Abstract
Background
Excess weight is paradoxically associated with better cardiovascular disease (CVD) outcomes and mortality in end-stage renal disease (ESRD) patients treated with hemodialysis. This association has been observed in chronic kidney disease (CKD) as well. One potential explanation for this inverse relationship is that the usual positive correlation between severity of CVD risk factors and higher body mass index (BMI) is reversed in CKD. To test this hypothesis, we determined the relationship between BMI and CVD risk factors in patients with and without CKD.
Methods
This was a cross-sectional study of the nationally representative US National Health and Nutrition Examination Survey (NHANES) 1999–2006. CKD was defined as glomerular filtration rate <60 ml/min per 1.73 m2. Covariates were age, race/ethnicity, sex and use of relevant prescription medications. Outcome variables were total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, systolic blood pressure (SBP), diastolic blood pressure (DBP), C-reactive protein (CRP) and fasting glucose (FG).
Results
There were 1,895 and 32,431 patients with and without CKD, respectively. Those with CKD were older and had higher BMI. The shapes of the association between BMI and total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, SBP, CRP and FG were similar in those with or without CKD. In a sensitivity analysis excluding patients taking relevant prescription medications, our results did not differ substantially.
Conclusions
CKD did not alter the shapes of the association between higher BMI and CVD risk factors. Inverse associations between BMI and CVD risk factors are unlikely to explain why CKD patients with higher BMI may have better outcomes.
Keywords: BMI, Cardiovascular, CKD, Risk factors
Introduction
Although excess weight is associated with increased mortality in the general population, among end-stage renal disease (ESRD) patients treated with hemodialysis, the relationship between excess weight and death appears to be reversed (1–4). ESRD patients with higher body mass index (BMI) actually suffer fewer cardiovascular events and survive longer compared with their leaner counterparts (1–5). Several mechanisms have been postulated to explain this reverse relation between excess weight and mortality, including more stable hemodynamic status, alterations in circulating cytokines and malnutrition-inflammation syndromes (6, 7).
In the predialysis chronic kidney disease (CKD) population, several studies (8–13) have also suggested an inverse relationship between excess weight and adverse outcomes. Among 920 patients with advanced CKD, a BMI (calculated as kg/m2) greater than 30 was associated with lower mortality (13). In the Atherosclerosis Risk in Communities (ARIC) cohort, higher BMI was associated with lower mortality in those with stage 3 CKD (11).
One potential explanation for the reverse association between BMI and cardiovascular disease and mortality in patients with CKD may be that the usual positive association between higher BMI and worse cardiovascular risk factors – such as higher lipid levels, elevated blood pressure, more inflammation and high fasting glucose levels – are disrupted. Here, in a nationally representative study sample, we test the hypothesis that the associations between excess weight and selected cardiovascular disease risk factors among patients with CKD are inversed compared with these associations observed among people without CKD.
Subjects and methods
Study design and study population
This was a cross-sectional study of the National Health and Nutrition Examination Survey (NHANES) 1999–2006. NHA-NES is a cross-sectional nationally representative complex survey of the noninstitutionalized civilian population in the United States.
In NHANES 1999–2006, 39,352 adults completed both the medical evaluation and study interview. Exclusion criteria for our study were unavailable serum creatinine measurement, unavailable height or weight, or BMI <18.5 kg/m2. Participants with BMI <18.5 kg/m2 were omitted because our research question focused on excess weight. Additionally, there were too few CKD participants in this range to make meaningful between-group comparisons.
Predictors
BMI was a predictor variable that was obtained from the physical examination component of NHANES 1999–2006 and was calculated as weight (in kilograms) divided by height (in meters) squared (BMI = weight/height2). BMI was measured as a continuous variable for our analysis.
