Abstract
Background:
Metastatic Ewing’s sarcoma to the central nervous system is an uncommon condition and debate concerning the true origin of its metastases is still up to date. To the best of our knowledge, only two cases of dural metastatic Ewing’s sarcoma have been published in the English medical literature. We present an additional case in a 24-year-old female and discuss the pathogenesis of these unusual tumors with review of the relevant literature concerning their treatment and outcome.
Case Description:
A 24-year-old female with previous history of pelvis Ewing’s sarcoma and recently discovered lung metastases, presented with moderate headache for the past 2 weeks and weakness in her left leg for the past 2 days. Computed tomography scan and magnetic resonance imaging revealed an extra-axial right frontoparietal mass invading the superior sagittal sinus but with clear delineation with brain parenchyma. Imaging features were suggestive of a meningioma as no abnormalities in the skull abutting to the tumor were noted. The patient underwent surgical removal of her tumor. Near total resection was achieved and histological examination showed evidence of metastatic Ewing’s sarcoma. Postoperative adjuvant radiation and chemotherapy were administered. The patient improved well postoperatively with full recovery of her motor weakness. She is symptom free with no signs of progression, at most recent follow-up, 8 months after surgery.
Conclusion:
Despite its rarity, metastatic Ewing’s sarcoma must be considered in the differential diagnosis of extra-axial dural masses particularly meningiomas.
Keywords: Cranial, Ewing’s sarcoma, meningeal tumor, metastasis
INTRODUCTION
Ewing’s sarcoma is a malignant bone tumor, accounting for approximately 10% of primary bone malignancies [33] and usually occurring in the first or second decade of life [13] with no sex predilection. [1] Metastases of Ewing’s sarcoma to the central nervous system (CNS) are uncommon [19] and metastases that originate from the dura are exceedingly rare.
To the best of our knowledge, only two cases have been published in the English medical literature. [19,21] We present an additional case of metastatic dural Ewing’s sarcoma mimicking a parasagittal meningioma, in a 24-year old female and discuss the pathogenesis of these unusual tumors with review the relevant literature concerning their treatment and outcome.
CASE REPORT
A 24-year-old right-handed female was admitted to our hospital complaining of a 2-week history of moderate headache and weakness in her left leg for the past 2 days. She did not present with nausea, vomiting, dizziness, or seizures but reported an underestimated weight loss in the last 3 months.
Her medical history was significant for a Pelvis Ewing’s sarcoma, operated with complete surgical removal and stabilization one and a half year ago [Figure 1], and was recently diagnosed as having small lung metastases.
Figure 1.
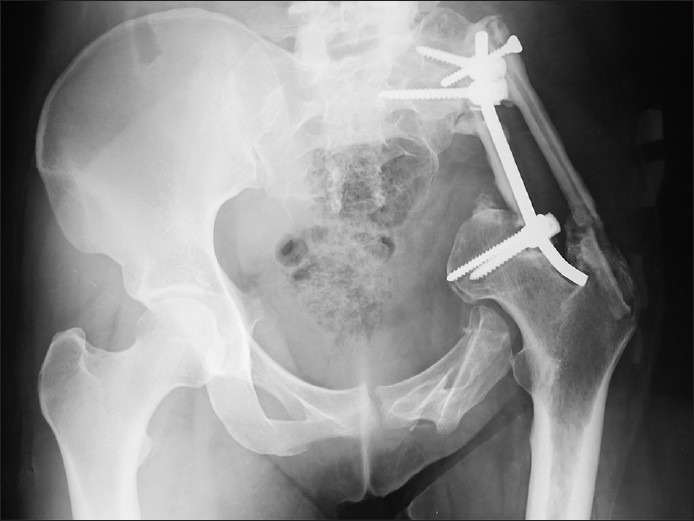
Pelvis X-rays showing left iliac wing reconstruction with tibial autografts and stabilization with screw-rod type osteosynthesis
On admission, the patient’s neurological examination revealed no cognitive deficits but a left sided leg monoparesis with motor strength of 3/5. The deep tendon reflexes in her left leg were hyperreactive and the gait and standing position were impossible. Fundoscopic examination showed a grade II papilledema. The physical examination disclosed neither local signs of relapse at the primary site nor other clinical signs of metastatic spread.
