Abstract
In the peripheral immune system, IL-2 is essential for immune homeostasis, normal T regulatory cell function, and self-tolerance. IL-2 knockout (IL-2KO) mice develop spontaneous autoimmunity characterized by increased T cell trafficking to multiple organs. The IL-2 gene is also expressed in the brain, and in vitro studies have shown that IL-2 is a potent modulator of acetylcholine release from septohippocampal neurons and exerts trophic effects on septal neurons in culture. We previously described the apparent loss of cholinergic cell bodies in the medial septum of IL-2KO mice. Here we investigated if loss of brain-derived IL-2, or autoimmunity stemming from loss of peripheral IL-2, is responsible for the alteration in choline acetyltransferase (ChAT) expression in the medial septum of IL-2KO mice. To accomplish this objective, we compared ChAT-positive neurons between wild-type (WT) mice, IL-2KO, and congenic mice with a double gene deletion for the IL-2 and the recombinase activating gene-2 (RAG-2) which are referred to as IL-2KO/RAG-2KO mice (congenic mice which lack mature T and B cells as well as peripheral and brain-derived IL-2). We found that the loss of ChAT staining did not coincide with an overall loss of cells in the medial septum, suggesting that loss of brain IL-2 results in a change in cholinergic phenotype unrelated to cell death. No differences were noted in the endogenous expression of cytokines and chemokines tested in the medial septum. Evaluation of BDNF and NGF levels between WT and IL-2KO mice in medial septal homogenates revealed that IL-2KO mice have markedly higher levels of NGF in the medial septum compared to WT mice. Our findings suggest that brain-derived IL-2 plays an essential role in the maintainance of septohippocampal projection neurons in vivo.
Keywords: Interleukin-2, IL-2, knockout, neuroimmunology, congenic mice, T cells, autoimmunity, brain, medial septum, cholinergic, neurotrophins, cytokines, chemokines
Interleukin-2 (IL-2) has been implicated in the pathogenesis of several major neurological and neuropsychiatric disorders including multiple sclerosis, Alzheimer’s disease and schizophrenia [10, 20]. The indispensable role of IL-2 for normal immune system functioning was discovered when IL-2 knockout (IL-2KO) mice demonstrated that IL-2 deficiency results in increased T cell trafficking and autoimmunity to multiple organ systems [12, 17, 24], and by research showing that IL-2 is essential for immune homeostasis, normal T regulatory cell function, and self-tolerance [21, 27]. IL-2 is also expressed by brain cells. IL-2 receptors are enriched in the septohippocampal system where the cytokine has been shown to have trophic effects on fetal septal and hippocampal neurons, and have potent effects on acetylcholine release from septohippocampal cholinergic neurons [1, 11, 22, 23, 26]. In addition to IL-2’s actions in the immune and central nervous systems, we have found that loss of brain IL-2 gene expression results in dysregulation of the brain’s endogenous neuroimmunological milieu (e.g., alterations in the normal balance of cytokines and chemokines), and that such effects may be involved in initiating processes that lead to central nervous system (CNS) autoimmunity [4, 13–15].
We found previously that compared to wild-type (WT) littermates, adult IL-2 deficient mice had a marked reduction of choline acetyltransferase (ChAT) positive medial septum/diagonal band of Broca (MS/vDB) cell bodies [3]. This loss of ChAT-positive neurons was selective for medial septum, as the cholinergic phenotype of WT and IL-2KO mice did not differ in the number of ChAT-positive neurons in the striatum, and GABAergic neurons in the MS/vDB did not differ between WT and IL-2KO mice [2]. Central versus peripheral immunological contributions on brain development and neuropathology are not well understood. Neuroimmunology studies revealed that T lymphocytes can have important effects on CNS neurons, and normal peripheral T cell function has been found to be essential for the preservation of the phenotype of injured motoneurons [7, 16, 25]. We previously found in IL-2KO mice that there is a marked infiltration of T cells to the brain that mirrors, in relative magnitude, the progression of autoimmunity in the periphery [13]. In the present study, we sought to test the hypothesis that the loss of quantifiable medial septal cholinergic neurons in IL-2KO mice is due to the loss of cholinergic phenotype rather than neuronal cell loss, and that the loss of phenotype is due to loss of brain-derived IL-2 rather than changes in neuroimmune status or T cell infiltration. In experiment 1, we sought to determine if the loss ChAT-positive neurons in the medial septum was due to loss of central (brain-derived) IL-2, peripheral IL-2 (autoimmunity), or a combination of both factors. To accomplish this objective, in experiment 1 we compared ChAT-positive neurons between WT mice, IL-2KO and congenic mice with a double gene deletion for the IL-2 and the recombinase activating gene-2 (RAG-2) – referred to as IL-2KO/RAG-2KO. These double knockout IL-2KO/RAG-2KO congenic mice have peripheral immunodeficiency resulting from the absence of mature T and B cells associated with the loss of both RAG-2 gene alleles, and also have both IL-2 gene alleles deleted. In experiment 2, we determined if the loss of the IL-2 gene resulted in changes in the endogenous expression of cytokines and chemokines in the medial septum. In experiment 3, we quantified total neurons in the medial septum to test our working hypothesis that the marked reduction of ChAT-positive neurons in the medial septum of IL-2KO mice is due to the loss of the cholinergic phenotype, rather than neuronal cell loss. Exploring a potential mechanism for downregulation of cholinergic phenotype [18, 28, 29], in experiment 3 we also quantified BDNF and NGF in the medial septum to assess how levels of these neurotrophic factors correlate with changes in ChAT-positive neurons in the medial septum of IL-2KO mice.
