Abstract
BACKGROUND
Substantial phenotypic overlap exists between fragile X syndrome (FXS) and autism, suggesting that FMR1 (the gene causing FXS) poses a significant risk for autism. Cross-population comparisons of FXS and autism therefore offer a potentially valuable method for refining the range of phenotypes associated with variation in FMR1. This study adopted a broader phenotype approach, focusing on parents who are at increased genetic liability for autism or FXS. Women who were carriers of FMR1 in its premutation state were compared with mothers of individuals with autism, and controls in an attempt to determine whether subtle features of the broad autism phenotype may express at elevated rates among FMR1 premutation carriers.
METHODS
The principal personality and language features comprising the broad autism phenotype (i.e., rigid and aloof personality, and particular patterns of pragmatic language use) were assessed among 49 premutation carriers who were mothers of individuals with FXS, 89 mothers of individuals with autism, and 23 mothers of typically developing individuals.
RESULTS
Relative to controls, the autism and premutation parent groups showed elevated rates of certain personality and language characteristics which have been described as constituting the broad autism phenotype.
CONCLUSIONS
Findings suggest partially overlapping personality and language profiles among autism and premutation parent groups, with rigid personality style and patterns of pragmatic language use emerging as features most clearly shared between groups. These results provide further evidence for the overlap of autism and FXS, and may implicate FMR1 in some of the subtle features comprising the broad autism phenotype.
Keywords: Autism, fragile X syndrome, fragile X premutation, FMR1, language, broad autism phenotype
As currently defined (1), there is no single “autism” but rather different etiological processes that converge in a similar clinical-behavioral endpoint. A known genetic cause can be identified in 10–20% of cases of autism, with ~10% of these being single-gene disorders associated with autism (2). The observation that autism occurs at elevated rates in certain single-gene disorders has prompted investigation of associated monogenic conditions as a paradigm for identifying gene and brain pathways potentially involved in the behavioral phenotype of autism. Fragile X syndrome (FXS) is the most common single-gene disorder associated with autism (3–7). Approximately 30% of individuals with FXS meet full diagnostic criteria for autism, and an additional 20% meet criteria for pervasive developmental disorder, not otherwise specified (PDD-NOS) (3, 5, 7–9). Of individuals diagnosed with autism 3 – 6% also have FXS (10, 11). Furthermore, the FMR1 premutation (i.e., CGG repeat length of the 5′ UTR of the FMR1 gene between 55–200) appears to confer risk to autism among carrier relatives of individuals with FXS (3, 12–15). Clifford et al. (3) reported 14% of boys and 5% of girls carrying the premutation had an autism spectrum disorder. Examining rates of autism in clinical referrals and their relatives, Farzin et al. (14) found that 73% of boys presenting clinically and 7% of males identified through cascade testing showed an autism spectrum disorder.
Because much is known about the molecular and neurobiological basis of FXS, and given its strong phenotypic overlap with autism, comparisons of autism and FXS have been pursued as a method for defining specific phenotypes associated with known genetic variation, in this case variation in the fragile X mental retardation gene (FMR1) (16) (17, 18). The protein encoded by FMR1, the Fragile X Mental Retardation Protein (FMRP), regulates a number of pathways associated autism (19–23), leading to specific hypotheses regarding role of dosage-sensitive genes encoding proteins involved in synaptic plasticity (23). Knowledge of the neuropathology and neurobiological abnormalities in FXS is also leading to the development of targeted treatment of symptoms in FXS, many of which could provide promising avenues for treating symptoms of autism as well (16).
In spite of such promising directions, definitive links between symptom overlap, underlying genetic variation, and neuropathology related to autism have not been established, and there exists some debate concerning the clinical validity of autism in FXS (7, 24), with suggestion that autism symptoms observed in FXS are a result of more severe impairment and intellectual disability overall. In line with this argument, some studies of brain morphology in autism and FXS have suggested distinct neuroanatomical profiles in these groups (25–27), demonstrating that different brain changes could give rise to similar behavioral phenotypes.
The study of relatives who are at increased genetic liability (and who in the case of FXS, will be carriers of FMR1 in its premutation state) may complement studies of individuals affected with autism and FXS, and help to address how FMR1 may play a role in the broad spectrum of autism related features. Phenotypes among unaffected relatives are less likely to be obscured by comorbidities, such as intellectual disability, and can offer a glimpse at the distilled expression of genetic liability, thereby affording more straightforward examination of gene-behavior relationships. Adopting such an approach, this study builds on the well-documented observation that genetic liability to autism appears to manifest among unaffected relatives through features that are milder but qualitatively similar to the defining characteristics of autism, described as constituting a broad autism phenotype (BAP) (28–32). Such features include social reticence/aloof personality, inflexibility/rigid personality, overly-conscientiousness, and particular profiles of pragmatic (social) language. These features are similar in quality to the component features of autism (social impairment, restricted interests and behaviors, and problems with social communication, respectively) but are typically subtly expressed among relatives, and tend not to be associated with functional impairment (33).
The premutation occurs in approximately 1 in 250 females and 1 in 800 males (34, 35). Unlike in FXS, in the premutation methylation does not typically occur, although higher repeat sizes have been associated with decreases in FMRP (12) and deficits of FMRP have been found in brain tissue of premutation mice (36). The primary known molecular mechanism in the premutation involves elevated mRNA, which leads to gain of function effect and toxicity to cells (37). RNA toxicity is implicated in both fragile X-associated tremor/ataxia syndrome (FXTAS) and premutation ovarian failure (FXPOI) (38), although it is still unclear precisely how it may lead to additional phenotypes documented among carriers.
As noted previously, elevated rates of autism among premutation carriers have been reported. Additionally, women with premutation alleles have been shown to display social-emotional profiles reminiscent of the aloof personality trait of the BAP described among autism relatives (39). Higher rates of detail-oriented, perfectionistic traits (as well as more significant psychopathology) have also been reported in both male and female premutation carriers (38, 40), supporting the hypothesis that variation in FMR1 could give rise to features associated with the BAP. This study investigated this hypothesis by comparing mothers of individuals with FXS who were confirmed carriers of the FMR1 premutation allele with mothers of individuals with autism (with and without the BAP), and controls along directly assessed, clinically defined personality and language features of the BAP. We also examined correlations between BAP features in parents and autism symptoms in children using a quantitative measure of autism traits.