The computed tomography (CT) scan showed an extra-axial, dural based, heterogeneously hyperdense, and enhancing lesion in the right frontoparietal parasagittal region, with moderate circumferential edema and mass effect [Figure 2]. No abnormalities in the skull abutting to the tumor were noted [Figure 3] and imaging features were strongly suggestive of a meningioma.
Figure 2.
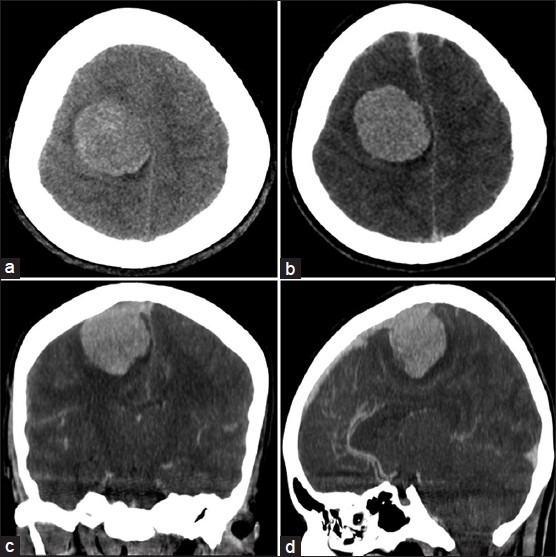
Preoperative plain CT scan (a) and postcontrast CT scan images (b), (c) and (d) showing a rounded, well-defined, heterogeneously hyperdense, and enhancing lesion in the right frontoparietal region, with circumferential edema and mass effect
Figure 3.
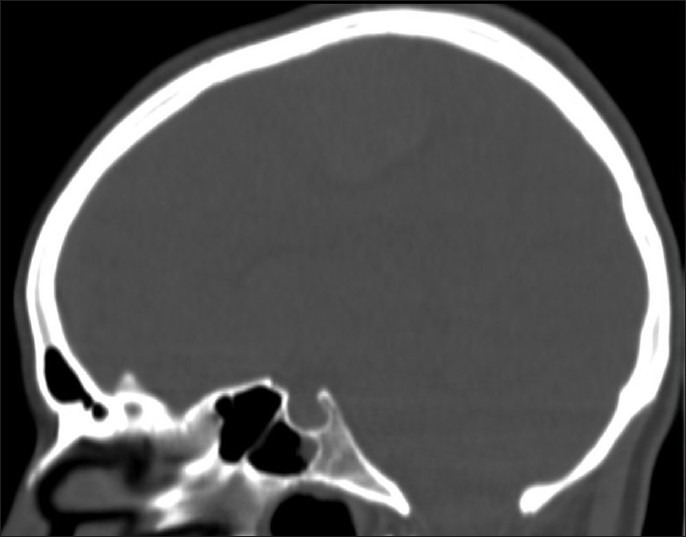
Bone window CT scan in sagittal view obtained at the time of admission showing no osteolytic changes of the calvarial bone
Magnetic resonance imaging (MRI) delineated better the tumor with its marked surrounding edema. The tumor appeared hypointense on T1-weighted MRI scans, hyperintense on T2-weighted MRI scans and displayed important enhancement with gadolinium. The tumor was attached to the dura of the convexity with present dural tail sign and invaded partially the superior sagittal sinus increasing evidence of a meningeal tumor [Figure 4].
Figure 4.
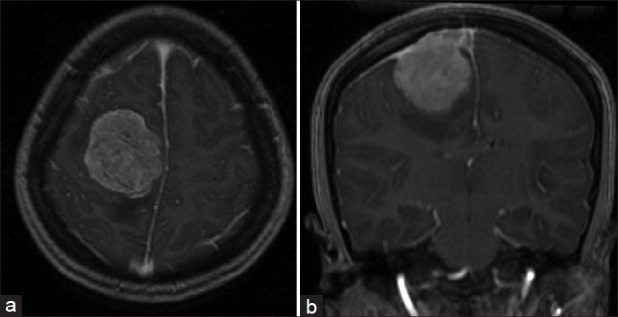
Preoperative axial (a) and coronal (b) postgadolinium T1-weighted MRI images, showing a strongly enhancing right frontoparietal tumor with sagittal venous sinus invasion. Note the dural tail indicating a meningeal tumor
The patient underwent a right frontoparietal craniotomy with an amazing discovery: The tumor was associated with a small lobulated epidural extension tightly attached to the skull [Figure 5]. Macroscopic examination showed a reddish gray, rubbery in consistency and vascular tumor of dural origin. The tumor invaded the superior sagittal sinus but has clear delineation with cerebral parenchyma allowing near total excision; only a small portion involving the superior sagittal sinus was left and the remaining dural edges were coagulated.
