Abstract
Objective:
The objective of this study was to evaluate the antibacterial effect of two hybrid restoratives, namely resin modified glass ionomer cement (GC Fuji II™ LC, GC Corporation, Tokyo, Japan) and giomer (Beautifil-II, Shofu Inc., Kyoto, Japan) against Streptococcus mutans [Microbial Type Culture Collection (MTCC), 890].
Materials and Methods:
The antibacterial effect was evaluated using an agar diffusion test. The prepared wells in petri dishes were completely filled with chlorhexidine (positive control group), resin modified glass ionomer cement and giomer respectively. Prepared bacterial suspension was poured over the petri dish and was spread evenly using the plate spreader. The culture plates were placed in the incubator for 24 h at 37°C. The antibacterial activity was evaluated after 24 h, 48 h, and 7 days for each group in triplicates.
Results and Conclusion:
The results of the antibacterial effect of the tested materials were collected, statistically analyzed using the ANOVA test to determine the difference between the mean diameters of the inhibition zone produced. The mean zone of bacterial inhibition was found to be more with the giomer specimens at all time periods. However, this inhibitory activity showed a gradual decrease over a period of 7 days and the maximum inhibition was evident after 24 h with both the test materials.
Keywords: Giomer, resin modified glass ionomer cement, Streptococcus mutans
INTRODUCTION
The fundamental requirements of dental materials for restorative treatment include mechanical, physical, and adhesive properties that have been greatly exalted as a result of numerous investigations with many materials exhibiting enhanced clinical performance. Such improvement of restorative materials and the altering professional perceptions also come along with raising cognizance that caries treatment is not merely a technique, but requires a biomimetic approach. Among others, one of the bioactive functions proposed for restorative materials is antibacterial activity. The current trend in restorative dentistry has been greatly influenced by better understanding of the caries process, advances in the dental materials science and increased demand for bioactive restorations, which prevent the recurrence of carious lesions.
Recurrent dental caries has been associated with deteriorating restorative materials, providing a potential pathway to the cariogenic microbes. Glass ionomer cements (GIC) have been widely used as a caries preventive aid.[1] However, there is a perpetual evolution of restorative materials and techniques in dentistry, which has paved way for the development of various fluoride releasing dental materials. In this pursuit for newer restorative provisions, hybrid restorative materials such as resin modified glass ionomer cements (RMGIC), compomers and giomers were developed to combine the fluoride releasing properties of GIC and esthetics of composite resins.
Hybrid materials combining the technologies of glass ionomers and resin composites have been developed to help overcome problems of conventional GIC's such as moisture sensitivity, low initial mechanical properties, and inferior translucency; and at the same time maintain their clinical advantages such as fluoride release and adhesiveness.[2]
Although various mechanisms have been proposed to explain the antibacterial activity of GIC,[3,4] there is little information regarding this activity in hybrid restorative materials. Hence, the purpose of the present study was to evaluate the antibacterial effect of two hybrid restoratives against Streptococcus mutans.
MATERIALS AND METHODS
An agar diffusion test was used for evaluation of antibacterial effect against S. mutans (MTCC 890). All the procedures were carried out under aseptic conditions in a laminar airflow chamber. The hybrid restorative materials used were RMGIC (GC Fuji II™ LC, GC Corporation, Tokyo, Japan) and giomer (Beautifil-II, Shofu Inc., Kyoto, Japan).
Indicator strains of S. mutans (MTCC 890) in the form of lyophilized culture were obtained (Institute of Microbial Technology, IMTECH, Chandigarh). They were grown in 15 ml of Luria Bertani broth (HiMedia Laboratory Pvt. Ltd., Mumbai, India) separately for 48 h at 37°C according to physiological characteristics of the microorganism. The resultant bacteria were again placed in 5 ml of Brain Heart Infusion broth (HiMedia Laboratory Pvt. Ltd., Mumbai, India) for 24 h at 37°C to form a suspension (inoculum), corresponding to 106 colony forming units/mL using the McFarland scale.
