Abstract
Background:
With decompressive craniectomy for ischemic stroke, traumatic brain injury, and skull-infiltrating tumors, the need for cranioplasty has increased. Different materials for custom-made cranioplasties have been evaluated, but a gold standard could not yet be established. We report our experience with the new custom-made titanium CRANIOTOP®cranioplasty (CL Instruments, Germany).
Methods:
A total of 50 consecutive patients received a CRANIOTOP cranioplasty within a 2 year interval. We reviewed the charts for time between initial surgery and cranioplasty, indication, complications, operative time, and cosmetic outcome. Postoperative imaging (computed tomography [CT] scan n = 48, magnetic resonance imaging (MRI) n = 5) was screened for fitting accuracy and for hemorrhages.
Results:
The most common indication for craniectomy were diffuse edema due to traumatic brain injury (n = 17, 34%) and ischemic stroke (n = 12, 24%). All patients were satisfied with the cosmetic result. In the postoperative CT scan accurate fitting was confirmed in all patients, the postoperative MRI was free of artifacts. Surgical revision was necessary in five patients because of empyema (n = 2), wound exposure (n = 2), and one cerebrospinal fluid fistula. Thus, the surgical morbidity was 10%.
Conclusion:
With due consideration of the limitations of this retrospective study, we feel the present data allow concluding that the custom-made titanium cranioplasty CRANIOTOP®is safe and feasible.
Keywords: CL Instruments, craniectomy, CRANIOTOP, titanium cranioplasty
BACKGROUND
Craniectomy implies plastic reconstruction of the skull. Cranioplasty restores and preserves cranial function, reshapes the neuro- and viscerocranium and prevents complications. [11] With emerging evidence for the benefit of early decompressive craniectomy for ischemic stroke, [25] diffuse traumatic brain injury [24] and skull-infiltrating tumors, [20] the need for cranioplasty has dramatically increased.
Good biocompatibility, appropriate defect closure with accurate fitting of the plastic reconstruction to the osseous rims, and particularly a satisfying cosmetic result are important goals. [11,22] Furthermore, in patients with skull-penetrating tumors like meningeomas or metastases, subsequent radiographic assessment must be warranted. [1,3,7]
Various materials have been evaluated and computerized virtually designed implants have found increasingly wider use. Among these materials are autologous bone, polymethyl methacrylate (PMMA), ceramics, hydroxyapatite, polyether ether ketone (PEEK), carbon-fiber-reinforced polymer (CFRP), and titanium. [2,21]
Titanium is a nonferrous metal of low atomic number, generating no relevant artifacts in computed tomography (CT) and magnetic resonance imaging (MRI). [8] Titanium for cranioplasty has been evaluated in previous study and some authors recommended this material as the method of choice for secondary cranioplasty. [4,6,12]
In this retrospective series, we analyzed the feasibility of the new preformed titanium CRANIOTOP® cranioplasty (CL Instruments, Germany) that we have utilized for the past 2 years. To our knowledge, this is the first report evaluating this novel cranioplasty.
MATERIALS AND METHODS
We had no conflict of interest. The study was approved by our local ethics committee.
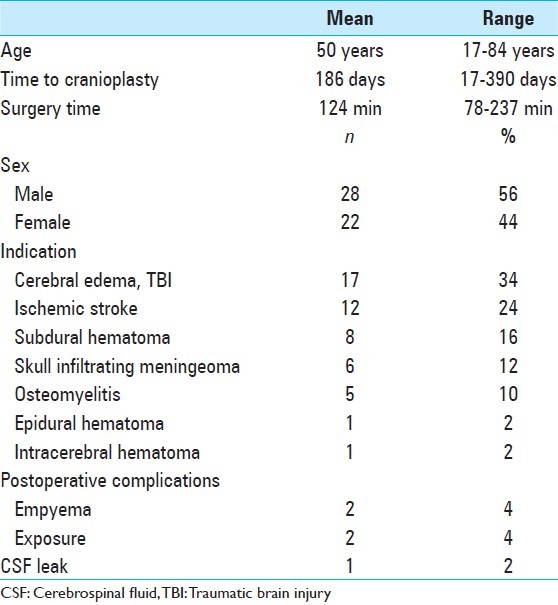
Between November 2010 and December 2012, 50 consecutive patients (22 female, 28 male; mean age 50 years) received a CRANIOTOP cranioplasty in our neurosurgical department. We reviewed the charts for demographical data, time between initial surgery and secondary cranioplasty, indication for cranioplasty, pre- and postoperative radiographic data, surgical approach, peri- and postoperative complications, operative time, postoperative morbidity, and cosmetic outcome. Mean follow-up was 8 weeks and no patient was lost to follow-up.
