Abstract
Aim:
The aim of this study was to assess the antibacterial efficacy of three different herbal irrigants against Enterococcus faecalis.
Materials and Methods:
Single rooted teeth were extracted due to orthodontic and periodontal reasons. The teeth were then inoculated with E. faecalis. The teeth were randomly divided into three experimental groups and two control groups of six samples each. Group 1 specimens were treated with 5.2% sodium hypochlorite (NaOCL) for 30 min followed by 5 mmol/L Ethylenediaminetetraacetic acid (EDTA) for 5 min and saline as final irrigant. Group 2 specimens were treated with and 5.2% NaOCl for 30 min as final irrigant. Group 3 were treated with Morinda citrifolia (MC) for 30 min as final irrigant. Group 4 were treated with Azadiracta indica (AI) as final irrigant. Group 5 were treated with green tea (GT) for 30 min as final irrigant. The dentin specimens were carefully spread onto a microscope slide and stained with BacLight and examined in a confocal laser scanning microscope set to monitor fluorescein isothiocyanate and propidium iodide. A total of nine fields were examined for each treatment and the bacteria presented were counted.
Statistical Analysis:
Using the one-way ANOVA with multiple comparison, significantly less bacteria were found adhering to the samples treated with Neem followed by NaOCL, GT, MC, Saline.
Results:
AI treatment produced the maximum reduction in adherence of E. faecalis to dentin (9.30%) followed by NaOCl (12.50%), GT (27.30%), MC (44.20%) and saline (86.70%).
Conclusion:
Neem is effective in preventing adhesion of E. faecalis to dentin.
Keywords: Adherence, confocal laser scanning microscope, Enterococcus faecalis, Neem
INTRODUCTION
Failure of endodontic treatment is commonly associated with the presence of persistent microorganism. Enterococcus faecalis can form a calcified biofilm in tough environmental conditions within the root canals.[1] Biofilm formation on tissues and biomaterial surfaces can lead to biofilm-mediated infections, which are difficult to treat as a result of the increased antimicrobial resistance of biofilm bacteria.[2] The adherence of bacteria to a solid surface forms the initial and the most important step in the formation of biofilm.[2,3]
E. faecalis possess different virulence factors that enable them to adhere to dentin and invade dentinal tubules.[4] Enterococci also express factors that aid their adhesion to host cells and extracellular matrix, which in turn facilitates tissue invasion, causes immunomodulation and produces toxin mediated damage.[5] The E. faecalis collagen binding protein, Ace and a serine protease, targets the extracellular matrix proteins of host cells and allows adherence to type I collagen.[5]
Sodium hypochlorite (NaOCl) is one of the most commonly used endodontic irrigant because of its ability to destroy a broad spectrum of microbes,[3] but it has some undesirable characteristics such as tissue toxicity, allergic potential, and disagreeable taste, which has prompted researchers to look for other alternatives.[6] The literature has shown that various natural plant extracts, a source of bioactive compounds has antimicrobial, and therapeutic effects suggesting its potential to be used as an endodontic irrigant.[6,7]
Literature has shown that Neem has antimicrobial and therapeutic effects suggesting its potential to be used as an endodontic irrigant.[8] Murray et al. also suggested that Morinda citrifolia (MC) juice can be formulated for use as an intracanal irrigant.[6] Similarly Prabhakar et al. evaluated green tea (GT) to be safe, having antioxidant, anti-inflammatory, and radical scavenging activity, which is an advantage over the traditional root canal irrigants.[9]
The aim of this study was to study the effects of various herbal extracts namely MC (noni), Azadiracta indica (AI) (Neem) and GT as a final rinse on the adherence of E. faecalis to root canal dentin using the confocal laser scanning microscopy (CLSM)-based bacterial adherence assay.
MATERIALS AND METHODS
Sample preparation
A pre-existing collection of donated human teeth was used after ethics institutional review board approval. Fifty non-carious single-rooted teeth maintained in phosphate-buffered saline (PBS) solution were used. The teeth were sterilized using a steam autoclave at 121°C for 20 min. The crown was decoronated at the level of cementenamel junction and the apical 3 mm were ground using a diamond rotary bone-cutting saw (Marterials Science, NW Ltd., Settle, England, UK) to obtain uniform root sections of about the 8-mm length. The tooth specimens were then vertically sectioned with microtome to obtain a thickness of 50 microns.