This study focused on women because of the known inheritance pattern of FXS – i.e., all mothers of a child with FXS are carriers of the FMR1 premutation allele, unless they themselves have the full mutation. The autism and control parent groups were restricted to women to control for sex. By comparing these groups along established measures of the BAP, we aimed to help define the range of phenotypic characteristics associated with autism that could be linked with FMR1 variation. Evidence of the BAP among FMR1 premutation carriers would support the hypothesis that FMR1 variation may play a role in autism and contribute to current debates concerning the validity of autism in the context of FXS.
Methods
Participants
Participants included 49 mothers of children with FXS (premutation carriers), 89 mothers of children with autism, and 23 mothers of children with typical development. All participants were native speakers of English. There as no significant difference between groups in age, with the average age for all groups falling in the early- to mid-40s, means and standard deviations as follows: premutation carrier parents= 42.8 (8.2), autism parents = 45.6 (7.2), and control parents = 41.8 (8.3). Groups were also matched on race and SES (income).
FMR1 premutation group
Mothers of individuals with FXS (premutation carriers) were recruited from the Research Participant Registry Core of the Carolina Institute for Developmental Disabilities at UNC. Although both men and women can be carriers of premutation FMR1, to optimize recruitment and avoid drawing blood to determine carrier status of men, only mothers of children with FXS were included (i.e., all mothers of a child with full mutation FXS are premutation carriers). While men can be carriers and pass on premutation FMR1 to their daughters, FMR1 appears stable in males, and does not expand to the full mutation state in off-spring). Participants were ascertained through a child with confirmed full mutation expansion of the FMR1 5′ UTR CGG repeat of ≥200 copies. Medical records were obtained to confirm premutation alleles (i.e., between 55 and 200 CGG repeats) in mothers. Two individuals were identified as having the full mutation, and were excluded, resulting in a total of 49 women who were included in the study.
Autism parent group
Eighty-nine mothers of individuals with idiopathic autism participated in this study. The sample comprised a previously tested group of 46 mothers who had participated in a family study of the neuropsychological basis of autism and the BAP (41). An additional group of 43 mothers of individuals with autism were recruited 22 from the local the community and 21 from midwestern regions of the United States, in order to increase sample size for comparison with our all-female premutation parent group. For previously tested parents, a diagnosis of autism was established through diagnostic medical records and confirmed through administration of the ADOS and ADI. For the newly recruited group, diagnostic records were also obtained to confirm diagnoses of idiopathic autism. Autism symptomatology was also queried using the Autism Family History Interview (28) to confirm the presence of developmental delays and behaviors consistent with a diagnosis of autism. All children were screened for evidence of gross central nervous system impairment, and for FXS and other monogenetic conditions associated with autism.
Control parent group
A total of 23 mothers of typically developing children were included in the control group. Data for four mothers who had been previously tested for a prior study (41) were included. An additional 19 mothers of typically developing individuals were recruited from the community to serve as a comparison group. Mothers were recruited via brochures distributed throughout the community (e.g., at local child care centers) and mass email to campus employees. The Autism Family History Interview (28) was administered to probe developmental histories for all children, and for the participants’ own developmental history to confirm typical language, social and motor development, and no family history of autism or FXS or any language or cognitive delays. Only mothers of children who were at least 3 years of age were included to ensure that children were of a sufficient age to assess the presence of any language or cognitive delays.
Assessment of personality traits of the BAP
The Modified Personality Assessment Schedule (MPAS; (42)) was used to assess the personality traits of the BAP. This instrument has been used repeatedly to investigate personality features in the BAP (e.g., 31, 32, 43), and is incorporated within a broader, semi-structured interview that begins by soliciting autobiographical accounts used for rating pragmatic language (below) and substantiating personality assessments in the formal interview (e.g., questions about childhood friendships, experiences in school, jobs, spousal relationships). Subjects are then guided through a number of questions to probe personality characteristics relevant to autism and the BAP, namely, rigid personality, overly-conscientiousness, and socially aloof disposition. These traits correspond to the ritualistic/repetitive and social symptom domains of autism, respectively. Concrete behavioral examples are solicited to substantiate trait endorsement. Ratings are assigned by raters blind to group status using a three-point scale (absent, mild, or present and striking) based on operational definitions of each trait. All samples were rated independently by two coders who were blind to group status (JK served as primary coder, with one of two secondary coders). Coders underwent extensive training, including blinded coding of interviews from an extensive video library of practice interviews, discussion of constructs and item definitions, and consensus coding meetings of practice tapes prior to rating interviews from study participants. Disagreements were resolved through discussion and consensus scores were produced. Inter-rater reliability was good, with 87% agreement for aloof (κ = .37), 90% for rigid/inflexible (κ = .58), and 94% for overly-conscientious (κ = .90).
Whereas this interview can include both subject and informant assessments (involving the same questions addressed to either the subject, or an informant spouse or close friend), owing to time constraints, only the subject portion of this interview was administered in this study. Thus, all premutation carriers, control parents, and newly ascertained autism parents were administered the subject portion of the MPAS only. Interviews conducted with the 46 previously tested autism parents involved both subject and informant interviews, but to ensure comparability across samples only ratings of subject interviews were examined here. Furthermore, interviews from 15% of the previously coded autism sample were randomly selected and re-rated (using only the subject portion of the interviews) by the primary rater (JK), who was blind to all previous scores and group status. Agreement was as follows: aloof: 100% (κ = 1.0); rigid: 91.6% (κ = .63); and overly-conscientious: 100% (κ = 1.0). Because of the high agreement between the primary rater and the previously completed MPAS scores, scores for all autism parent participants were combined for analyses using subject interview ratings.
