Abstract
8-oxoguanine-DNA glycosylase (OGG-1) is a base excision DNA repair enzyme; however, its function in modulating allergic diseases remains undefined. Using OGG-1 knockout (KO) mice, we show that this protein impacts allergic airway inflammation following sensitization and challenge by ovalbumin (OVA). OGG-1 KO mice exhibited less inflammatory cell infiltration and reduced oxidative stress in the lungs after OVA challenge compared to WT mice. The KO phenotype included decreased IL-4, IL-6, IL-10, and IL-17 in lung tissues. In addition, OGG-1 KO mice showed decreased expression and phosphorylation of STAT6 as well as NF-κB. Down-regulation of OGG-1 by siRNA lowered ROS and IL-4 levels but increased INF-γ production in cultured epithelial cells following exposure to house dust mite (HDM) extracts. OGG-1 may affect the levels of oxidative stress and proinflammatory cytokines during asthmatic conditions. OGG-1-deficiency negatively regulates allergen-induced airway inflammatory response.
Keywords: knockout mice, Th2 cytokines, HDM extracts, oxidative stress, airway inflammation
Introduction
Asthma is characterized by airway inflammation and hyper-responsiveness (1). The pathogenesis of the allergic inflammation involves multiple mediators, cell types, and pathways (2). Although the skewing of Th2 cytokines is considered the key pathogenic factor for asthma (3), oxidative stress may also be a crucial contributor to asthma development. Accumulating evidence demonstrates that levels of oxidative stress are increased both in children and in adults with asthma, not only in their lungs but also in the blood (4). Oxidative stress triggers inflammation and can also result from inflammation (5). Allergen exposure induces airway inflammation accompanying a rapid increase in reactive oxygen species (ROS). ROS are secondary messengers involved in the induction of NF-κB, a transcriptional activator that induces pro-inflammatory cytokines (6). For example, intracellular ROS production modulates the gene expression of asthma-associated Th2 cytokine IL-4 (7). Oxidative stress may augment airway inflammation independent of the adaptive immune response (8) through multiple intracellular signaling pathways (5, 9). Ever-diverse cellular or molecular sources of ROS (e.g., NADPH oxidases, mitochondria, environmental exposures) are being identified (10). Furthermore, oxidative DNA damage in peripheral blood lymphocytes was shown to be significantly higher in asthma patients than in healthy subjects (11), thus DNA repair mechanisms may be associated with asthma development (12).
Base DNA damage induced by oxidation is repaired by the base excision DNA repair (BER) pathway that is initiated by mammalian 8-oxoguanine DNA glycosylase (OGG-1). Briefly, OGG-1 excises 7, -dihydro-8-oxoguanine 8 (8-oxoG), which is formed by oxidative damage of the DNA base guanine and has the propensity to mispair with adenine during DNA replication. OGG-1 attacks the N-glycosidic bond of 8-oxoG site using the active-site Lys249 nucleophile to form a transient Schiff base (13). Once 8-oxoG is removed, OGG-1 eliminates the damaged base, yielding an apurinic/apyrimidinic (AP) site (14). The AP site is then removed by AP endonuclease 1 (APE1) which makes incisions in the phosphodiester at the 5′-end of the lesion. This creates an intermediate involving a single-strand break that is filled by polymerase β and linked together by ligase I (15). OGG-1 has been shown to play important roles in preventing the accumulation of oxidative DNA damage (16). To effectively repair damaged DNA, the BER pathway interacts with other DNA repair pathways or cell signaling proteins, such as poly(ADP-ribose) polymerase 1 (PARP1) (15, 17). PARP1 has been shown to regulate inflammatory responses in various diseases (18). Since OGG-1 is down-regulated in pathological conditions (19, 20) and OGG-1 knockout (KO) mice display accumulated 8-oxoG (21), over-expression of OGG-1 is thought to alleviate toxicity caused by chemotherapeutics, hyperoxia (high concentrations of oxygen), and other oxidants (22-25). Similar effects can be achieved through over-expression of E. coli formamidopyrimidine [fapy]-DNA glycosylase (Fpg), a functional homolog of OGG-1. OGG-1 may also mitigate neurodegenerative processes by interacting with nuclear excision repair proteins CSB and XPB (26). As oxidative stress, proinflammatory cytokines, and OGG-1 were increased in rat lungs after exposure to particular matter (27), OGG-1 may be associated with oxidative stress in allergen-induced airway inflammation.
OGG-1 KO mice showed a decrease in lipopolysaccharide (LPS)-induced neutrophil infiltration and oxidative stress as compared to wild-type (WT) mice, suggesting its role in regulating inflammation (28). OGG-1 deficiency also protects the gastric mucosa against inflammatory lesions under H. pylori infection (29) and regulates inflammatory response to P. aeruginosa invasion (13). In addition, OGG-1 plays a role in diabetic autoimmune models of inflammation (28). OGG-1 deficient mice exhibited an increase in 8-oxoG in the liver, but not in the spleen and kidneys, indicating that OGG-1's role in oxidative stress varies with different organs (21). However, whether OGG-1 modulates airway allergic inflammation remains undetermined. Due to its importance in repairing oxidative DNA damage, we hypothesized that OGG-1 may play a role in oxidation-induced inflammation in asthmatic conditions. Our data showed that OGG-1 deficiency impacted the development of allergic inflammation in an ovalbumin-induced asthma model.
Methods
Animals
OGG-1 KO mice and wild-type (WT) mice on a J129/C57BL/6 genetic background were generated as described in reference (30) and generously provided by S. Ackerman (Jackson laboratory). Mice were maintained under specific pathogen–free conditions and were used at 6 to 8 weeks of age. Genotyping was performed by PCR and western blot. Mice were housed in University of North Dakota (UND) Center of Biomedical Research under pathogen- and allergen-free conditions. All animal experiments were performed in accordance with the guidelines of the UND Institutional Animal Care and Use Committee.
Sensitization and challenge protocol
OGG-1 KO and WT mice (6 mice for each group) were randomly grouped and sensitized by i.p. injection with 20 μg of ovalbumin (OVA) in aluminum hydroxide on 0 and 14 day. Mice were challenged with intranasal instillation 10 μg OVA in 50 μl PBS buffer at 28 day. Mice were also given 50 μl aluminum hydroxide alone or 50 μl PBS as controls. 24 hours after OVA challenge, mice were killed with an overdose of ketamine (31).
Histological evaluation
The left lungs were homogenized for biochemical analysis, and the right lungs were fixed with 10% neutral-buffered formalin for H & E stain using a standard histological method (32). The tissues were assessed for general morphology and cellular infiltration. Images were obtained using a Nikon Eclipse Microscope (80i)(33). The degree of cellular infiltration was scored by previously-described methods (34, 35). A value of 0 indicates no detectable inflammatory cells; 1 indicates 1-3 cell thick; 2 indicates 4-9 cell thick; and 3 indicates more than 10 cell thick in most bronchi. Inflammation scores were expressed as a mean value from at least 7 randomly selected tissue section areas per mouse. Two different investigators (blinded) scored the inflammation data in order to obtain objective results.
Lung tissue homogenization and cytokines assay
Lungs were removed and crushed in PBS buffer. The suspension was sonicated 3 times for 30 s each and centrifuged at 3000 g for 5 min. The supernatants were collected and quantified by Bio-Rad protein assay (Bio-Rad Laboratories). Cytokine levels were determined in triplicate of total lung lysates from each animal by ELISA (26). The ELISA kits of TNF-α, IL-2, IFN-γ, IL-4, IL-6, IL-10, IL-22, and IL-17 were purchased from R&D Systems (Minneapolis, MD) or eBiosciences (San Diego, USA).