Phytochemical preparation
Fresh plants of MC, AI and GT (Yucca Enterprise, Mumbai) were powdered and prepared for extraction. A weighed quantity of 500 g of the powdered herbal plants were macerated with 500 ml of 99% ethanol and filtered using a double filter paper and then centrifuged at 10,000 rpm for 20 min. It was stored at 4°C until required.
A dilution assay was first performed in order to determine the minimum inhibitory concentrations of the phytochemical extracts. E. faecalis growth was determined by the presence of turbidity in different concentrations and the minimum inhibitory concentration was determined to be 0.33 mg/ml for AI, GT and 1.25 mg/ml for MC.
Preparation of E.faecalis biofilm
One colony of E. faecalis American Type Culture Collection (ATCC 29212) was raised in Tryptone Bile X-glucuronide agar (Merck) and was transferred to 50 ml of All Culture media (Sigma Aldrich, St. Louis, MO). The culture was allowed to grow overnight under stationary aerobic conditions at 37°C.
Irrigation procedure
The prepared teeth (n = 50) were divided randomly into 5 groups of 10 each. All the specimens were treated with 5.25% NaOCL for 30 min followed by 5 mmol/L Ethylenediaminetetraacetic acid (EDTA) for 5 min and the final irrigants to be tested were used for 30 min. In Group 1 specimens, saline was used as the final irrigant. In Group 2 specimens, 5.25% NaOCl was used as the final irrigant. In Group 3 specimens, MC was used as the final irrigant. In Group 4 specimens, AI was used as the final irrigant and in Group 5, GT was used as the final irrigant.
The treated dentin blocks were then washed with distilled water. The dentin blocks were inoculated with 200 μL of E. Faecalis (106 cells/ml colony-forming units) at 37°C for 1 h. After the incubation period, the root canals of dentin blocks were washed with 1 ml of PBS to remove any non-adherent bacteria of the root canal walls.
CLSM-based bacterial adherence assay
The dentin specimens were carefully spread onto a microscope slide and stained with BacLight (Invitrogen, Molecular Probes, Carlsbad, CA) and examined in a CLSM (Carl Zeiss, Oberkochen, Germany), which were set to monitor fluorescein isothiocyanate and propidium iodide. The BacLight stain has two fluorescent dyes, fluorescein isothiocyanate and propidium diode with emission of 480/500 nm and 490/635 nm respectively. Photographs were produced on a Sony 5200 MB video printer (Sony Corp, Tokyo). Total of nine fields were examined for each treatment, and the bacteria presented were counted. The nine fields were made up of three fields each from three similarly treated samples, inoculated by bacteria from the same culture. Only bacteria in focus of each optical section were counted and were carried out carefully by one operator using a manual digital counter.
The results of the bacterial adherence assay were determined statistically by one-way analysis of variance with post-hoc Tukey.
RESULTS
The histogram depicting the bacterial count for different treatment groups is shown in Figure 1. Using one way ANOVA with multiple comparison, significantly less bacteria were found adhering to the samples treated with AI followed by NaOCL, GT, MC, saline. There were conspiously high numbers of bacteria on dentin when saline (86.70%) was used. AI treatment produced the maximum reduction in adherence of E. faecalis to dentin (9.30%) followed by NaOCl (12.50%), GT (27.30%), MC (44.20%) and saline (86.70%). CLSM images of collagen membranes treated with the different groups and inoculated with bacteria are shown in Figures 2–6. No statistical difference was seen between AI and NaOCl.
Figure 1.
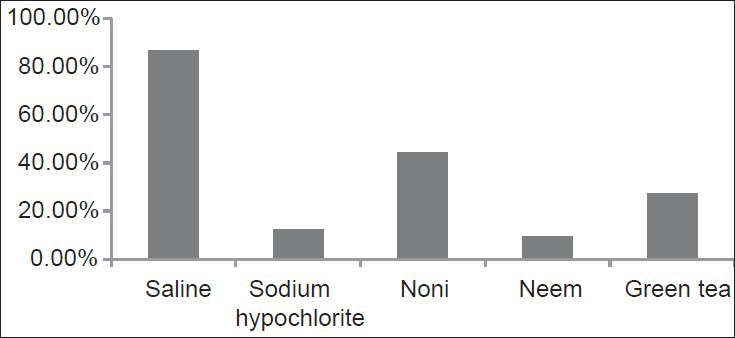
Percentage of adherent Enterococcus faecalis remaining in dentin after different final irrigant treatment
Figure 2.