CKD was defined as glomerular filtration rate (eGFR) <60 ml/min per 1.73 m2 to correspond to CKD stages 3–5 per the National Kidney Foundation staging system criteria (14). eGFR was estimated using the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation (15). Standard adjustments as recommended by the NHANES analytic guidelines were applied to serum creatinine laboratory measurements to account for variations in technique across survey years (16). Patients were excluded if they reported needing dialysis within the last 12 months.
Outcomes
Outcome variables included total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, systolic blood pressure (SBP), diastolic blood pressure (DBP), C-reactive protein (CRP) and fasting glucose (FG). Serum samples were obtained during the exam and assays and frozen. LDL cholesterol, triglycerides and FG were measured in the subsample of participants whose exams were scheduled in the morning and reported having fasted prior to the exam. All laboratory outcome variables were measured continuously and reported in standard units. SBP and DBP were measured in seated patients who had rested at least 5 minutes, via a standardized protocol in which study physicians were trained and certified. The average of at least 3 consecutive readings was calculated.
Covariates
Covariates included age, race/ethnicity and sex. Age was determined by self-report at the time of examination and was reported as years. Race/ethnicity was determined by self report and categorized as: (i) Mexican-American, (ii) other Hispanic, (iii) non-Hispanic white, (iv) non-Hispanic black or (v) other. Laboratory data were obtained by standard NHANES techniques (16). Use of medications for hypertension, dyslipidemia and diabetes was based on self-report from questionnaires in which participants were asked, “Do you take pills for condition [X].” Use of statin medications was ascertained from review of the participants’ prescription medication bottles.
Statistical analysis
All analyses were performed using the complex survey functions in STATA version 10.0 (Stata, College Station, TX, USA). In accord with the NHANES analytic guidelines, sample weights, strata and primary sampling unit variables were used to account for unequal probabilities of selection and the multistage, stratified sample design (16). Observations from the 1999–2002, 2003–2004 and 2005–2006 surveys were pooled, and overall 8-year weights were calculated. Subsample fasting weights for LDL cholesterol, triglycerides and FG measurements were calculated. Two-sided p values <0.05 were considered statistically significant.
We first used t- and F-tests for survey data to assess unadjusted differences in continuous and dichotomous variables by CKD status. We then used linear regression to determine whether the association between BMI and cardiovascular risk factors differed by CKD status. We adjusted for age, sex and race/ethnicity. The associations between BMI and total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, SBP, DBP, CRP and FG were clearly nonlinear. Accordingly, the association between BMI and each risk factor was modeled within the CKD and non-CKD groups using a restricted cubic spline with knots at BMI values of 22, 24, 28, 32 and 39. F-Tests were used to assess differences in mean levels of the outcome by overall CKD status.
In exploratory analyses, we also tested for interactions of CKD with age, sex and race/ethnicity, but found no persuasive evidence for such effects. In addition, we performed sensitivity analyses excluding participants taking medications likely to affect each outcome, including antihypertensive medications (for SBP and DBP), lipid-lowering agents (for total cholesterol, LDL cholesterol, HDL cholesterol and triglycerides), statins (for CRP) (17–25) and insulin or oral hypoglycemic agents (for FG). We also repeated our analysis using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation instead of the MDRD Study equation to calculate eGFR (26).
Results
In the study sample, there were 1,895 participants with CKD and 32,431 participants without CKD after the exclusion criteria were applied. Of those with CKD, 1,733 had stage 3 CKD (eGFR 30–59 ml/min per 1.73 m2), 108 had stage 4 CKD (eGFR 15–29 ml/min per 1.73 m2) and 54 had stage 5 CKD (eGFR <15 ml/min per 1.73 m2). Those with CKD overall were older, more likely to be female, less likely to be African-American, had lower levels of education and lower household income compared with those without CKD. Additionally, those with CKD had a higher mean BMI and a higher prevalence of diabetes and coronary artery disease (Tab. I).
Participants with CKD had higher total cholesterol, triglycerides, SBP, CRP and FG (Tab. II).