The test specimens for both the dental materials were prepared using a custom made teflon ring mould with a diameter of 6.5 mm and thickness of 2 mm. The capsules of RMGIC (GC Fuji II LC™, GC Corporation, Tokyo, Japan) were activated and mixed in amalgamator for 10 s according to manufacturer's instructions. The specimens were made by placing the respective materials into the mould over a glass slab sandwiched between two mylar strips. The material was light cured for 40 s on each side. The giomer (Beautifil-II, Shofu Inc., Kyoto, Japan) specimens were prepared similarly by incremental insertion into the mold with the plastic instruments followed by sandwiching between two mylar strips and light curing for 40 s on each side. Ten microliters of aqueous 0.2% chlorhexidine digluconate (Hexidine mouthwash-ICPA Health Products Ltd., India) was applied on sterile filter paper discs, which was used as a positive control. All specimens were sterilized by autoclaving at 121°C at 15 lbs pressure for 15 min.
For the agar diffusion test, a base layer containing 15 ml Brain Heart Infusion agar (HiMedia Laboratory Pvt. Ltd., Mumbai, India) was evenly spread to a thickness of 5 mm in sterile petri dish. After solidification of the culture medium, in each petri dish three wells measuring 6.5 mm in diameter were made using the blunt end of a micropipette tip. The wells were completely filled with the chlorhexidine, RMGIC and giomer respectively A total of 100 ul of bacterial suspension (620OD = 0.4) was poured with micropipette and it was spread evenly using the plate spreader. The culture plates were placed in the incubator for 24 h at 37°C. The antibacterial activity was evaluated at 24 h, 48 h and 7 days for each group in triplicates. After incubation, the plates were taken out of the incubator and the zones of bacterial inhibition were recorded in millimeters using a digital caliper. Measurements were taken at the greatest distance between two points at the outer limit of inhibition halo formed around the wells. This measurement was repeated three times and the mean was calculated for each well.
RESULTS
The results of the antibacterial effect of the tested materials were collected, statistically analyzed using the ANOVA test to determine the difference between the mean of the diameters of the inhibition zone produced. The data generated during the study was processed with the aid of PASW 18.0 Software. The significance level was chosen to be 0.05 and the mean zones of inhibition produced were recorded after 24 h, 48 h, and 7 days for both the test materials [Tables 1 and 2]. The comparison of mean zone of inhibition obtained at different time intervals indicated no statistically significant difference. However, there was a general trend of decrease in zone of inhibition, which indicated lowering of antibacterial activity as a function of time with both materials. The comparative assessment of the mean zone of inhibition obtained with the RMGIC and giomer indicated significant differences [Table 3]. The material giomer exhibited greater zone of inhibition at all time periods indicating a higher antibacterial activity.
Table 1.
Mean zone of inhibition observed as a function of use of RMGIC against Streptococcus mutans (MTCC 890)
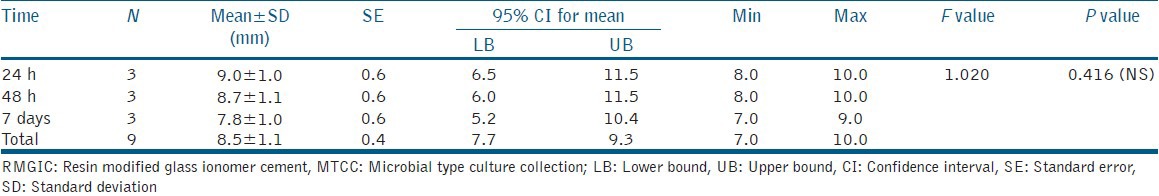
Table 2.
