Abstract
Context:
During biomechanical preparation, a smear layer is formed which occludes the openings of dentinal tubules and disfavors the penetration of irrigants. Hence, such layers should be removed. It becomes more challenging when we approach the apical third of the root canal.
Aim:
The aim was to compare the efficacy of different irrigants including ethylenediaminetetraacetic acid (EDTA), EDTA along with ultrasonication, citric acid, and mixture of tetracycline isomer, an acid, and a detergent (MTAD) as final irrigants where sodium hypochlorite (NaOCl) was used in each experimental group during root canal preparation with special emphasis on the apical third.
Settings and Design:
Forty-five human upper anterior teeth were selected and divided into one control group (group 1) and four experimental groups (group 2 to group 5), each containing nine teeth. All the four experimental groups were irrigated with 5.25% NaOCl solution during preparation, whereas test irrigants (5 mL) as the final solution used in each experimental group were 17% EDTA, 17% EDTA along with ultrasonication, 25% citric acid, and MTAD, respectively. The samples were prepared and observed under a scanning electron microscope (SEM). The photomicrographs were recorded and evaluated with a scoring system.
Statistical Analysis Used:
Data were analyzed using Kruskal-Wallis test and Dunn's test (P = 0.05).
Results:
None of the combined irrigants was found completely effective. All the test irrigants including MTAD worked well in the middle and cervical third, whereas MTAD showed excellent results in the apical third as compared to the other groups.
Keywords: Apical third, irrigating solution, MTAD, root canal, smear layer
INTRODUCTION
During preparation of the root canal, that is, enlarging and shaping of root canal with files and other instruments, some dentinal shavings are formed. These dentinal shavings associated with dentinal tubular organic materials and micro-organisms form a layer called smear layer. This layer blocks the openings of the dentinal tubules.
The objective of biomechanical preparation is not only to remove the pulp and dentinal debris and to prepare the conical shape of the root canal but also to remove the smear layer and to clear the dentinal opening. Lots of bacteria harbor inside these tubules and these tubules need to be sterilized, as advocated by Shovelton (1962). Moreover, the smear layer has the potential to protect bacteria within the dentinal tubules; therefore, it may be prudent to remove the smear layer from infected root canals. Further, removal of the smear layer allows better penetration of the irrigants into the dentinal tubules, better flow of cement sealer through empty spaces, and ultimately provides better adhesion between the obturating material and the dentinal wall.
The irrigating process has three objectives as advocated by the Walker:[1] 1) Dissolution of remnant tissue, 2) antimicrobial action, and 3) lubrication of the canal. Many researchers have pointed out that the smear layer is difficult to remove. One of the irrigants should be a chelating agent to remove it more effectively. Considering all the objectives of irrigants, a protocol of various combined irrigants has been advocated since long. Earlier, Yamda et al.[2] had advocated that irrigation with 17% ethylenediaminetetraacetic acid (EDTA) followed by 5.25% sodium hypochlorite (NaOCl) produced very clean surface of root canals.
According to Leol[3] and Baumgartner et al.,[4] citric acid in combination with NaOCl is very effective in removing the smear layer from the surface of the middle and coronal third of the root canal, whereas removal from the apical region remains unpredictable.[5,6]
The apical third of the root canal is the most difficult portion to clean possibly because of its narrower dimension. As per previous studies by Ciucchi et al.,[7] Takeda et al.,[8] and Ahmad et al.,[9] the use of ultrasonic stirring has been suggested to improve irrigation in the root canal and Ahmad et al. had demonstrated that the main mechanism responsible for ultrasonic debridement was acoustic streaming.[9]
As previously documented by Torabinejad et al.,[10] MTAD (BioPure, DENTSPLY, (Tulsa Dental, Johnson City, TN) is the irrigating solution of the mixture of tetracycline isomer (doxycycline), an acid (citric acid), and a detergent (sodium lauryl sulfate/Tween-80). The effectiveness of MTAD to remove the smear layer completely is synergistically improved when 2.5% NaOCl is used prior to the use of MTAD.
The aim of this study was to compare the effects of different irrigating groups including 17% EDTA, EDTA along with ultrasonication, 25% citric acid, and MTAD as final irrigants to remove the smear layer where NaOCl solution was used in each experimental group during preparation of the root canal, with special emphasis on the apical third of the root canal.