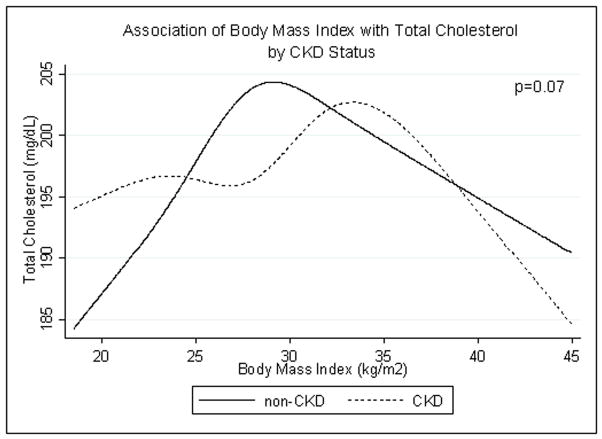
We found no evidence that the association between total cholesterol and BMI was modified by CKD status (p=0.07) (Fig. 1). In both groups, total cholesterol increased with BMI and then decreased above BMI >30 kg/m2. LDL cholesterol also did not clearly differ by CKD status (p=0.054); LDL rose with increasing BMI and declined at the highest BMI levels for both groups. HDL cholesterol was lower in those with CKD at basically all BMI levels (p<0.001). In both groups, HDL cholesterol decreased with increasing BMI. Triglyceride level was higher in those with CKD (p=0.01), but rose with increasing BMI among both groups.
Fig. 1.
Age-, race/ethnicity- and sex-adjusted spline functions demonstrating the cross-sectional association of body mass index and total cholesterol in patients with (dotted line) and without (solid line) chronic kidney disease (CKD).
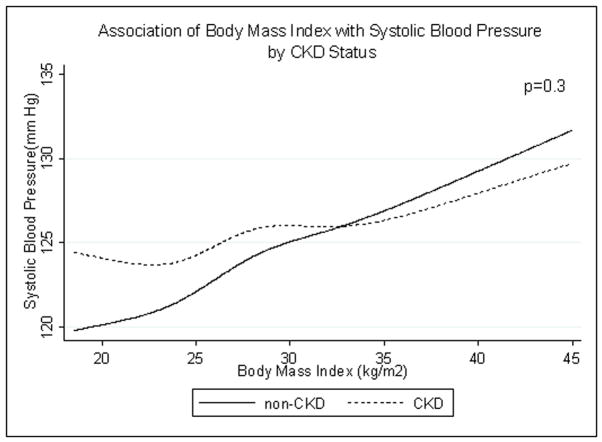
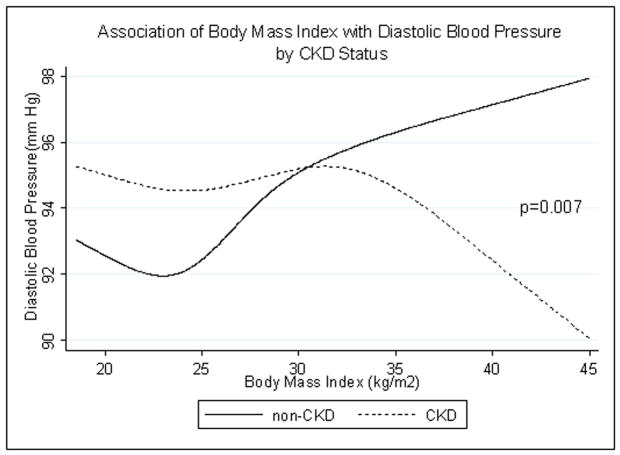
SBP did not differ by CKD status (p=0.3). In both groups, SBP rose with increasing BMI (Fig. 2). In participants with CKD, DBP decreased at very high BMI while in participants without CKD, DBP rose with increasing BMI (interaction p=0.007) (Fig. 3).
Fig. 2.