Mean zone of inhibition observed as a function of use of giomer against Streptococcus mutans (MTCC 890)
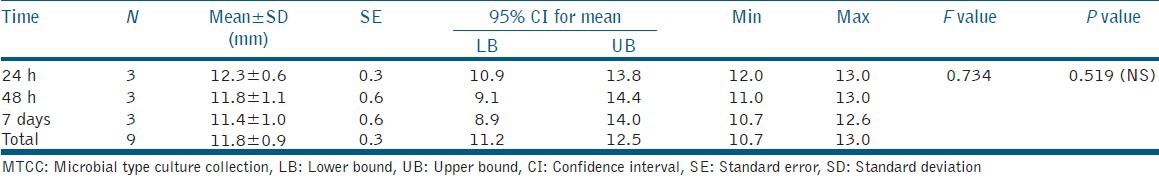
Table 3.
Comparative assessment of mean zone of inhibition obtained with RMGIC and giomer against Streptococcus mutans (MTCC 890)
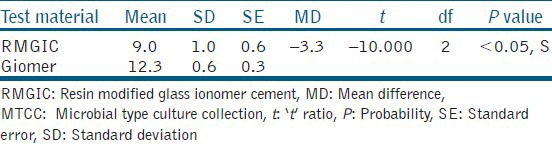
DISCUSSION
Hybrid tooth colored restorative materials have many advantages such as the ability to bond chemically to tooth, biocompatibility, and good cavity seal.[5] Furthermore, the restorative margins of GIC and hybrid restorations have been found to have lower levels of S. mutans and plaque, which implies that plaque formed on these restorations has less potential to induce recurrent caries.[6,7]
Antibacterial properties of restorative cements have been evaluated in the past, and the bactericidal effects are often attributed to their low pH and/or release of fluoride. A variety of mechanisms are involved in the anti-cariogenic effects of fluoride on the teeth.[8,9,10] Fluoride inhibits production of bacterial acids and glucans produced by S. mutans, which is known to be the primary etiologic factor for carious lesions, and therefore has a routine use in testing the antimicrobial activity of restorative materials.[11,12]
In the present study, the antibacterial activity was evaluated using the agar diffusion test. This assay allows bacteria to be screened in a routine, economical, and easy way for the detection of resistance.[13] In our study, the wells made in the agar were approximately 6.5 mm in diameter, whereas in most other studies, the diameter of the wells used was 4 mm. It is believed that 6.5 mm wells incorporate more amounts of cement and provide a much larger surface area for the diffusion of the soluble antibacterial agents and would ease in the determination of the antibacterial effect of the materials under study.[14] The agar medium used in the present study was Brain Heart Infusion agar. However, many different agar media have been used, which can bear an influence on the solubility of the antibacterial agents released by the dental cements leading to varied results. The results of the present study showed that both of the test materials possessed strong inhibitory effect on the bacterial growth. However, the inhibiting zone produced by both test materials was smaller than chlorhexidine, which produced an average inhibition zone of 15 mm. Because of its widespread clinical use and a common point of reference for comparisons with others studies, chlorhexidine was chosen as the positive control in our study.[15]
The effects of glass ionomer based restoratives on cariogenic bacteria are known, probably resulting from the release of fluoride, but this information is not reliable.[16] According to Vermeersch et al.,[17] the low pH of GICs, during setting may contribute more to their antibacterial properties than their fluoride-leaching capabilities. Yap et al.,[12] reported that there was no antibacterial activity despite the presence of fluoride in the agar around the set materials. However, varying results could be obtained, as it is known that the diffusibility of an antimicrobial agent depends on its size, form of filler particles, and its concentration in the material. In addition, the diffusibility of ions (F−, Ca++, Al+++, OH−) from GIC depends on the pH of the environment.
In the present study, a gradual decrease in antibacterial activity was observed for both the materials as a function of time. The maximum effect was observed after 24 h. Vermeersch et al.[17] reported that the GIC Fuji II LC and Ketac-Fil showed an inhibition halo only during hardening, probably by the generation of a low pH around the test specimens. Conversely, in the present study, prolonged inhibitory activity was observed against S. mutans with both test materials. However, a gradual decrease in the antibacterial activity was observed from 24 h to 1 week. A study of the short-term and long-term fluoride release from four RMGIC materials into water reported values ranging from 0.15 μg to 0.46 μg in the first 24 h.[18] High level of fluoride release on the 1st day may be caused by the initial superficial rinsing effect, although the constant fluoride release during the following days occurs because of fluoride ability to diffuse through cement pores and fractures.