SUBJECTS AND METHODS
Sample selection and criteria
Forty-five human upper anterior teeth freshly extracted because of periodontal reason were considered for this study. The teeth were randomly selected from the patients. Teeth with mature root apex, similar anatomic characteristics, and relatively straight roots (degree of root curvature <30, according to Schneider's[11] classification) were selected for this study.
They were divided into one control group and four experimental groups. Hence, for each group, nine teeth were taken. The extracted teeth were rinsed under running distilled water and kept in saline.
Smear layer production and irrigation protocols
Within seven days, these anatomical crowns were decapitated, leaving behind an average root length of 12 mm. The length of root canal was measured by placing a #10 K-file. The tip of the file that was visible from the apex was recorded and the working length of the specimen was just 1 mm short of the recorded length. Biomechanical preparation of teeth was done by crown-down technique using ProTaper rotary files. After instrumentation, each canal was flushed with 3 mL sterile water. The apical foramen of each tooth was plugged with modeling compound to prevent the irrigating solution from passing from the apical foramen.
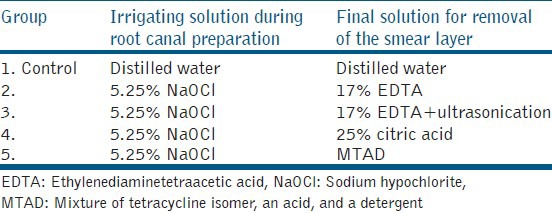
Following instrumentation, the root canal was irrigated with the test solutions (5 mL) each. The group-wise irrigation protocols used in this study have been described in Table 1.
Table 1.
Substances used and the irrigation protocol
Preparation of SEM samples
The root canals were then dried with paper points and the coronal openings were blocked with cotton pellets to prevent entry of any foreign material into the canals. Sectioning of the teeth was done with a diamond disc. Two furrows were made on the buccal and lingual aspects of each tooth without damaging the root canal. The two section parts were split with a bone chisel. One half of each root was discarded and the other half was sectioned into three parts, that is, cervical third, middle third, and apical third. These section parts were immersed for tissue fixation in 2% gluteraldehyde with phosphate buffer (pH = 7.3) for 12 hours. The specimens were then washed in 20 mL of phosphate buffer for 60 minutes and dehydrated by dipping the sections into 50, 75, and 100% ethyl alcohol for 24 hours.
The roots were then left overnight in a dessicator. The temperature was maintained at 60°C. The specimens also had to be coated with a thin conductive metal film. The metal film offers a high secondary emission coefficient. Palldium-gold was used for this purpose. Now the specimens were ready for observation under a scanning electron microscope (SEM). The photomicrographs of the specimens were obtained in different magnifications (×750, ×1500, ×2000, ×3000, ×5000).
Evaluation of SEM
To evaluate the degree of removal of the smear layer, the scoring system described by Takeda et al.[8] was used with modifications.[12] The photomicrographs were quantitatively evaluated to rate the cleanliness with regard to the presence of debris, smear layer, and opening of dentinal tubules on a scale of 1-4.
Briefly,
Score 1 : No smear layer and debris at all, with all tubules cleaned and opened
Score 2 : A few areas covered by a smear layer and debris, with most tubules cleaned and opened
Score 3 : Smear layer and debris covering almost all the surface, with few tubules opened
Score 4 : Smear layer and debris covering all the surfaces.
Statistical analysis
Data were analyzed by the Kruskal-Wallis test and the Dunn's test for pair-wise comparisons. All the statistical analyses were set with a significance level of P = 0.05.
RESULTS
The results of the smear layer scores for each group with their mean, standard deviation (SD), median, and standard error (SE) values are listed in Table 2, and their group-wise comparisons with post-hoc analysis (Dunn's test) in the apical, middle, and cervical third have been shown in Table 3. Also, the SEM photomicrographs of each group have been shown in Figure 1.
Table 2.
Mean, standard deviation, median, and standard error values of smear layer scores
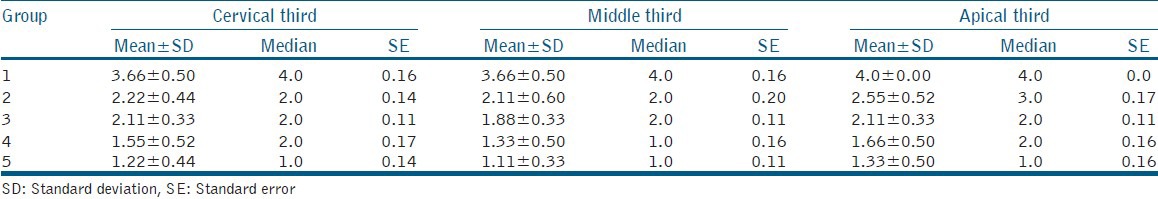
Table 3.
