Abstract
Objective:
Home bleaching agents may not be safe for composite resins. The purpose of this study was to evaluate the effects of 10 and 20% Opalescence® PF home bleaching agents on the surface roughness and hardness of universal nanocomposite (Filtek Z350), anterior nanocomposite (KeLFiL), and nanohybrid composite (TPH 3).
Materials and Methods:
Fifty-four composite resin samples with 18 samples for each type of composite resin were prepared using acrylic molds (4 × 2 mm). Each type of composite resin was further divided into three groups [n = 6 controls were placed in distilled water for 14 days and the other two groups of n = 6 were bleached with 10 and 20% carbamide peroxide (CP), respectively for 14 days]. Surface hardness of the composite resin was tested with a Vickers hardness tester, whereas surface roughness was tested with atomic force microscopy (AFM).
Results:
There were significant changes in the surface hardness of KeLFiL and TPH 3. However, all the tested materials showed no significant changes in the surface roughness.
Conclusion:
After 14 days of home bleaching treatment, there was no adverse effect on the surface roughness of all three composite resins, although the surface hardness for KeLFiL and TPH 3 were significantly reduced.
Keywords: Bleaching, composite, hardness, surface roughness
INTRODUCTION
Since the introduction of tooth whitening by Haywood and Heymann in 1989, this trend is getting more popular.[1] There are a few types of bleaching methods, but all of them share a common principle of the decomposition of peroxides from hydrogen peroxide or its compounds such as carbamide peroxide (CP) into unstable free radicals. These radicals further breakdown into large pigmented molecules either through oxidation or reduction reaction. The oxidation or reduction process changes the chemical structure of the interacting organic substances of the tooth, which results in the change in color.[2] Furthermore, at 45% concentration of CP in different intracoronal tooth-bleaching procedures, Maleknejad (2012) reported an increase in the diameter of dentinal tubules, and it also promoted alterations in the mineral content of the dentine.[3]
Types of bleaching methods include nonvital bleaching, in-office professional bleaching, and home bleaching. Nightguard home bleaching uses a relatively low level of whitening agent and is applied to the teeth via a custom-fabricated mouthguard and is worn at night for a duration of at least two weeks.[4]
A lot of research has been carried out to identify the effects of bleaching on the tooth surface and dental restorative materials. Jacob (2007) reported that bleaching with CP may alter the marginal leakage of resin composite restoration; however, amalgam restorations were not adversely affected in vitro.[5]
It was reported that the bleaching agent, regardless of the whitening products used, will reduce the microhardness of the enamel and promote an increase in surface roughness.[6] These days, composite resin is one of the most popular dental restorative materials. It has a superior aesthetic, conservative tooth preparation compared to dental amalgam. Hence, it is widely used for anterior and also posterior restorations.
Due to this, it is important to know the effects of bleaching on composite restorative material. Some studies concluded that application of home bleaching agent gels may cause surface softening of composite resins,[7] whereas others stated that there are no significant changes in surface microhardness found after the application of 15% CP on composite resin.[8] Furthermore, a study was done to evaluate the effects of bleaching on toothbrush abrasion in three types of composites. It was concluded that nano-filled resin composites are to be used for restoration when bleaching treatment is required.[9]
There is a significant and positive correlation found between surface roughness and vital bacterial adhesion.[10] It appears that bleaching agents may affect the adherence of certain cariogenic micro-organisms to the outer surfaces of composite resin restorations.[11] Roughness has a major impact on aesthetic appearance, discoloration of restorations, plaque accumulation, secondary caries, and gingival irritation.[12]
The experimental composite tested in this study was a locally produced dental restoration nanocomposite (KelFil). This nanocomposite was produced by the School of Dental Sciences (PPSG), Universiti Sains Malaysia (USM), Kelantan, Malaysia. The nanosilica fillers, synthesized locally by a sol-gel process, were in the size range of 10–20 nm. The experimental nanocomposite (ENC2) contained 35 wt% of nanosilica fillers.[13] This material (ENC2) will be called KeLFiL (brand name) hereafter in this article. The nanosilica fillers obtained were relatively monodispersed, spherical with low agglomeration.