Postoperative imaging (CT scan n = 48, MRI n = 5) within 24 hours after surgery was screened for fitting accuracy, restoration of skull contour, artifacts, and for any relevant hemorrhages.
Fabrication and operative technique
For each patient a thin sliced CT-scan was obtained, utilizing a standardized imaging protocol (helical CT, length of acquisition 1 mm, Gantry tilt 0°). The DICOM data was then transmitted to the manufacturer (CL Instruments, Germany). A 1:1 scale model of the skull, including the craniectomy [Figure 1], was customized after 3-dimensional data reconstruction [Figure 2a and b] and rapid prototyping with a 3D-plotter (Z-Cooperation, MA, USA). The implant solely consisted of titanium with minimal thickness of 0.5 mm [Figure 3]. The delay between image acquisition and shipment of the cranioplasty was approximately 14 days.
Figure 1.

3-D model
Figure 2.
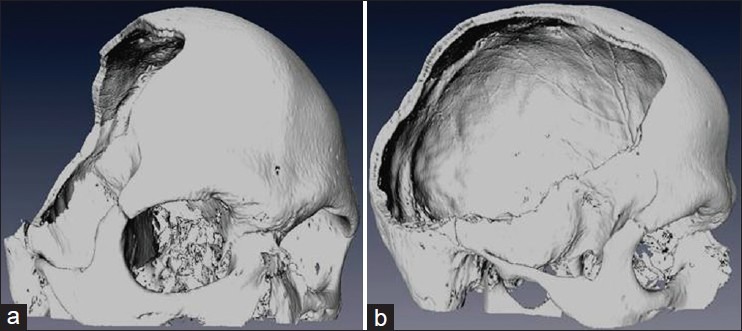
(a) 3-dimensional data reconstruction, oblique view (b) 3-dimensional data reconstruction, lateral view
Figure 3.
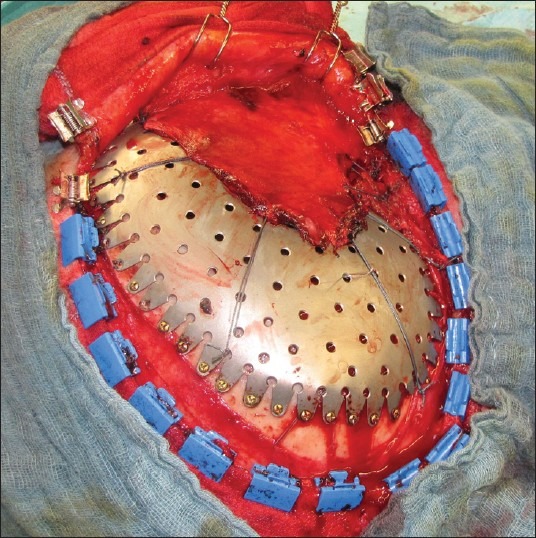
Intraoperative view: Tenting sutures, temporalis muscle and application of mini screws
After skin incision and dissection of the scalp, full exposure of the defect without dissection of the dura was performed. If necessary, lumbar cerebrospinal fluid (CSF) drainage was implanted preoperatively. Central and peripheral dural tenting sutures were applied and fixed with the plastic [Figure 3]. When accurate fitting was achieved, mini skull screws (length 4 mm, Ψ 1.5 mm; minimum 4, maximum 10) were applied that penetrated the external table. At the end of surgery, a soft suction drain was placed. CT- and MRI-scans were performed within 24 hours following surgery.