Mice used in these experiments were 8–12 weeks of age, and were matched for age and balanced for sex. IL-2KO mice were bred in our colony using IL-2 heterozygote by IL-2 heterozygote crosses as described previously [13]. IL-2KO/RAG-2KO mice were bred in our colony using recombinase activating gene 2 knockout (RAG-2KO) mice that were originally obtained from Taconic farms. The RAG-2 protein is necessary for the recombination of T cell receptors and immunoglobulins, therefore, RAG-2KO mice fail to develop a mature and functional T and B cells. The breeding of these congenic mice was performed as described previously by our lab, where IL-2 heterozygotic mice where bred with RAG-2KO mice, producing mice with both IL-2 and RAG-2 alleles deleted - referred to here as IL-2KO/RAG-2KO [14]. All mice used in study were on C57BL/6 background. Genotypes of mice were determined by PCR as described previously [14]. Statistical analyses for these studies were performed using analysis of variance (ANOVA), and post-hoc comparisons were performed using Fisher’s post-hoc analysis.
Mice were anesthetized by a 0.5mg/ml ketamine cocktail in a 3:3:1 ratio (ketamine/xylazine/acepromazine) and were perfused with 4% buffered formaldehyde. Brains were dissected, post-fixed for 2 h, and cryoprotected in 30% sucrose overnight. Tissue was snap frozen in isopentane and stored at −80°C. Coronal sections were cut through the brain and brainstem at a thickness of 40µm. Sections were collected in 0.1 M phosphate buffered saline (PBS) and immediately used in staining protocol. Tissue sections were incubated in normal goat serum (Vector; 1:30 in PBS) for 1 hour at room temperature followed by overnight incubation at 4°C with the primary antibodies rabbit anti-ChAT (Chemicon; 1:2000 in PBS with 0.3% Triton-X-100 and 1% normal goat serum (NGS)), or rabbit anti-beta-III tubulin (Chemicon; 1:1000 in PBS with 0.3% Triton-X-100 and 1% NGS, 200 µl/well). Sections were washed and incubated overnight in the secondary antibody, biotinylated goat anti-rabbit IgG (Sigma B-7389; 1:1000 dilution in PBS with 0.3% Triton-X-100 and 1% NGS). The sections were then washed and incubated in ExtrAvidin (Sigma E-2886; 1:1000 in PBS) for 2 h. The sections were developed in 0.5 mg/ml 3,3′-diaminobenzidine (DAB), 0.2 mg/ml urea H2O2 for approximately 5 min and were placed on slides, dehydrated in graded ethanol washes, cleared in two changes of xylenes, and coverslipped.