Age-, race/ethnicity- and sex-adjusted spline functions demonstrating the cross-sectional association of body mass index and systolic blood pressure in patients with (dotted line) and without (solid line) chronic kidney disease (CKD).
Fig. 3.
Age-, race/ethnicity- and sex-adjusted spline functions demonstrating the cross-sectional association of body mass index and diastolic blood pressure in patients with (dotted line) and without (solid line) chronic kidney disease (CKD).
The association between BMI and CRP for the CKD and non-CKD groups did not differ by CKD status (p=0.095). CRP rose with increasing BMI for both groups.
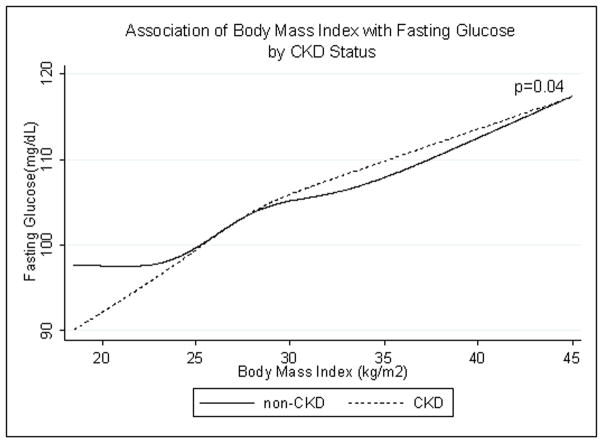
The association between BMI and FG for the CKD and non-CKD groups is shown in Figure 4. Interaction by CKD status was borderline statistically significant (p=0.04). But FG rose with increasing BMI in both those with and without CKD.
Fig. 4.
Age-, race/ethnicity- and sex-adjusted spline functions demonstrating the cross-sectional association of body mass index and fasting glucose in patients with (dotted line) and without (solid line) chronic kidney disease (CKD).
Sensitivity analyses
In a sensitivity analysis excluding subjects on relevant medication use, our findings did not change (results not shown). In addition, using the CKD-EPI equation to calculate eGFR and define CKD yielded similar results (results not shown) (26).
Discussion
The purpose of our study was to evaluate whether CKD modifies the shape of the association between BMI and select cardiovascular risk factors in a nationally representative population. Our results suggest that in patients with CKD, high BMI was also associated with worsening cardiovascular risk factor profiles. Thus we found no support for our hypothesis that these expected associations were reversed in patients with CKD, relative to the general population.
We did note that the association between DBP and BMI differed in the 2 groups. These results likely reflect the increased vascular stiffness in patients with CKD and excess weight (27–35). Low DBP has been shown to be associated with adverse outcomes (36, 37), therefore this does not likely explain why overweight patients with CKD have better survival.
In both participants with and without CKD, we also found a decrease in total cholesterol and LDL cholesterol at very high BMI. This is consistent with previous publications (38–40). The mechanism for this association remains unclear.
Several prior studies have also examined the association between higher BMI and cardiovascular risk factors among predialysis CKD populations (41). In an analysis of the ARIC cohort, the presence of CKD did not reverse the associations of body size with metabolic syndrome and markers of inflammation (11). These findings are thus consistent with our results, but our study examined a broader array of cardiovascular disease risk factors, compared those patients with CKD to those without and relied on a nationally representative cohort.
We did not find that the expected associations between BMI and select cardiovascular risk factors were reversed in CKD. There are other potential mechanisms to explain the inverse association between higher BMI and cardiovascular disease and mortality. Perhaps the adverse effect of high FG level or lower HDL cholesterol or higher SBP is not the same in CKD patients as it is in non-CKD patients.
Our study had several strengths. This was a nationally representative large sample of subjects from the US population. Our results were similar using both the MDRD equation and the CKD-EPI equation to estimated eGFR. We did sensitivity analyses to ensure that our results were not due to confounding by medication use.