The aim of this study was to study the effects of home bleaching on the hardness and surface morphology of a universal nanocomposite, an anterior nanocomposite, and a nanohybrid composite.
MATERIALS AND METHODS
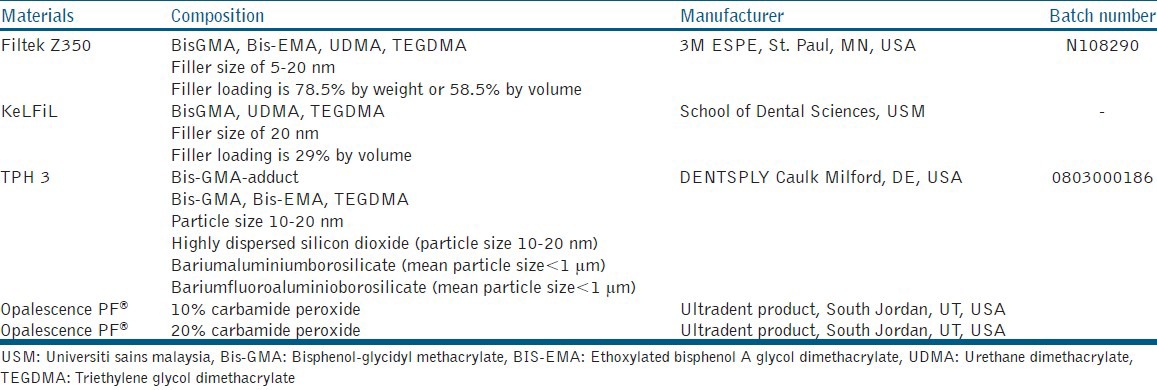
Two commercially available resin-based composites, based on bisphenol A-glycidyl methacrylate/triethyleneglycol dimethacrylate/ethoxylated bisphenol-A-dimethacrylate (Bis-GMA)/ethoxylated bisphenol-A dimethacrylate (Bis-EMA)/triethylene glycol dimethacrylate (TEGDMA)] resin chemistry (Filtek Z350; 3M ESPE, St. Paul, MN, USA and TPH 3; DENTSPLY Caulk, Milford, DE, USA) and one experimental resin-based composite based on Bis-GMA/urethane dimethacrylate (UDMA)/TEGDMA resin chemistry (KeLFiL) were used in the present study [Table 1]. All composites were packed into acrylic molds (4 mm diameter × 2 mm thick) which were placed on a mylar strip. The excess composites were removed using another mylar strip on top of the mold and pressed flat with a glass slab. Eighteen samples of each composite material were prepared, with a total at 54 samples. All samples were light-cured for 20 seconds from each top and bottom surface using Elipar Freelight 2 (3M ESPE St. Paul, MN, USA) according to instructions of the manufacturer. All samples were then polished using Sof-Lex™ (3M ESPE, St. Paul, MN, USA). Four different roughness of disks were used, which were coarse (55 μm), medium (40 μm), fine (24 μm), and ultrafine (8 μm), using a mandrel on a slow-speed handpiece, 10 seconds per disk without water. Conscious efforts were made to standardize strokes, downward forces, and number of strokes for each disk. All samples were stored in a distilled water bath at 37° C for 24 hours prior to the treatment procedure.
Table 1.
Composites resin and bleaching agents tested
The samples from each composite resin material were further divided into three groups; Groups 1, 2 and 3 with n = 6. Samples in group 1 as the control group were not bleached but stored in shaking water bath SW23 (Julabo, Seelbach, Germany) for 14 days at 37 °C of distilled water. Samples in group 2 were subjected to Opalescence® PF (Ultradent Product, South Jordan, UT, USA) 10% CP for eight hours per day, for 14 days according to the suggested wear time of the manufacturer.[9] Samples in group 3 were bleached with Opalescence® PF (Ultradent Product, South Jordan, UT, USA) 20% CP for two hours per day for a total of 14 days. In groups 2 and 3, prior to each bleaching procedure, the samples were taken out from the distilled water bath, air-dried with an air jet spray for 60 seconds. The bleaching agent was applied on one surface of the sample using a microbrush (Kerr Corporation, California, USA) and left for the duration suggested by the manufacturer. After each bleaching procedure, the samples were washed with a water jet spray for 60 seconds before they were stored back in distilled water bath and ready for the next bleaching procedure the next day.
