Abstract
Hepatic progenitor cells (HPCs) are bipotential cells residing in normal liver. Their proliferation is observed in reactive conditions of the liver and in primary liver cancers. The observation that some hepatocellular carcinomas (HCCs) express a biliary-like immunophenotype has led to the identification of HPCs in HCC. Accumulating evidence suggests that HPCs play a role as the cell of origin in a variety of primary liver cancers. This has led to the development of revolutionary concepts in hepatocarcinogenesis. In this article, the role and significance of HPCs in HCC, including its classification, are summarized and discussed.
Keywords: Classification, Hepatic progenitor cells, Hepatocarcinogenesis, Hepatocellular carcinoma, Primary liver cancer
A Brief History of the Classification of Primary Liver Cancers
Edmondson et al. put forward a classification of primary liver cancers in 1954 in their paper entitled “Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies” [1]. After that, it was long accepted that primary liver cancers most commonly showed evidence of hepatocytic or biliary differentiation, possibly derived from and recapitulating their respective normal counterparts. In the decades following Edmondson's landmark study, data accumulated that pointed to hepatocarcinogenesis being a multistep process. In 1995, the International Working Party proposed a consensus nomenclature of hepatocellular nodules, introducing and consolidating the concept of precursor lesions of HCC, namely dysplastic foci, low-grade dysplastic nodules (LGDN), and high-grade dysplastic nodules (HGDN) [2]. During this period, the WHO classification for HCC focused on describing the architectural pattern, grading, cytological variants, and some variants such as fibrolamellar carcinoma and sarcomatoid HCC [3].
Growing Evidence for the Presence of HPCs in HCC
Within the framework of the established classification, researchers observed some variations in terms of the differentiation of primary liver cancers, and these findings were reported. In 1996, Wu et al. studied a number of primary liver cancers and found that in some cases, tumors histologically established as HCC expressed biliary differentiation by positive immunohistochemical staining for AE1/3 and CK19 [4]. Kim et al. reported a series of 13 cases in their article on primary liver carcinoma of intermediate phenotype. Morphologically, the tumors comprised “intermediate cells” with small, uniform, round-to-oval cells and scant cytoplasm among a fibrous stroma. Immunohistochemically, many of the tumors showed simultaneous expression of hepatocytic and biliary features and co-expressed c-kit [5]. Intermediate (hepatobiliary) cells are defined by Roskams et al. as those displaying both hepatocytic and biliary differentiation in diseased liver, with a size of between 6 and 40 μm, and showing dual immunophenotype [6].
Durnez et al. studied the immunohistochemical expression of cytokeratins CK7 and CK19 in 109 clinical HCC samples. The majority (72%) of cases were CK7-/CK19-, while 6% was CK7-/CK19+, 12% CK7+/CK19-, and 10% CK7+/CK19+ [7]. CK7 and CK19 are cytokeratin markers for biliary differentiation and are theoretically not expressed in normal or neoplastic hepatocytes. Expression of CK7 and/or CK19 in HCC suggests a biliary trait or an intermediate hepatocyte/HPC phenotype. These findings were echoed by a gene expression study in which 14 of 70 (20%) HCCs were found to have the traits of cholangiocarcinoma (CC) and enriched embryonic stem cell-like features [8].
The Nature of HPCs
HPCs exist in normal liver tissues in a reserved compartment. In humans, this is called the progenitor cell compartment and resides in the canals of Hering [6]. HPCs are referred to as oval cells in animal models due to their appearance under the microscope. Oval cells possess the potential to differentiate toward the hepatocytic or biliary phenotype. Histologically, these cells adopt the appearance of small epithelial cells with an oval nucleus and scant cytoplasm. Immunohistochemically, they express markers such as OV-6 and chromogranin A [9, 10, 11]. As reservoir cells, HPCs were shown to be activated in a wide range of liver diseases and in conditions such as submassive necrosis, chronic viral hepatitis, and fatty liver disease [9, 11, 12, 13]. The presence of HPCs in primary liver cancers therefore raised the suspicion that they may be implicated in hepatocarcinogenesis. Related theories emerged that included maturation arrest and dedifferentiation as mechanisms [14]. Based on this postulation, it was reasonable to raise the question whether HCC precursor lesions LGDN and HGDN share the HPC phenotype. In fact, HPC features were identified in precursor lesions including dysplastic foci less than 1 mm in diameter [14], and this might fit into the multistep hepatocarcinogenesis model in a proportion of HCCs [15] (fig. 1a).