Our study had several limitations as well. Many of demographic characteristics of participants with CKD were different compared with those without CKD (Tab. I); however, this is likely representative of the US CKD population. More specific information about participant comorbid diseases is lacking in this dataset. Most of the CKD participants had stage 3 CKD. We were thus underpowered to examine the impact of different stages of CKD, and it is possible that persons with more advanced stages of CKD exhibit different associations between BMI and cardiovascular risk factors. We were not able to determine the etiology of CKD in this dataset. The use of BMI as a marker of adiposity is imperfect; however, it is the primary marker that has been used to demonstrate this reverse association between BMI and mortality. Medication use was based on self-report, and residual confounding may exist even after adjustment for medication use. Only one serum creatinine measurement was available. Finally, this was a cross-sectional study, which limits causal inference. We also did not have information on outcomes such as cardiovascular events and mortality.
In conclusion, our results show that CKD does not significantly modify the association between higher BMI and cardiovascular risk factors. Additional studies are needed to explain why higher BMI has been observed to be associated with reduced risk of adverse outcomes among patients with CKD.
TABLE I.
BASELINE CHARACTERISTICS OF STUDY POPULATION BY CHRONIC KIDNEY DISEASE (CKD) STATUS
| CKD (n=1,895) | Non-CKD (n=32,431) | p Value | |
|---|---|---|---|
| Mean age ± SD (years) | 68.7 ± 13.4 | 43.5 ± 16.3 | <0.001 |
| Female (%) | 61.7 | 50.7 | <0.001 |
| Race | <0.001 | ||
| White (%) | 84.1 | 67.9 | |
| African-American (%) | 7.5 | 12.1 | |
| Married (%) | 55.0 | 52.2 | <0.001 |
| Education | <0.001 | ||
| Less than high school (%) | 28.1 | 19.2 | |
| High school or more (%) | 71.8 | 80.6 | |
| Household income, US dollars | <0.001 | ||
| <$20,000 (%) | 29.6 | 17.5 | |
| $20,000–$44,999 (%) | 33.3 | 28.5 | |
| $45,000–$74,999 (%) | 19.4 | 24.3 | |
| ≥$75,000 (%) | 14.6 | 27.5 | |
| Mean estimated glomerular filtration rate ± SD (ml/min/1.73 m2) | 48.6 ± 10.8 | 92.6 ± 18.1 | |
| Mean body mass index ± SD (calculated as kg/m2) | 28.7 ± 5.7 | 27.5 ± 6.0 | <0.001 |
| Body mass index categories (calculated as kg/m2) (%) | <0.001 | ||
| Normal (18.5–24.9) | 27.4 | 39.6 | |
| Overweight (25–29.9) | 38.1 | 31.6 | |
| Obese (30–30.9) | 28.7 | 24.2 | |
| Very obese (≥40) | 5.8 | 4.6 | |
| Diabetes (%) | 19.1 | 5.2 | <0.001 |
| Coronary artery disease (%) | 12.9 | 2.8 | <0.001 |
| Current tobacco use (%) | 21.5 | 42.9 | <0.001 |
TABLE II.