All samples were subjected to surface roughness testing using atomic force microscopy (AFM) (Ambios Technology, California, USA). The mean surface roughness was assessed with contact mode. Five different areas were selected randomly with a scan area of 40 × 40 μm and resolution 512 × 512 pixels to obtain surface roughness values (Ra). Ra analysis was done by ScanAtomic SPM control software. Then, three-dimensional (3D) images with 10 × 10 μm sizes were acquired for each group of materials.
After surface roughness testing, all samples were subjected to hardness testing using a Vickers Hardness Tester, Model VM50 (FIE, Maharashtra State, India). The samples were placed underneath the indenter and 1 kg load was applied for a dwell time of 15 seconds. Every sample was indented three times at three different points, and the mean readings were recorded.
Statistical analysis
Data collected were analyzed using SPSS version 16.0. All statistical analysis was conducted at a significance level of P < 0.05 using Mann-Whitney test and Kruskal-Wallis test.
RESULTS
The results of the statistical analysis using Kruskal-Wallis Test are presented in Tables 2 and 3. Table 4 reveals the result of Mann-Whitney Test analysis of KeLFiL and Table 5 is the result of Mann-Whitney Test of TPH 3.
Table 2.
Median roughness number (Ra, nm) and interquartile range of the three tested composite resins after 14 days of bleaching with 10 and 20% CP
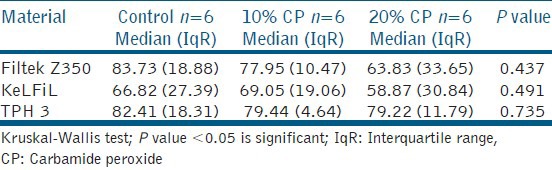
Table 3.
Median vickers hardness number and interquartile range of the three tested composite resins after 14 days of bleaching with 10 and 20% CP
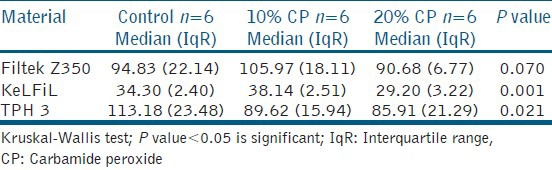
Table 4.
Median vickers hardness number and interquartile range for KeLFiL after 14 days of bleaching with 10 and 20% CP
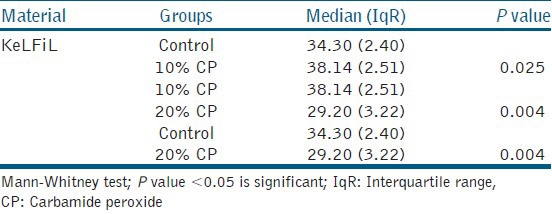
Table 5.
Median vickers hardness number and interquartile range for TPH 3 after 14 days of bleaching with 10 and 20% CP
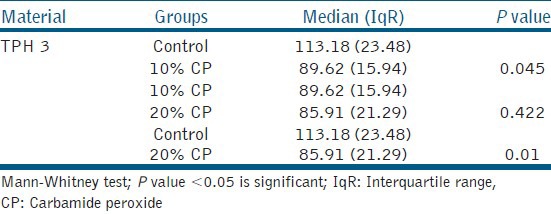
Table 2 shows the roughness number (Ra) of the three types of composites that were bleached with 10 and 20% CP home bleaching agents. There was no significant change in roughness in all three composites after 14 days of bleaching with 10 and 20% CP compared to the control group.
Table 3 illustrates the results of all the samples that were subjected to the Vickers hardness test. KeLFiL showed significant change in hardness with a P value of 0.001 and there was also a significant change in TPH 3 with a P value of 0.021.