Figure 5.
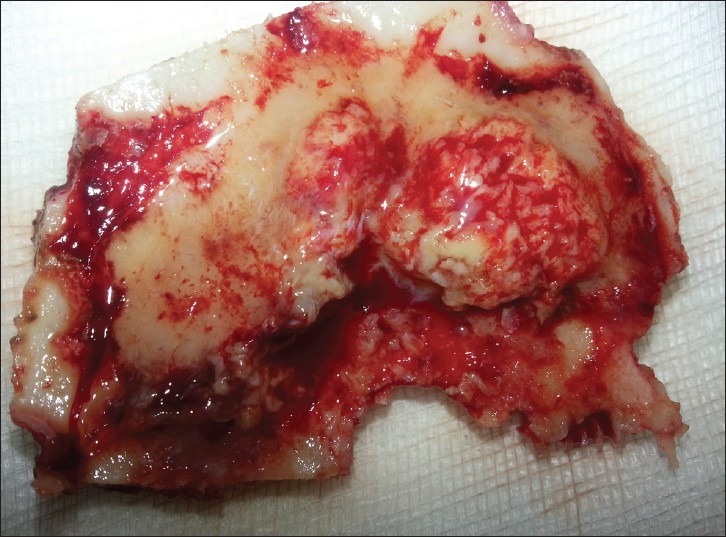
Intraoperative photograph of the bone flap showing extradural lobulated tumor tissue tightly attached to the inner calvarial bone
The definitive histological diagnosis was metastatic Ewing’s sarcoma. The tumor was composed of densely packed, small round, dark staining cells with scanty cytoplasm on light microscopy [Figure 6]. A strong MIC-2 antigen expression was noted on immunohistochemical study.
Figure 6.
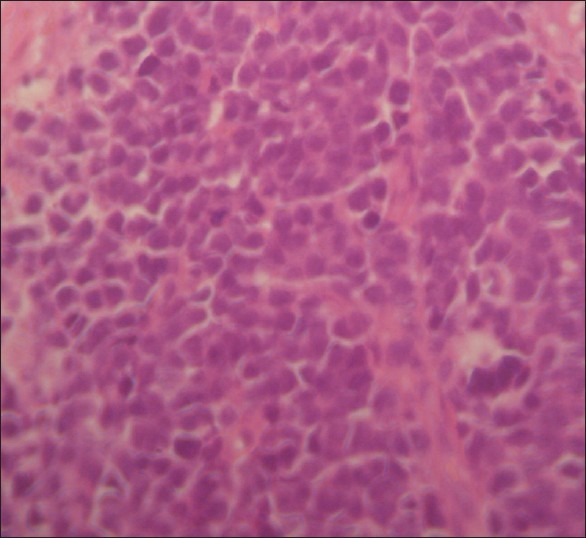
Hematoxylin and eosin-stained tumor specimen showing densely packed, small round cells with scanty clear cytoplasm and regular vesicular and hyper chromatic nuclei; magnification, ×200
The patient’s immediate postoperative course was uneventful, and she recovered quickly. Her neurological condition improved continuously, and follow-up examinations showed that the monoparesis had disappeared completely. A postoperative CT scan confirmed the extent of tumor resection with a postoperative craniotomy defect [Figure 7].
Figure 7.