Fig. 1.
(a) Schematic showing activation of progenitor cells in precursor lesions and hepatocellular carcinoma. (b) Model of the involvement of liver stem cells in multistep hepatocarcinogenesis. Hit=an oncogenic genetic event.
The Evolution of HCC Classification
In 2009, based on the classification of hepatocellular nodules put forward by the International Working Party in 1995, the International Consensus Group for Hepatocellular Neoplasia further elaborated on the histological features of small hepatocellular nodules (<2 cm) in a background of cirrhosis [16]. The group also stated the five histological criteria for early HCC and reinstated stromal invasion as the most useful diagnostic criteria to distinguish early HCC from HGDN. These findings were included in the latest WHO “blue book” [17]. In the same latest WHO classification handbook, the entity of combined hepatocellular-cholangiocarcinoma (CHCC-CC) was illustrated and enriched in a single chapter. The histological traces of stem cells/progenitor cells were demonstrated in terms of morphology and immunostaining. CHCC-CC is believed to carry a poorer prognosis than pure HCC [17].
The Increasing Importance of CHCC-CC
CHCC-CC is distinct because it displays the morphological features of both HCC and CC. The initial theories regarding the histogenesis of this particular entity were (i) that it was a collision tumor, (ii) it involved dedifferentiation of a mature cell tumor; and (iii) the tumor originated from HPCs [18].
In a typical case of CHCC-CC, a transition/intermediate area exists between areas of classic HCC and CC. The transition areas feature small, uniform, oval-shaped cells with scant cytoplasm and hyperchromatic nuclei. Some other cases had tubules and cords in an “antlerlike” pattern. Immunohistochemically, most transition areas express CK7 and CK19 in addition to progenitor cell markers EpCAM and c-kit [19]. Theise et al. also demonstrated that HPCs histologically and immunohistochemically merge with the HCC and CC components in cases of CHCC-CC [20]. From the viewpoint of genetics, there was evidence from the allelic loss pattern that CHCC-CC represented a single clone, and that the distinct morphology was probably a result of divergent differentiation [21]. CHCC-CC is genetically closer to CC than to HCC, as has been shown by molecular studies investigating the loss of heterozygosity and mutations in p53 and ß-catenin [22].
Another histological indication that primary liver cancers may originate in HPCs came from the study of cholangiolocellular carcinoma (CLC). CLC was first described as a subtype of CC. Morphologically, the tumor shows a ductular reaction-like pattern within a fibrous stroma. Immunophenotypically, CLC shows cholangiocellular differentiation with expression of CK7 and CK19. What makes CLC distinct is the co-expression of neural cell adhesion molecule (NCAM), an HPC marker. In a recent case series of 30 CLCs, a component of classic HCC was identified in all cases [23]. CLC has been described in the latest WHO “blue book” as “combined hepatocellular-cholangiocarcinoma with stem-cell features, cholangiolocellular type” [17].
The Prognostic Significance of the HPC Signature in HCC
Multiple studies have shown that the HPC signature is associated with poor prognosis in patients with HCC [24, 25]. In a cohort study of 137 human HCCs by Kim et al. [26], the immunohistochemical expression rates of CK19, EpCAM, c-kit, and CD133 were 18.2%, 35.0%, 34.3%, and 24.8%, respectively. In a second cohort of 237 HCC cases, CK19+ cases were associated with more frequent microvascular invasion, less frequent capsule formation, and a fibrous stroma; CK19 expression was an independent prognostic factor for disease-free survival after tumor resection in this cohort [26].