RESULTS
The most common indication for craniectomy were diffuse edema due to traumatic brain injury (n = 17, 34%) and ischemic stroke of the middle cerebral artery territory (n = 12, 24%). Other reasons for craniectomy were acute subdural hematoma (n = 8, 16%), skull-infiltrating tumors (n = 6, 12%), osteomyelitis after craniotomy (n = 5, 10%), epidural hematoma (n = 1, 2%), and intracerebral hematoma (n = 1, 2%).
The mean time interval between initial craniectomy and cranioplasty was 186 days (range 17-390 days), the mean time of surgery was 124 minutes (range 78-237 minutes).
During the follow-up, all patients were satisfied with the cosmetic result. No revision for cosmetic reasons was required.
In the postoperative CT scan (n = 46) accurate fitting, restoring the skull contour, was confirmed in all patients [Figure 4]. We encountered no relevant postoperative hemorrhage.
Figure 4.
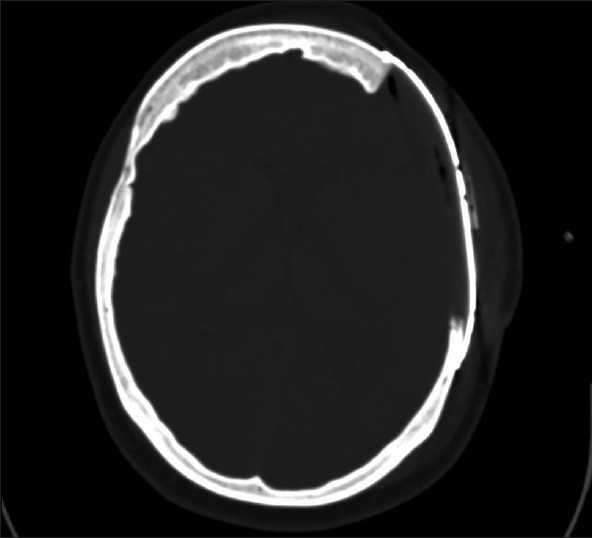
Postoperative CT, axial view displayed accurate fitting of the titanium plate
Postoperative MRI was obtained in four patients with skull- and dura-infiltrating tumors. In these four cases, the postoperative MRI was free of any artifacts, allowing assessment of residual tumor or tumor progression during the long-term follow-up [Figure 5a and b].
Figure 5a.
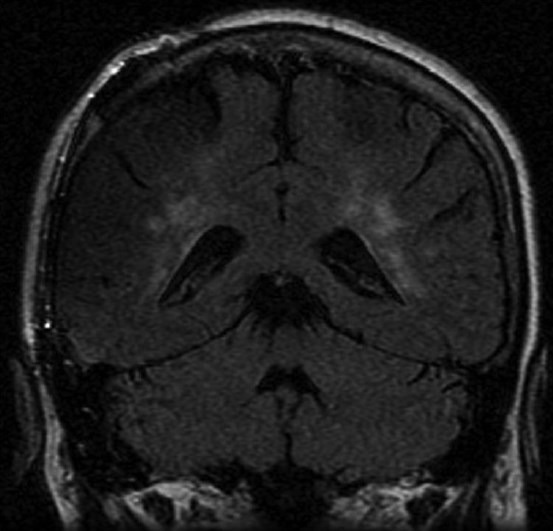
Postoperative MRI allows assessment of adjacent structures (in a patient after resection of a skull-infiltrating meningeoma right temporally)
Figure 5b.