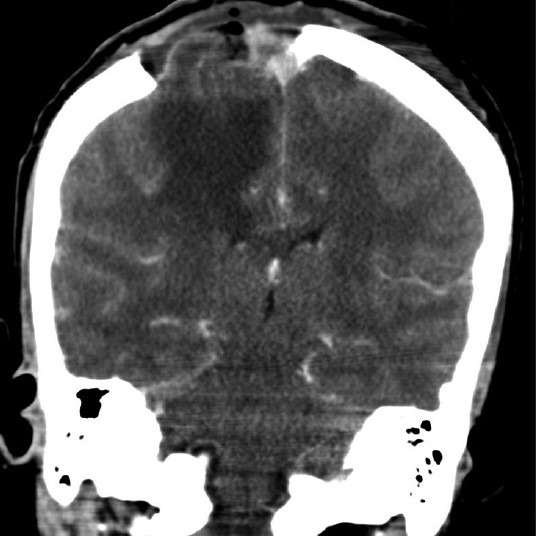
Postoperative coronal CT scan showing near total tumor removal with craniotomy defect
The patient was administered a 55 Gy radiation therapy along with an adjunctive high dose chemotherapy associating alkylating agents and doxorubicin. Clinical status remained unchanged till the most recent follow-up examination, 8 months after surgery.
DISCUSSION
Ewing’s sarcoma accounts for 10% of primary bone malignancies and usually occurs in the first or second decade of life [13] with no sex predilection. [1]
It was first described by James Ewing in 1921, who attributed its origin to bone endothelial cells [10] but now is considered as a part of Ewing’s sarcoma family of tumors (ESFTs), which is a classification used to describe a heterogeneous group of small round blue cell neoplasms of neuroectodermal origin. [2,15,19]
This malignant tumor often presents as a solitary mass affecting mainly long bones and the pelvic girdle [5,12,25,28] with a high propensity to metastasize to the lungs (38%), bone (31%), and bone marrow (11%). [19,30]
Ewing’s sarcoma can also metastasize to the CNS with a relatively low incidence (6.3% of cases). [38] There are two reported principal modes of metastatic spread of Ewing’s sarcoma to the CNS. The first is direct extension from the skull, which may be the site of both primary and secondary Ewing’s sarcoma. [24] Alternatively, it may reach the CNS via hematogenous spread. [19]
The main question raised in this case is: What is the true origin of such tumor? Is it bone or dura?
The pathogenesis includes two conceivable possibilities for meningeal involvement in this type of tumor presentation: (1) dural metastasis of the pelvic Ewing’s sarcoma; (2) direct extension of a skull metastasis with dural breakthrough and massive intradural extension.
What is already known is that, when located within the skull (less than 1% of cases), [4,8,14] these tumors tend to pass the cortical of the bone of origin to spread into the adjacent soft tissues [33] but in such reported presentations, dural break through was associated with diffuse massive epidural masses [17,21] responsible of what so called volcano-shape radiological feature. [17]
In contrast, dural metastasis may mimic skull metastasis. [16]
This and regard of our patient’s tumor presentation, make us believe that the tumor really originates from the dura in the present case. And that Ewing’s sarcoma should be considered in the differential diagnosis of extra-axial dural masses particularly meningiomas.
Dural metastases accounts for 9% of all CNS metastases. However, dural metastasis of Ewing’s sarcoma has rarely been reported on. To the best of our knowledge, only two cases have been published in the English medical literature. [19,21] Kleinschmidt-DeMasters [21] was the first to describe a dural metastasis of Ewing’s sarcoma in his 2001 surgical and autopsy series but dural involvement was associated with a massive epidural plaque-like nodule. Such a finding and the lack of imaging investigations makes it difficult to determine the true origin of the dural lesion in that case. Kim et al. [19] in their 2008 radiology report, presented the second published case. In that report, the lesion was a purely intracranial dural mass, which was considered as a metastatic tumor because of a subsequent recurrence uncommon with primary Ewing’s sarcoma. What makes our case unique is that not only it was associated with a small epidural involvement not visible in radiological examinations but also evidence of metastatic spread is already established.
Metastases are present in about 25% of Ewing’s sarcoma cases at diagnosis [9] and in 75-80% within the first 2 years of evolution [8] and in the natural history of sarcoma it is reported that the time between diagnosis and brain metastasis is usually between 3 months and 1 year [34] Such metastases most often follow lung metastasis (57-80% of patients), which is verified in the present case.