Table 4 shows that there was a significant change in KeLFiL after 14 days of bleaching. There was a significant increase in the surface hardness after bleaching with 10% CP home bleaching agent. However, there was a significant decrease after bleaching with 20% CP home bleaching agent. There was also a significant decrease in the surface hardness of KeLFiL after bleaching with 20% CP when compared to the group of samples bleached with 10% CP.
Table 5 reveals a significant change in the surface hardness of TPH 3 after being subjected to 14 days of bleaching. There was a significant decrease in the surface hardness in the groups bleached with 10 and 20% CP compared to the control group. However, there was no significant change when the group was bleached with 10% CP compared to the group bleached with 20% CP.
DISCUSSION
In this study, Filtek Z350, a commercialized and widely used nanocomposite did not show any significant changes in surface hardness when compared to its control group, and also to groups of samples bleached with either 10 or 20% CP. This result was consistent with the conclusion from other studies.[2,14,15]
However, there were significant changes in the Vickers hardness number for both KeLFiL and TPH 3 that had been subjected to 14 days of bleaching with either 10 or 20% CP. For KeLFiL, there was a significant increase in hardness after bleaching for 14 days with 10% CP but a significant decrease in hardness with 20% CP.
The observation maybe explained by this proposed theory. After the curing process of a composite resin, a postpolymerization process continues to occur up to a certain period of time, which increases the hardness of the composite.[16] In this study, the bleaching process was possibly being carried out at the same time as the postpolymerization process. The postpolymerization process in KeLFiL was observed with the 10% CP, and it was more prominent than the bleaching process; hence the increase in its surface hardness. This pattern was also observed in Filtek Z350; however, it was not significant. Nevertheless, with 20% CP, the bleaching process was more dominant than the postpolymerization process in all the three composites tested. The increase in hardness with 10% CP was observed in both Filtek Z350 and KeLFiL, and it maybe due to the similarities in their filler structure.
In TPH 3, there was a significant decrease when comparison was made between the control group and 10% CP group, and also between the control group and 20% CP. This significant decrease is consistent with the conclusion drawn from other studies.[17]
As a general rule, the higher the filler loading, the greater the physical properties of the composite resins.[18] KeLFiL is a nanocomposite which was designed as an anterior composite. It has a lower filler loading of 29% by volume compared to Filtek Z350 with the filler loading of 78.5% by weight or 59.5% by volume which is suitable for anterior and also posterior composite resin restorations. The lower filler loading of KeLFiL may lead to loosely packed inorganic fillers, which in turn caused the significant decrease in the Vickers hardness number.
For TPH 3, there was a significant decrease in Vickers hardness number in samples that were subjected to 14 days of bleaching with both 10 and 20% CP. TPH 3 is a nanohybrid composite for anterior and posterior restorations. It was concluded that nanohybrid resins generally presented inferior properties compared to the nanofilled composite.[19] TPH 3 has 58% volume filler loading,[20] which is similar to Filtek Z350. The possible explanation for the significant reduction in the surface hardness might be due to the presence of Bis-GMA monomer. Resin composites are reported to be highly susceptible to chemical softening due to the presence of Bis-GMA monomer if the chemicals have a solubility parameter ranging from 1.82 × 104 to 2.97 × 104 (J/m3)½.[21] In the case of TPH 3, the content of Bis-GMA in its matrix might be higher than in Filtek Z350. Another possible explanation is the degree to which the filler is bonded to the resin matrix.[22]
Although there was a significant decrease in the Vickers hardness numbers of KeLFiL and TPH 3, there were no significant changes in the roughness number in all specimens after 14 days of bleaching with either 10 or 20% CP Opalescence® PF home bleaching agents. This is consistent with the conclusion that no statistically significant differences were detected for any material.[23]
The cantilever sensor of AFM senses any irregularities at the surface of the specimen; however, as all the composites tested have similar nano-sized fillers, approximately 20 nm, there was no significant difference between them. Furthermore, all samples were polished with the same polishing system, Sof-Lex™ disks which may contribute to a similar surface finish in the composite resins even after bleaching treatment. The 3D images of AFM [Figures 1–3] show that there was no plucking out of filler particles after the bleaching treatment.