Assessment of pragmatic language features of the BAP
Pragmatic language was assessed from videotaped conversational samples from a semi-structured interview, in which the participant was asked questions about their life history. To ensure similar topics across interviews a series of probe questions were used that focused on common topics (e.g., “How did you meet your spouse?”). Interviewers were trained to elicit responses sufficient for coding relevant pragmatic language skills by following the participant’s conversational lead, commenting, and offering information as one would during a natural conversation.
Conversational samples were assessed for pragmatic language violations with the Pragmatic Rating Scale (PRS; (44)), which was designed to capture social language use among relatives of individuals with autism. This measure has been used in several family studies of autism and has been shown to reliably distinguish relatives of individuals with autism from controls (e.g., 32, 43). Items are rated on a 3-point scale, with a score of ‘0’ indicating no evidence of the language feature in question, ‘1’ indicating present but mild, and ‘2’ indicating present to a large degree. Objective criteria for each code were used to rate the frequency and severity of each item. As with coding of personality features, all samples were rated by two independent raters who were blind to group status. Where disagreements occurred, consensus scores were determined through discussion. Reliability was conducted prior to consensus discussions using two-way mixed Intra-Class Correlations, and indicated an ICC (3,1) of .626 with the primary rater and secondary rater #1, and an ICC (3,1) of .716 with the primary rater and secondary rater #2. Pragmatic language data were was not available for previously recruited autism parents due to changes in pragmatic language coding methodology for the present study.
Assessment of autism features in children
In order to obtain a quantitative measure of autism symptoms and severity in children for parent-child correlations, parents were administered the Social Responsiveness Scale (SRS) (45). The SRS is an informant-based questionnaire that assesses autism features, and social reciprocity in particular, on a single dimension, in affected and unaffected individuals.
Results
Personality features of the BAP
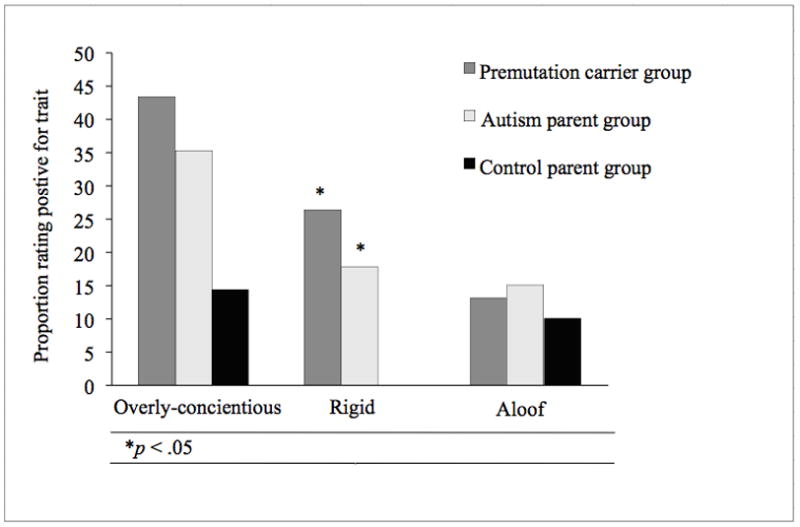
Personality features measured by the MPAS scores were analyzed as dichotomized variables by combining scores of 0 and 1 as ‘absent’ versus scores of 2 indicating the presence of a trait. All traits were more common among the autism and premutation carrier parent groups than in controls, and were relatively equally prevalent in the autism and premutation groups (see mean prevalence rates in Figure 1). Rates of personality characteristics between the parent groups were compared using simple frequency analysis with two-tailed tests. The relatively small sample size of the control group attenuated between-group differences, potentially masking significant effects. Nonetheless, the control group was significantly less likely to exhibit the rigid trait than either the autism (χ2 = 5.61, p < .05) or the premutation group (χ2 = 7.66, p < .05). The difference between control and premutation groups on overly conscientious was notable, but non-significant (χ2 = 3.64, p = .057). No significant differences were detected for the aloof trait. The autism and premutation parent groups did not differ significantly on any of the traits.
Figure 1.
Pragmatic language features of the BAP
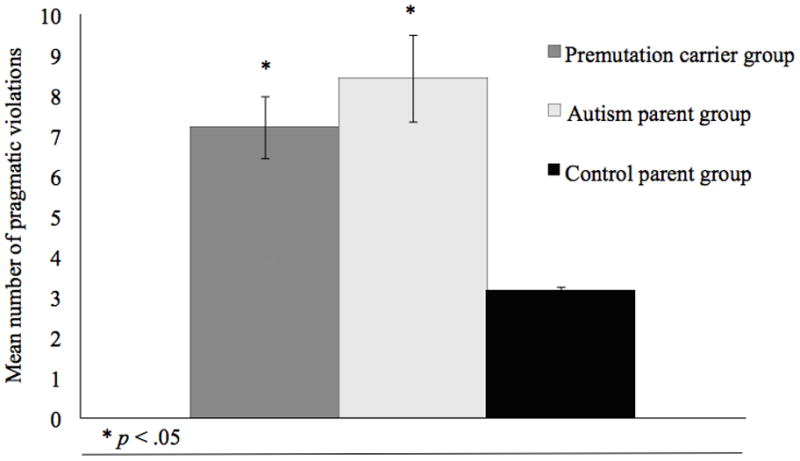
Analysis of variance was used to examine mean differences in the frequency of pragmatic language violations across groups. As illustrated in Figure 2, group differences were detected in the total frequency of pragmatic language violations (F (2, 94) = 7.36, p < .001), with both the autism and premutation parent groups scoring higher on the PRS (i.e., committing more pragmatic language violations) than controls (p values < .05, adjusted for multiple tests using Tukey-Kramer; (46)). The autism and premutation carrier parent groups did not differ significantly in the total frequency of pragmatic language violations.
Figure 2.