Generally the tumor presents with features of raised intracranial pressure as well as focal neurological deficits [4,8,14,26] and the imaging characteristics of this rare tumor in an extra-axial location consists of high attenuation on CT, which can show the intracranial or extra cranial extension of the tumor as well as even slight bone destruction. [24,37] MRI will show low signal intensity on T2-weighted sequences, [24] which usually suggest hyper cellularity, well known in this category of tumors and by providing multiplanar views it will also demonstrate the tumor extent, which is of great help in surgical planning. [39] Actually, positron emission tomography-CT is increasingly used in the staging of cancers, but has not been found as sensitive as MRI in the evaluation of brain metastases. [11] Imaging features of our patient’s tumor were more suggestive of a meningioma than a metastatic Ewing’s sarcoma.
The diagnosis of CNS Ewing’s sarcoma is based on histological and immunohistochemical analysis and apart from conventional assessment, staining for neural markers (NSE, Neurofilaments, S-100 and synaptophysin) should be performed. [2] Histological features of our patient’s tumor were typical of Ewing’s sarcoma.
The differential diagnosis of intracranial small round cell tumors apart of Ewing’s sarcoma eloquently discussed by Navas-Palacinos et al. [27] includes nonHodgkin lymphoma, metastatic neuroblastoma, and rhabdomyosarcoma. [6,8] In the present case, our initial differential diagnosis included more common tumors such as meningioma though age or medical history of the patient was not in favor. In fact, the combination of its infrequent location in the intradural compartment, along with the lack of distinctive imaging characteristics, compounds to the diagnostic pitfalls encountered with Ewing’s sarcoma in such locations.
The optimal management of metastatic Ewing’s sarcoma is still a challenge. Improved understanding of the biology of this tumor, as well as early detection and better multimodalities therapy has significantly improved survival for patients with Ewing’s sarcoma affecting the CNS and literature suggests that timely radical resection followed by chemo radiation is of paramount importance to accomplish a favorable outcome. [33,35] Unfortunately, presurgical chemotherapy to downstage the tumor, as for extra cranial Ewing’s sarcoma, may not be possible in this situation, as the majority of patients presents with rapidly increasing lesions with raised intracranial pressure. [33] Furthermore, in metastatic disease, chemotherapy after intensive local treatment achieves disease-free survival rates lower than 10-20% at 5 years, [3,7,9,20,40] rates that have not well improved by increasing the dose or with adjunction of alkylating agents or doxorubicin. [16,22,29] Recently, several nonrandomized trials have assessed the value of more intensive, time-compressed or high dose chemotherapy approaches, followed by autologous stem cell rescue in advanced cases like ours, with promising results, but evidence of benefit, resulting from trials, is pending. [23]
Ewing’s sarcoma is a radiation sensitive tumor [41] and it is necessary to administer full dose radiation therapy after an incomplete surgical resection. [43] but toxicities of radiation combined with aggressive chemotherapy still requires further definition. To that aim, proton radiation therapy is actually conducted, offering nearly the same results with improved dose localization and safety for normal tissue [32] but its availability is lacking.
There has been a wealth of recent advances in understanding the biology of Ewing’s sarcoma and the unique biological factors responsible for tumor genesis are currently the target of trials to develop highly selective, safe, and efficacious therapies. Nowadays, molecular and biologically directed therapies are the attractive and emerging treatment modalities of Ewing’s sarcoma as targeting the autocrine signaling pathways, including the insulin-like growth factor has been shown to decrease proliferation and increase apoptosis. [18,31,36] But its use according to van de Luijtgaarden et al., [42] could be more effective in first line than in refractory or recurrent diseases, like ours, which are the ones in need of new therapeutic options.
CONCLUSION
Metastatic Ewing’s sarcoma to the CNS is a rare and challenging condition requiring a multidisciplinary team approach.
The case reported in this paper appears to represent a genuine example of occurrence of metastatic Ewing’s sarcoma in the dura and that it should be included in the differential diagnosis of extra-axial dural masses particularly meningiomas.
We believe that in the era of personalized cancer therapy, strategies to develop drugs for uncommon tumors such as Ewing’s sarcoma and to individualize therapy will be crucial.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/1/96/115487
Disclaimer: The authors of this article has no conflict of interest to disclose, and has adhered to SNI's policies regarding human/animal rights, and informed consent. Advertisers in SNI did not ask for, nor did they receive access to this article prior to publication
Contributor Information
Atef Ben Nsir, Email: atefbn@hotmail.fr.
Mohamed Boughamoura, Email: Boughamoura.mohamed@yahoo.fr.