Others have demonstrated that expression of AE1/3 and CK19 in HCC correlated with poorer cellular differentiation, a high proliferative marker index, and poor survival [4]. It has also been reported that, after liver transplantation, CK19 expression is associated with a higher rate of recurrence compared to CK19– tumors [7]. In a gene expression study, a cholangiocarcinoma-like trait was an independent prognostic factor in HCC [8].
The prognostic significance of HPC markers extends beyond tumor tissues. Non-tumoral ductular reaction and its proliferative index, which signifies HPC activation, were independent prognostic factors for overall and disease-free survival in CHCC-CC patients after resection [27]. In addition, soluble NCAM in patient sera was shown to be associated with poor prognosis [28].
There have also been studies indicating that the presence of HPCs in chronic liver diseases predicts HCC occurrence. The frequency of expression of stem cell markers was found to correlate with the frequency of HCC arising in the background of various chronic liver diseases such as HBV, HCV, alcoholic steatohepatitis, and non-alcoholic steatohepatitis [29]. Ziol et al. prospectively studied the expression of progenitor cell markers in a cohort of 150 HCV-related cirrhotic liver biopsy cases and found that immunohistochemical expression of intermediate cell markers was associated independently with the occurrence of HCC [30].
Supporting Evidence from Basic Research on the Role of HPCs in HCC
In recent years, the study of cancer stem cells (CSCs) or tumor-initiating cells (TICs) in HCC has been a hot topic of basic research, and many results and much data have recently been published. HPCs, first characterized histologically under a microscope, are believed to represent liver stem cells. Upon genetic deregulation of the self-renewal pathway, HPC/liver stem cells may transform into CSCs or TICs that drive tumor initiation [31] (fig. 1b). The characteristics of CSCs in HCC have been reviewed by Chiba et al. [32], and the various markers of liver TICs have been summarized by our group [33] (table 1). These studies will help our understanding of how HPCs play a role in the tumorigenesis of HCC.
Table 1.
Summary of TIC/CSC markers for therapeutic purposes
| TIC/CSC markers | Inhibitors |
|---|---|
| CD13 | CD13 inhibitor ubenimex, anti-CD13 antibody |
| CD24 | Anti-CD24 antibody |
| CD44 | Anti-CD44 antibody, RNA interference, antisense oligonucleotides |
| CD90 | Anti-CD90 antibody |
| CD133 | Anti-CD133 antibody, lupeol, antisense oligonucleotides |
| DLK1 | RNA interference targeting DLK1 |
| EpCAM | Bispecific antibody EpCAMxCD3, RNA interference, GSK-3ß inhibitor BIO |
| GEP | Anti-GEP antibody, RNA interference |
| OV6 | RNA interference targeting ß-catenin |
| SP | RNA interference targeting BMI-1 |
Characterization of HPCs/CSCs in HCC Cell Lines
In 2006, Chiba et al. identified CSC-like properties in HCC cell lines by means of side-population cell analysis and sorting analysis [34]. In the same year, Haraguchi et al. identified and characterized stem cells in HCC cell lines [35]. The tumorigenic role of HPCs/stem-cell-marker-positive cells has been demonstrated. The EpCAM+ population among HCC cell lines confers a higher tumorigenic activity [36]. EpCAM+ cells showed a higher ability to self-renew and differentiate and were capable of higher tumorigenicity [37]. CD133+ cells in the Huh7 cell line have higher proliferative potential in vitro and higher tumorigenic activity in vivo [38]. In addition, in HCC cell lines, CD133+ cells have a higher tumorigenicity and clonogenicity than CD133– cells do [39]. OV6+ TICs exhibited greater potential in terms of invasiveness and metastasis in in vivo and in vitro studies [40].