Factor Analysis of the PRS
Exploratory factor analysis (EFA) was conducted to investigate potential factors underlying the patterns of pragmatic language violations, and to explore whether patterns of pragmatic language errors were qualitatively similar across groups. EFA models were run in Mplus and fit under full information maximum likelihood estimation with a geomin rotation. Geomin is an oblique rotation which allows for correlated factors. The eigenvalues for the first three factors were 6.3, 4.3, and 1.9. The eigenvalues for the fourth and fifth factors were greater than one, a common criterion for the inclusion of those factors. However, examination of the scree plot showed a distinct leveling after three factors, thus a three factor model was determined to be the most appropriate.
As noted in methods, PRS items had three response categories (0, 1, 2) based on severity, and these codes were treated as categorical variables for EFA. In estimating factor analytic models with categorical data, polychoric correlations are used to produce the asymptotic covariance matrix for analysis (Joreskog, 1994). Mplus automates this procedure and it is done internally. Four variables were dropped from the analysis due to high collinearity with other variables in the model: “Unclear Intent”; “No background information”; and “Cannot clarify message.”
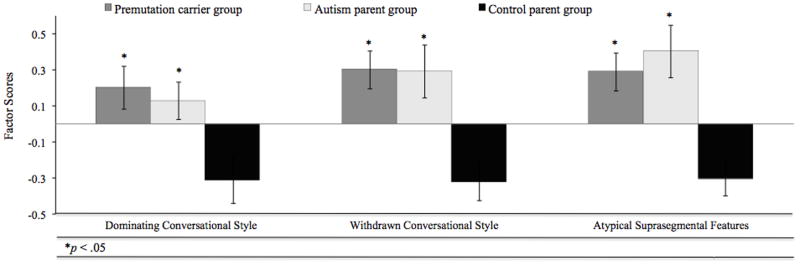
All three models had strong model fit (see Table 1). Although the two and three factor solutions did not provide statistically better fit than the one factor solution, the three factor solution provided the most theoretically meaningful constructs. The pattern of correlations among these variables, particularly the near zero correlation of factor one and factor two, as well as the relatively small correlation between factor one and factor three, further indicate that these factors are separate constructs, supporting a three-factor model. Factor loadings are presented in Table 2. Items associated with conversational dominance and verbosity tended to load together on a “Dominating” factor. A “Withdrawn” factor included items reflecting a failure to offer sufficient information in conversation. Finally, features related to the suprasegmental characteristics of speech (e.g., speech rate, volume) loaded on a third factor, “Atypical Suprasegmental”, although pedantic speech and lack of reciprocity in conversation also loaded on this factor.
Table 1.
Tests of model fit for factor analysis examining types of pragmatic language violations
| Chi-Square (df) | RMSEA | CFI | |
|---|---|---|---|
| 1 Factor | 655.74 (152)*** | .19 | .83 |
| 2 Factor | 160.56 (134) | .05 | .99 |
| 3 Factor | 112.05 (117) | .00 | 1.00 |
Table 2.
Factor loadings for three factor solution examining pragmatic language items
| Dominating Conversational Style Loading (SE) | Withdrawn Conversational Style Loading (SE) | Atypical Suprasegmental Style Loading (SE) | |
|---|---|---|---|
| Overly detailed | 0.82 (0.08) | −0.04 (0.13) | 0.07 (0.21) |
| Vague | −0.62 (0.14) | 0.73 (0.14) | 0.00 (0.01) |
| Tangential | 0.91 (0.08) | −0.03 (0.13) | 0.15 (0.2) |
| Overly frank | 0.70 (0.12) | 0.21 (0.17) | 0.00 (0.04) |
| Informal | 0.16 (0.15) | 0.62 (0.16) | −0.05 (0.25) |
| Pedantic | 0.14 (0.16) | −0.51 (0.19) | 0.54 (0.18) |
| Overly talkative | 0.89 (0.09) | −0.02 (0.06) | 0.25 (0.24) |
| Overly succinct | −0.65 (0.10) | 0.54 (0.14) | 0.11 (0.12) |
| No reciprocation | 0.01 (0.02) | 0.72 (0.16) | 0.47 (0.19) |
| Odd humor | 0.36 (0.16) | 0.21 (0.16) | 0.27 (0.21) |
| Topic preoccupation | 0.77 (0.09) | 0.21 (0.17) | 0.24 (0.14) |
| Atypical eye contact | −0.24 (0.25) | 0.07 (0.22) | 0.67 (0.16) |
| Interruptions | 0.38 (0.13) | 0.04 (0.14) | 0.43 (0.17) |
| Too loud | 0.59 (0.12) | 0.09 (0.22) | 0.07 (0.19) |
| Too soft | −0.73 (0.15) | −0.11 (0.29) | 0.68 (0.28) |
| Rate too fast/slow | −0.16 (0.21) | 0.01 (0.11) | 0.87 (0.18) |
| Atypical intonation | −0.42 (0.19) | 0.02 (0.12) | 0.69 (0.15) |
| Atypical rhythm | 0.00 (0.02) | −0.44 (0.27) | 0.69 (0.16) |
| Reformulations | 0.21 (0.16) | 0.00 (0.14) | 0.36 (0.17) |
In order to assess whether groups committed similar types of pragmatic language violations, factor scores were derived for each participant and compared across groups. Factor scores differed significantly for groups for each of the three factors: “Dominating” (F (2, 94) = 3.213, p < .05), “Withdrawn” (F (2, 94) = 6.67, p < .005), and “Atypical Suprasegmental” conversational styles (F (2, 94) = 7.44, p < .005). In each case, both the premutation and autism parent groups differed significantly from controls (p <.05), but did not differ from one another (p values >.50) (see Figure 3).
Figure 3.
Parent-Child Correlations
Autism symptoms in children assessed by the SRS were examined in relationship to personality and language features of the BAP among parents. Within the premutation group, a significant positive association was detected between children’s SRS scores and the presence of the rigid personality trait (r = .33, p < .05), and the frequency of pragmatic language violations (r = .46, p < .05) in parents. That is, premutation carriers who displayed the rigid personality feature and who exhibited language characteristics of the BAP tended to have children with more severe autism symptoms. No parent-child correlations were detected for the autism or control groups.