The inclusion of fluorite and/or cryolite as fluxes for firing purposes in the RMGIC may enhance the release of F − ions into the matrix during the setting reaction in the initial 24 h period. Furthermore, the liquid component of RMGIC conventionally contains hydroxyl-ethyl methacrylate, which in part may aid the antibacterial effect by providing a low initial pH. The initial mix of the cement is also of low pH (2.2-3.6) and rises to neutrality during progress of the setting reaction. This early acidity may play a major role in its antibacterial effect. Current studies show that the growth of S. mutans colonies significantly decrease at pH 5.1 and completely appears at pH 4.8 or lower level.[17,19] Accordingly, remarkable strong antibacterial effect exhibited by giomer may be owing to its similar low pH during initial mixing and setting. The pH rises to higher levels after 24 h, which could explain the decreased antibacterial action after hardening of the material. A study has shown that the amount of total and free fluoride release from giomer is higher than compomer and resin composite and concluded that the extent of glass ionomer matrix of the glass filler play a significant role for fluoride releasing and recharging abilities of the resin based materials.[20]
In the present study, another explanation for the difference in fluoride release between the RMGIC and the giomer could be owing to the porosity of the materials that may have a great influence on the amount of fluoride release. The greater resin content of RMGIC may act as a barrier for diffusion of water and fluoride. This was in accordance with the results of the present study as giomer was found to have better antibacterial effect.
When evaluated clinically, giomer restorations exhibited superior surface finish compared to RMGIC, which was attributed to the microstructure and mean particle size of the restorative materials. The mean particle size of RMGIC (Fuji II LC) is shown to be 4.5-4.8 μm, whereas for giomer it is 20-40 nm.[21]
Fluoride release can be affected by factors such as material solubility, solution acidity and/or the presence of surface coatings, the type and brand of material, fluoride concentration of glass, acidity of environment and handling of material. The three key factors that affect fluoride release from dental materials in the oral cavity are (i) composition of the material (ii) manipulation of material and (iii) kinetics of the oral environment.[22] Our results implies that the level of fluoride released as well as the physical properties of the material may influence the ability of fluoride containing restorative materials to inhibit growth of caries-producing bacteria.
CONCLUSION
Based on the results of the present study, it can be concluded that the test materials, RMGIC and giomer showed inhibitory activity against S. mutans. This activity appears to be variable and dependent on factors such as the chemical composition, low pH, release of F−, and other ions from the restorative materials. Thus, it can be established with the continuous urge for novelties in dentistry, ranging from changing professional perceptions to altering demands from the patient and the enormous progress in material sciences, the quest for an ideal aesthetic biomimetic restorative material still continues. Further, research regarding these bioactive materials is still required.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Loyola-Rodriguez JP, Garcia-Godoy F, Lindquist R. Growth inhibition of glass ionomer cements on mutans Streptococci. Pediatr Dent. 1994;16:346–9. [PubMed] [Google Scholar]
- 2.Arora V, Bogra P. Giomer-A new hybrid aesthetic restorative material. J Conserv Dent. 2002;5:149–55. [Google Scholar]
- 3.DeSchepper EJ, White RR, von der Lehr W. Antibacterial effects of glass ionomers. Am J Dent. 1989;2:51–6. [PubMed] [Google Scholar]