Septal homogenates were analyzed from IL-2KO and WT mice to compare cytokine levels in the septum as described previously [4]. The brains of saline perfused mice were removed, snap frozen, and then allowed to equilibrate to −20 °C. The brains were sectioned on a cryostat at −20 °C at 400 µm thickness and the septum was dissected from sections with a 0.75 mm micropunch on a −20 °C freezing platform. The dissected tissue was weighed on a microgram scale and then transferred to 25 µl of homogenizing solution (500 mM NaH2PO4/Na2HPO4 buffer and 0.2% TX-100 in H2O with anti-protease complete TM cocktail (Boehringer) per mg of wet weight tissue). The tissue was sonicated in the homogenizing solution for 30 s on ice and centrifuged at 16,000 × g for 15 min at 4 °C. The supernatant was collected and stored at −20 °C for Luminex analysis. Multiplex microsphere cytokine analysis was performed to measure a number of cytokines in the septum of IL-2KO and WT mice using Lincoplex mouse cytokine (Linco, Research, Inc) and Luminex 100 LabMAP system (Upstate Biotechnology) kits. Assays were performed according to the manufacturer's instructions, and cytokine concentrations were calculated using the Softmax program and the linear range on the standard curve (3.2–10,000 pg/ml). Altogether, we attempted to detect a total of 22 different cytokines and chemokines. In medial septal homogenates, among all animal subjects tested (WT and IL-2KO combined for experiment 2, where cytokine and chemokine assessments were made) there were detectable levels for the following cytokines and chemokines. Weight adjusted values (mean ± S.E.M.) used for statistical analyses were: IL-12 (8.98 ± .72), IL-15 (7.72 ± .69), IL-7 (5.51 ± .50), IL-9 (20.41 ± 6.39), interferon-gamma inducible protein of 10 kD: IP-10 (13.70 ± 1.25), and monocyte chemoattractant protein-1: MCP-1 (11.50 ± 1.11). Two cytokines were detectable but were below the linear range of the standard curve; these were IL-1α (2.14. ± .19), and IL-6 (2.83 ± .25). The remainder of the cytokines and chemokines tested could not be detected, these were: IFN-γ, TNF-α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17, kerotinocyte-derived chemokines (KC), granulocyte-stimulating factor (G-CSF), macrophage inflammatory protein-1 alpha (MIP-1), and RANTES. Enzyme-linked immunosorbent assay (ELISA) measurements of NGF and BDNF were analyzed in homogenates from the medial septum using a commercially available Emax immunoassay system according to the manufacturer's instructions (Promega) [2]. The data were reported as pg of protein per mg wet weight tissue.
For quantification of stained neuronal somata of the medial septum cells were counted using the software MCID 5.1 and the three-dimensional counting box (optical dissector) method described by Williams and Rakic [30] as described previously by our lab using 40 µm sections [2, 3]. All stereology was performed using a CCD High Resolution Sony camera and a Zeiss Axioplan 2 microscope with a motorized x–y stage made by Imaging Research, Inc. The latter is capable of making movements as fine as 0.1 µm. Every third section through the anterior-posterior extent of the septal region was sampled. The regions to be counted were outlined at 10× magnification and the size of the counting boxes were generated to be approximately 5% of the most rostral, and therefore, smallest, area of the medial septum (defined by the section where the corpus collosum first joins in the midline). The size of the outlined count regions, but not the counting box, varied depending on where the individual section was taken from the rostral to caudal extent of the medial septum. The defined counting box was approximately 2–2.5% of the outlined count area of the largest single section of the medial septum. Quantification of ChAT positive neurons was performed on 20 µm sections stored at −80°C that were used to assess T cells and microglia from other brain regions for other ongoing studies in our lab, and the neuronal assessments were done as described previously in our lab for comparing relative difference between groups [8]. Planimetric counting methods were used due to section thickness. A total 30 sections throughout the entire medial septum per animal were collected. Eight sections per animal (approximately 1/4 of the entire medial septum), were used to quantify the number of cholinergic neurons. Sections were chosen throughout the medial septum at a fixed interval with every fourth section selected for quantification. ChAT-immunostained histological sections were processed for cell number quantification within the region of interest encompassing the individual left and right medial septal nuclei defined by a triangular shape that extended, dorsoventrally, from the apex of the medial septum to an imaginary line connecting the lower limits of the anterior commissures on each hemisphere and, medio-laterally, from the midline to the outer limits of the medial septal area [19]. Briefly, color images were taken with a SPOT digitial camera at 10× magnification. ImageJ (National Institutes of Health) was used to view the images and perform the planimetric cell counting.