Post hoc analysis (Dunn's test) for cervical third, middle third, and apical third of root canal
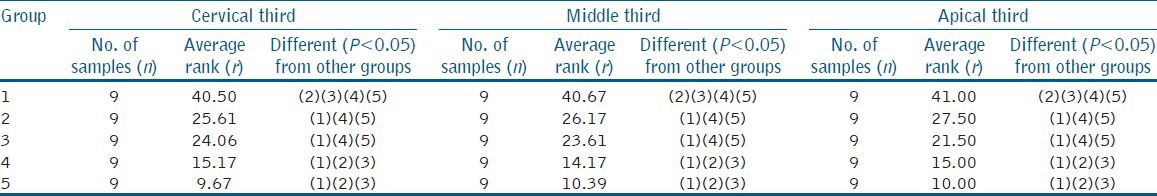
Figure 1.
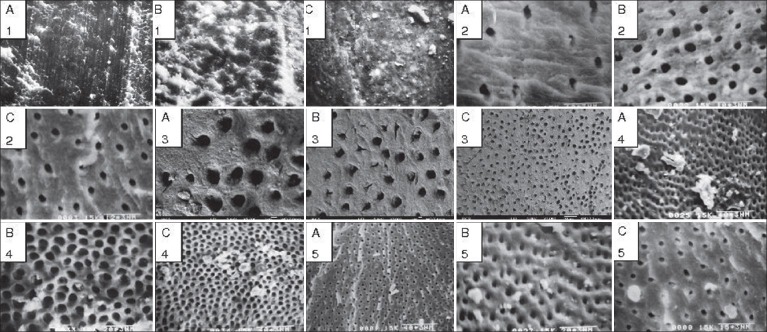
Scanning electron microscope (SEM) photomicrographs of specimens of all the five groups with configurations (on the top left) A, B, and C representing the apical, middle, and cervical third portion of the root canal, respectively, whereas 1, 2, 3, 4, and 5 represent their respective groups; these photographs were obtained at different magnifi cations of ×750, ×1500, ×2000, ×3000, and ×5000
Group 1 (control): Examination of the surface of root canal walls showed the presence of a heavy smear layer throughout the entire length of canals. Also, there was an extensive amount of debris fixed to all root canal surfaces. The control group showed a statistically significant difference in the amount of smear layer remaining at all the three levels of canals in comparison to the experimental groups (P < 0.001).
Group 2 (5.25% NaOCl + 17% EDTA): Compared to group 1, a lighter smear layer was noted on the surface of most samples in the coronal and middle third of the roots but in most samples in the apical third of the roots, smear layer and debris covered almost all the surface, with a few tubules opened.
Group 3 (5.25% NaOCl + 17% EDTA with ultrasonication): Almost all the specimens showed a lighter smear layer and some debris remaining in the apical region of canals compared to EDTA alone as final irrigant. However, ultrasonication produced an erosive effect especially in the middle and coronal third of the root canal. Although this group showed better clearance than group 2, statistically, the difference was not found significant.
Group 4 (5.25% NaOCl + 25% citric acid): The specimens of this group were significantly clearer when compared to the earlier groups, that is, group 1, 2, and 3 (P < 0.05). In higher magnification, the canals surfaces were almost free of the smear layer; the middle third of the canal especially exhibited a much cleaner surface than the other two portions. Many dentinal tubules were patent but some debris was noted in the apical region.
Group 5 (5.25% NaOCl + MTAD): All the specimens (except three) in the coronal and middle third of the canals had no smear layer. Also, only a few samples showed a smear layer in the apical region. This group showed a statistically significant difference with the groups 1, 2, and 3 at all the 3 levels (P < 0.05). Also, this group produced better clearance of the smear layer than group 4 at all the three levels but the difference was not found statistically significant.