Thiobarbituric acid reactive substances (TBARS) assay in lung tissue
To determine lipid peroxidation, the TBARS (a substrate to detect ROS released from lipids) assay was used (36). Briefly, lung tissue samples were crushed in RIPA lysis buffer (1% sodium deoxycholate, 1% Triton X-100, 5 mM iodoacetamide, 1% bovine hemoglobin, and 0.025% NaN3). The suspension was sonicated 3 times for 30 s and centrifuged at 3000 g for 10 min at 4°C. 100 μl of supernatant was added with 200 μl ice cold 10% trichloroacetic acid to precipitate proteins and incubated for 15 minutes on ice. The samples were centrifuged at 2200g for 15 minutes. 200μl supernatant was added equal volume of 0.67% thiobarbituric acid and incubated in boiling water bath for 10 minutes. Samples were measured at 532nm (26).
OGG-1 activity assay in lung tissue
OGG-1 activity assay was done as described previously (22). Oligonucleotides containing a single 8-oxoG residue (ATCACCGGC [8-oxoG] CCACACGAGCTG) were synthesized. The 5′-end was 32P-labelled by T4 polynucleotide kinase. Labeled probe and samples were diluted in OGG-1 activity buffer (10 mM HEPES-KOH (pH 7.4), 100 mM KCl, 10 mM EDTA, and 0.1 mg/ml BSA). Reaction mixtures contained 25 fmol probes and samples in a total volume of 13 μl. After incubating for 1 hour at 37°C, samples were added 5μl DNA loading dye to terminate. The products of the reaction were run on 20% denaturing DNA sequencing gels (23). The migration of radiolabeled DNA products was visualized and the extent of nicking was quantified.
Lung tissue immunohistochemistry and western blot
Lung tissues fixed with OCT (frozen at -70°C) were sectioned onto slides and immunohistochemistry stained using standard histological methods (32). The sections were fixed in cold acetone and blocked at room temperature. Tissue sections were incubated with phospho-STAT6 (signal transducer and activator of transcription) mouse monoclonal, STAT6 rabbit polyclonal, NF-κB mouse monoclonal, and phospho-NF-κB rabbit polyclonal antibodies (p65 Ser276, Santa Cruz Biotechnology, Inc.). FITC– conjugated goat anti–mouse antibodies or TRITC- conjugated goat anti–rabbit antibodies were used to bind primary antibodies. Tissue sections were viewed with Zeiss 510 META confocal microscope (37). The results were quantified using Image J software (38). Lung tissues were homogenized in RIPA lysis buffer for western blot analysis. Lysates (20 μg) were run on 10% SDS polyacrylamide gel at 100 V for 2 hours and transferred to microporous polyvinylidene difluoride (PVDF) membrane at 100 mA for 2 hours. The membrane were blotted with OGG-1/2 goat polyclonal (Cat # sc-12074), GAPDH mouse monoclonal antibodies, and those antibodies described above (Santa Cruz Biotechnology, Inc.) and processed via enhanced chemiluminescence (Pierce) (39).
Measurement of ROS in cultured epithelial cells
MLE-12 cells (Murine lung epithelial cell line) were grown to 85% confluence in DMEM and DMEM/F12 culture media (1:1), respectively, and were transfected with mouse OGG-1 siRNA (sc-44850, Santa Cruz Biotechnology, Inc) with LipofectAmine 2000 according to the manufacturer's instruction. We obtained 70-90% knockdown efficiency as determined by western blotting. After 24 hours, the cells were loaded with 50 μM H2DCF-DA (Molecular Probes) at 37°C for 30 minutes. After removing excess probes, cells were treated with 10μg, 20μg, or 50μg of house dust mite (HDM) extracts in serum-free culture medium for 4 hours. The change in fluorescence intensity was assessed in fluorescence microplate reader (Bio-Tek) at 488-nm excitation and 530-nm emission (26).
Studies of dust mite effect on cultured epithelial cells
To define the impact of OGG1-deficiency on allergic inflammation, MLE-12 cells grown to 85% confluence were transfected with mouse OGG-1 siRNA. After 24 hours, 50μg HDM extracts in serum-free culture medium were added to cells for 4 hours, and cell lysates were collected to assess NF-κB, STAT6, and OGG-1. Cytokine secretion in the culture supernatant was determined using ELISA.
Statistical analysis
The results were expressed as means ±S.E. Statistical analysis was performed by one way ANOVA (Turkey's post-hoc) or Mann-Whitney test and the significance level was defined as P < 0.05 between two groups (40). The data were analyzed statistically by SPSS 10.0 software.
Results
Effect of OGG-1 deficiency on inflammatory cell infiltration in OVA-challenged mice
After sensitizing (day 0) and challenging (day 14, day 28) with OVA (see methods), mice were sacrificed 24 h later and OGG-1 WT mice showed a significant inflammatory response in lungs and airways compared to KO mice. Specifically, OGG-1 WT mice exhibited an increased inflammatory cell infiltration in the airway, around blood vessels and alveoli following OVA challenge (Figure 1A and C). In contrast, OGG-1 KO mice showed much less inflammatory cell infiltration in the airway, around blood vessels, and in alveoli (Figure 1B and 1D). The degree of inflammatory cell infiltration was then scored as previously described (35, 41), and the data showed that infiltration in the airway system was decreased in OVA-challenged OGG-1 KO mice compared to WT mice (*p<0.043, Mann-Whitney-Wilcoxon analysis) (Figure 1E). WT control mice, OGG-1 KO control mice, and aluminum hydroxide-only (vehicle) mice had no apparent inflammatory cell infiltration in lung tissues (Figure 1A and 1B). Correlating to the changes in the airway and alveoli, similar patterns of the inflammatory cell penetration in the BAL fluid were also observed (Figure 1F), suggesting that inflammatory cell infiltration may be a critical contributor to oxidation and subsequent inflammation.
Figure 1. OGG-1 deficiency decreases infiltration of inflammatory cells.
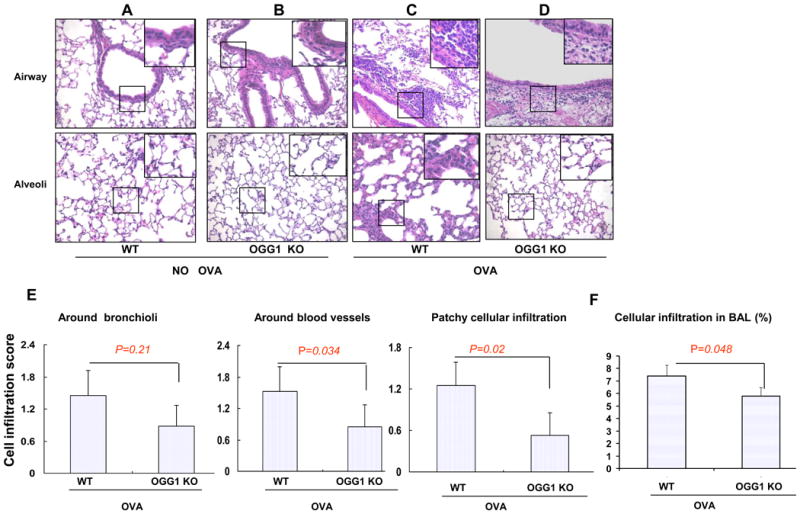
AD) Lung samples were stained with hematoxylin and eosin (H&E). WT mice and OGG-1 KO mice were sensitized and challenged by OVA in aluminum hydroxide. OGG-1 KO and WT mice were also treated and challenged by aluminum hydroxide alone or PBS. E) The degree of cell infiltration was scored in OVA-challenged WT mice and OGG-1 KO mice. OVA-challenged OGG-1 KO mice showed less infiltration of inflammatory cells than WT mice (p<0.043, Mann-Whitney-Wilcoxon test). F) Inflammatory cell penetration in the BAL fluid (Mann-Whitney test). Results are representative of three experiments and data are shown with mean± standard error.