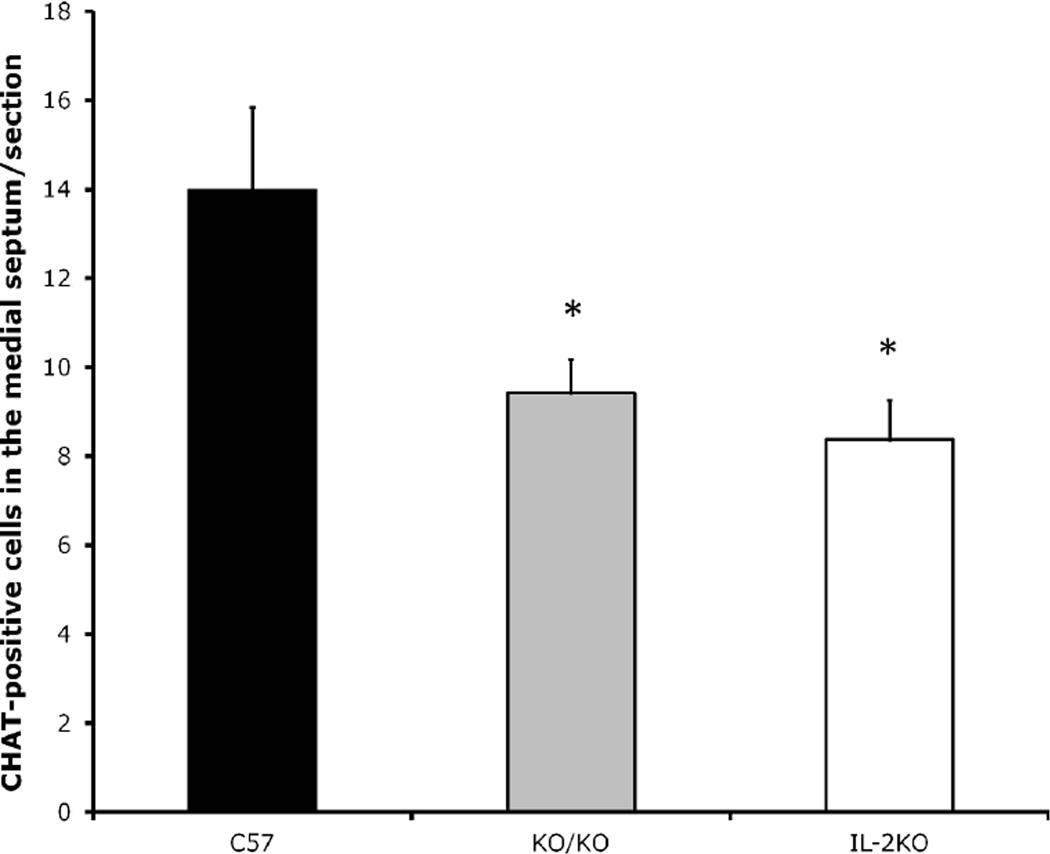
In experiment 1, we compared ChAT-positive medial septal neurons between WT, IL-2KO and congenic IL-2KO/RAG-2KO mice. As seen in Figure 1, there was a significant main effect of subject group [F(2,10)=38.9, p< .05]. Post-hoc analsyes comfirmed that IL-2KO and congenic IL-2KO/RAG-2KO mice did not differ from one another, however, both of these subject groups had significantly lower ChAT-positive neuron numbers than WT mice (p<.05).
Figure 1.
Quantification of ChAT+ cells/section (20µm) in the mouse medial septum of subject groups at 8 weeks of age. Bars represent the mean ± S.E.M. for WT (C57), IL-2KO/RAG-2KO (KO/KO), and IL-2KO mice. N=5 mice/group. *p< .05.
In experiment 2, to determine if the loss of the IL-2 gene resulted in changes in the endogenous expression of cytokines and chemokines in the medial septum, we compared levels of cytokines and chemokines between WT (n=8) and IL-2KO (n=7) mice. As noted earlier (see methods above), in medial septal homogenates, there were detectable levels of IL-12, IL-15, IL-7, IL-9, IP-10, and MCP-1. There were not differences between WT and IL-2KO for any of these cytokines or chemokines. Two cytokines, IL-1α and IL-6, we detectable but below the linear range of the standard curve; for comparative purposes ANOVA analyses were performed for these two cytokines as well, and confirmed that they did not differ between WT and IL-2KO mice.