Discussion
The substantial phenotypic and etiologic heterogeneity in autism has hampered efforts to identify the causes of this serious neurodevelopmental disorder. Fragile X syndrome (FXS) and other single-gene disorders showing phenotypic overlap with autism provide a means of studying the autism phenotype in the context of a known genetic condition, where the identification of a candidate gene can provide a foothold for understanding gene-behavior relationships in autism (16). This study focused on carrier relatives, comparing blind ratings of directly assessed personality and language features of the broad autism phenotype (BAP) in mothers of children with FXS with mothers of children with autism and controls, with the aim of identifying features of the BAP that overlap with the FMR1 premutation phenotype, and which may be linked to variation in the FMR1 gene.
Findings indicated that some, but not all BAP characteristics occurred at significantly elevated rates among FMR1 premutation carrier women. Significantly higher rates of rigid personality were noted among the premutation and autism parent groups than controls. These groups also differed in their use of pragmatic language, with the autism and premutation groups committing significantly more pragmatic language violations than controls. Notably, comparisons of factor scores across the three factors (Dominating, Withdrawn, and Atypical Suprasegmental conversational styles) showed that the premutation and autism parent groups committed the same types of pragmatic language violations. Pragmatic language impairment is a hallmark of autism, and among the most consistently reported features of the BAP, making it noteworthy that premutation carriers showed both similar rates and types of errors as autism parents. Finally, significant parent-child correlations were detected in the premutation carrier group, where the presence of rigid personality, and the frequency of pragmatic language violations in mothers were both positively correlated with severity of autism symptoms in children with FXS. Similar associations were not detected in the autism parent group, perhaps owing to reduced variation in children’s scores on the SRS (i.e., all children had autism and scored within a limited range on the SRS).
Rates of aloof and overly-conscientious features did not differ significantly across groups, although it should be noted that both the premutation and autism parent groups exhibited rates of aloof (~15%) and overly-conscientious (~35–45%) that were comparable to those reported in prior studies of the BAP (e.g., 32) and elevated relative to controls (10% aloof, 14% overly-conscientious). The relatively small control group included here likely limited statistical power to detect differences. This study’s exclusive focus on women may also be an important consideration in evaluating the frequency of BAP traits in all groups, given that some studies have shown that features of the BAP may be more common among males (e.g., 47). Or it may simply be the case that autism-related phenotypes are not identical in these groups, with overlap confined to the pragmatic language and rigid/perfectionistic personality features. Because this study was the first to compare directly BAP features in premutation carriers and autism parents, and was thus exploratory in nature, the elevated (though nonsignificant) rates of each of the personality features studied should be studied further, particularly given that the aloof and overly-conscientious characteristics bear striking similarity to personality features previously documented among premutation carriers (38–40).
Whether the BAP is evident among FMR1 premutation carriers in a more limited way, or should it prove to be more extensively expressed upon further study, evidence of BAP traits among premutation carriers supports the validity of autism in FXS. These findings also add to knowledge of the range of phenotypes associated with the FMR1 premutation, once thought not to have any phenotypic expression. The clinical features of the BAP may also guide investigations of neuropsychological endophenotypes in autism and FXS. In parents of individuals with autism, for instance, social features of the BAP have been linked with performance on social cognitive tasks known to tap amygdala function, and which also differentiate individuals with autism from controls (41, 48). Imaging studies of men with the premutation have reported decreased amygdala volume, accompanied by decreased amygdala activation to social cognitive tasks (49, 50). Hessl et al. (50) also reported an association between reduced amygdala activation and decreased FMRP (above and beyond associations with elevated mRNA), providing important evidence of an impact of reduced FMRP without methylation in premutation carriers. Structural and functional differences have been observed in the hippocampus and prefrontal cortex (51–53) (with additional, more extensive cerebral and cellular abnormalities present in premutation carriers with FXTAS e.g., (52, 54)). Direct comparisons of cerebral structure and function in premutation carriers and parents of individuals with autism/the BAP could be particularly informative in defining neurological endophenotypes in the BAP and premutation, related to FMR1 and/or its interactors.
An important question concerns how exactly FMR1 variation may lead to features of the BAP, or autism for that matter. Evidence from a considerable literature indicates that FMR1 mutations in autism are not common, and most studies employing conventional methods for measuring and analyzing FMRP (the Fragile X Mental Retardation Protein encoded by FMR1 and implicated in the cognitive phenotype of FXS) have reported that FMRP is not altered among autistic individuals (though see reports from postmortem studies showing lower levels of FMRP in cerebella and superior frontal cortex in autistic individuals, compared to brains from matched controls (55, 56)). Thus, the mechanisms by which FMR1 variation leads to autistic-like phenotypes are not straightforward.
It may be the case that FMR1 variation causes a similar diathesis of symptoms in relatives as those genes thought to cause autism. Alternatively, FMR1 may act in an additive or epistatic manner with other autism susceptibility loci to produce features of autism and the BAP (7, 18). This possibility is consistent with the observation that mutations in FMR1 are associated with autism in only a portion of FXS cases. Similarly, studies of FMR1 knockout mice indicate that social phenotypes associated with autism only appear on certain genetic backgrounds (57), suggesting the importance of modifier genes. Our finding that autism symptoms in children with FXS (measured by the Social Responsiveness Scale) correlated with the presence of BAP features in premutation carrier parents supports the possible role of modifier genes in autism. Perhaps the subgroup of parent-child dyads showing the BAP in parents and symptoms of autism in children carried additional genetic variants contributing to these phenotypes.
Although these questions cannot be addressed directly with the present data, evidence exists to support the convergence of common biological pathways in FXS and autism. For instance, FMRP regulates the translation of many genes involved in synaptic plasticity which are also associated with autism (16). Darnell et al. (23) recently compared 842 FMRP known target genes with 117 autism candidate genes in the SFARI Gene database (a reference of all known human genes associated with autism) and found significant overlap with 28 FMRP targets, including well studied autism risk genes such as NLGN3, NRXN1, SHANK3, PTEN, TSC2, and NF1. Together, these molecular targets, and emerging findings on the wide-ranging role of FMRP in regulating synaptic function, with complementary studies of neuroanatomical functioning in FXS, the premutation, and autism may provide a guided path for investigating mechanisms underlying features of autism and the BAP.