Effect of OGG-1 deficiency on cytokine production in allergen-challenged mice
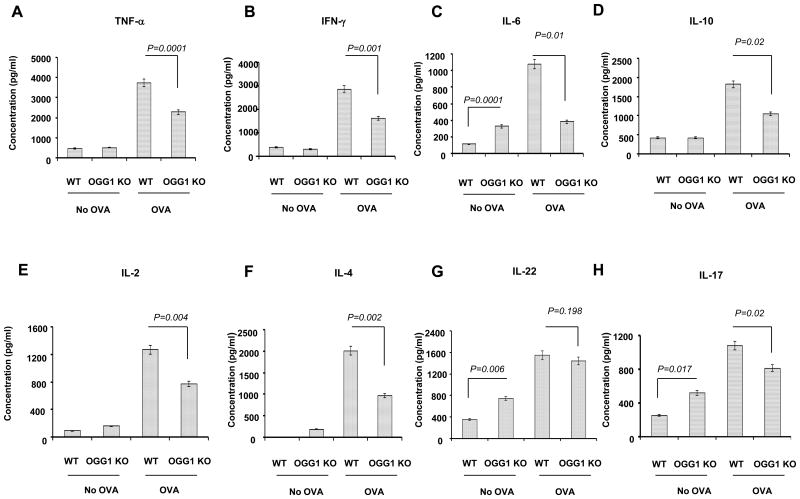
Prior to OVA immunization and challenge, OGG-1 KO mice showed increased levels of IL-6, IL-17, and IL-22 compared to WT mice as determined by ELISA. After OVA immunization and stimulation, WT mice exhibited increased levels of IL-4, IL-6, IL-10, IL-2, and IL-12, TNF-α, and IFN-γ. Similarly, WT mice showed increased IL-17 and IL-22 (Figure 2). In contrast, OGG-1 KO mice demonstrated decreased levels of IL-4, IL-6, IL-10, IL-2, IL-12, TNF-α, and IFN-γ as well as IL-17 after OVA challenge. However, OVA challenge did not alter IL-22 levels in OGG-1 KO mice (Figure 2). These data suggest that OGG-1 deficiency significantly decreased the airway inflammatory response.
Figure 2. OGG-1 deficiency modulates cytokine levels in OVA-challenged mice.
Cytokine levels were determined in lung homogenates by ELISA. A) TNF-α; B) IFN-γ, C) IL-6; D) IL-10; E) IL-2; F) IL-4; G) IL-22; and H) IL-17. Results are representative of three experiments and data are shown with mean±standard error (One way ANOVA).
Effect of OVA challenge on OGG-1 activity in mice
To define the direct role of OGG1, we measured 8-oxodG-DNA repair activity specific for OGG-1 in lung homogenates. We found that OVA-challenged WT mice exhibited an increase in OGG-1 associated DNA repair activity compared to control mice (Figure 3A). Quantification with densitometry showed an increase in OGG-1 associated DNA repair activity in OVA challenged WT mice (Figure 3B), indicating that OGG-1 participates in the response to allergic challenge in the lung. DNA repair activity was observed even in the OVA-challenged KO mice, as similarly reported by others (42); this activity may be due to the contribution of other DNA repair enzymes.
Figure 3. OVA challenge alters OGG-1 enzymatic activity in mice.
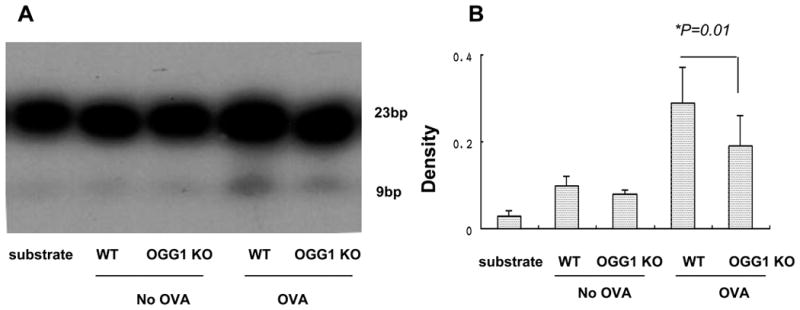
The oligonucleotides were γ[32P] labeled using T4 polynucleotide kinase. A radio-labeled synthetic short double-stranded oligonucleotide was incubated with nuclear extracts. A) The results were analyzed by denaturing DNA polyacrylamide gels. B) Densitometry analysis (relative to controls) of OGG-1 incision activity (One way ANOVA). Results are representative of three experiments and data are shown with mean± standard error.
Effect of OGG-1 deficiency on STAT6 and NF-κB levels in allergen-challenged mice
To determine the molecular mechanism of OGG-1 in regulating allergic inflammation, we analyzed a variety of cell signaling proteins in lung lysates by western blotting. OVA challenge resulted in increased expression of OGG-1 and STAT6 as well as phosphorylation of STAT6 and NF-κB (Ser276 of p65) in WT mice (Figure 4A and B). Recently, bacterial oxidants were also reported to induce significant OGG-1 expression and enzymatic activity (13, 43). However, nuclear OGG-1 activity increase (Fig. 3) did not seem stoichiometric to the protein levels. The explanation may be two-fold: 1) mitochondrial OGG (OGG-2 or OGG-1β) does not seem to exhibit in vitro incision activity (44); and 2) other repair enzymes may contribute to the enzymatic activity. OVA challenge induced less expression of STAT6 and phosphorylation of NF-κB and STAT6 in OGG-1 KO mice versus WT mice (p>0.05, Figure 4A and 4B), whereas STAT3 expression remained unchanged (not shown). The expression of NF-κB in OGG-1 KO mice was also increased, but phosphorylation was drastically decreased compared to that in WT mice. These data suggest that OGG-1 may regulate cytokine expression through the STAT6/NF-κB pathway. We then determined the expression and localization of STAT6 and NF-κB using immunohistochemistry. Following OVA challenge, WT mice showed increased expression as well as phosphorylation of STAT6 and NF-κB in the airway epithelium (Figure 4C). However, OGG-1 KO mice exhibited significantly decreased expression as well as phosphorylation of STAT6 and NF-κB in lung tissues (Figure 4C), whereas the changes in STAT3 were insignificant (data not shown). These data suggest that OGG-1-deficiency down-regulates STAT6 and NF-κB, thus diminishing inflammatory responses.
Figure 4. OGG-1 deficiency impacts activation of STAT6 and NF-κB in allergic airway inflammation.
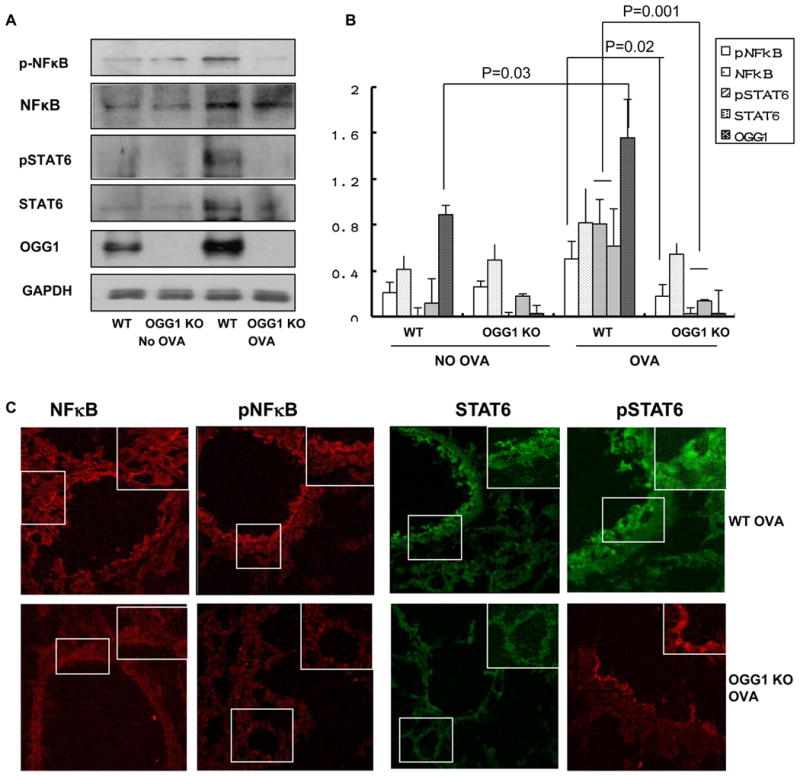
A) Phosphorylation of STAT6 and NF-κB in lung homogenates from OGG-1 WT and KO mice were determined by western blotting. B) Densitometry analysis of pSTAT6 and pNF-κB in lung tissues (change for A, One way ANOVA). C) Frozen lung sections were stained with polyclonal antibodies against pSTAT6 and pNF-κB followed by incubation with FITC-conjugated secondary antibody (green) or TRITC-conjugated secondary antibody (red). Sections were observed using confocal microscopy (Original magnification X400). Results are representative of three experiments and data are shown with mean± standard error.