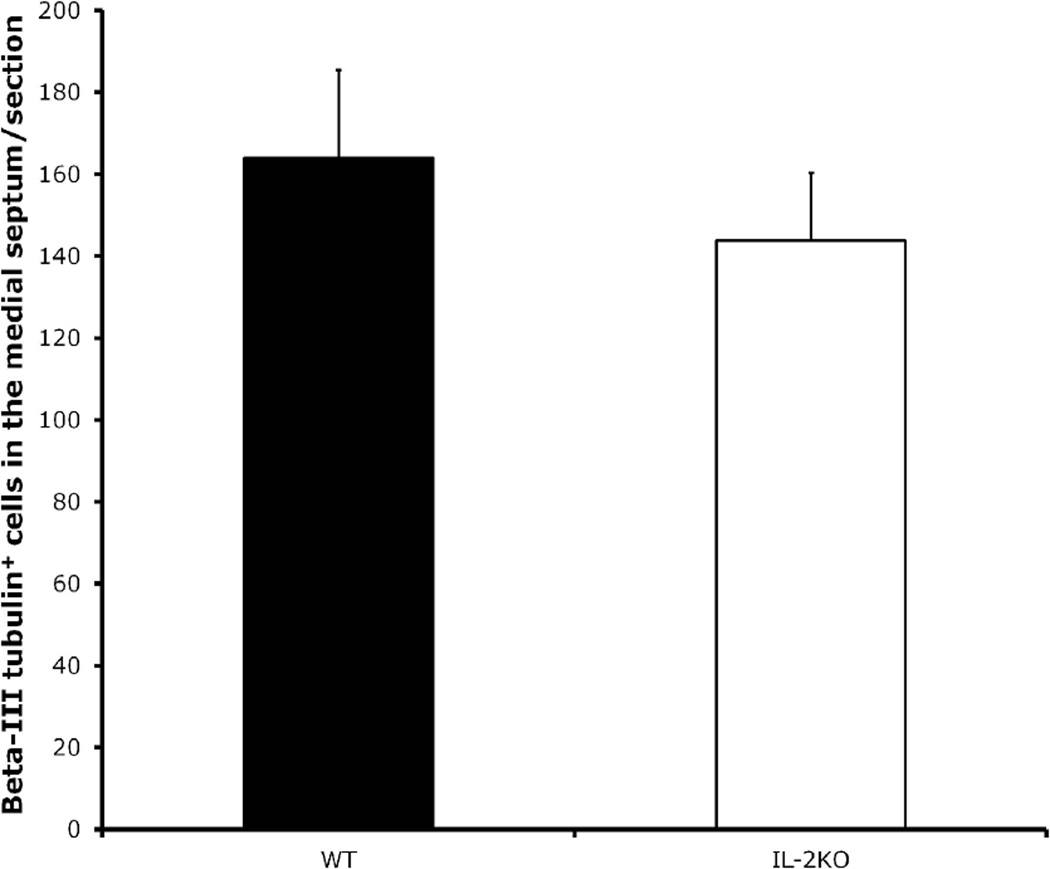
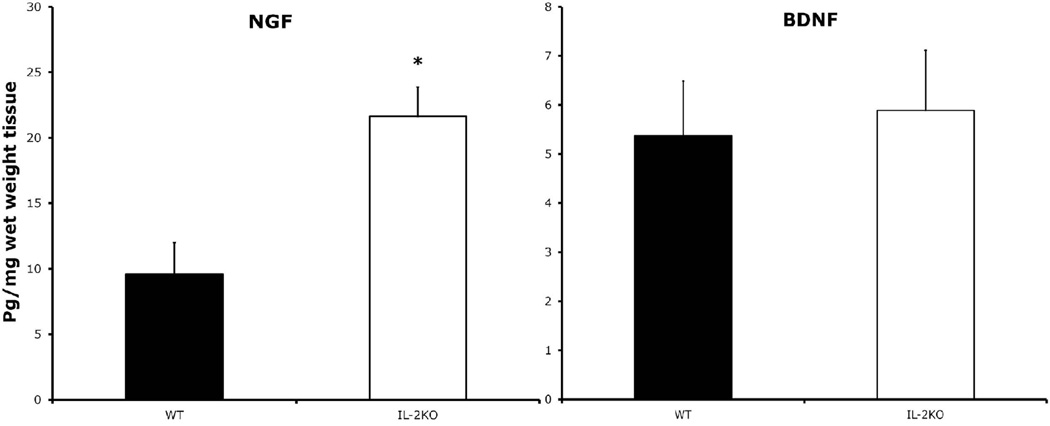
In experiment 3, we quantified total neurons in the medial septum by counting cells positive for the pan-neuronal marker beta-III tubulin between WT (n=5) and IL-2KO (n=5) mice, to test the hypothesis that the marked reduction of ChAT-positive neurons in the medial septum of IL-2KO mice is due to the loss of the cholinergic phenotype. As seen in Figure 2, we found that there were not differences in beta-III tubulin stained neurons between the WT and IL-2KO mice. We also compared BDNF and NGF levels between WT and IL-2KO mice in medial septal homogenates. Figure 3 shows the results of the comparison of these neurotrophins between the groups. As seen in Figure 3, IL-2KO mice had markedly higher levels of NGF in the medial septum compared to WT mice [F(1,11)=13.3, p<.005]. For BDNF, however, there were no differences between these subject groups.
Figure 2.
Quantification of beta-III tubulin+ cells/section (40µm) in the medial septum of the subject groups at 8 weeks of age. Bars represent the mean ± S.E.M. for WT and IL-2KO mice. N=5 mice/group.
