, Silva AC, Raman M, Merkle H, Koretsky AP. Functional MRI of the rodent somatosensory pathway using multislice echo planar imaging. Magn Reson Med. 2004;52(1):89–99. doi: 10.1002/mrm.20114. [DOI] [PubMed] [Google Scholar]
- 28.Keilholz SD, Silva AC, Raman M, Merkle H, Koretsky AP. BOLD and CBV-weighted functional magnetic resonance imaging of the rat somatosensory system. Magn Reson Med. 2006;55(2):316–324. doi: 10.1002/mrm.20744. [DOI] [PubMed] [Google Scholar]
- 29.Silva AC, Koretsky AP. Laminar specificity of functional MRI onset imes during somatosensory stimulation in rat. Proc Natl Acad Sci USA. 2002;99(23):15182–15187. doi: 10.1073/pnas.222561899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 31.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44(1):162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 32.Hu X, Le TH, Parrish T, Erhard P. Retrospective estimation and correction of physiological fluctuation in functional MRI. Magn Reson Med. 1995;34(2):201–212. doi: 10.1002/mrm.1910340211. [DOI] [PubMed] [Google Scholar]
- 33.Peltier SJ, Kerssens C, Hamann SB, Sebel PS, Byas-Smith M, Hu X. Functional connectivity changes with concentration of sevoflurane anesthesia. NeuroReport. 2005;16(3):285–288. doi: 10.1097/00001756-200502280-00017. [DOI] [PubMed] [Google Scholar]
- 34.Kiviniemi VJ, Haanpaa H, Kantola JH, Jauhiainen J, Vainionpaa V, Alahuhta S, et al. Midazolam sedation increases fluctuation and synchrony of the resting brain BOLD signal. Magn Reson Imaging. 2005;23(4):531–537. doi: 10.1016/j.mri.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Luckl J, Keating J, Greenberg JH. alpha-Chloralose is a suitable anesthetic for chronic focal cerebral ischemia studies in the rat: a comparative study. Brain Res. 2008;1191:157–167. doi: 10.1016/j.brainres.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jang HS, Choi HS, Lee SH, Jang KH, Lee M-G. Evaluation of the anaesthetic effects of medetomidine and ketamine in rats and their reversal with atipamezole. Vet Anaest Analgesia. 2009;36:319–327. doi: 10.1111/j.1467-2995.2009.00463.x. [DOI] [PubMed] [Google Scholar]
- 37.Ferron J-F, Kroeger D, Chever O, Amzica F. Cortical inhibition during burst suppression induced with isoflurane anesthesia. J Neuroscience. 2009;29(31):9850–9860. doi: 10.1523/JNEUROSCI.5176-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maandag NJG, Coman D, Sanganahalli BG, Herman P, Smith AJ, Blumenfeld H, et al. Energetics of neuronal signaling and fMRI activity. PNAS. 2007;104(51):20546–20551. doi: 10.1073/pnas.0709515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osol GHW. Spontaneous vasomotion in pressurized cerebral arteries from genetically hypertensive rats. Am J Physiol. 1988;254:H28–H33. doi: 10.1152/ajpheart.1988.254.1.H28. [DOI] [PubMed] [Google Scholar]
- 40.Vern BA, Leheta BJ, Juel VC, LaGuardia J, Graupe P, Schuette WH. Interhemispheric synchrony of slow oscillations of cortical blood volume and cytochrome aa3 redox state in unanesthetized rabbits. Brain Res. 1997;775(1–2):233–239. doi: 10.1016/s0006-8993(97)01028-7. [DOI] [PubMed] [Google Scholar]
- 41.Kleinfeld D, Mitra PP, Helmchen F, Denk W. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc Natl Acad Sci USA. 1998;95(26):15741–15746. doi: 10.1073/pnas.95.26.15741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayhew JEW, Askew S, Zheng Y, Porrill J, Westby GWM, Redgrave P, et al. Cerebral vasomotion: a 0.1-Hz oscillation in reflected light imaging of neural activity. NeuroImage. 1996;4(3):183–193. doi: 10.1006/nimg.1996.0069. [DOI] [PubMed] [Google Scholar]