Effect of OGG-1 deficiency on oxidative stress in mice and ROS levels in cells
Since increased oxidation can also cause airway tissue damage by lipid degradation, we evaluated lipid peroxidation in lung homogenates using a thiobarbituric acid reactive substances (TBARS) assay. We found an increase in TBARS levels in OVA-challenged WT mice as compared to control WT and KO mice. The data suggest that OVA-challenged OGG-1 KO mice showed a decrease in TBARS levels compared to WT mice (Figure 5A). It should be noted that inflammatory cells may also contribute to the increase in TBARS levels at 24 h post OVA challenge. Importantly, previous studies suggest that OGG-1 may be involved in regulating ROS levels (45). To determine whether OGG-1 regulates oxidative stress response in allergen-exposure conditions, OGG-1 siRNA was transfected to MLE-12 cells, which are widely used for studying respiratory mechanisms (13, 36, 46). Alveolar type II pneumocytes were recently found to express cystic fibrosis transmembrane conductance regulator (CFTR) and may regulate the ion and fluid transport in the upper airway (47). House dust mite extracts (HDM), inducers of airway asthmatic pathology and ROS (48), were used to challenge cultured cells. Complementing the OVA animal model, the dust mite in vitro model may probe a broader role of OGG-1 in allergic conditions. HDM significantly increased ROS levels in MLE-12 cells in a dose-dependent manner. We transfected MLE-12 cells with OGG-1 siRNA and obtained 70-90% knockdown efficiency as determined by western blotting. As expected, down-regulation of OGG-1 by siRNA markedly decreased ROS levels in MLE-12 cells (p<0.01, Figure 5B). These data indicate that OGG-1 knockdown decreased oxidative stress responses, consistent with the results from OVA-challenged OGG-1 KO mice. To further determine the link between OGG-1 and ROS, we pre-treated MLE-12 cells with anti-oxidants (N-acetyl cysteine, 10 mM and vitamin C, 10 mM) for 2 h before HDM challenge. Compared with the sham control, ROS levels were inhibited by anti-oxidants (Figure 5C) and OGG-1 levels were also decreased (Figure 5D). These results suggest that OGG-1 may increase following allergen stimulation to regulate inflammatory response and oxidation, whereas inhibition of ROS by antioxidants may offset the response by OGG-1. Combining with the observations from KO mice, our data suggest that OGG-1 is associated with ROS levels.
Figure 5. OGG-1 deficiency modifies oxidative stress in mice and ROS levels in cells.
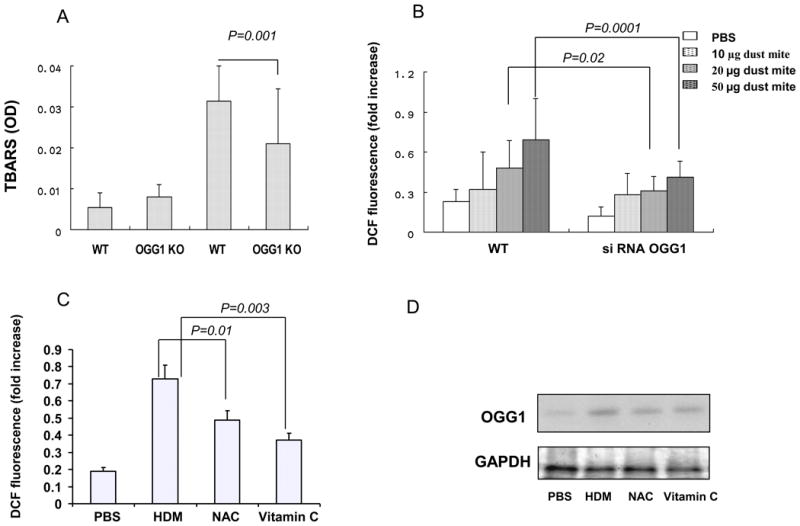
A) OGG-1 KO mice showed decreased lipid peroxidation (TBARS) levels compared with WT type mice following OVA challenge (Mann-Whitney test). B) OGG-1 siRNA decreased ROS levels induced by HDM extract compared to control MLE-12 cells (Mann-Whitney test). C) Effect of antioxidants on ROS levels (Mann-Whitney test). N-acetyle cysteine (NAC, 10 mM) and vitamin C (10 mM) were added to pretreat MLE-12 cells for 2 h before the challenge with HDM extract (50 μg/ml). D) Effect of antioxidants (as panel C) on OGG-1 levels determined by western blotting. Results are representative of three experiments and data are shown with mean± standard error.
Effect of OGG1-knockdown in epithelial cells on HDM-induced cytokines
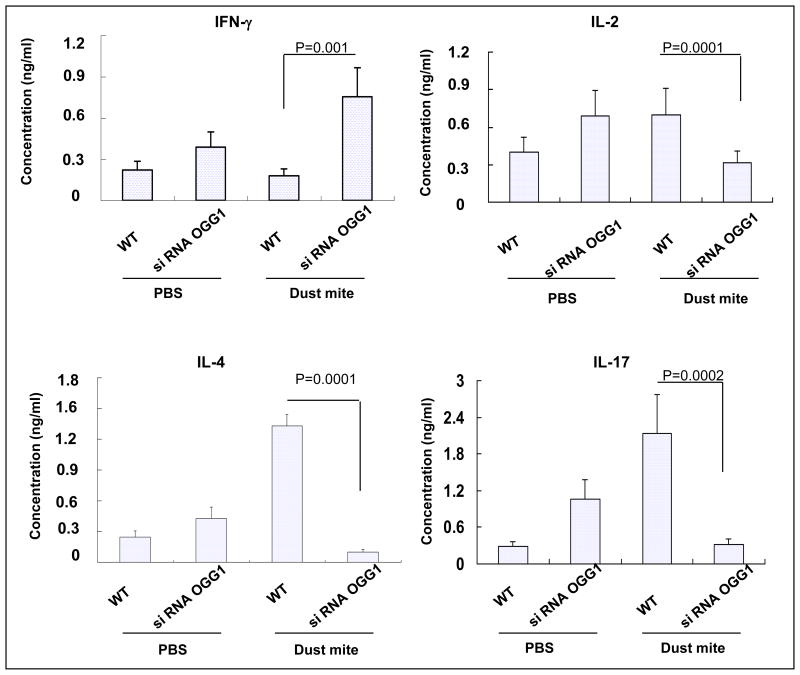
To dissect the impact of OGG-1 on cytokine production, we treated MLE-12 with 50 μg HDM and found that the levels of IL-2, IL-4, and IL-17 were increased as compared to sham controls (p<0.01, Figure 6). However, IFN-γ was not changed in HDM-treated MLE-12 cells (p>0.05). To determine whether OGG-1-deficiency regulates allergen-induced cytokine responses, we transfected MLE-12 cells with OGG-1 siRNA. After HDM treatment, OGG-1 knockdown in these cells significantly increased IFN-γ, but decreased IL-2, IL-4, and IL-17 compared to WT MLE-12 (p<0.01). These results demonstrate that OGG-1 down-regulation decreased IL-4, indicating a potential role for OGG-1 in asthmatic inflammation (Figure 6).
Figure 6. OGG-1 knockdown increases IFN-γ but decreases IL-2, IL-4, and IL-17 in MLE-12 cells.
Following HDM treatment, supernatant cytokines from OGG-1 siRNA-transfected MLE-12 cells were measured by ELISA compared to transfection control cells (Mann-Whitney test). Results are representative of three experiments and data are shown with mean± standard error.