Figure 3.
Comparison of NGF (left) and BDNF (right) protein levels in the medial septum of IL-2KO mice (n=7) vs. WT littermates (n=6). Bars represent mean ± S.E.M. * p<.005.
Consistent with our hypothesis, the results of these quantitative experiments show that the reduction in stained neurons in the medial septum is not due to cell loss, but to a change in cholinergic phenotype. Since IL-2KO mice do not produce brain IL-2, but have peripheral T cell dysregulation and autoimmunity from the loss of IL-2 in the peripheral immune system, we needed to determine if the reduction in medial septal ChAT-positive neurons was the result of the peripheral immune alterations associated with the loss of peripheral IL-2. Using IL-2KO/RAG-2KO mice that lack a functional immune system, we established that the loss of ChAT-positive cells in the medial septum is due to loss of brain-derived IL-2 rather than the peripheral immune dysregulation present in IL-2KO mice [13]. Quantitative comparisons of total cells in the medial septum of IL-2KO and WT mice revealed that the reduction in ChAT-positive cells in the medial septum is indicative of a change in cholinergic phenotype rather than cell death. Loss of the cholinergic phenotype by medial septal neurons has been recognized since the seminal study by Hagg and colleagues [9], who demonstrated that loss of medial septal cholinergic phenotype occurs following axotomy, and is reversible with intracerebroventricular infusion of NGF [9].
Surprisingly, although there were no differences in detectable cytokines and chemokines in the medial septum, we found previously that IL-2KO mice had alterations in the hippocampus [4]. The most parsimonious explanation is that loss of IL-2 modifies the neuroimmunological environment differently in different regions of the brain. As noted earlier, a number of chemokines and cytokines including some with known effects in the brain (e.g., IL-1β) were not detectable. Assessments of chemokines and cytokines in the septohippocampal system have been much more commonly been made by real-time PCR, a significantly more sensitive method. It may be possible to modify such protein measurements of chemokines and cytokines in the medial septum to increase the sensitivity of the assay (e.g., using a more concentrated brain homogenate, spiking the brain homogenate sample with known amounts of the protein of interest to raise the total level into the detectable range and then subtracting known from measured protein levels).
The neurotrophic factor that is specifically responsible for maintaining cholinergic phenotype, NGF, was elevated while BDNF levels were not abnormal in the absence of IL-2. IL-2 has been found to modify the expression of neurotrophin receptors in lymphocytes [5], however, the effects of IL-2 on NGF in the brain is unknown. Septal cholinergic neurons account for most of the cholinergic innervation of the hippocampus and play a key role in the regulation of hippocampal synaptic activity [18]. Since neuronal expression of neurotrophins is controlled by some neurotransmitters and there is a topographical correlation between neurotrophin expression and cholinergic terminal distribution from the cholinergic basal forebrain [31], it has been investigated whether cholinergic afferents regulate neurotrophin gene expression in the hippocampus. When cholinergic neurons were selectively and completely destroyed by intraventricular injection of 192 IgG-saporin, resulting in a cholinergic deafferentation of the hippocampus, there were no siginificant changes in NGF or BDNF mRNA levels from 1 week to 5 months after the lesion [31]. Similarly, in a developmental study using hippocampal slice culture, changes in neurotrophin expression in the excised hippocampus over time reflected the changes that occur in vivo [6]. These results suggest that cholinergic afferents may not play a significant role in maintaining basal levels of neurotrophin gene expression in the hippocampus, and that perhaps loss of IL-2, rather than the consequence of changes in cholinergic functionality, may be responsible for changes in neurotrophic environment. This dysregulation of hippocampal and medial septal neurotrophins may be, in part, responsible for the failure of cholinergic neuronal maintenance seen in the Ms/vDB of IL-2KO mice. Further studies are needed to determine the purported point of convergence of the IL-2 and neurotrophin signaling pathways.
In summary, the reduction of cholinergic cells in the medial septum of IL-2KO mice is due to loss of brain-derived IL-2 rather than neuroimmunological processes initiated by the peripheral T cell dysregulation and autoimmuny that develops in these mice. The loss of ChAT staining in the medial septum of IL-2KO mice did not coincide with loss of total neurons, suggesting that the failure to visualize these cells by ChAT immunohistochemistry is due to a down regulation of the protein and a consequent change in cholinergic phenotype. Lastly, we detected an increase in NGF in the medial septum that mirrored the increase we previously reported in the hippocampus [2]. This dysregulation of the neurotrophin environment in the septohippocampal pathway, in response to loss of brain-derived IL-2, is a likely candidate in the etiology of the observed changes in phenotype, or conversely, may play some compensatory function in providing cholinergic support. If the latter is true, and IL-2 deficiency has direct consequences on cholinergic function, we are compelled to hypothesize that endogenously produced brain IL-2 has a biologically significant role in maintaining cholinergic circuitry in the septohippocampal system. Given the effects of medial septal cholinergic projection neurons on aspects of cognition, and the supportive actions of neurotrophic factors on septohippocampal system function, our findings suggest that alterations in brain-derived IL-2 may play a role in neurodegenerative diseases (e.g., Alzheimer’s disease) [2–4, 9–10].