DISCUSSION
The endodontic instrumentation methods currently in use produce a smear layer that covers the root canal surfaces. The smear layer contains inorganic and organic substances which include the fragments of odontoblastic processes, micro-organisms, their by-products, and necrotic materials, and because of its potential contamination and adverse effects on the outcome of root canal therapy, it seems reasonable to suggest removal of the smear layer for disinfection of the entire root canal system.[13]
The apical third of the root canal system is particularly difficult to clean because of the typically challenging complexity of the root canal morphology, making irrigant delivery and activation less effective. Current methods of removal of the smear layer include chemical, ultrasonic, and laser methods, none of which is totally effective or has received universal acceptance.[13]
A recent review of the smear layer by Violich and Chandler[14] indicates that removal of the smear layer results in a more thorough disinfection of the root canal system and better adaptation of filling materials to the canal walls.
Irrigation plays a major role in successful debridement and disinfection. The most widely used irrigant for root canal therapy is NaOCl at a concentration of 0.5 to 5.25%. The tissue-dissolving capacity and microbicidal activity of NaOCl make it an excellent irrigating solution, but it has only a limited effect on dissolution of the smear layer.[15]
Also, acid solutions have been recommended for removing the smear layer including sodium salt of EDTA at a concentration of 15-17%,[16] citric acid at concentrations of 10, 25, and 50%,[17] and orthophosphoric acid at concentrations of 10, 32, and 37%.[16]
Recently, MTAD has been introduced to dentistry as a final irrigant for removal of the smear layer.[10] It has proved to be effective in eliminating resistant micro-organisms and providing sustained antimicrobial activity.[18]
The purpose of the current study was to compare the effectiveness of various irrigating solutions including 17% EDTA, 25% citric acid, and MTAD as final irrigants combined with 5.25% NaOCl as irrigants while cleaning and shaping in the removal of the smear layer from all the three regions of the root canal especially in the apical third. Moreover, in one experimental group, we included ultrasonication along with EDTA as a final irrigating solution and it has been shown that the use of ultrasonication with 17% EDTA facilitates the removal of smear layer in the apical region of the root canal.[19]
An SEM was used to assess the effectiveness of such irrigants to remove the smear layer and the erosion caused in the dentinal tubules. In group 1 (control), distilled water was completely ineffective in removing the smear layer from all the three regions, as it showed the presence of a heavy smear layer throughout the entire length of the root canal.
Yamada et al.(1983)[2] and Baumgartner and Mader (1987)[5] showed that alternating the use of EDTA and NaOCl is an effective method for removal of the smear layer. This supports our finding for group 2. The smear layer was effectively removed from the middle and coronal third, whereas this seemed less effective in the apical third of the canal where a few areas of smear layer with tubules containing debris were observed in most of the samples. This finding is probably because of inadequate penetration of the solution into the apical portion of the canal during irrigation.
In group 3, the ultrasonication of 17% EDTA as the final irrigant resulted in better removal of the smear layer and debris, although the difference was not statistically significant when compared with group 2. This was probably due to adequate penetration of the solution into the apical portion of canal and this is more likely to be due to the use of a small file with ultrasonic stirring or the introduction of the solution into the apical region to promote the interaction of the chelating agent with the dentinal wall.[20]
Van der Sluis et al.[21] found ultrasonication to be more effective for the removal of artificially placed dentin debris, although not to a significant degree. This finding is in agreement with the present result and is also supported by the study done by Kocani et al.[22]
Along with better removal of the smear layer in the apical third than group 2, group 3 demonstrated a more erosive effect in the root canal surface especially in the middle and coronal third. The fact that 17% EDTA dissolves the mineral content of dentin and there is free oscillation of the ultrasonic file in the coronal third which contain large dentinal tubules may explain the higher dentinal erosion in this region.[23]
Citric acid, a crystalline organic acid, is antibacterial, and concentrations of 10, 25, and 50% citric acid have been demonstrated to remove the smear layer by Baumgartner et al. (1984).[4] They evaluated the amount of superficial debris and the smear layer that remained on the canal wall after a combination of NaOCl and citric acid and concluded that citric acid or a combination of these, irrespective of the order in which they were used was more effective than NaOCl alone to remove the smear layer from the surface of the instrumented canal.
In the present study, the instrumented canals irrigated with 25% citric acid showed the canal surfaces which were almost free of the smear layer. The middle third of the canal especially exhibited a much cleaner surface than the other two portions and this may be attributed to availability of better volume and better penetration of the acid in this region. Perez-Heredia et al. (2006),[24] who used 15% EDTA and 15% citric acid, found better results for the cervical and middle third, compared with the apical one-third.