Effect of OGG1-knockdown in epithelial cells on expression and activation of STAT6 and NF-κB
To further confirm the role of OGG-1 in cytokine release and the related pathway, we treated MLE-12 cells with HDM and showed an increase in phospho-STAT6 and phospho-NF-κB at 1 h and 4 h. HDM treatment also increased OGG-1 expression in MLE-12 cells at 0.5 h (Figure 7A), consistent with a previous study showing that OGG-1 can increase as early as 10 min (49). The OGG-1/2 antibodies from Santa Cruz react with both OGG-1 (39 kD) and OGG-2 (47 kD) isoforms (44). To determine the effect of OGG-1 on STAT6 and NF-κB, OGG-1 siRNA was transfected to MLE-12 cells. OGG-1 siRNA transfection inhibited the expression of STAT6 and OGG-1 as well as phosphorylation and nuclear translocation of STAT6 and NF-κB compared to OGG-1 WT cells or scrambled siRNA transfected cells (Figure 7B). STAT1 and STAT3 levels were also examined but no significant changes were observed (data not shown). Therefore, OGG-1 siRNA diminished inflammatory response to HDM challenge in cell culture (Figure 7C and 7D). Dust mite treatment induced marked NF-κB nuclear translocation, which was abolished by OGG-1 siRNA transfection (Figure 7E), suggesting that NF-κB activation may contribute to the dysregulated cytokine response. Taken together, our studies identified a novel pathway involving OGG-1 regulation of airway inflammatory response by affecting, at least in part, the STAT6-NF-κB axis and key cytokines such as IL-4 (Figure 7F). OGG-1 may also impact allergic inflammation and oxidation directly or through other mechanisms.
Figure 7. OGG-1 knockdown decreases pSTAT6 and pNF-κB levels in MLE-12 cells.
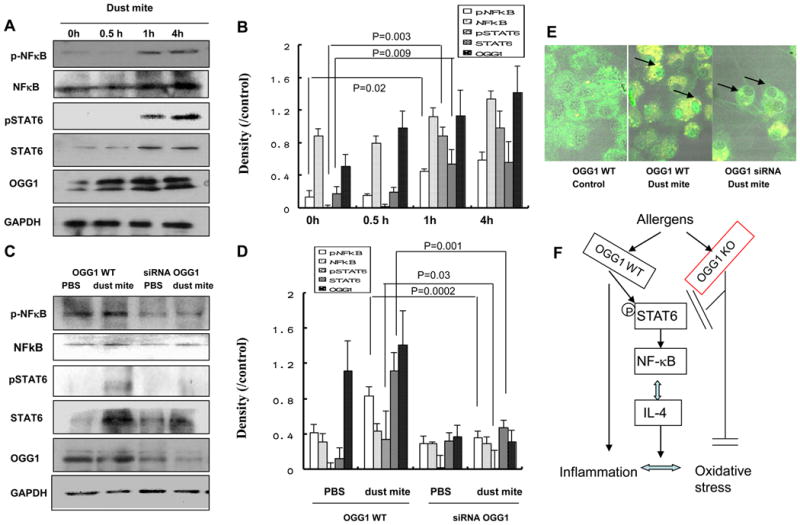
Expression of OGG-1, STAT6, and NF-κB was determined by western blotting in MLE-12 cells following HDM treatment. A) Levels of OGG-1, pSTAT6, and pNF-κB were altered at 0 h, 0.5 h, 1 h, and 4 h in HDM extract-treated MLE-12 cells. B) Relative density (change for A, Mann-Whitney test) of OGG-1, pSTAT6 and pNF-κB at 0 h, 0.5 h, 1 h, and 4 h in MLE-12 cells. C) Levels of pSTAT6 and pNF-κB were significantly down-regulated in OGG-1 siRNA-transfected MLE-12 cells. D) Relative density (change for C, Mann-Whitney test) of OGG-1, pSTAT6, and pNF-κB in OGG-1 WT and OGG-1 siRNA MLE-12 cells. Results are representative of three experiments and data are shown with mean±standard error. E) NF-κB nuclear translocation upon dust mite treatment but abolished by OGG1 siRNA transfection. Cells were pretreated with dust mite for 1 h and immunofluorescence staining with NF-κB p65 antibody. F) Diagram showing the cell signaling pathway with OGG-1 deficiency.
Discussion
Deficiency in OGG-1, a DNA repair enzyme for oxidation damage, is associated with decreased inflammatory lesions and genotoxicity in bacterial infection (13, 28). However, it is unknown whether OGG-1 can contribute to airway allergic response. Our current study is the first to evaluate its role in an asthmatic model, demonstrating a decrease in inflammatory cell infiltration in the lung and airway of OGG-1 KO mouse following OVA challenge. Our observations indicate that OGG-1 deficiency drastically alleviated airway inflammation, particularly lowering cytokine IL-4 production through a STAT6-NF-κB pathway. In addition, we show that the role of OGG-1 in asthmatic conditions may be associated with the regulation of ROS levels.
It is believed that Th2 type cells play a critical role in development of allergic disease. Th2 type cells secrete IL-4, IL-5, IL-10, and IL-13, whereas Th1 type cells produce IL-2, IL-12, IFN-γ, and TNF-α (50). Recently, Th17 type cells have also been shown to play a role in regulating neutrophilic and macrophage inflammation in the lung, in turn suggesting a potential role for Th17 type cells in severe, steroid-insensitive asthma, as well as chronic obstructive pulmonary disease (COPD) (51). IL-22 is a member of the IL-10 cytokine family that also plays an important role in inflammatory responses; however, it is unclear whether IL-22 is involved in asthma pathogenesis (52). Our study demonstrates that TNF-α, IFN-γ, IL-2, IL-4, IL-6, IL-10, and IL-17 were all significantly decreased in OGG-1 KO mice (P<0.01), suggesting that OGG-1 broadly impacts asthmatic inflammation. On the other hand, the levels of IL-22 in OGG-1-deficient mice were not significantly changed, suggesting that IL-22 may not be associated with allergic pathogenesis in this model. It has been reported that under inflammatory conditions OGG-1 activity is decreased in parallel with a significant increase in 8-oxoG levels (53). However, we found that OGG-1 repair activity and expression levels were significantly increased in lung tissues in OVA-challenged WT mice, indicating that airway allergic inflammation may be different from other inflammatory conditions.
Binding of a cytokine to its receptor leads to the activation of members of the JAK family of receptor-associated kinases. These kinases subsequently activate STATs via tyrosine phosphorylation (54). In addition, activation of STAT6 may be critical in allergic inflammation as PARP-1 was shown to affect ovalbumin-induced IL-5 expression through the STAT6 pathway (55). However, DNA repair proteins are not previously linked to asthma. We found that OVA-challenged OGG-1 KO mice exhibited lowered STAT6 expression and phosphorylation, resulting in increased IL-4. Previous studies have shown that STAT6 may stimulate the secretion of IL-4 and IL-13, which aggravates inflammatory cell penetration, triggers airway hyper-responsiveness, and eventually leads to airway remodeling (56). Studies also indicate that a tandem NF-κB/Rel binding motif is required for the gamma 3 evolutionary conserved sequence responsiveness to IL-4, while a STAT6-binding site is also critical for up-regulating IL-4. Thus, we speculate that STAT6 activation may affect NF-κB, whose phosphorylation occurs predominantly in the epithelium of conducting airway diseases (57). Indeed, we found that decreased allergic airway inflammation is associated with decreased NF-κB phosphorylation in lung tissues in OGG-1 KO mice.
The balance between antioxidants and oxidants is critical to maintaining normal physiological functions in the lung. Increases in oxidants or decreases in antioxidants disrupt this balance. This is the case in asthmatic airways owing to an excess accumulation of ROS (58). The resulting oxidative stress may also aggravate airway inflammation and hyper-reactivity in asthma (59). In turn, accelerated inflammation intensifies oxidation. In our study, OVA-challenged WT mice exhibited increased TBARS levels, while OVA-challenged OGG-1 KO mice displayed a significant decrease in TBARS levels. In addition, we found that allergic airway inflammation enhanced OGG-1 repair activity in OVA-challenged WT mice. These results demonstrated that OGG-1-deficiency down-regulates oxidative stress during allergic inflammation. Lower oxidative stress might result from decreased inflammatory response in OGG-1 KO mice in response to OVA challenge. This is consistent with previous studies showing that OGG-1 may impact oxidative stress and ROS levels (45).