Dissecting Heterogeneous Liver Cancer Stem Cells (L-CSCs)
Given the vast number of cancer stem cell markers identified in HCC, recent research has suggested heterogeneity in L-CSCs; a large amount of work on further categorization according to expression profiles has been carried out. Some results have confirmed that subgroups of L-CSCs, in terms of expression of markers, possess more pronounced “sternness” features, including the ability to proliferate and generate tumors and to confer increased chemoresistance. For example, CD133+/CD44+ HCC cells displayed higher clonogenicity in vitro, higher tumorigenicity in vivo, and higher chemoresistance than the CD133+/CD44– subgroup did [41]. CD90+/CD44+ L-CSCs behaved more aggressively than CD90+/CD44– L-CSCs did [42]. These findings, while requiring more supporting data, possibly provide some enlightenment on the choice of targeted therapy to deliver the most cost-effective treatment regimen.
Regulations and Signaling Pathways of L-CSC
The signaling pathways through which L-CSCs maintain and exert their properties have been summarized [43]. While signaling is not explicitly discussed in this article, some examples will help to introduce the scope of this topic. It has been proposed that the control of stem cell proliferation is associated with Notch, Wnt, and TGF-β signaling pathways [44]. In addition, TP53-mutated tumors have been shown to be associated with expression of stem cell markers CD24 and AFP [45].
Perspectives
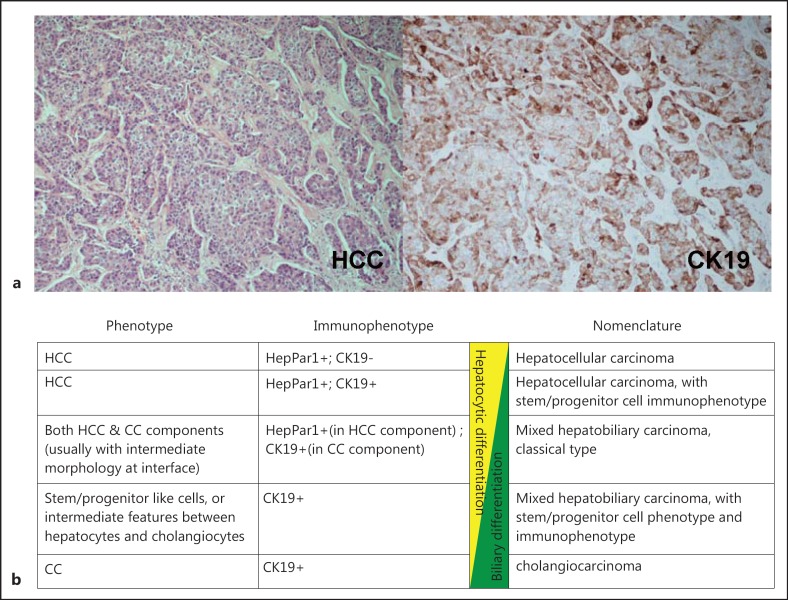
Further understanding of the role of HPCs in the tumorigenesis of HCC will help to stratify patients with chronic liver diseases according to their chances of developing HCC, as well as adding prognostication information for patients who have already developed HCC. Targeted therapy for patients with precursor or malignant lesions expressing HPC signatures may possibly lower the risk of HCC development and progression, respectively. Characterization of HPC in HCC may also shed light on altering the high chemoresistance of HCC to conventional systemic chemotherapy. In terms of histological classification of primary liver cancers, it has been suggested that a proportion of tumors that display intermediate features or stem/progenitor cell phenotypes according to morphological and immunohistochemical criteria should be labeled “mixed hepatobiliary carcinomas” [46]. This upcoming classification will hopefully simplify the picture and reflect the constantly emerging data from studies on the differentiation lineage of HCC (fig. 2).
Fig. 2.
(a) HCC with classic histology (left) and with immunohistochemical expression of CK19 (right). (b) Emerging classification of primary liver cancer based on tumor cell differentiation by morphology and immunophenotype.
Conflict of Interest
There are no conflicts of interest to report with respect to this article.
Acknowledgment
IOL Ng is Loke Yew Professor in Pathology.