Finally, it is important to consider the significance of findings for understanding of the clinical presentation of the FMR1 premutation, as the presence of BAP features among premutation carriers may have implications for clinical practice and research. These findings contribute to a growing literature documenting a range of phenotypes associated with the premutation in humans (12, 14, 38, 50–52, 54, 58–68). Understanding the nature and degree of phenotypic expression of the premutation can inform screening efforts for individuals who are carriers. Although the features of the BAP do not typically constitute any significant functional impairment, such features may influence social behavior and communication in ways that could be helpful for clinicians to understand in order to best to support these individuals in clinical discussions of their children, and their own psychiatric health. The presence of the BAP in premutation carriers might also be important to consider in studies of neurobiology and molecular genetics in the premutation, where knowledge of clinically distinct subgroups may help to specify mechanisms underlying various premutation-associated phenotypes. In sum, results reported here point to the promise of studying relatives who are at increased genetic liability in both FXS and autism, and suggest several lines of further inquiry that could prove promising in the discovery of gene-brain-behavior relationships in both FMR1-related conditions and autism.
Acknowledgments
We are grateful to the families who participated in this research, and acknowledge support from the following sources: R03 MH079998-01, 1R01DC010191-01A1, the National Science Foundation BCS-0820394 (ML), R01 HD038819 and R01 HD038819-09S1 (GM), and #U54 MH66418 (JP).
References Cited
- 1.American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4. Washington DC: 1994. [Google Scholar]
- 2.Kielinen M, Rantala H, Timonen E, Linna S-L, Moilanen I. Associated medical disorders and disabilities in children with autistic disorder: A population-based study. Autism. 2004;8(1):49–60. doi: 10.1177/1362361304040638. [DOI] [PubMed] [Google Scholar]
- 3.Clifford S, Dissanayake C, Bui QM, Huggins R, Taylor AK, Loesch DZ. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. Journal of Autism and Developmental Disorders. 2007;37(4):738–47. doi: 10.1007/s10803-006-0205-z. [DOI] [PubMed] [Google Scholar]
- 4.Hall SS, Lightbody AA, Reiss AL. Compulsive, self-injurious, and autistic behavior in children and adolescents with fragile X syndrome. American Journal of Mental Retardation. 2008;113(1):44–53. doi: 10.1352/0895-8017(2008)113[44:CSAABI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, et al. Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. American Journal of Medical Genetics. 2004;129(3):225–34. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- 6.Philofsky A, Hepburn SL, Hayes A, Hagerman R, Rogers SJ. Linguistic and cognitive functioning and autism symptoms in young children with fragile X syndrome. American Journal of Mental Retardation: AJMR. 2004;109(3):208–18. doi: 10.1352/0895-8017(2004)109<208:LACFAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: Symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. Journal of Developmental Behavioral Pediatrics. 2001;22(6):409–17. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Hatton DD, Skinner M, Sideris J, Mankowski JB, Bailey DB, Roberts JE, et al. Autistic behavior in children with fragile X syndrome: Prevalence, stability, and the impact of FMRP. American Journal of Medical Genetics. 2006;140(17):1804–13. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- 9.Loesch DZ, Bui Q, Dissanayake C, Clifford S, Gould E, Bulhak-Paterson D, et al. Molecular and cognitive predictors of the continuum of autistic behaviours in fragile X. Neuroscience and Behavioral Reviews. 2007;31:315–26. doi: 10.1016/j.neubiorev.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey AJ, Bolton P, Butler L, Le Couteur A. Prevalence of the Fragile X anomaly amongst autistic twins and singletons. Journal of Child Psychology and Psychiatry. 1993;34(5):673–88. doi: 10.1111/j.1469-7610.1993.tb01064.x. [DOI] [PubMed] [Google Scholar]
- 11.Dykens EM, Volkmar F, Cohen D. Medical Conditions Associated with Autism. In: Cohen DJ, Volkmar FR, editors. Handbook of Autism and Pervasive Developmental Disorders. 2. New York: John Wiley & Sons, Inc; 1997. pp. 388–410. [Google Scholar]
- 12.Tassone F, Hagerman RJT, AK, Mills JB, Harris SW. Clinical involvement and protein expression in individuals with the FMR1 premutation. American Journal of Medical Genetics. 2000;91:144–52. doi: 10.1002/(sici)1096-8628(20000313)91:2<144::aid-ajmg14>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Goodlin-Jones BL, Tassone F, Gane LW, Hagerman RJ. Autistic spectrum disorder and the fragile X premutation. Journal of developmental and behavioral pediatrics : JDBP. 2004;25(6):392–8. doi: 10.1097/00004703-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Farzin F, Perry H, Hessl D, Loesch D, Cohen J, Bacalman S, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. Journal of Developmental and Behavioral Pediatrics. 2006;27(Supplement 2):S137–44. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- 15.Aziz M, Stathopulu E, Callias M, Taylor C, Turk J, Oostra B, et al. Clinical features of boys with fragile X premutations and intermediate alleles. Am J Med Genet B. 2003;121:119–27. doi: 10.1002/ajmg.b.20030. [DOI] [PubMed] [Google Scholar]