Oxidative stress is reportedly associated with allergic inflammatory responses and exposure to environmental oxidants (60). The pollen NADPH oxidases, for example, rapidly increase ROS levels in the lung epithelium (61). Cell types involved in regulating oxidative stress appear to be complex, as Bacsi et al., found that OGG-1 KO fibroblast cells consist of two subpopulations: low ROS cells (∼84%) and high ROS cells (16±5%) (45). We also observed that HDM extracts induced a significant increase in ROS levels in MLE-12 cells in a dose dependent manner. To further define the role of OGG-1 in oxidative stress, we used OGG-1 siRNA strategy and assessed ROS production in cell culture. Down-regulation of OGG-1 by siRNA decreased ROS levels in HDM-treated MLE-12, confirming the largely identical effect in OGG-1 KO mice. Furthermore, antioxidant pretreatment decreased ROS levels and lowered OGG-1 expression. In contrast to limited prior studies which indicate that antioxidant nutrients may impact DNA repair levels (62), our study directly dissected OGG-1 levels in relation to ROS levels in allergen challenge. These results suggest that OGG-1 may influence airway inflammation through the regulation of oxidative stress.
HDM extracts contain various proteins of known and unknown characteristics, including Der p 1, Der p 2, and Der p 5. Der p1 contains cysteine protease activity, which causes cytokine release, facilitates allergen translocation across epithelial cell layers (63), and increases neutrophil ROS levels (48). A recent study reported that HDM induced proinflammatory cytokines IL-6 and IL-8 in a dose- and time-dependent manner (64). Our study demonstrated a similar cytokine profile, namely induction of IL-2, IL-4, and IL-17 in MLE-12 cells. However, HDM extracts did not induce IFN-γ. In addition, OGG-1 siRNA significantly inhibited IL-2, IL-4, and IL-17, while enhancing IFN-γ. It is worth noting that there are differences in cytokine expression between the OVA and HDM models as HDM extracts induce inflammation, largely as a result of their protease activities (65). Nevertheless, our HDM in vitro model identified a potential regulatory role for OGG-1 in ROS levels because anti-oxidants prevented the increase in ROS and OGG-1 levels following HDM challenge. One important finding of this study is the link between oxidative stress and OGG-1 in asthmatic conditions. Der p1 promotes activation of NF-κB by interfering with the function of its cytoplasmic inhibitor IκBα (66). Our study also showed that NF-κB was phosphorylated and translocated to the nucleus, which may up-regulate IL-4 cytokine. OGG-1 inhibition decreased phosphorylation and nuclear translocation of NF-κB and expression of STAT6, which may down-regulate IL-4. These results indicate that OGG-1 plays a critical role in development of allergen-induced inflammatory responses through the regulation of cytokine levels. Consistent with our animal and in vitro studies, we recently noticed that OGG-1 was increased in airway tissue from chronic asthmatic patients (data not shown), suggesting that OGG-1 may be a true regulator in both asthmatic oxidation and inflammation. OGG-1 regulation of oxidation and cytokine production is fascinating and warrants further assessment.
The present study demonstrated for the first time that OGG-1 is involved in regulating allergic inflammation. OGG-1 KO mice exhibited decreased allergic airway inflammatory response and oxidative stress. This regulatory role is associated with down-regulation of cytokines IL-4, IL-6, and IL-17. The reduction in airway allergic inflammation also accompanies the regulation of STAT6 and NF-κB in OVA-challenged mice. Using an siRNA approach to knockdown OGG-1, we confirmed similar roles for OGG-1 in regulating cytokine release and oxidative stress in cultured epithelial cells. Taken together, our results indicate that OGG-1-deficiency plays a negative regulatory role in allergen-induced airway inflammatory response.
Acknowledgments
Declaration of all sources of funding: This project was supported by NIH ES014690, FAMRI, and American Heart Association Scientist Development Grant (National Office) to MW and NHLBI to HG.
Abbreviations
- OGG-1
8-OxoG-DNA glycosylase
- ROS
Reactive oxygen species
- KO
knockout mice
- WT
wild-type
- OVA
ovalbumin
- TBARS
Thiobarbituric acid reactive substances
- siRNA
small interference RNA
- H2DCF-DA
dichlorodihydrofluorescein diacetate
- HDM
house dust mite
Footnotes
Author contributions: G.L., H.G., and M.W. designed research; G.L., K.Y., J.F., M.G., H.Z., and M.W. performed research; W.B., A.K.B., and H.G. provided critical reagents; G.L., H.G., and M.W. analyzed data and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ohno I. Bronchial asthma and psychological stress. Rinsho Byori. 2010;58:292–299. [PubMed] [Google Scholar]
- 2.Broide DH. Molecular and cellular mechanisms of allergic disease. J Allergy Clin Immunol. 2001;108:S65–71. doi: 10.1067/mai.2001.116436. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal DK, Shao Z. Pathogenesis of Allergic Airway Inflammation. Curr Allergy Asthma Rep. 2010;10:39–48. doi: 10.1007/s11882-009-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadeem A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol. 2003;111:72–78. doi: 10.1067/mai.2003.17. [DOI] [PubMed] [Google Scholar]
- 5.Dozor AJ. The role of oxidative stress in the pathogenesis and treatment of asthma. Ann N Y Acad Sci. 2010;1203:133–137. doi: 10.1111/j.1749-6632.2010.05562.x. [DOI] [PubMed] [Google Scholar]
- 6.Gomez-Mejiba SE, Zhai Z, Akram H, Pye QN, Hensley K, Kurien BT, Scofield RH, Ramirez DC. Inhalation of Environmental Stressors & Chronic Inflammation: Autoimmunity and Neurodegeneration. Mutat Res. 2009;674:62–72. doi: 10.1016/j.mrgentox.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z, Turner DR, Oliveira DB. IL-4 gene expression up-regulated by mercury in rat mast cells: a role of oxidant stress in IL-4 transcription. Int Immunol. 2001;13:297–304. doi: 10.1093/intimm/13.3.297. [DOI] [PubMed] [Google Scholar]
- 8.Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, Mitra S, Goldblum RM, Sur S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen induced allergic airway inflammation. J Clin Invest. 2005;115:2169–2179. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho YS, Moon HB. The role of oxidative stress in the pathogenesis of asthma. Allergy Asthma Immunol Res. 2010;2:183–187. doi: 10.4168/aair.2010.2.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkham P, Rahman I. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol Ther. 2006;111:476–494. doi: 10.1016/j.pharmthera.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Zeyrek D, Cakmak A, Atas A, Kocyigit A, Erel O. DNA damage in children with asthma bronchiale and its association with oxidative and antioxidative measurements. Pediatr Allergy Immunol. 2009;20:370–376. doi: 10.1111/j.1399-3038.2008.00780.x. [DOI] [PubMed] [Google Scholar]
- 12.Batar B, Guven M, Onaran I, Tutluoglu B, Kanigur-Sultuybek G. DNA repair gene XRCC1 polymorphisms and the risk of asthma in a Turkish population. Allergy Asthma Proc. 2010;31:349–354. doi: 10.2500/aap.2010.31.3332. [DOI] [PubMed] [Google Scholar]