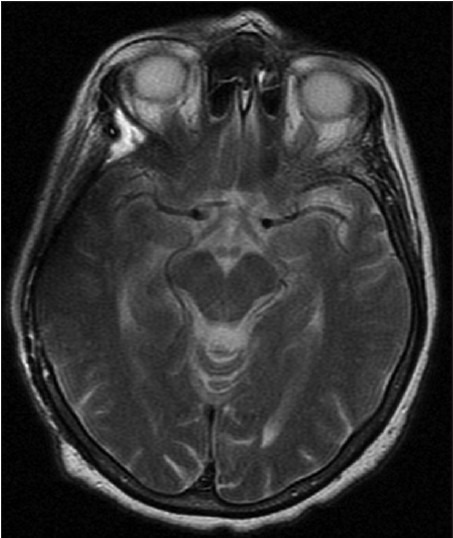
Postoperative MRI, axial plane
Two patients developed a subdural empyema postoperatively, in two patients an early wound dehiscence with exposure of the titanium plate occurred, and one patient had a new postoperative CSF fistula. In these five patients, explantation of the cranioplasty was necessary. Thus, the perioperative morbidity was 10%. We registered no neurological deterioration, no new seizures or neuropathies (chronic pain after wound healing), postoperatively. The implanted material was well tolerated in all cases.
The clinical characteristics and postoperative findings are presented in [Table 1].
Table 1.
Clinical characteristics and postoperative findings
DISCUSSION
Cranioplasty is a fundamental neurosurgical procedure that was first described in the 16thcentury. [19] During the past century, various materials and techniques have appeared; the choice of material is frequently influenced by its cost as well as the surgeon’s experience and preferences.
Particularly in children, the reimplantation of the autologous bone flap should be performed whenever possible to avoid any growth-related dislocation. However, in adults, presently available data does not demonstrate superiority of autologous bone. Some authors even consider the patient’s own bone flap to be inferior as it is prone to osteolysis and infection. [17,18]
A very common and cheap material is PMMA, but often the cosmetic outcome is poor, especially when the cranioplasty involves parts of the forehead and of the viscerocranium. [5,10,16] Moreover, the minimal size of the bone flap for decompressive craniectomy should be 12 × 12 cm. [24,25] A defect of that size becomes time-consuming and difficult to reconstruct in free-handed fashion with PMMA. Therefore, the utilization of pre-formed cranioplasties may be preferred. Materials available for computerized remodeling are ceramics, hydroxyapatite, PEEK, CFRP, and titanium. [2,4,9,13,22]
The osteo-inductive potency of hydroxyapatite makes this material interesting for the clinical use in cranial reconstruction. However, the high infection rate of up to 22.4%, [18] especially when covering large defects, made some authors reject this material. Ceramic materials often have too much volume and are difficult to attach to the adjacent bone. They also typically require more extensive dissection. [23] For custom-made titanium cranioplasties, a small volume of implant material is needed. [6,15] The plate is simply laid over the defect and fixed with mini-screws. Thus, it is not necessary to dissect the dura and the osseous rims.
The titanium cranioplasty CRANIOTOP®was evaluated in a previous mini-series (n = 2). [20] In these two cases, the cranioplasty was applied in patients with skull-infiltrating meningeomas. The cosmetic results were satisfying and the assessment of the postoperative CT and MRI was uncomplicated. In the present study, 50 patients are presented, 4 patients received postoperative MRI and 46 patients had a postoperative CT. Both imaging modalities were free of artifacts and allowed assessment of adjacent bone, meninges and brain parenchyma.
The cosmetic result was good in all patients and replacement of the cranioplasty for cosmetic reasons was not necessary. However, in five patients the cranioplasty had to be explanted, thus the procedure-related overall morbidity in our cohort is 10% (n = 5) with an infection rate of 4% (n = 2). Severe headache, seizures or allergic reactions were not registered. Both overall morbidity and infection rate are within the range reported in a recent review of literature by Cabraja et al., the infection rate for titanium cranioplasty ranged between 0% and 4.5%. [6] In a very recent review of 127 custom-made titanium cranioplasties in 113 patients, the group of Wiggins found an infection rate of 16%. [26] Generally, the overall morbidity for cranioplasty is 0-34%. [6,14]
Further, the cost of the CRANIOTOP®cranioplasty (3000-4500€, depending on the size) is reasonable. Osteo-inductive materials as hydroxyapatite are approximately 2.5-times more expensive.