Figure 1.
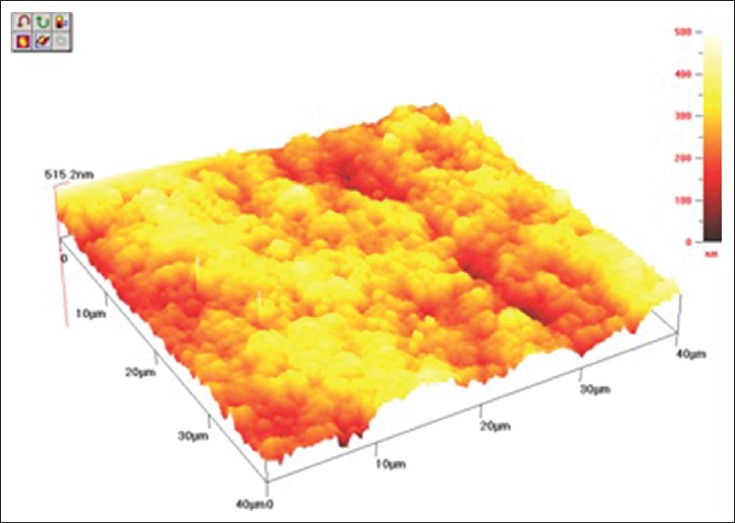
AFM topography 40 × 40 μm of Z350 after bleaching with 20% CP
Figure 3.
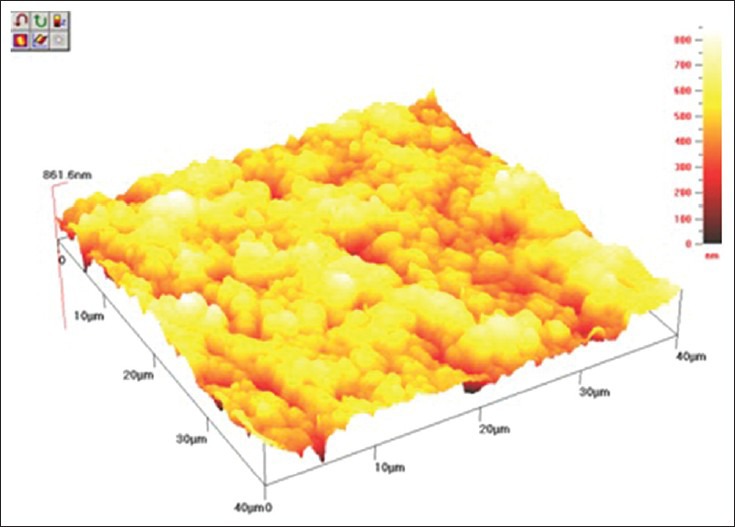
AFM topography 40 × 40 μm of TPH 3 after bleaching with 20% CP
Figure 2.