- 13.Wu M, Huang H, Zhang W, Kannan S, Weaver A, Mckibben M, Herington D, Zeng H, Gao H. Host DNA repair proteins in response to P. aeruginosa in lung epithelial cells and in mice. Infect Immun. 2011;79:75–87. doi: 10.1128/IAI.00815-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nash HM, Bruner SD, Scharer OD, Kawate T, Addona TA, Spooner E, Lane WS, Verdine GL. Cloning of a yeast 8-oxoguanine DNA glycosylase reveals the existence of a base excision repair protein superfamily. Curr Biol. 1996;6:968–980. doi: 10.1016/s0960-9822(02)00641-3. [DOI] [PubMed] [Google Scholar]
- 15.Izumi T, Wiederhold LR, Roy G, Roy R, Jaiswal A, Bhakat KK, Mitra S. Mammalian DNA base excision repair proteins: their interactions and role in repair of oxidative DNA damage. Toxicology. 2003;193:43–65. doi: 10.1016/s0300-483x(03)00289-0. [DOI] [PubMed] [Google Scholar]
- 16.Youn CK, Song PI, Kim MH, Kim JS, Hyun JW, Choi SJ, Yoon SP, Chung MH, Chang IY, You HJ. Human 8-Oxoguanine DNA Glycosylase Suppresses the Oxidative Stress-Induced Apoptosis through a p53-Mediated Signaling Pathway in human fibroblasts. Mol Cancer Res. 2007;5:1083–1098. doi: 10.1158/1541-7786.MCR-06-0432. [DOI] [PubMed] [Google Scholar]
- 17.Lu AL, Li X, Gu Y, Wright PM, Chang DY. Repair of oxidative DNA damage: mechanisms and functions. Cell Biochem Biophys. 2001;35:141–170. doi: 10.1385/CBB:35:2:141. [DOI] [PubMed] [Google Scholar]
- 18.Virág L, Szabó C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 19.Cardozo-Pelaez F, Cox DP, Bolin C. Lack of the DNA repair enzyme OGG1 sensitizes dopamine neurons to manganese toxicity during development. Gene Expr. 2005;12:315–323. doi: 10.3727/000000005783992007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodges NJ, Chipman JK. Down-regulation of the DNA-repair endonuclease 8-oxo-guanine DNA glycosylase 1 (hOGG1) by sodium dichromate in cultured human A549 lung carcinoma cells. Carcinogenesis. 2002;23:55–60. doi: 10.1093/carcin/23.1.55. [DOI] [PubMed] [Google Scholar]
- 21.Osterod M, Hollenbach S, Hengstler JG, Barnes DE, Lindahl T, Epe B. Age-related and tissue-specific accumulation of oxidative DNA base damage in 7,8-dihydro-8-oxoguanine-DNA glycosylase (Ogg1) deficient mice. Carcinogenesis. 2001;22:1459–1463. doi: 10.1093/carcin/22.9.1459. [DOI] [PubMed] [Google Scholar]
- 22.He Y, Xu Y, Wu M, Kobune M, Kelley MR, Martin WJ., II E. coli FPG and human Ogg1 reduce DNA damage and cytotoxicity by BCNU in human lung cells. Am J Physiol Lung Cell Mol Physiol. 2002;182:L50–55. doi: 10.1152/ajplung.00316.2001. [DOI] [PubMed] [Google Scholar]
- 23.Wu M, He Y, Xu Y, Kobune M, Kelley MR, Martin WJ., II Protection of human lung cells against hyperoxia using the DNA base excision repair genes hOgg1 and Fpg. Am J Respir Crit Care Med. 2002;166:192–199. doi: 10.1164/rccm.200112-130OC. [DOI] [PubMed] [Google Scholar]
- 24.Dobson AW, Grishko V, LeDoux SP, Kelley MR, Wilson GL, Gillespie MN. Enhanced mtDNA repair capacity protects pulmonary artery endothelial cells from oxidant-mediated death. Am J Physiol Lung Cell Mol Physiol. 2002;283:L205–210. doi: 10.1152/ajplung.00443.2001. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Hansen WK, Rosenquist TA, Williams DA, Limp-Foster M, Kelley MR. Protection of mammalian cells against chemotherapeutic agents thiotepa, 1,3-N,N′- Bis(2-chloroethyl)-N- nitrosourea, and mafosfamide using the DNA Base excision repair genes Fpg and α-hOgg1: Implications for protective gene therapy applications. J Pharmacol Exp Ther. 2001;296:825–831. [PubMed] [Google Scholar]
- 26.Wu M, Audet A, Cusic J, Seeger D, Cochran R, Ghribi O. Broad DNA repair responses in neural injury are associated with activation of the IL-6 pathway in cholesterol-fed rabbits. J Neurochem. 2009;111:1011–1021. doi: 10.1111/j.1471-4159.2009.06390.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Danielsen PH, Loft S, Jacobsen NR, Jensen KA, Autrup H, Ravanat JL, Wallin H, Møller P. Oxidative stress, inflammation, and DNA damage in rats after intratracheal instillation or oral exposure to ambient air and wood smoke particulate matter. Toxicol Sci. 2010;118:574–585. doi: 10.1093/toxsci/kfq290. [DOI] [PubMed] [Google Scholar]
- 28.Mabley JG, Pacher P, Deb A, Wallace R, Elder RH, Szabo C. Potential role for 8-oxoguanine DNA glycosylase in regulating inflammation. FASEB J. 2005;19:290–292. doi: 10.1096/fj.04-2278fje. [DOI] [PubMed] [Google Scholar]
- 29.Touati E, Michel V, Thiberge JM, Avé P, Huerre M, Bourgade F, Klungland A, Labigne A. Deficiency in OGG-1 Protects against Inflammation and Mutagenic Effects Associated with H. pylori Infection in Mouse. Helicobacter. 2006;11:494–505. doi: 10.1111/j.1523-5378.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 30.Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, Seeberg E, Lindahl T, Barnes DE. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci U S A. 1999;96:13300–13305. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu M, Hussain S, He HY, Pasula R, Smith PA, Martin WJ., II Genetically engineered macrophages expressing IFN-γ restore alveolar immune function in. scid mice Proc Natl Acad Sci U S A. 2001;98:14589–14594. doi: 10.1073/pnas.251451498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu M, Pasula R, Smith PA, Martin WJ., II Mapping alveolar binding sites in vivo using phage display peptide libraries. Gene Ther. 2003;10:1429–1436. doi: 10.1038/sj.gt.3302009. [DOI] [PubMed] [Google Scholar]
- 33.Loo BWJ, Meyer-Ilse W, Rothman SS. Automatic image acquisition, calibration and montage assembly for biological X-ray microscopy. J Microsc. 2000;197:185–201. doi: 10.1046/j.1365-2818.2000.00644.x. [DOI] [PubMed] [Google Scholar]
- 34.Suzaki Y, Hamada K, Nomi T, Ito T, Sho M, Kai Y, Nakajima Y, Kimura H. A small-molecule compound targeting CCR5 and CXCR3 prevents airway hyperresponsiveness and inflammation. Eur Respir J. 2008;31:783–789. doi: 10.1183/09031936.00111507. [DOI] [PubMed] [Google Scholar]
- 35.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kannan S, Huang H, Seeger D, Audet A, Chen Y, Huang C, Gao H, Li S, Wu M. Alveolar epithelial type II cells activate alveolar macrophages and mitigate P. Aeruginosa infection. Plos One. 2009;4:e4891. doi: 10.1371/journal.pone.0004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu M, Sherwin T, Brown WL, Stockley PG. Delivery of antisense oligonucleotides to leukaemia cells by RNA bacteriophage capsids. Nanomedicine. 2005;1:67–76. doi: 10.1016/j.nano.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Kannan S, Audet A, Huang H, Chen LJ, Wu M. Cholesterol-Rich Membrane Rafts and Lyn Are Involved in Phagocytosis during Pseudomonas aeruginosa Infection. J Immunol. 2008;180:2396–2408. doi: 10.4049/jimmunol.180.4.2396. [DOI] [PubMed] [Google Scholar]
- 39.Wu M, Stockley PG, Martin WJ., II An improved Western blotting effectively reduces the background. Electrophoresis. 2002;23:2373–2376. doi: 10.1002/1522-2683(200208)23:15<2373::AID-ELPS2373>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 40.Wu M, Kelley MR, Hansen WK, Martin WJ., II Reduction of BCNU toxicity to lung cells by high-level expression of O6-methylguanine-DNA methyltransferease. Am J Physiol Lung Cell Mol Physiol. 2001;280:L755–L761. doi: 10.1152/ajplung.2001.280.4.L755. [DOI] [PubMed] [Google Scholar]