The present analysis was designed as a feasibility study, confirming the clinical use and manageability of the CRANIOTOP®cranioplasty. Whether this type of cranioplasty provides superior qualities than other cranioplasties must be evaluated prospectively.
LIMITATIONS
This series carries the limitations of a retrospective review. Follow-up was also limited. However, our patient population is predominantly regional and would have likely presented with important events beyond the reported follow-up.
Moreover, the cosmetic result was evaluated by subjective patient assessment and radiographic evaluation of accuracy; additional evaluation with standardized, objective tools could provide more robust data.
Despite some methodological weaknesses of our study, the custom-made titanium cranioplasty CRANIOTOP®is safe and feasible.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/1/88/114811
Disclaimer: The authors of this article has no conflict of interest to disclose, and has adhered to SNI's policies regarding human/animal rights, and informed consent. Advertisers in SNI did not ask for, nor did they receive access to this article prior to publication
Contributor Information
Julius Höhne, Email: julius.hoehne@ukr.de.
Alexander Brawanski, Email: alexander.brawanski@ukr.de.
Holger G. Gassner, Email: holger.gassner@ukr.de.
Karl-Michael. Schebesch, Email: karl.michael.schebesch@ukr.de.
REFERENCES
- 1.Alexiou GA, Gogou P, Markoula S, Kyritsis AP. Management of meningiomas. Clin Neurol Neurosurg. 2010;112:177–82. doi: 10.1016/j.clineuro.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Aydin S, Kucukyuruk B, Abuzayed B, Sanus GZ. Cranioplasty: Review of materials and techniques. J Neurosci Rural Pract. 2011;2:162–7. doi: 10.4103/0976-3147.83584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bikmaz K, Mrak R, Al-Mefty O. Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg. 2007;107:905–12. doi: 10.3171/JNS-07/11/0905. [DOI] [PubMed] [Google Scholar]
- 4.Blake GB, MacFarlane MR, Hinton JW. Titanium in reconstructive surgery of the skull and face. Br J Plast Surg. 1990;43:528–35. doi: 10.1016/0007-1226(90)90115-g. [DOI] [PubMed] [Google Scholar]
- 5.Bloch O, McDermott MW. In situ cranioplasty for hyperostosing meningiomas of the cranial vault. Can J Neurol Sci. 2011;38:59–64. doi: 10.1017/s0317167100011082. [DOI] [PubMed] [Google Scholar]
- 6.Cabraja M, Klein M, Lehmann TN. Long-term results following titanium cranioplasty of large skull defects. Neurosurg Focus. 2009;26:E10. doi: 10.3171/2009.3.FOCUS091. [DOI] [PubMed] [Google Scholar]
- 7.Cannon PS, Rutherford SA, Richardson PL, King A, Leatherbarrow B. The surgical management and outcomes for spheno-orbital meningiomas: A 7-year review of multi-disciplinary practice. Orbit. 2009;28:371–6. doi: 10.3109/01676830903104645. [DOI] [PubMed] [Google Scholar]
- 8.Chandler CL, Uttley D, Archer DJ, MacVicar D. Imaging after titanium cranioplasty. Br J Neurosurg. 1994;8:409–14. doi: 10.3109/02688699408995107. [DOI] [PubMed] [Google Scholar]
- 9.Chim H, Schantz JT. New frontiers in calvarial reconstruction: Integrating computer-assisted design and tissue engineering in cranioplasty. Plast Reconstr Surg. 2005;116:1726–41. doi: 10.1097/01.prs.0000182386.78775.cd. [DOI] [PubMed] [Google Scholar]
- 10.D′Urso PS, Redmond MJ. A method for the resection of cranial tumours and skull reconstruction. Br J Neurosurg. 2000;14:555–9. doi: 10.1080/02688690020005581. [DOI] [PubMed] [Google Scholar]