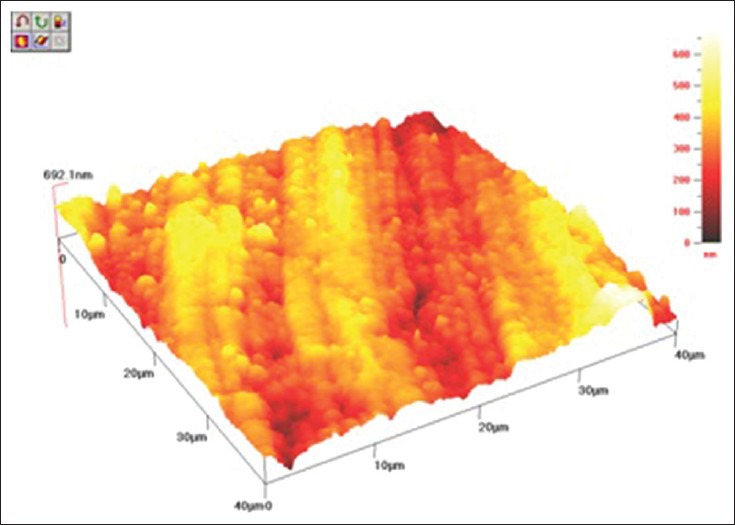
AFM topography 40 × 40 μm of KeLFiL after bleaching with 20% CP
Generally, the surface roughness of all composites tested has a reading below 0.2 μm or 200 nm. Ra above 0.2 μm results in an increase in the accumulation of plaque and a higher risk for caries and periodontal inflammation.[24] According to Chung in 1994, when Ra was lower than 1μm, the surfaces were visibly smooth. Therefore, all composites surfaces evaluated after bleaching treatment have demonstrated a smooth surface, which from the clinical point of view, present no risk of accumulation of plaque.[25]
From this study, it can be inferred that following bleaching, the experimental anterior composite and nanohybrid composite may need to be replaced, due to the reduction in their surface hardness. However, this has to be further investigated with in vitro studies evaluating the effects of saliva, and controlled clinical trials are necessary to determine any clinical implication.
CONCLUSIONS
Based on the result of this study, it can be concluded that 14 days of bleaching with 10 or 20% CP home bleaching agents did not cause any changes in surface roughness of the three tested composites. The AFM evaluation of surface roughness showed that both bleaching agents yielded Ra values below 200 nm for all materials tested, which poses no risk of the accumulation of plaque. The performance of KeLFiL after bleaching treatment is similar to that of the commercial nanocomposites. The AFM evaluation of surface roughness observed in the 3D images promises to be an effective technique. Nanocomposite and nanohybrid composite resins bleached with 10 or 20% CP bleaching agents result in the same outcome. On the other hand, the surface hardness is dependent on the material.
ACKNOWLEDGMENT
The authors would like to thank the Universiti Sains Malaysia for the financial support given through the RU Grant 1001/PPSG/813012.
Footnotes
Source of Support: USM RU Grant 1001/PPSG/813012
Conflict of Interest: None declared
REFERENCES
- 1.Haywood VB, Heymann HO. Nightguard vital bleaching. Quintessence Int. 1989;20:173–6. [PubMed] [Google Scholar]
- 2.Costa SX, Becker AB, Rastelli AN, Loffredo LC, Andrade MF, Bagnato VS. Effect of four bleaching regimens on color changes and microhardness of dental nanofilled composite. Int J Dent 2009. 2009 doi: 10.1155/2009/313845. 313845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maleknejad F, Ameri H, Kianfar I. Effect of intracoronal bleaching agents on ultrastructure and mineral content of dentin. J Conserv Dent. 2012;15:174–7. doi: 10.4103/0972-0707.94586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenwall L. London: Martin Dunitz Ltd; 2001. Bleaching techniques in restorative dentistry-an illustrated guide. [Google Scholar]
- 5.Jacob AS, Dhanya Kumar NM. Effect of pre and post operative bleaching on microleakage of amalgam and composite restoration using 10% carbamide peroxide-an in vitro study. J Conserv Dent. 2007;10:33–7. [Google Scholar]
- 6.Pinto CF, Oliveira R, Cavalli V, Giannini M. Peroxide bleaching agent effects on enamel surface microhardness, roughness and morphology. Braz Oral Res. 2004;18:306–11. doi: 10.1590/s1806-83242004000400006. [DOI] [PubMed] [Google Scholar]
- 7.Bailey SJ, Swift EJ., Jr Effects of home bleaching products on composite resins. Quintessence Int. 1992;23:489–94. [PubMed] [Google Scholar]