References
- 1.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Terminology of nodular hepatocellular lesions International Working Party. Hepatology. 1995;22:983–993. doi: 10.1016/0270-9139(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton SR, Aaltonen LA, World Health Organization . World Health Organization Classification of Tumours. 3rd ed. Lyon Oxford: IARC Press; Oxford University Press (distributor); 2000. International Agency for Research on Cancer: Pathology and genetics of tumours of the digestive system; pp. 159–166. [Google Scholar]
- 4.Wu PC, Fang JW, Lau VK, Lai CL, Lo CK, Lau JY. Classification of hepatocellular carcinoma according to hepatocellular and biliary differentiation markers. Clinical and biological implications. Am J Pathol. 1996;149:1167–1175. [PMC free article] [PubMed] [Google Scholar]
- 5.Kim H, Park C, Han KH, Choi J, Kim YB, Kim JK, Park YN. Primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype. J Hepatol. 2004;40:298–304. doi: 10.1016/j.jhep.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, Brunt EM, Crawford JM, Crosby HA, Desmet V, Finegold MJ, Geller SA, Gouw AS, Hytiroglou P, Knisely AS, Kojiro M, Lefkowitch JH, Nakanuma Y, Olynyk JK, Park YN, Portmann B, Saxena R, Scheuer PJ, Strain AJ, Thung SN, Wanless IR, West AB. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–1745. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- 7.Durnez A, Verslype C, Nevens F, Fevery J, Aerts R, Pirenne J, Lesaffre E, Libbrecht L, Desmet V, Roskams T. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology. 2006;49:138–151. doi: 10.1111/j.1365-2559.2006.02468.x. [DOI] [PubMed] [Google Scholar]
- 8.Woo HG, Lee JH, Yoon JH, Kim CY, Lee HS, Jang JJ, Yi NJ, Suh KS, Lee KU, Park ES, Thorgeirsson SS, Kim YJ. Identification of a cholangiocarcinoma-like gene expression trait in hepatocellular carcinoma. Cancer Res. 2010;70:3034–3041. doi: 10.1158/0008-5472.CAN-09-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roskams T, De Vos R, Van Eyken P, Myazaki H, Van Damme B, Desmet V. Hepatic OV-6 expression in human liver disease and rat experiments: evidence for hepatic progenitor cells in man. J Hepatol. 1998;29:455–463. doi: 10.1016/s0168-8278(98)80065-2. [DOI] [PubMed] [Google Scholar]
- 10.Alison MR, Lovell MJ. Liver cancer: the role of stem cells. Cell Prolif. 2005;38:407–421. doi: 10.1111/j.1365-2184.2005.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roskams TA, Libbrecht L, Desmet VJ. Progenitor cells in diseased human liver. Semin Liver Dis. 2003;23:385–396. doi: 10.1055/s-2004-815564. [DOI] [PubMed] [Google Scholar]
- 12.Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, Crawford JM. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425–1433. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]
- 13.Roskams T, Yang SQ, Koteish A, Durnez A, DeVos R, Huang X, Achten R, Verslype C, Diehl AM. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163:1301–1311. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006;25:3818–3822. doi: 10.1038/sj.onc.1209558. [DOI] [PubMed] [Google Scholar]
- 15.Alison MR. Liver stem cells: implications for hepatocarcinogenesis. Stem Cell Rev. 2005;1:253–260. doi: 10.1385/SCR:1:3:253. [DOI] [PubMed] [Google Scholar]
- 16.International Consensus Group for Hepatocellular Neoplasia Pathologic diagnosis of early hepatocellular carcinoma: a report of the International Consensus Group for Hepatocellular neoplasia. Hepatology. 2009;49:658–664. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- 17.Bosman FT, Carneiro F, Hruban RH, Theise ND, World Health Organization . World Health Organization Classification of Tumours. ed 4. Lyon: International Agency for Research on Cancer; 2010. International Agency for Research on Cancer: WHO Classification of Tumours of the Digestive System; pp. 225–227. [Google Scholar]