- 4.Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials: Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 2007;23:343–62. doi: 10.1016/j.dental.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Davidovich E, Weiss E, Fuks AB, Beyth N. Surface antibacterial properties of glass ionomer cements used in atraumatic restorative treatment. J Am Dent Assoc. 2007;138:1347–52. doi: 10.14219/jada.archive.2007.0051. [DOI] [PubMed] [Google Scholar]
- 6.Svanberg M, Mjör IA, Orstavik D. mutans Streptococci in plaque from margins of amalgam, composite, and glass-ionomer restorations. J Dent Res. 1990;69:861–4. doi: 10.1177/00220345900690030601. [DOI] [PubMed] [Google Scholar]
- 7.Svanberg M, Krasse B. Comparative recovery of mutans Streptococci on two selective media. Caries Res. 1990;24:36–8. doi: 10.1159/000261235. [DOI] [PubMed] [Google Scholar]
- 8.Ten Cate JM, Featherstone JD. Physicochemical aspects of fluoride-enamel interactions. In: Fejerskov O, Ekstrand J, Burt BA, editors. Fluoride in Dentistry. 2nd ed. Copenhagen: Munksgaard; 1996. pp. 252–72. [Google Scholar]
- 9.Forss H, Jokinen J, Spets-Happonen S, Seppä L, Luoma H. Fluoride and mutans Streptococci in plaque grown on glass ionomer and composite. Caries Res. 1991;25:454–8. doi: 10.1159/000261410. [DOI] [PubMed] [Google Scholar]
- 10.Lewinstein I, Matalon S, Slutzkey S, Weiss EI. Antibacterial properties of aged dental cements evaluated by direct-contact and agar diffusion tests. J Prosthet Dent. 2005;93:364–71. doi: 10.1016/j.prosdent.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Burgess JO. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials. 2003;24:2451–61. doi: 10.1016/s0142-9612(02)00638-5. [DOI] [PubMed] [Google Scholar]
- 12.Yap AU, Khor E, Foo SH. Fluoride release and antibacterial properties of new-generation tooth-colored restoratives. Oper Dent. 1999;24:297–305. [PubMed] [Google Scholar]
- 13.Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin Infect Dis. 2009;49:1749–55. doi: 10.1086/647952. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues MA, Barbosa SL, Gonzaga LL. Nanosilver application in dental cements. ISRN Nanotechnol 2012. 2012:1–6. [Google Scholar]
- 15.Herrera M, Carrión P, Baca P, Liébana J, Castillo A. In vitro antibacterial activity of glass-ionomer cements. Microbios. 2001;104:141–8. [PubMed] [Google Scholar]
- 16.Weerheijm KL, de Soet JJ, van Amerongen WE, de Graaff J. The effect of glass-ionomer cement on carious dentine: An in vivo study. Caries Res. 1993;27:417–23. doi: 10.1159/000261573. [DOI] [PubMed] [Google Scholar]
- 17.Vermeersch G, Leloup G, Delmée M, Vreven J. Antibacterial activity of glass-ionomer cements, compomers and resin composites: Relationship between acidity and material setting phase. J Oral Rehabil. 2005;32:368–74. doi: 10.1111/j.1365-2842.2004.01300.x. [DOI] [PubMed] [Google Scholar]
- 18.Mousavinasab SM, Meyers I. Fluoride release by glass ionomer cements, compomer and giomer. Dent Res J (Isfahan) 2009;6:75–81. [PMC free article] [PubMed] [Google Scholar]
- 19.DeSchepper EJ, Thrasher MR, Thurmond BA. Antibacterial effects of light-cured liners. Am J Dent. 1989;2:74–6. [PubMed] [Google Scholar]
- 20.Itota T, Carrick TE, Yoshiyama M, McCabe JF. Fluoride release and recharge in giomer, compomer and resin composite. Dent Mater. 2004;20:789–95. doi: 10.1016/j.dental.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Jyothi K, Annapurna S, Kumar AS, Venugopal P, Jayashankara C. Clinical evaluation of giomer and resin-modified glass ionomer cement in class V noncarious cervical lesions: An in vivo study. J Conserv Dent. 2011;14:409–13. doi: 10.4103/0972-0707.87214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemamalathi Ballal S, Kandaswamy D, Gupta T. A dynamic methodology for measuring fluoride release from restorative materials. J Conserv Dent. 2006;9:113–6. [Google Scholar]