Hightlights.
Significant loss of choline acetyltransferase (ChAT) positive neurons in medial septum of IL-2KO mice
No difference in total cell number between IL-2KO mice and IL-2WT mice in medial septum
Decrease in ChAT staining due to loss of cholinergic phenotype in medial septum of IL-2KO mice rather than cell death
IL-2KO mice mice have higher levels of NGF in medial septum than IL-2WT
No difference in detectable cytokines and chemokines in medial septum of IL-2KO mice
Acknowledgements
Funding for this study was provided by: NIH RO1 NS055018 (JMP)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Awatsuji H, Furukawa Y, Nakajima M, Furukawa S, Hayashi K. Interleukin-2 as a neurotrophic factor for supporting the survival of neurons cultured from various regions of fetal rat brain. Journal of neuroscience research. 1993;35:305–311. doi: 10.1002/jnr.490350310. [DOI] [PubMed] [Google Scholar]
- 2.Beck RD, Jr, King MA, Ha GK, Cushman JD, Huang Z, Petitto JM. IL-2 deficiency results in altered septal and hippocampal cytoarchitecture: relation to development and neurotrophins. Journal of neuroimmunology. 2005;160:146–153. doi: 10.1016/j.jneuroim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Beck RD, Jr, King MA, Huang Z, Petitto JM. Alterations in septohippocampal cholinergic neurons resulting from interleukin-2 gene knockout. Brain research. 2002;955:16–23. doi: 10.1016/s0006-8993(02)03295-x. [DOI] [PubMed] [Google Scholar]
- 4.Beck RD, Jr, Wasserfall C, Ha GK, Cushman JD, Huang Z, Atkinson MA, Petitto JM. Changes in hippocampal IL-15, related cytokines, and neurogenesis in IL-2 deficient mice. Brain research. 2005;1041:223–230. doi: 10.1016/j.brainres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Besser M, Wank R. Cutting edge: clonally restricted production of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3 mRNA by human immune cells and Th1/Th2-polarized expression of their receptors. J Immunol. 1999;162:6303–6306. [PubMed] [Google Scholar]
- 6.Forster E, Otten U, Frotscher M. Developmental neurotrophin expression in slice cultures of rat hippocampus. Neuroscience letters. 1993;155:216–219. doi: 10.1016/0304-3940(93)90711-s. [DOI] [PubMed] [Google Scholar]
- 7.Ha GK, Huang Z, Petitto JM. Prior facial motor neuron injury elicits endogenous T cell memory: relation to neuroregeneration. Journal of neuroimmunology. 2007;183:111–117. doi: 10.1016/j.jneuroim.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ha GK, Huang Z, Streit WJ, Petitto JM. Endogenous T lymphocytes and microglial reactivity in the axotomized facial motor nucleus of mice: effect of genetic background and the RAG2 gene. Journal of neuroimmunology. 2006;172:1–8. doi: 10.1016/j.jneuroim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Hagg T, Manthorpe M, Vahlsing HL, Varon S. Delayed treatment with nerve growth factor reverses the apparent loss of cholinergic neurons after acute brain damage. Experimental neurology. 1988;101:303–312. doi: 10.1016/0014-4886(88)90013-1. [DOI] [PubMed] [Google Scholar]
- 10.Hanisch UK, Quirion R. Interleukin-2 as a neuroregulatory cytokine. Brain research. 1995;21:246–284. doi: 10.1016/0165-0173(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 11.Hanisch UK, Seto D, Quirion R. Modulation of hippocampal acetylcholine release: a potent central action of interleukin-2. J Neurosci. 1993;13:3368–3374. doi: 10.1523/JNEUROSCI.13-08-03368.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horak I. Immunodeficiency in IL-2-knockout mice. Clinical immunology and immunopathology. 1995;76:S172–S173. doi: 10.1016/s0090-1229(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 13.Huang Z, Dauer DJ, Ha GK, Lewis MH, Petitto JM. Interleukin-2 deficiency-induced T cell autoimmunity in the mouse brain. Neuroscience letters. 2009;463:44–48. doi: 10.1016/j.neulet.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Z, Meola D, Petitto JM. Dissecting the effects of endogenous brain IL-2 and normal versus autoreactive T lymphocytes on microglial responsiveness and T cell trafficking in response to axonal injury. Neuroscience letters. 