- 18.Yeh MM. Pathology of combined hepatocellular-cholangiocarcinoma. J Gastroenterol Hepatol. 2010;25:1485–1492. doi: 10.1111/j.1440-1746.2010.06430.x. [DOI] [PubMed] [Google Scholar]
- 19.Park HS, Bae JS, Jang KY, Lee JH, Yu HC, Jung JH, Cho BH, Chung MJ, Moon WS. Clinicopathologic study on combined hepatocellular carcinoma and cholangiocarcinoma: with emphasis on the intermediate cell morphology. J Korean Med Sci. 2011;26:1023–1030. doi: 10.3346/jkms.2011.26.8.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theise ND, Yao JL, Harada K, Hytiroglou P, Portmann B, Thung SN, Tsui W, Ohta H, Nakanuma Y. Hepatic ‘stem cell’ malignancies in adults: four cases. Histopathology. 2003;43:263–271. doi: 10.1046/j.1365-2559.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 21.Fujii H, Zhu XG, Matsumoto T, Inagaki M, Tokusashi Y, Miyokawa N, Fukusato T, Uekusa T, Takagaki T, Kadowaki N, Shirai T. Genetic classification of combined hepatocellular-cholangiocarcinoma. Hum Pathol. 2000;31:1011–1017. doi: 10.1053/hupa.2000.9782. [DOI] [PubMed] [Google Scholar]
- 22.Cazals-Hatem D, Rebouissou S, Bioulac-Sage P, Bluteau O, Blanche H, Franco D, Monges G, Belghiti J, Sa Cunha A, Laurent-Puig P, Degott C, Zucman-Rossi J. Clinical and molecular analysis of combined hepatocellular-cholangiocarcinomas. J Hepatol. 2004;41:292–298. doi: 10.1016/j.jhep.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 23.Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, Yano H, Suzuki T, Matsuda M, Fujii H, Desmet VJ, Kojiro M, Roskams T. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47:1544–1556. doi: 10.1002/hep.22238. [DOI] [PubMed] [Google Scholar]
- 24.Yang XR, Xu Y, Yu B, Zhou J, Qiu SJ, Shi GM, Zhang BH, Wu WZ, Shi YH, Wu B, Yang GH, Ji Y, Fan J. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut. 2010;59:953–962. doi: 10.1136/gut.2008.176271. [DOI] [PubMed] [Google Scholar]
- 25.Yeh CT, Kuo CJ, Lai MW, Chen TC, Lin CY, Yeh TS, Lee WC. CD133-positive hepatocellular carcinoma in an area endemic for hepatitis B virus infection. BMC Cancer. 2009;9:324. doi: 10.1186/1471-2407-9-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H, Choi GH, Na DC, Ahn EY, Kim GI, Lee JE, Cho JY, Yoo JE, Choi JS, Park YN. Human hepatocellular carcinomas with “Stemness”-related marker expression: keratin 19 expression and a poor prognosis. Hepatology. 2011;54:1707–1717. doi: 10.1002/hep.24559. [DOI] [PubMed] [Google Scholar]
- 27.Cai X, Zhai J, Kaplan DE, Zhang Y, Zhou L, Chen X, Qian G, Zhao Q, Li Y, Gao L, Cong W, Zhu M, Yan Z, Shi L, Wu D, Wei L, Shen F, Wu M. Background progenitor activation is associated with recurrence after hepatectomy of combined hepatocellular-cholangiocarcinoma. Hepatology. 2012;56:1804–1816. doi: 10.1002/hep.25874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuchiya A, Kamimura H, Tamura Y, Takamura M, Yamagiwa S, Suda T, Nomoto M, Aoyagi Y. Hepatocellular carcinoma with progenitor cell features distinguishable by the hepatic stem/progenitor cell marker NCAM. Cancer Lett. 2011;309:95–103. doi: 10.1016/j.canlet.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Oliva J, French BA, Qing X, French SW. The identification of stem cells in human liver diseases and hepatocellular carcinoma. Exp Mol Pathol. 2010;88:331–340. doi: 10.1016/j.yexmp.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziol M, Nault JC, Aout M, Barget N, Tepper M, Martin A, Trinchet JC, Ganne-Carrie N, Vicaut E, Beaugrand M, N'Kontchou G. Intermediate hepatobiliary cells predict an increased risk of hepatocarcinogenesis in patients with hepatitis C virus-related cirrhosis. Gastroenterology. 2010;139:335–343. doi: 10.1053/j.gastro.2010.04.012. e2. [DOI] [PubMed] [Google Scholar]