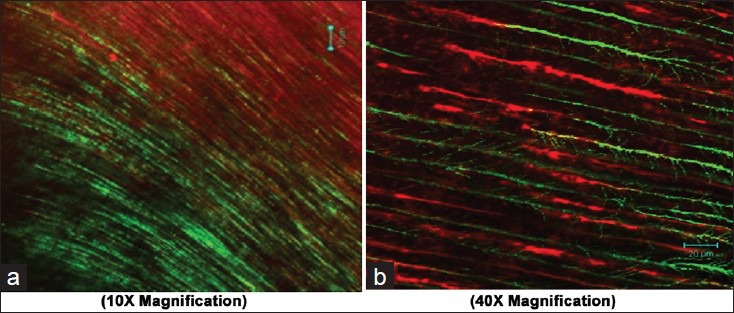
Adherence of Enterococcus faecalis after using saline as final irrigant (a 10×; b 40X magnification)
Figure 6.
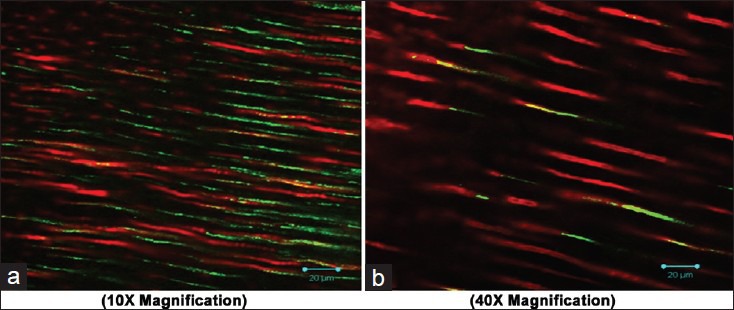
Adherence of Enterococcus faecalis after using Green tea as final irrigant (a 10×; b 40X magnification)
Figure 3.
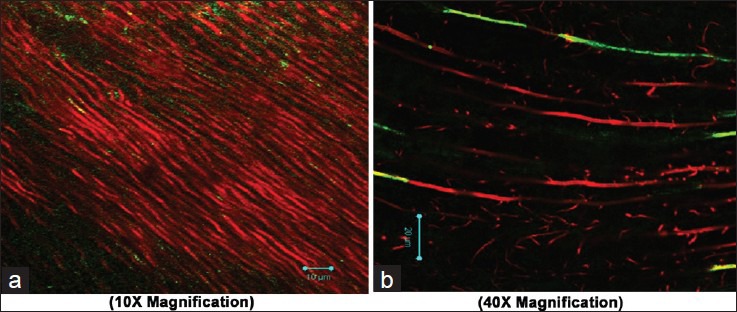
Adherence of Enterococcus faecalis after using sodium hypochlorite as final irrigant (a 10×; b 40X magnification)
Figure 4.
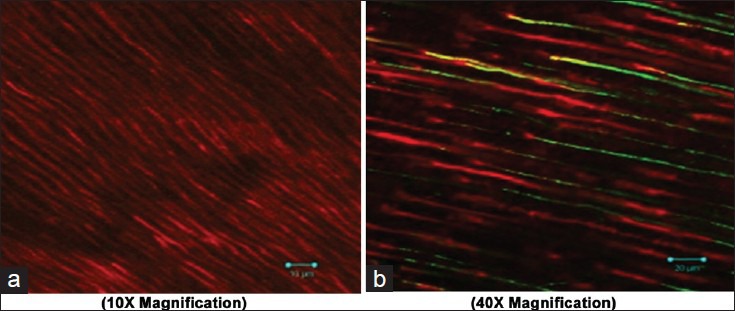
Adherence of Enterococcus faecalis after using Neem as final irrigant (a 10×; b 40X magnification)
Figure 5.