- 8.Yu H, Li Q, Hussain M, Wang Y. Effects of bleaching gels on the surface microhardness of tooth-colored. J Dent. 2008;36:261–7. doi: 10.1016/j.jdent.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Hajizadeh H, Ameri H, Eslami S, Mirzaeepoor B. The effect of bleaching on toothbrush abrasion of resin composites. J Conserv Dent. 2013;16:17–20. doi: 10.4103/0972-0707.105292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aykent F, Yondem I, Ozyesil AG, Gunal SK, Avunduk MC, Ozkan S. Effect of different finishing techniques for restorative materials on surface roughness and bacterial adhesion. J Prosthet Dent. 2010;103:221–7. doi: 10.1016/S0022-3913(10)60034-0. [DOI] [PubMed] [Google Scholar]
- 11.Mor C, Steinberg D, Dogan H, Rotstein I. Bacterial adherence to bleached surfaces of composite resin in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:582–6. doi: 10.1016/s1079-2104(98)90350-x. [DOI] [PubMed] [Google Scholar]
- 12.Janus J, Fauxpoint G, Arntz Y, Pelletier H, Etienne O. Surface roughness and morphology of three nanocomposites after two different polishing treatments by a multitechnique approach. Dent Mater. 2010;26:416–25. doi: 10.1016/j.dental.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Rahim TN, Mohamad D, Ismail AR, Akil HM. Synthesis of nanosilica fillers for experimental dental nanocomposites and their characterisations. J Phys Sci. 2011;22:93–105. [Google Scholar]
- 14.Mujdeci A, Gokay O. Effect of bleaching agent on the microhardness of tooth-colored restorative materials. J Prosthet Dent. 2006;95:286–9. doi: 10.1016/j.prosdent.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Godoy F, Garcia-Godoy A, Garcia-Godoy F. Effect of bleaching gels on the surface roughness, hardness and micromorphology of composites. Gen Dent. 2002;50:247–50. [PubMed] [Google Scholar]
- 16.Mohamad D, Young RJ, Mann AB, Watts DC. Post-Polymerization of dental resins composite evaluated with Nanoindentation and Micro-Raman spectroscopy. Arch Orofac Sci. 2007;2:26–31. [Google Scholar]
- 17.Hannig C, Duong S, Becker K, Brunner E, Kahler E, Attin T. Effect of bleaching on subsurface microhardness of composite and a polyacid modified composite. Dent Mater. 2007;23:198–203. doi: 10.1016/j.dental.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Craig R. Dental Materials. St. Louis, Missouri: Mosby; 1993. Properties and Manipulation. [Google Scholar]
- 19.Moraes RR, Gonçalves LS, Lancellotti AC, Consani S, Correr-Sobrinho L, Sinhoreti MA. Nanohybrid resin composites: Nanofiller loaded materials or traditional microhybrid resins? Oper Dent. 2009;34:551–7. doi: 10.2341/08-043-L. [DOI] [PubMed] [Google Scholar]
- 20.Hafez R, Ahmed D, Yousry M, El-Badrawy W, El-Mowafy O. Effect of In-Office Bleaching on Color and Surface Roughness of Composite Restoratives. Eur J Dent. 2010;4:118–27. [PMC free article] [PubMed] [Google Scholar]
- 21.Wu W, McKinney JE. Influence of chemicals on wear of dental composites. J Dent Res. 1982;61:1180–3. doi: 10.1177/00220345820610101501. [DOI] [PubMed] [Google Scholar]
- 22.Taher NM. The effect of bleaching agents on the surface hardness of tooth colored restorative materials. J Contemp Dent Pract. 2005;6:18–26. [PubMed] [Google Scholar]
- 23.de A Silva MF, Davies RM, Stewart B, DeVizio W, Tonholo J, da Silva JG, Junior, et al. Effect of whitening gels on the surface roughness of restorative materials in situ. Dent Mater. 2006;22:919–24. doi: 10.1016/j.dental.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 24.Bollen CM, Lambrechts P, Quirynen M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent Mater. 1997;13:258–69. doi: 10.1016/s0109-5641(97)80038-3. [DOI] [PubMed] [Google Scholar]
- 25.Chung KH. Effects of finishing and polishing procedures on the surface texture of resin composites. Dent Mater. 1994;10:325–30. doi: 10.1016/0109-5641(94)90041-8. [DOI] [PubMed] [Google Scholar]