- 11.Dujovny M, Aviles A, Agner C, Fernandez P, Charbel FT. Cranioplasty: Cosmetic or therapeutic? Surg Neurol. 1997;47:238–41. doi: 10.1016/s0090-3019(96)00013-4. [DOI] [PubMed] [Google Scholar]
- 12.Eufinger H, Wehmoller M. Individual prefabricated titanium implants in reconstructive craniofacial surgery: Clinical and technical aspects of the first 22 cases. Plast Reconstr Surg. 1998;102:300–8. doi: 10.1097/00006534-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Goiato MC, Anchieta RB, Pita MS, dos Santos DM. Reconstruction of skull defects: Currently available materials. J Craniofac Surg. 2009;20:1512–8. doi: 10.1097/SCS.0b013e3181b09b9a. [DOI] [PubMed] [Google Scholar]
- 14.Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: Analysis of 62 cases. Neurosurg Focus. 2009;26:E9. doi: 10.3171/2009.3.FOCUS0962. [DOI] [PubMed] [Google Scholar]
- 15.Hill CS, Luoma AM, Wilson SR, Kitchen N. Titanium cranioplasty and the prediction of complications. Br J Neurosurg. 2012;26:832–7. doi: 10.3109/02688697.2012.692839. [DOI] [PubMed] [Google Scholar]
- 16.Marbacher S, Coluccia D, Fathi AR, Andereggen L, Beck J, Fandino J. Intraoperative patient-specific reconstruction of partial bone flap defects after convexity meningioma resection. World Neurosurg. 2013;79:124–30. doi: 10.1016/j.wneu.2011.05.057. [DOI] [PubMed] [Google Scholar]
- 17.Matsuno A, Tanaka H, Iwamuro H, Takanashi S, Miyawaki S, Nakashima M, et al. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir (Wien) 2006;148:535–40. doi: 10.1007/s00701-006-0740-6. [DOI] [PubMed] [Google Scholar]
- 18.Moreira-Gonzalez A, Jackson IT, Miyawaki T, Barakat K, DiNick V. Clinical outcome in cranioplasty: Critical review in long-term follow-up. J Craniofac Surg. 2003;14:144–53. doi: 10.1097/00001665-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Sanan A, Haines SJ. Repairing holes in the head: A history of cranioplasty. Neurosurgery. 1997;40:588–603. doi: 10.1097/00006123-199703000-00033. [DOI] [PubMed] [Google Scholar]
- 20.Schebesch KM, Hohne J, Gassner HG, Brawanski A. Preformed titanium cranioplasty after resection of skull base meningiomas-A technical note. J Craniomaxillofac Surg. 2013 doi: 10.1016/j.jcms.2013.01.030. [In press] [DOI] [PubMed] [Google Scholar]
- 21.Schmiedek H. chapt. 134. 6th ed. Singapore: Elsevier Science; 2000. Operative Neurosurgical Technique: Cranioplasty: Indications, Technique and Prognosis. 2. [Google Scholar]
- 22.Spetzger U, Vougioukas V, Schipper J. Materials and techniques for osseous skull reconstruction. Minim Invasive Ther Allied Technol. 2010;19:110–21. doi: 10.3109/13645701003644087. [DOI] [PubMed] [Google Scholar]
- 23.Staffa G, Barbanera A, Faiola A, Fricia M, Limoni P, Mottaran R, et al. Custom made bioceramic implants in complex and large cranial reconstruction: A two-year follow-up. J Craniomaxillofac Surg. 2012;40:e65–70. doi: 10.1016/j.jcms.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Tagliaferri F, Zani G, Iaccarino C, Ferro S, Ridolfi L, Basaglia N, et al. Decompressive craniectomies, facts and fiction: A retrospective analysis of 526 cases. Acta Neurochir (Wien) 2012;154:919–26. doi: 10.1007/s00701-012-1318-0. [DOI] [PubMed] [Google Scholar]
- 25.Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–22. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- 26.Wiggins A, Austerberry R, Morrison D, Ho KM, Honeybul S. Cranioplasty with custom-made titanium plates-14 years experience. Neurosurgery. 2013;72:248–56. doi: 10.1227/NEU.0b013e31827b98f3. [DOI] [PubMed] [Google Scholar]