CARDIOVASCULAR RISK FACTORS IN STUDY POPULATION BY CHRONIC KIDNEY DISEASE (CKD) STATUS
| Cardiovascular risk factor | CKD | Non-CKD | p Value |
|---|---|---|---|
| Total cholesterol, mean ± SD (mg/dL) | 205.7 ± 44.6 (n=1,862) | 195.6 ± 42.0 (n=24,471) | <0.001 |
| LDL cholesterol, mean ± SD (mg/dL) | 116.3 ± 36.8 (n=775) | 115.7 ± 34.1 (n=10,923) | 0.9 |
| HDL cholesterol, mean ± SD (mg/dL) | 52.5 ± 15.8 (n=1,861) | 52.2 ± 15.3 (n=24,467) | 0.5 |
| Triglycerides, mean ± SD (mg/dL) | 166.3 ± 101.3 (n=861) | 138.1 ± 106.7 (n=11,610) | <0.001 |
| Systolic blood pressure, mean ± SD (mm Hg) | 136.0 ± 22.6 (n=1,163) | 119.6 ± 16.9 (n=17,458) | <0.001 |
| Diastolic blood pressure, mean ± SD (mm Hg) | 93.6 ± 17.1 (n=1,129) | 94.2 ± 15.1 (n=17,343)) | 0.3 |
| CRP, mean ± SD (mg/L) | 0.58 ± 1.05 (n=1,849) | 0.41 ± 0.78 (n=23,095) | <0.001 |
| Fasting glucose (mg/dL) | 112.0 ± 35.2 (n=856) | 100.8 ± 29.1 (n=11,215) | <0.001 |
| Taking lipid-lowering agents, no. (%) | 507 (26.7%) | 1,907 (5.9%) | 0.007 |
| Taking antihypertensives, no. (%) | 1,092 (57.6%) | 3,588 (11.1%) | <0.001 |
| Taking insulin, no. (%) of those with self-reported diabetes | 115 (35.0%) | 312 (24.8%) | <0.001 |
| Taking oral diabetic medications, no. (%) of those with self-reported diabetes | 206 (63.8%) | 863 (69.4%) | 0.1 |
CRP = C-reactive protein.
Acknowledgments
Financial support: This research was supported by the American Kidney Fund and the US National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) K23DK088865.
Footnotes
Conflict of interest statement: None.
References
- 1.Brown CD, Higgins M, Donato KA, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8:605–619. doi: 10.1038/oby.2000.79. [DOI] [PubMed] [Google Scholar]
- 2.Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int. 1999;55:1560–1567. doi: 10.1046/j.1523-1755.1999.00389.x. [DOI] [PubMed] [Google Scholar]
- 3.Kopple JD, Zhu X, Lew NL, Lowrie EG. Body weight-for-height relationships predict mortality in maintenance hemo-dialysis patients. Kidney Int. 1999;56:1136–1148. doi: 10.1046/j.1523-1755.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 4.Leavey SF, McCullough K, Hecking E, Goodkin D, Port FK, Young EW. Body mass index and mortality in ‘healthier’ as compared with ‘sicker’ haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2001;16:2386–2394. doi: 10.1093/ndt/16.12.2386. [DOI] [PubMed] [Google Scholar]
- 5.Johansen KL, Young B, Kaysen GA, Chertow GM. Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr. 2004;80:324–332. doi: 10.1093/ajcn/80.2.324. [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K. Causes and consequences of the reverse epidemiology of body mass index in dialysis patients. J Ren Nutr. 2005;15:142–147. doi: 10.1053/j.jrn.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis. 2007;49:581–591. doi: 10.1053/j.ajkd.2007.02.277. [DOI] [PubMed] [Google Scholar]
- 9.Kovesdy CP, Anderson JE. Reverse epidemiology in patients with chronic kidney disease who are not yet on dialysis. Semin Dial. 2007;20:566–569. doi: 10.1111/j.1525-139X.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 10.Elsayed EF, Tighiouart H, Weiner DE, et al. Waist-to-hip ratio and body mass index as risk factors for cardiovascular events in CKD. Am J Kidney Dis. 2008;52:49–57. doi: 10.1053/j.ajkd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwan BC, Murtaugh MA, Beddhu S. Associations of body size with metabolic syndrome and mortality in moderate chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:992–998. doi: 10.2215/CJN.04221206. [DOI] [PubMed] [Google Scholar]
- 12.Obermayr RP, Temml C, Gutjahr G, et al. Body mass index modifies the risk of cardiovascular death in proteinuric chronic kidney disease. Nephrol Dial Transplant. 2009;24:2421–2428. doi: 10.1093/ndt/gfp075. [DOI] [PubMed] [Google Scholar]