- 16.Wang LW, Berry-Kravis E, Hagerman RJ. Fragile X: leading the way for targeted treatments in autism. Neurotherapeutics. 2010;7(3):264–74. doi: 10.1016/j.nurt.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krueger DD, Bear MF. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annual Review of Medicine. 2011;62:411–29. doi: 10.1146/annurev-med-061109-134644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagerman R, Hoem G, Hagerman P. Fragile X and autism: Intertwined at the molecular level leading to targeted treatments. Mol Autism. 2010;1(1):12. doi: 10.1186/2040-2392-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends in Neurosciences. 2004;27(7):370–7. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(11):7746–50. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Hulst C, Kooy RF. The GABAA receptor: a novel target for treatment of fragile X? Trends in Neurosciences. 2007;30(8):425–31. doi: 10.1016/j.tins.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 22.D’Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, et al. Decreased expression of the GABAA receptor in fragile X syndrome. Brain research. 2006;1121(1):238–45. doi: 10.1016/j.brainres.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 23.Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146(2):247–61. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall SS, Lightbody AA, Hirt M, Rezvani A, Reiss AL. Autism in fragile X syndrome: a category mistake? Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(9):921–33. doi: 10.1016/j.jaac.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoeft F, Walter E, Lightbody AA, Hazlett HC, Chang C, Piven J, et al. Neuroanatomical differences in toddler boys with fragile x syndrome and idiopathic autism. Arch Gen Psychiat. 2011;68(3):295–305. doi: 10.1001/archgenpsychiatry.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazlett HC, Poe MD, Lightbody AA, Gerig G, Macfall JR, Ross AK, et al. Teasing apart the heterogeneity of autism: Same behavior, different brains in toddlers with fragile X syndrome and autism. J Neurodev Disord. 2009;1(1):81–90. doi: 10.1007/s11689-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson LB, Tregellas JR, Hagerman RJ, Rogers SJ, Rojas DC. A voxel-based morphometry comparison of regional gray matter between fragile X syndrome and autism. Psychiatry Research. 2009;174(2):138–45. doi: 10.1016/j.pscychresns.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolton P, Macdonald H, Pickles A, Rios P, Goode S, Crowson M, et al. A Case-Control Family History Study of Autism. Journal of Child Psychology and Psychiatry. 1994;35(5):877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- 29.Piven J, Wzorek M, Landa R, Lainhart J, Bolton P, Chase GA, et al. Personality characteristics of the parents of individuals with autism. Psychological Medicine. 1994;24(3):783–95. doi: 10.1017/s0033291700027938. [DOI] [PubMed] [Google Scholar]
- 30.Fombonne E, Bolton P, Prior J, Jordan H, Rutter M. A family study of autism: cognitive patterns and levels in parents and siblings. Journal of Child Psychology and Psychiatry. 1997;38(6):667–83. doi: 10.1111/j.1469-7610.1997.tb01694.x. [DOI] [PubMed] [Google Scholar]
- 31.Murphy M, Bolton P, Pickles A, Fombonne E, Piven J, Rutter M. Personality traits of the relatives of autistic probands. Psychological Medicine. 2000;30(6):1411–24. doi: 10.1017/s0033291799002949. [DOI] [PubMed] [Google Scholar]
- 32.Losh M, Childress D, Lam K, Piven J. Defining key features of the broad autism phenotype: A comparison across parents of multiple- and single-incidence autism families. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B(4):424–33. doi: 10.1002/ajmg.b.30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Losh M, Adolphs R, Piven J. The Broad Autism Phenotype. In: Dawson G, Amaral D, Geschwind D, editors. Autism Spectrum Disorders. Oxford: Oxford University Press; 2011. pp. 457–76. [Google Scholar]
- 34.Rousseau F, Rouillard P, Morel ML, Khandjian EW, Morgan K. Prevalence of carriers of premutation-size alleles of the FMRI gene-and implications for the population genetics of the fragile X syndrome. American Journal of Human Genetics. 1995;57(5):1006–18. [PMC free article] [PubMed] [Google Scholar]
- 35.Dombrowski C, Levesque S, Morel ML, Rouillard P, KM, Rousseau F. Premutation and intermediate-size FMR1 alleles in 10572 males from the general population: loss of an AGG interruption is a late event in the generation of fragile X syndrome alleles. Human Molecular Genetics. 2002;11(4):371–8. doi: 10.1093/hmg/11.4.371. [DOI] [PubMed] [Google Scholar]
- 36.Qin M, Entezam A, Usdin K, Huang T, Liu ZH, Hoffman GE, et al. A mouse model of the fragile X premutation: effects on behavior, dendrite morphology, and regional rates of cerebral protein synthesis. Neurobiology of Disease. 2011;42(1):85–98. doi: 10.1016/j.nbd.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 messenger RNA in carrier males: A new mechanism of involvement in the fragile X syndrome. American Journal of Human Genetics. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourgeois J, Coffey S, Rivera SM, Hessl D, Gane L, Tassone F, et al. Fragile X premutation disorders: Expanding the psychiatric perspective. Journal of Clinical Psychiatry. 2009;70:852–62. doi: 10.4088/JCP.08m04476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franke P, Leboyer M, Gansicke M, Weiffenbacj O. Genotype-phenotype relationship in female carriers of the premutation and full mutation of FMR-1. Psychiatry Research. 1998;90:113–27. doi: 10.1016/s0165-1781(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 40.Hessl D, Rivera SM, Reiss AL. The neuroanatomy and neuroendocrinology of fragile X syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(1):17–24. doi: 10.1002/mrdd.20004. [DOI] [PubMed] [Google Scholar]
- 41.Losh M, Adolphs R, Poe MD, Couture S, Penn D, Baranek GT, et al. Neuropsychological profile of autism and the broad autism phenotype. Arch Gen Psychiat. 2009;66(5):518–26. doi: 10.1001/archgenpsychiatry.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tyrer P. Personality assessment schedule. In: Tyrer P, editor. Personality disorders: diagnosis, management, and course. London: Butterworth and Company; 1988. [Google Scholar]
- 43.Piven J, Palmer P, Landa R, Santangelo SJD, Childress D. Personality and language characteristics in parents from multiple-incidence autism families. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 1997;74(4):398–411. [PubMed] [Google Scholar]
- 44.Landa R, Piven J, Wzorek MM, Gayle JO, Chase GA, Folstein SE. Social language use in parents of autistic individuals. Psychological Medicine. 1992;22(1):245–54. doi: 10.1017/s0033291700032918. [DOI] [PubMed] [Google Scholar]