Mezri Maatouk, Email: m.mezri@gmail.com.
Mohamed Kilani, Email: kilanineurochirurgien@gmail.com.
Nejib Hattab, Email: nejib.hattab@rns.tn.
REFERENCES
- 1.Agrawal A, Dulani R, Mahadevan A, Vagaha SJ, Vagha J, Shankar SK. Primary Ewing’s sarcoma of the frontal bone with intracranial extension. J Cancer Res Ther. 2009;5:208–9. doi: 10.4103/0973-1482.57129. [DOI] [PubMed] [Google Scholar]
- 2.Antuña García MJ. Ewing’s sarcoma family of tumors. Clin Transl Oncol. 2005;7:262–9. doi: 10.1007/BF02710174. [DOI] [PubMed] [Google Scholar]
- 3.Arpaci E, Yetisyigit T, Seker M, Uncu D, Uyeturk U, Oksuzoglu B, et al. Prognostic factors and clinical outcome of patients with Ewing’s sarcoma family of tumors in adults: Multicentric study of the Anatolian Society of Medical Oncology. Med Oncol. 2013;30:469. doi: 10.1007/s12032-013-0469-z. [DOI] [PubMed] [Google Scholar]
- 4.Bricha M, Jroundi L, Boujida N, El Hassani MR, Chakir N, Jiddane M. Primary Ewing sarcoma of the skull vault. J Radiol. 2007;88:1899–901. doi: 10.1016/s0221-0363(07)78370-1. [DOI] [PubMed] [Google Scholar]
- 5.Carvajal R, Meyers P. Ewing’s sarcoma and primitive neuroectodermal family of tumors. Hematol Oncol Clin North Am. 2005;19:501–25. doi: 10.1016/j.hoc.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Choudhury KB, Sharma S, Kothari R, Majumder A. Primary extraosseous intracranial Ewing’s sarcoma: Case report and literature review. Indian J Med Paediatr Oncol. 2011;32:118–21. doi: 10.4103/0971-5851.89798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson MJ. Ewing’s sarcoma of the temporal bone.A case report. Oral Surg Oral Med Oral Pathol. 1991;72:534–6. doi: 10.1016/0030-4220(91)90489-y. [DOI] [PubMed] [Google Scholar]
- 8.Desai KI, Nadkarni TD, Goel A, Muzumdar DP, Naresh KN, Nair CN. Primary Ewing’s sarcoma of the cranium. Neurosurgery. 2000;46:62–8. [PubMed] [Google Scholar]
- 9.Esiashvili N, Goodman M, Marcus RB., Jr Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol. 2008;30:425–30. doi: 10.1097/MPH.0b013e31816e22f3. [DOI] [PubMed] [Google Scholar]
- 10.Ewing J. Diffuse endothelioma of bone. Proc NY Pathol Soc. 1921;21:17–24. [Google Scholar]
- 11.Fink KR, Fink JR. Imaging of brain metastases. Surg Neurol Int. 2013;4:209–19. doi: 10.4103/2152-7806.111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzer PM, Steffey WR. Brain and bone scans in primary Ewing’s sarcoma of the petrous bone. J Neurosurg. 1976;44:608–12. doi: 10.3171/jns.1976.44.5.0608. [DOI] [PubMed] [Google Scholar]
- 13.Fraumeni JF, Jr, Glass AG. Rarity of Ewing’s sarcoma among U.S. Negro children. 1980;1:366, 7. doi: 10.1016/s0140-6736(70)90754-3. [DOI] [PubMed] [Google Scholar]
- 14.Garg A, Ahmad FU, Suri A, Mahapatra AK, Mehta VS, Atri S, et al. Primary Ewing’s sarcoma of the occipital bone presenting as hydrocephalus and blindness. Pediatr Neurosurg. 2007;43:170–3. doi: 10.1159/000098397. [DOI] [PubMed] [Google Scholar]
- 15.Grier HE. The Ewing family of tumors.Ewing’s sarcoma and primitive neuroectodermal tumors. Pediatr Clin North Am. 1997;44:991–1004. doi: 10.1016/s0031-3955(05)70541-1. [DOI] [PubMed] [Google Scholar]