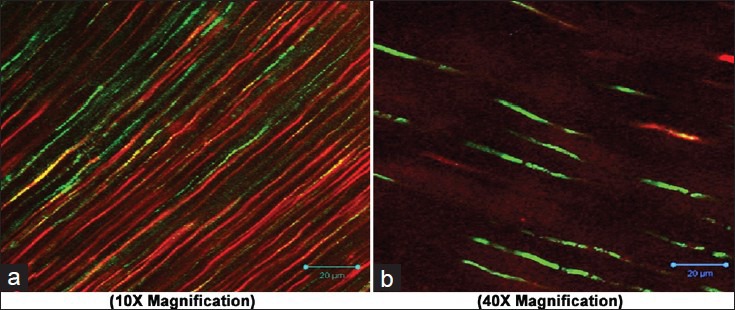
Adherence of Enterococcus faecalis after using Morinda citrifolia as final irrigant (a 10×; b 40X magnification)
DISCUSSION
Adherence is considered to be the first step for bacterial colonization of host tissue, including tubule invasion.[10] The strain of E. faecalis has shown an ability to infect dentinal tubules,[11] to tolerate high pH[12] and to survive in obturated canals.[13] It is the most prevalent bacterial strain in endodontic cases with persistent endodontic lesions.
Dentinal tubules within intact dentine are normally inaccessible for systematic investigation. These dentinal tubule walls may be a selective site for bacterial adherence and alteration of dentine components may influence the colonization.[14] Thus, for the adherence study, human tooth roots were split longitudinally to expose tubule surfaces for CLSM analysis.
After root canal treatment, residual bacteria reseed the chemically treated root-canal dentine and makes use of dentin to adhere and survive tough environmental conditions. NaOCl are known to be cytotoxic to tissues and a need for replacement with a more biocompatible irrigant is necessitated.[6] Pharmacological studies acknowledged the value of medicinal plants as a potential source of bioactive compounds.[15,16] MC, AI, and GT have a broad range of antimicrobial activity and has been suggested as a natural endodontic irrigating solution. This study highlighted on the adherence capability of E. faecalis to dentin after the use of these 3 herbals as final irrigants.
In order of adherence of E. faecalis to dentin, the most to least effective irrigants are: AI, NaOCl, GT, MC, and saline. The negative control group with saline was the least effective irrigating solution. The presence of active constituents such as nimbidin, nimbin, nimbolide, gedunin, azadirachtin, mahmoodin, margolone, and cyclictrisulphide contributes to the antibacterial activity of AI.[8,17] These active constituents uncouples mitochondrial oxidative phosphorylation; thus, inhibiting the respiratory chain.[8] This resulted in its anti-adherence activity by altering the bacterial adhesion and the ability of the microorganism to colonize thereby causing maximum reduction in adherence of E. faecalis to dentin.[18]
The antimicrobial action of GT might be attributed to its flavonoid content by inhibition of bacterial enzyme gyrase by binding to Adenosine triphosphate B sub unit.[19] MC, commercially known as noni has a broad range of therapeutic effects including antibacterial, antifungal, antiviral, antitumor, antihelminthic, analgesic, hypotensive, anti-inflammatory, and immune enhancing effects.[6] The antibacterial property is due to the antibacterial components L-asperuloside and alizarin, which might be the reason for the antibacterial property.[19] Although AI, GT and MC exhibited antibacterial activity, AI showed relatively lesser adherence of E. faecalis to dentin.
The major advantages of using herbal alternatives are easy availability, cost-effectiveness, increased shelf life, low toxicity, and lack of microbial resistance reported so far. This may be due to the fact that Neem elaborates a vast array of biologically active compounds that are chemically diverse and hinders the vitality of the bacteria by inhibiting the respiratory chain.[8,20] This has an added advantage over the traditional root canal irrigants.[8,17,18,21] Hence, the medicinal and antimicrobial properties of the plant AI can be an alternative to NaOCl as a final root canal irrigation.