2012;526:138–143. doi: 10.1016/j.neulet.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Z, Meola D, Petitto JM. Loss of CNS IL-2 gene expression modifies brain T lymphocyte trafficking: response of normal versus autoreactive Treg-deficient T cells. Neuroscience letters. 2011;499:213–218. doi: 10.1016/j.neulet.2011.05.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones KJ, Serpe CJ, Byram SC, Deboy CA, Sanders VM. Role of the immune system in the maintenance of mouse facial motoneuron viability after nerve injury. Brain, behavior, and immunity. 2005;19:12–19. doi: 10.1016/j.bbi.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I. Science. Vol. 262. New York, N.Y: 1993. Immune responses in interleukin-2-deficient mice; pp. 1059–1061. [DOI] [PubMed] [Google Scholar]
- 18.Lazo OM, Mauna JC, Pissani CA, Inestrosa NC, Bronfman FC. Axotomy-induced neurotrophic withdrawal causes the loss of phenotypic differentiation and downregulation of NGF signalling, but not death of septal cholinergic neurons. Molecular neurodegeneration. 2010:5. doi: 10.1186/1750-1326-5-5. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Coviella I, Mellott TJ, Schnitzler AC, Blusztajn JK. BMP9 protects septal neurons from axotomy-evoked loss of cholinergic phenotype. PloS one. 2011;6:e21166. doi: 10.1371/journal.pone.0021166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merrill JE. Interleukin-2 effects in the central nervous system. Annals of the New York Academy of Sciences. 1990;594:188–199. doi: 10.1111/j.1749-6632.1990.tb40478.x. [DOI] [PubMed] [Google Scholar]
- 21.Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol. 2004;172:3983–3988. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- 22.Petitto JM, Huang Z. Cloning the full-length IL-2/15 receptor-beta cDNA sequence from mouse brain: evidence of enrichment in hippocampal formation neurons. Regulatory peptides. 2001;98:77–87. doi: 10.1016/s0167-0115(00)00229-9. [DOI] [PubMed] [Google Scholar]
- 23.Sarder M, Saito H, Abe K. Interleukin-2 promotes survival and neurite extension of cultured neurons from fetal rat brain. Brain research. 1993;625:347–350. doi: 10.1016/0006-8993(93)91080-c. [DOI] [PubMed] [Google Scholar]
- 24.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz M, Moalem G. Beneficial immune activity after CNS injury: prospects for vaccination. Journal of neuroimmunology. 2001;113:185–192. doi: 10.1016/s0165-5728(00)00447-1. [DOI] [PubMed] [Google Scholar]
- 26.Seto D, Kar S, Quirion R. Evidence for direct and indirect mechanisms in the potent modulatory action of interleukin-2 on the release of acetylcholine in rat hippocampal slices. British journal of pharmacology. 1997;120:1151–1157. doi: 10.1038/sj.bjp.0701002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turka LA, Walsh PT. IL-2 signaling and CD4+ CD25+ Foxp3+ regulatory T cells. Front Biosci. 2008;13:1440–1446. doi: 10.2741/2773. [DOI] [PubMed] [Google Scholar]
- 28.van der Zee CE, Hagg T. Delayed NGF infusion fails to reverse axotomy-induced degeneration of basal forebrain cholinergic neurons in adult p75(LNTR)-deficient mice. Neuroscience. 2002;110:641–651. doi: 10.1016/s0306-4522(01)00606-6. [DOI] [PubMed] [Google Scholar]
- 29.Ward NL, Hagg T. BDNF is needed for postnatal maturation of basal forebrain and neostriatum cholinergic neurons in vivo. Experimental neurology. 2000;162:297–310. doi: 10.1006/exnr.1999.7346. [DOI] [PubMed] [Google Scholar]
- 30.Williams RW, Rakic P. Three-dimensional counting: an accurate and direct method to estimate numbers of cells in sectioned material. The Journal of comparative neurology. 1988;278:344–352. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]
- 31.Yu J, Pizzo DP, Hutton LA, Perez-Polo JR. Role of the cholinergic system in the regulation of neurotrophin synthesis. Brain research. 1995;705:247–252. doi: 10.1016/0006-8993(95)01169-2. [DOI] [PubMed] [Google Scholar]