- 16.Hattori T, Yamakawa H, Nakayama N, Kuroda T, Andoh T, Sakai N, et al. Skull metastasis of Ewing’s sarcoma--three case reports. Neurol Med Chir (Tokyo) 1999;39:946–9. doi: 10.2176/nmc.39.946. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim GM, Fallah A, Shahideh M, Tabori U, Rutka JT. Primary Ewing’s sarcoma affecting the central nervous system: A review and proposedprognostic considerations. J Clin Neurosci. 2012;19:203–9. doi: 10.1016/j.jocn.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Kelleher FC, Thomas DM. Molecular pathogenesis and targeted therapeutics in Ewing sarcoma/primitive neuroectodermal tumours. Clin Sarcoma Res. 2012;2:6. doi: 10.1186/2045-3329-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim EY, Lee SK, Kim DJ, Kim J, Lee KS, Jung W, et al. Intracranial dural metastasis of Ewing’s sarcoma: A case report. Korean J Radiol. 2008;9:76–9. doi: 10.3348/kjr.2008.9.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinsella TJ, Miser JS, Waller B, Venzon D, Glatstein E, Weaver-McClure L, et al. Long-term follow up of Ewing’s sarcoma of bone treated with combined modality therapy. Int J Radiat Oncol Biol Phys. 1991;20:389–96. doi: 10.1016/0360-3016(91)90047-8. [DOI] [PubMed] [Google Scholar]
- 21.Kleinschmidt-DeMasters BK. Dural metastases.A retrospective surgical and autopsy series. Arch Pathol Lab Med. 2001;125:880–7. doi: 10.5858/2001-125-0880-DM. [DOI] [PubMed] [Google Scholar]
- 22.Kolb EA, Kushner BH, Gorlick R, Laverdiere C, Healey JH, LaQuaglia MP, et al. Long-term eventfree survival after intensive chemotherapy for Ewing’s family of tumors in children and young adults. J Clin Oncol. 2003;21:3423–30. doi: 10.1200/JCO.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 23.Ladenstein R, Pötschger U, Le Deley MC, Whelan J, Paulussen M, Oberlin O, et al. Primary disseminated multifocal Ewing sarcoma: Results of the Euro-EWING 99 trial. J Clin Oncol. 2010;28:3284–91. doi: 10.1200/JCO.2009.22.9864. [DOI] [PubMed] [Google Scholar]
- 24.Li WY, Brock P, Saunders DE. Imaging characteristics of primary cranial Ewing sarcoma. Pediatr Radiol. 2005;35:612–8. doi: 10.1007/s00247-005-1438-2. [DOI] [PubMed] [Google Scholar]
- 25.Mazur MA, Gururangan S, Bridge JA, Cummings TJ, Mukundan S, Fuchs H, et al. Intracranial Ewing sarcoma. Pediatr Blood Cancer. 2005;45:850–6. doi: 10.1002/pbc.20430. [DOI] [PubMed] [Google Scholar]
- 26.Naama O, Ajja A, Gazzaz M, Albouzidi A, Belhachmi A, Asri A, et al. Primary Ewing sarcoma of the skull base with cerebral extension.A case report. J Neuroradiol. 2007;34:68–9. doi: 10.1016/j.neurad.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Navas-Palacinos JJ, Aparicio-Duque R, Valdes MD. On the histogenesis of Ewing’s sarcoma.An ultrastructural, immunohistochemical, and cytochemicalstudy. Cancer. 1984;53:1882–901. doi: 10.1002/1097-0142(19840501)53:9<1882::aid-cncr2820530915>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 28.Paulussen M, Ahrens S, Braun-Munzinger G, Craft AW, Dockhorn-Dworniczak B, Dörffel W, et al. EICESS 92 (European Intergroup Cooperative Ewing’s Sarcoma Study) preliminary results. Klin Padiatr. 1999;211:276–83. doi: 10.1055/s-2008-1043800. [DOI] [PubMed] [Google Scholar]
- 29.Pekala JS, Gururangan S, Provenzale JM, Mukundan S., Jr Central nervous system extraosseous Ewing sarcoma: Radiologic manifestations of this newly defined pathologic entity. AJNR Am J Neuroradiol. 2006;27:580–3. [PMC free article] [PubMed] [Google Scholar]