- 41.Li GP, Liu ZG, Qiu J, Ran PX, Zhong NS. DNA vaccine encoding Der p 2 allergen generates immunologic protection in recombinant Der p 2 allergen-induced allergic airway inflammation mice model. Chin Med J (Engl) 2005;118:534–540. [PubMed] [Google Scholar]
- 42.de Souza-Pinto NC, Eide L, Hogue BA, Thybo T, Stevnsner T, Seeberg E, Klungland A, Bohr VA. Repair of 8-oxodeoxyguanosine lesions in mitochondrial dna depends on the oxoguanine dna glycosylase (OGG1) gene and 8-oxoguanine accumulates in the mitochondrial dna of OGG1-defective mice. Cancer Res. 2001;61:5378–5381. [PubMed] [Google Scholar]
- 43.Bartz RR, Suliman HB, Fu P, Welty-Wolf K, Carraway MS, MacGarvey NC, Withers CM, Sweeney TE, Piantadosi CA. Staphylococcus aureus sepsis and mitochondrial accrual of the 8-oxoguanine DNA glycosylase DNA repair enzyme in mice. Am J Respir Crit Care Med. 2011;183:226–233. doi: 10.1164/rccm.200911-1709OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashiguchi K, Stuart JA, de Souza-Pinto NC, Bohr VA. The C-terminal alphaO helix of human Ogg1 is essential for 8-oxoguanine DNA glycosylase activity: the mitochondrial beta-Ogg1 lacks this domain and does not have glycosylase activity. Nucleic Acids Res. 2004;32:5596–5608. doi: 10.1093/nar/gkh863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bacsi A, Chodaczek G, Hazra TK, Konkel D, Boldogh I. Increased ROS generation in subsets of OGG-1 knockout fibroblast cells. Mech Ageing Dev. 2007;128:637–649. doi: 10.1016/j.mad.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacDonald JI, Possmayer F. Stimulation of phosphatidylcholine biosynthesis in mouse MLE-12 type-II cells by conditioned medium from cortisol-treated rat fetal lung fibroblasts. Biochem J. 1995;312:425–431. doi: 10.1042/bj3120425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fang X, Song Y, Hirsch J, Galietta LJ, Pedemonte N, Zemans RL, Dolganov G, Verkman AS, Matthay MA. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L242–249. doi: 10.1152/ajplung.00178.2005. [DOI] [PubMed] [Google Scholar]
- 48.Fukunaga M, Gon Y, Nunomura S, Inoue T, Yoshioka M, Hashimoto S, Ra C. Protease-mediated house dust mite allergen-induced reactive oxygen species production by neutrophils. Int Arch Allergy Immunol. 2011;155:104–109. doi: 10.1159/000327492. [DOI] [PubMed] [Google Scholar]
- 49.Simone S, Gorin Y, Velagapudi C, Abboud HE, Habib SL. Mechanism of oxidative DNA damage in diabetes: tuberin inactivation and downregulation of DNA repair enzyme 8-oxo-7,8-dihydro-2′-deoxyguanosine-DNA glycosylase. Diabetes. 2008;57:2626–2636. doi: 10.2337/db07-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazzarella G, Bianco A, Catena E, De Palma R, Abbate GF. Th1/Th2 lymphocyte polarization in asthma. Allergy. 2000;55:6–9. doi: 10.1034/j.1398-9995.2000.00511.x. [DOI] [PubMed] [Google Scholar]
- 51.Alcorn JF, Crowe CR, Kolls JK. TH17 Cells in Asthma and COPD. Annu Rev Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 52.Arasteh J, Pourpak Z, Ebtekar M, Pourfathollah AA, Hassan ZM, Farahmandian T, Mahmoudzadeh NH. Evaluation of the Effect of IL-22 on Human Cord Blood CD4+ T Cells. Iran J Allergy Asthma Immunol. 2010;9:59–67. [PubMed] [Google Scholar]
- 53.Jaiswal M, LaRusso NF, Shapiro RA, Billiar TR, Gores GJ. Nitric oxide-mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology. 2001;120:190–199. doi: 10.1053/gast.2001.20875. [DOI] [PubMed] [Google Scholar]
- 54.Pernis AB, Rothman PB. JAK-STAT signaling in asthma. J Clin Invest. 2002;109:1279–1283. doi: 10.1172/JCI15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Datta R, Naura AS, Zerfaoui M, Errami Y, Oumouna M, Kim H, Ju J, Ronchi VP, Haas AL, Boulares AH. PARP-1 deficiency blocks IL-5 expression through calpain-dependent degradation of STAT-6 in a murine asthma model. Allergy. 2011;66:853–861. doi: 10.1111/j.1398-9995.2011.02549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoshino A, Tsuji T, Matsuzaki J, Jinushi T, Ashino S, Teramura T, Chamoto K, Tanaka Y, Asakura Y, Sakurai T, et al. STAT6-mediated signaling in Th2-dependent allergic asthma: critical role for the development of eosinophilia, airway hyper-responsiveness and mucus hypersecretion, distinct from its role in Th2 differentiation. Int Immunol. 2004;16:1497–1505. doi: 10.1093/intimm/dxh151. [DOI] [PubMed] [Google Scholar]
- 57.Janssen-Heininger YM, Poynter ME, Aesif SW, Pantano C, Ather JL, Reynaert NL, Ckless K, Anathy V, van der Velden J, Irvin CG, et al. Nuclear factor kappaB, airway epithelium, and asthma: avenues for redox control. Proc Am Thorac Soc. 2009;6:249–255. doi: 10.1513/pats.200806-054RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crapo JD. Oxidative stress as an initiator of cytokine release and cell damage. Eur Respir J. 2003;44:4s–6s. doi: 10.1183/09031936.03.00000203a. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L, Wang M, Kang X, Boontheung P, Li N, Nel AE, Loo JA. Oxidative Stress and Asthma: Proteome Analysis of Chitinase-like Proteins and FIZZ1 in Lung Tissue and Bronchoalveolar Lavage Fluid. J Proteome Res. 2009;8:1631–1618. doi: 10.1021/pr800685h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leem JH, Kim JH, Lee KH, Hong Y, Lee KH, Kang D, Kwon HJ. Asthma attack associated with oxidative stress by exposure to ETS and PAH. J Asthma. 2005;42:463–467. doi: 10.1080/02770900500200733. [DOI] [PubMed] [Google Scholar]
- 61.Dharajiya N, Choudhury BK, Bacsi A, Boldogh I, Alam R, Sur S. Inhibiting pollen reduced nicotinamide adenine dinucleotide phosphate oxidase-induced signal by intrapulmonary administration of antioxidants blocks allergic airway inflammation. J Allergy Clin Immunol. 2007;119:646–653. doi: 10.1016/j.jaci.2006.11.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Collins AR, Harrington V, Drew J, Melvin R. Nutritional modulation of DNA repair in a human intervention study. Carcinogenesis. 2003;24:511–515. doi: 10.1093/carcin/24.3.511. [DOI] [PubMed] [Google Scholar]
- 63.Kauffman HF, Tamm M, Timmerman JA, Borger P. House dust mite major allergens Der p 1 and Der p 5 activate human airway-derived epithelial cells by protease-dependent and protease-independent mechanisms. Clin Mol Allergy. 2006;45 doi: 10.1186/1476-7961-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shin SH, Ye MK. Th2 responses elicited by nasal epithelial cells exposed to house dust mite extract. Clin Exp Otorhinolaryngol. 2009;2:175–180. doi: 10.3342/ceo.2009.2.4.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Osterlund C, Grönlund H, Gafvelin G, Bucht A. Non-Proteolytic Aeroallergens from Mites, Cat Dog Exert Adjuvant-Like Activation of Bronchial Epithelial Cells. Int Arch Allergy Immunol. 2010;155:111–118. doi: 10.1159/000318743. [DOI] [PubMed] [Google Scholar]
- 66.Stacey MA, Sun G, Vassalli G, Marini M, Bellini A, Mattoli S. The allergen Der p1 induces NF-kappaB activation through interference with IkappaB alpha function in asthmatic bronchial epithelial cells. Biochem Biophys Res Commun. 1997;236:522–526. doi: 10.1006/bbrc.1997.6997. [DOI] [PubMed] [Google Scholar]