Torabinejad et al. (2003)[25] compared MTAD alone with a combination of various concentrations of NaOCl as an irrigant and MTAD as a final rinse. Apparently, NaOCl is needed as an irrigant to assist MTAD to remove the smear layer completely. This becomes more significant as the concentration of NaOCl increases from 0.65 to 5.25% and the effectiveness of NaOCl to remove the organic part of the smear layer becomes evident and significant at a higher concentration (1.3-5.25%).
Dineshkumar et al. (2012)[26] studied the effects of the three chelating agents including MTAD on the root dentin and found that these irrigants significantly reduced the microhardness of dentin than the control group because of its higher demineralizing action.
Here in this study, in group 5 with MTAD as the final irrigant, all the root canal apical surfaces were not completely devoid of the smear layer but there was a statistically significant clearance of root canal surfaces when compared with groups 1, 2, and 3. Although this group produced better results in removing the smear layer than group 4, it was not statistically significant.
CONCLUSION
Based on the findings of the present study, with respect to control, the procedure used in every group was competent enough to remove the smear layer. Most importantly, the results point toward the possibility of MTAD solution as a promising agent for significant removal of the smear layer from the apical third when used in accordance with the protocol of the manufacturer. An added advantage of the excellent disinfecting property of MTAD may also be considered while making a choice of irrigant for preparation of root canal.
ACKNOWLEDGMENT
The authors wish to express their indebtedness and sincere thanks to Professor (Dr.) B. B. Dutta, former Principal, Dr. R. Ahmed Dental College and Hospital, Kolkata for his guidance and support at every step in this venture.
Footnotes
Source of Support: Department of Conservative Dentistry and Endodontics, Dr. R. Ahmed Dental College and Hospital, Kolkata, and the Indian Association for the Cultivation of Science (IACS), Jadavpur, Kolkata
Conflict of Interest: None declared
REFERENCES
- 1.Walker A. A definite and dependable therapy for pulpless teeth. J Am Dent Assoc. 1936;23:1418–26. [Google Scholar]
- 2.Yamada RS, Annabelle A, Goldman M, Peck SL. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions: Part III. J Endod. 1983;4:137–42. doi: 10.1016/S0099-2399(83)80032-6. [DOI] [PubMed] [Google Scholar]
- 3.Loel DA. Use of acid cleanser in endodontic therapy. J Am Dent Assoc. 1975;90:148–51. doi: 10.14219/jada.archive.1975.0010. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner JC, Brown CM, Mader CI, Peters DD. A scanning electron Microscopic evaluation of root canal debridement using saline, sodium hypochlorite and citric acid. J Endod. 1984;10:525–31. doi: 10.1016/S0099-2399(84)80137-5. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner JC, Mader CI. A scanning electron microscopic evaluation of four root canal irrigating regimens. J Endod. 1987;13:147–57. doi: 10.1016/s0099-2399(87)80132-2. [DOI] [PubMed] [Google Scholar]
- 6.Abbott PV, Heijkoop PS, Cardaci SC, Hume WR, Heithersay GS. A SEM study of the effects of different irrigation sequences and ultrasonics. Int Endod J. 1991;24:308–16. doi: 10.1111/j.1365-2591.1991.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 7.Ciucchi B, Khettabi M, Holz J. The effectiveness of different endodontic irrigation procedures on the removal of smear layer: A scanning electron microscope study. Int Endod J. 1989;22:21–8. doi: 10.1111/j.1365-2591.1989.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 8.Takeda FH, Harashima T, Kimura Y, Matsumoto K. A comparative study of the removal of smear layer by three endodontic irrigants and two types of lasers. Int Endod J. 1999;32:32–9. doi: 10.1046/j.1365-2591.1999.00182.x. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad M, Pitt Ford TR, Crum LA. Ultrasonic debridement of root canals: Acoustic streaming and its possible role. J Endod. 1987;13:490–9. doi: 10.1016/s0099-2399(87)80016-x. [DOI] [PubMed] [Google Scholar]