CONCLUSION
Within the limitation of these experiments, we conclude that AI is effective in preventing adhesion of E. faecalis to dentin confirming the great potential of bioactive compounds and are useful for rationalizing the use of this plant as an endodontic irrigant. In vivo data may be helpful in determining the real potential usefulness of this plant for the treatment of root canal infections.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Sum C, Mohanty S, Gupta PK, Kishen A. Influence of endodontic chemical treatment on Enterococcus faecalis adherence to collagen studied with laser scanning confocal microscopy and optical tweezers: A preliminary study. J Biomed Opt. 2008;13 doi: 10.1117/1.2957972. 044017. [DOI] [PubMed] [Google Scholar]
- 2.Chavez de Paz LE. Redefining the persistent infection in root canals: Possible role of biofilm communities. J Endod. 2007;33:652–62. doi: 10.1016/j.joen.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Kishen A, Sum CP, Mathew S, Lim CT. Influence of irrigation regimens on the adherence of Enterococcus faecalis to root canal dentin. J Endod. 2008;34:850–4. doi: 10.1016/j.joen.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Hubble TS, Hatton JF, Nallapareddy SR, Murray BE, Gillespie MJ. Influence of Enterococcus faecalis proteases and the collagen-binding protein, Ace, on adhesion to dentin. Oral Microbiol Immunol. 2003;18:121–6. doi: 10.1034/j.1399-302x.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 5.Jett BD, Huycke MM, Gilmore MS. Virulence of Enterococci. Clin Microbiol Rev. 1994;7:462–78. doi: 10.1128/cmr.7.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray PE, Farber RM, Namerow KN, Kuttler S, Garcia-Godoy F. Evaluation of Morinda citrifolia as an endodontic irrigant. J Endod. 2008;34:66–70. doi: 10.1016/j.joen.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Kandaswamy D, Venkateshbabu N. Root canal irrigants. J Conserv Dent. 2010;13:256–64. doi: 10.4103/0972-0707.73378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. Biological activities and medicinal properties of neem (Azadirachta indica) Curr Sci. 2002;82:1336–45. [Google Scholar]
- 9.Prabhakar J, Senthilkumar M, Priya MS, Mahalakshmi K, Sehgal PK, Sukumaran VG. Evaluation of antimicrobial efficacy of herbal alternatives (Triphala and green tea polyphenols), MTAD, and 5% sodium hypochlorite against Enterococcus faecalis biofilm formed on tooth substrate: An in vitro study. J Endod. 2010;36:83–6. doi: 10.1016/j.joen.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 10.Patti JM, Allen BL, McGavin MJ, Höök M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 11.Haapasalo M, Orstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res. 1987;66:1375–9. doi: 10.1177/00220345870660081801. [DOI] [PubMed] [Google Scholar]
- 12.Chávez de Paz LE, Bergenholtz G, Dahlén G, Svensäter G. Response to alkaline stress by root canal bacteria in biofilms. Int Endod J. 2007;40:344–55. doi: 10.1111/j.1365-2591.2006.01226.x. [DOI] [PubMed] [Google Scholar]
- 13.Shin SJ, Jee SW, Song JS, Jung IY, Cha JH, Kim E. Comparison of regrowth of Enterococcus faecalis in dentinal tubules after sealing with gutta-percha or Resilon. J Endod. 2008;34:445–8. doi: 10.1016/j.joen.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Chivatxaranukul P, Dashper SG, Messer HH. Dentinal tubule invasion and adherence by Enterococcus faecalis. Int Endod J. 2008;41:873–82. doi: 10.1111/j.1365-2591.2008.01445.x. [DOI] [PubMed] [Google Scholar]
- 15.Essawi T, Srour M. Screening of some Palestinian medicinal plants for antibacterial activity. J Ethnopharmacol. 2000;70:343–9. doi: 10.1016/s0378-8741(99)00187-7. [DOI] [PubMed] [Google Scholar]
- 16.Prusti A, Mishra SR, Sahoo S, Mishra SK. Antibacterial activity of some indian medicinal plants. Ethnobotanical Leafl. 2008;12:227–30. [Google Scholar]
- 17.Ghosh A, Chakrabarti, Roy P, Bhadury S, Nag T, Sarkar S. Bioremidation of heavy metals from neem leaf extract by chelation with dithizone. Asian Pharm Clin Res. 2009;2:87–92. [Google Scholar]
- 18.Polaquini SR, Svidzinski TI, Kemmelmeier C, Gasparetto A. Effect of aqueous extract from Neem (Azadirachta indica A. Juss) on hydrophobicity, biofilm formation and adhesion in composite resin by Candida albicans. Arch Oral Biol. 2006;51:482–90. doi: 10.1016/j.archoralbio.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Kamat S, Rajeev K, Saraf P. Role of herbs in endodontics: An update. Endodontology. 2011;23:96–100. [Google Scholar]
- 20.Vinothkumar TS, Rubin MI, Kandaswamy D, Balaji L. In vitro evaluation of five different herbal extracts as an antimicrobial endodontic irrigant using real time quantitative polymerase chain reaction. J Conserv Dent. 2013;16:167–70. doi: 10.4103/0972-0707.108208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koona S, Buddida S. Antibacterial potential of the extracts of the leaves of Azadiracta indica Linn. Not Sci Biol. 2011;3:65–9. [Google Scholar]