- 45.Constantino JN. The Social Responsiveness Scale. Los Angeles: Western Psychological Services; 2005. [Google Scholar]
- 46.Kramer CY. Extension of multiple range tests to group means with unequal numbers of replications. Biometrics. 1956;12(3):307–10. [Google Scholar]
- 47.Baron-Cohen S, Hammer J. Parents of children with Asperger syndrome: what is the cognitive phenotype? Journal of Cognitive Neuroscience. 1997;9:548–54. doi: 10.1162/jocn.1997.9.4.548. [DOI] [PubMed] [Google Scholar]
- 48.Losh M, Piven J. Social-Cognition and the Broad Autism Phenotype: Identifying Genetically Meaningful Phenotypes. Journal of Child Psychology and Psychiatry. 2007;48:105–12. doi: 10.1111/j.1469-7610.2006.01594.x. [DOI] [PubMed] [Google Scholar]
- 49.Hessl D, Rivera S, Koldewyn K, Cordeiro L, Adams J, Tassone F, et al. Amygdala dysfunction in men with the fragile X premutation. Brain. 2007;130:404–16. doi: 10.1093/brain/awl338. [DOI] [PubMed] [Google Scholar]
- 50.Hessl D, Wang JM, Schneider A, Koldewyn K, Le L, Iwahashi C, et al. Decreased fragile X mental retardation protein expression underlies amygdala dysfunction in carriers of the fragile X premutation. Biol Psychiat. 2011;70(9):859–65. doi: 10.1016/j.biopsych.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adams PE, Adams JS, Nguyen DV, Hessl S, Brunberg JA, Tassone S, et al. Psychological symptoms correlate with reduced hippocampal volume in fragile X premutation carriers. American Journal of Medical Genetics. 2010;153B(3):775–85. doi: 10.1002/ajmg.b.31046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hashimoto R, Backer KC, Tassone F, Hagerman RJ, Rivera S. An fMRI study of the prefrontal activity during the performance of a working memory task in premutation carriers of the fragile X mental retardation 1 gene with and without fragile X-associated tremor/ataxia syndrome (FXTAS) Journal of Psychiatric Research. 2010;45(1):36–43. doi: 10.1016/j.jpsychires.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koldewyn K, Hessl D, Adams J, Tassone F, Hagerman PJ, Hagerman RJ, et al. Reduced hippocampal activation during recall is associated with elevated FMR1 mRNA and psychiatric symptoms in men with the fragile X premutation. Brain Imaging and Behavior. 2008;2(2):105–16. doi: 10.1007/s11682-008-9020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hashimoto R, Srivastava S, Tassone F, Hagerman RJ, Rivera S. Diffusion tensor imaging in male premutation carriers of the fragile X mental retardation gene. Movement Disorders. 2011;26(7):1329–36. doi: 10.1002/mds.23646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fatemi SH, Folsom TD. The role of fragile X mental retardation protein in major mental disorders. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fatemi SH, Kneeland RE, Liesch SB, Folsom TD. Fragile X mental retardation protein levels are decreased in major psychiatric disorders. Schizophrenia Research. 2010;124(1–3):246–7. doi: 10.1016/j.schres.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, et al. Social approach in genetically engineered mouse lines relevant to autism. Genes, Brain, and Behavior. 2009;8(2):129–42. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Selmeczy D, Koldewyn K, Wang JM, Harvey S, Hessl DR, Tassone F, et al. Investigation of amygdala volume in men with the fragile X premutation. Brain Imaging and Behavior. 2011 doi: 10.1007/s11682-011-9132-5. Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goodrich-Hunsaker NJ, Wong LM, McLennan Y, Srivastava S, Tassone F, Harvey D, et al. Young adult female fragile X premutation carriers show age- and genetically-modulated cognitive impairments. Brain Cognition. 2011 doi: 10.1016/j.bandc.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kogan CS, Cornish KM. Mapping self-reports of working memory deficits to executive dysfunction in Fragile X Mental Retardation 1 (FMR1) gene premutation carriers asymptomatic for FXTAS. Brain Cognition. 2010;73(3):236–43. doi: 10.1016/j.bandc.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 61.Keri S, Benedek G. The perception of biological and mechanical motion in female fragile X premutation carriers. Brain Cognition. 2010;72:197–201. doi: 10.1016/j.bandc.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 62.Hunter JE, Rohr JK, Sherman SL. Co-occurring diagnoses among FMR1 premutation allele carriers. Clinical Genetics. 2010;77(4):374–81. doi: 10.1111/j.1399-0004.2009.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roberts JE, Bailey DB, Mankowski J, Ford A, Weisenfeld LA, Heath TM, et al. Mood and anxiety disorders in females with the FMR1 premutation. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009;150B(1):130–9. doi: 10.1002/ajmg.b.30786. [DOI] [PubMed] [Google Scholar]
- 64.Hunter JE, Abramowitz A, Rusin M, Sherman SL. Is there evidence for neuropsychological and neurobehavioral phenotypes among adults without FXTAS who carry the FMR1 premutation? A review of current literature. Genetics in Medicine. 2009;11(2):79–89. doi: 10.1097/GIM.0b013e31818de6ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cornish KM, Kogan CS, Li L, Turk J, Hagerman RJ. Lifespan changes in working memory in fragile X premutation males. Brain Cognition. 2009;69(3):551–8. doi: 10.1016/j.bandc.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bailey DB, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. American Journal of Medical Genetics Part A. 2008;146A(16):2060–9. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- 67.Berry-Kravis E, Goetz CG, Leehey MA, Hagerman RJ, Zhang L, Li L, et al. Neuropathic features in fragile X premutation carriers. American Journal of Medical Genetics Part A. 2007;143(1):19–26. doi: 10.1002/ajmg.a.31559. [DOI] [PubMed] [Google Scholar]
- 68.Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, Holden JJA, Yang KT, Lee C, et al. Fragile X premutation is a significant risk factor for premature ovarian failure: The international collaborative POF in fragile X study—preliminary data. American Journal of Medical Genetics. 1999;83(4):322–5. [PMC free article] [PubMed] [Google Scholar]