- 10.Torabinejad M, Khademi AA, Babagoli J, Cho Y, Johnson WB, Bozhilov K, et al. A new solution for the removal of smear layer. J Endod. 2003;29:170–5. doi: 10.1097/00004770-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Schneider's SW. A comparison of canal preparation in straight and curved root canals. Oral Surg Oral Med Oral Pathol. 1971;32:271–5. doi: 10.1016/0030-4220(71)90230-1. [DOI] [PubMed] [Google Scholar]
- 12.Prado M, Gusman H, Gomes BP, Simão RA. Scanning electron microscopic investigation of the effectiveness of phosphoric acid in smear layer removal when compared with EDTA and citric acid. J Endod. 2011;37:255–8. doi: 10.1016/j.joen.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Torabinejad M, Handysides R, Khademi A, Bakland LK. Clinical implications of smear layer in endodontics: A review. Oral Radiol Endod. 2002;94:658–66. doi: 10.1067/moe.2002.128962. [DOI] [PubMed] [Google Scholar]
- 14.Violich DR, Chandler NP. The smear layer in endodontics: A review. Int Endod J. 2010;43:2–15. doi: 10.1111/j.1365-2591.2009.01627.x. [DOI] [PubMed] [Google Scholar]
- 15.Zehnder M, Kosicki D, Luder H, Sener B, Walumo T. Tissue dissolving capacity and antimicrobial effect of buffered and unbuffered hypochlorite solution. Oral Surg Oral Med Oral Path Oral Radiol Endod. 2002;94:756–62. doi: 10.1067/moe.2002.128961. [DOI] [PubMed] [Google Scholar]
- 16.Garberoglio R, Becce C. Smear layer removal by root canal irrigants: A comparative scanning electron microscopic study. Oral Surg Oral Med Oral Pathol. 1994;78:359–67. doi: 10.1016/0030-4220(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 17.Ferrer Luque CM, Gonzalez Lopez S, Navajas Rodriguez de Mondelo JM. Mechanical instrumentation of the root canals: A study using SEM and computerized image analysis. Bull Group Int Rech Sci Stomatol Odontol. 1996;39:111–7. [PubMed] [Google Scholar]
- 18.Newberry BM, Shabahang S, Johnson N, Aprecio RM, Torabinejad M. The antimicrobial effect of biopure MTAD on eight strains of Enterococcus faecalis: An in vitro investigation. J Endod. 2007;33:1352–4. doi: 10.1016/j.joen.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Kandaswamy D, Kandaswamy Venkateshbabu N. Root canal irrigants. J Cons Dent. 2010;13:256–64. doi: 10.4103/0972-0707.73378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krell KV, Johnson RJ, Madison S. Irrigation patterns during ultrasonic canal instrumentation. Part I. K- type files. J Endod. 1988;14:65–8. doi: 10.1016/S0099-2399(88)80003-7. [DOI] [PubMed] [Google Scholar]
- 21.Van der Sluis LW, Wu MK, Wasselink PR. The efficacy of ultrasonic irrigation to remove artificially placed dentin debris from human root canals prepared using instruments of varying taper. Int Endod J. 2005;38:764–8. doi: 10.1111/j.1365-2591.2005.01018.x. [DOI] [PubMed] [Google Scholar]
- 22.Kocani F, Kamberi B, Dragusha E. Manual sonic-air and ultrasonic instrumentation of root canal and irrigation with 5.25% sodium hypochlorite and 17% Ethylenediaminetetraacetic acid: A scanning electron microscope study. J Conserv Dent. 2012;15:118–22. doi: 10.4103/0972-0707.94575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beltz RE, Torabinejad M, Pouresmail M. Quantitative analysis of the solubilizing action of MTAD, sodium hypochlorite, and EDTA on bovine pulp and dentin. J Endod. 2003;29:334–7. doi: 10.1097/00004770-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Heredia M, Ferrer-Luque CM, Gonzalez-Rodriguez MP. The effectiveness of different acid irrigating solutions in root canal cleaning after hand and rotary instrumentation. J Endod. 2006;32:993–7. doi: 10.1016/j.joen.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Torabinejad M, Cho Y, Khademi AA, Bakland LK, Shabahang S. The effect of various concentrations of sodium hypochlorite on the ability of MTAD to remove the smear layer. J Endod. 2003;29:233–9. doi: 10.1097/00004770-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Dineshkumar MK, Vinothkumar TS, Arathi G, Shanthisree P, Kandaswamy D. Effect of ethylene diamine tetra-acetic acid, MTAD, and HEBP as a final rinse on the microhardness fo root dentin. J Conserv Dent. 2012;15:170–3. doi: 10.4103/0972-0707.94587. [DOI] [PMC free article] [PubMed] [Google Scholar]