- 30.Pizo P, Poplack D. Ewing’s sarcoma of bone and soft tissue: Principles and Practice of Pediatric Oncology. 3 rd ed. Philadelphia: JB Lippincott; 1996. pp. 840–1. [Google Scholar]
- 31.Rikhof B, de Jong S, Suurmeijer AJ, Meijer C, van der Graaf WT. The insulin-like growth factor system and sarcomas. J Pathol. 2009;217:469–82. doi: 10.1002/path.2499. [DOI] [PubMed] [Google Scholar]
- 32.Rombi B, DeLaney TF, MacDonald SM, Huang MS, Ebb DH, Liebsch NJ, et al. Proton radiotherapy for pediatric Ewing’s sarcoma: Initial clinical outcomes. Int J Radiat Oncol Biol Phys. 2012;82:1142–8. doi: 10.1016/j.ijrobp.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 33.Salunke PS, Gupta K, Malik V, Kumar N, Henke LE, Cai C, et al. Primary Ewing’s sarcoma of cranial bones: Analysis of ten patients. Acta Neurochir (Wien) 2011;153:1477–85. doi: 10.1007/s00701-011-1028-z. [DOI] [PubMed] [Google Scholar]
- 34.Salvati M, D′Elia A, Frati A, Santoro A. Multiple primary cranial Ewing’s sarcoma in adulthood: Case report. J Neurooncol. 2010;98:373–7. [Google Scholar]
- 35.Sato S, Mitsuyama T, Ishii A, Kawakami M, Kawamata T. Multiple primary cranial Ewing’s sarcoma in adulthood: Case report. Neurosurgery. 2009;64:384–6. doi: 10.1227/01.NEU.0000337128.67045.70. [DOI] [PubMed] [Google Scholar]
- 36.Scotlandi K, Benini S, Sarti M, Serra M, Lollini PL, Maurici D, et al. Insulin-like growth factor I receptor mediated circuit in Ewing’s sarcoma/peripheral neuroectodermal tumor: A possible therapeutic target. Cancer Res. 1996;56:4570–4. [PubMed] [Google Scholar]
- 37.Sharma A, Garg A, Mishra NK, Gaikwad SB, Sharma MC, Gupta V, et al. Primary Ewing’s sarcoma of the sphenoid bone with unusual imaging features: A case report. Clin Neurol Neurosurg. 2005;107:528–31. doi: 10.1016/j.clineuro.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Shuper A, Cohen IJ, Mor C, Ash S, Kornreich L, Zaizov R. Metastatic brain involvement in Ewing family of tumors in children. Neurology. 1998;51:1336–8. doi: 10.1212/wnl.51.5.1336. [DOI] [PubMed] [Google Scholar]
- 39.Singh P, Jain M, Singh DP, Kalra N, Khandelwal N, Suri S. MR findings of primary Ewing’s sarcoma of greater wing of sphenoid. Australas Radiol. 2002;46:409–11. doi: 10.1046/j.1440-1673.2002.01086.x. [DOI] [PubMed] [Google Scholar]
- 40.Subbiah V, Anderson P, Lazar AJ, Burdett E, Raymond K, Ludwig JA. Ewing’s sarcoma: Standard and experimental treatment options. Curr Treat Options Oncol. 2009;10:126–40. doi: 10.1007/s11864-009-0104-6. [DOI] [PubMed] [Google Scholar]
- 41.Thomas PR, Perez CA, Neff JR, Nesbit ME, Evans RG. The management of Ewing’s sarcoma: Role of radiotherapy in local tumor control. Cancer Treat Rep. 1984;68:703–10. [PubMed] [Google Scholar]
- 42.van de Luijtgaarden AC, Versleijen-Jonkers YM, Roeffen MH, Schreuder HW, Flucke UE, van der Graaf WT. Prognostic and therapeutic relevance of the IGF pathway in Ewing’s sarcoma patients. Target Oncol. 2013 doi: 10.1007/s11523-012-0248-3. [In press] [DOI] [PubMed] [Google Scholar]
- 43.Wunder JS, Paulian G, Huvos AG, Heller G, Meyers PA, Healey JH. The histological response to chemotherapy as a predictor of the oncological outcome of operative treatment of Ewing sarcoma. J Bone Joint Surg Am. 1998;80:1020–33. doi: 10.2106/00004623-199807000-00011. [DOI] [PubMed] [Google Scholar]