- 31.Lee TK, Castilho A, Ma S, Ng IO. Liver cancer stem cells: implications for a new therapeutic target. Liver Int. 2009;29:955–965. doi: 10.1111/j.1478-3231.2009.02040.x. [DOI] [PubMed] [Google Scholar]
- 32.Chiba T, Kamiya A, Yokosuka O, Iwama A. Cancer stem cells in hepatocellular carcinoma: Recent progress and perspective. Cancer Lett. 2009;286:145–153. doi: 10.1016/j.canlet.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 33.Lee TK, Cheung VC, Ng IO: Liver tumor-initiating cells as a therapeutic target for hepatocellular carcinoma. Cancer Lett 2012. [DOI] [PubMed]
- 34.Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, Nakauchi H, Taniguchi H. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240–251. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 35.Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 36.Kimura O, Takahashi T, Ishii N, Inoue Y, Ueno Y, Kogure T, Fukushima K, Shiina M, Yamagiwa Y, Kondo Y, Inoue J, Kakazu E, Iwasaki T, Kawagishi N, Shimosegawa T, Sugamura K. Characterization of the epithelial cell adhesion molecule (EpCAM)+ cell population in hepatocellular carcinoma cell lines. Cancer Sci. 2010;101:2145–2155. doi: 10.1111/j.1349-7006.2010.01661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, Reid LM, Minato H, Honda M, Kaneko S, Tang ZY, Wang XW. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820–824. doi: 10.1016/j.bbrc.2006.10.128. [DOI] [PubMed] [Google Scholar]
- 39.Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D, Yang S, Zheng S, Gu J. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444–1450. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- 40.Yang W, Wang C, Lin Y, Liu Q, Yu LX, Tang L, Yan HX, Fu J, Chen Y, Zhang HL, Zheng LY, He YQ, Li YQ, Wu FQ, Zou SS, Li Z, Wu MC, Feng GS, Wang HY. OV6(+) tumor-initiating cells contribute to tumor progression and invasion in human hepatocellular carcinoma. J Hepatol. 2012;57:613–620. doi: 10.1016/j.jhep.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J, Li J. Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int J Cancer. 2010;126:2067–2078. doi: 10.1002/ijc.24868. [DOI] [PubMed] [Google Scholar]
- 42.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Tong CM, Ma S, Guan XY. Biology of hepatic cancer stem cells. J Gastroenterol Hepatol. 2011;26:1229–1237. doi: 10.1111/j.1440-1746.2011.06762.x. [DOI] [PubMed] [Google Scholar]
- 44.Mishra L, Banker T, Murray J, Byers S, Thenappan A, He AR, Shetty K, Johnson L, Reddy EP. Liver stem cells and hepatocellular carcinoma. Hepatology. 2009;49:318–329. doi: 10.1002/hep.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woo HG, Wang XW, Budhu A, Kim YH, Kwon SM, Tang ZY, Sun Z, Harris CC, Thorgeirsson SS. Association of TP53 mutations with stem cell-like gene expression and survival of patients with hepatocellular carcinoma. Gastroenterology. 2011;140:1063–1070. doi: 10.1053/j.gastro.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roncalli M, Park YN, Di Tommaso L. Histopathological classification of hepatocellular carcinoma. Dig Liver Dis. 2010;42(Suppl 3):S228–S234. doi: 10.1016/S1590-8658(10)60510-5. [DOI] [PubMed] [Google Scholar]