- 13.Evans M, Fryzek JP, Elinder CG, et al. The natural history of chronic renal failure: results from an unselected, population-based, inception cohort in Sweden. Am J Kidney Dis. 2005;46:863–870. doi: 10.1053/j.ajkd.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 14.Shulman NB, Ford CE, Hall WD, et al. The Hypertension Detection and Follow-up Program Cooperative Group. Prognostic value of serum creatinine and effect of treatment of hypertension on renal function: results from the hypertension detection and follow-up program. Hypertension. 1989;13(Suppl):I80–I93. doi: 10.1161/01.hyp.13.5_suppl.i80. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Coresh J, Greene T, et al. Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 16.National Health and Nutrition Examination Survey Laboratory Procedure Manual. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2001. [Google Scholar]
- 17.Arnaud C, Braunersreuther V, Mach F. Toward immunomodulatory and anti-inflammatory properties of statins. Trends Cardiovasc Med. 2005;15:202–206. doi: 10.1016/j.tcm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Arnaud C, Veillard NR, Mach F. Cholesterol-independent effects of statins in inflammation, immunomodulation and atherosclerosis. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:127–134. doi: 10.2174/1568006043586198. [DOI] [PubMed] [Google Scholar]
- 19.Blanco-Colio LM, Tuñón J, Marín-Ventura JL, Egido J. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int. 2003;63:12–23. doi: 10.1046/j.1523-1755.2003.00744.x. [DOI] [PubMed] [Google Scholar]
- 20.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 21.Kleemann R, Kooistra T. HMG-CoA reductase inhibitors: effects on chronic subacute inflammation and onset of atherosclerosis induced by dietary cholesterol. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:441–453. doi: 10.2174/156800605774962077. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Raftery M, Yaqoob M, Fan SL. Anti-inflammatory effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors (statins) in peritoneal dialysis patients. Perit Dial Int. 2007;27:283–287. [PubMed] [Google Scholar]
- 23.Lahera V, Goicoechea M, de Vinuesa SG, et al. Endothelial dysfunction, oxidative stress and inflammation in atherosclerosis: beneficial effects of statins. Curr Med Chem. 2007;14:243–248. doi: 10.2174/092986707779313381. [DOI] [PubMed] [Google Scholar]
- 24.Montecucco F, Mach F. Update on statin-mediated anti-inflammatory activities in atherosclerosis. Semin Immunopathol. 2009;31:127–142. doi: 10.1007/s00281-009-0150-y. [DOI] [PubMed] [Google Scholar]
- 25.Schonbeck U, Libby P. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation. 2004;109(Suppl 1):11118–11126. doi: 10.1161/01.CIR.0000129505.34151.23. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ilyas B, Dhaun N, Markie D, et al. Renal function is associated with arterial stiffness and predicts outcome in patients with coronary artery disease. QJM. 2009;102:183–191. doi: 10.1093/qjmed/hcn171. [DOI] [PubMed] [Google Scholar]
- 28.Lacy P, Carr SJ, O’Brien D, et al. Reduced glomerular filtra-tion rate in pre-dialysis non-diabetic chronic kidney disease patients is associated with impaired baroreceptor sensitivity and reduced vascular compliance. Clin Sci (Lond) 2006;110:101–108. doi: 10.1042/CS20050192. [DOI] [PubMed] [Google Scholar]
- 29.Taal MW, Sigrist MK, Fakis A, Fluck RJ, McIntyre CW. Markers of arterial stiffness are risk factors for progression to end-stage renal disease among patients with chronic kidney disease stages 4 and 5. Nephron Clin Pract. 2007;107:c177–c181. doi: 10.1159/000110678. [DOI] [PubMed] [Google Scholar]
- 30.Tetzner F, Scholze A, Wittstock A, Zidek W, Tepel M. Impaired vascular reactivity in patients with chronic kidney disease. Am J Nephrol. 2008;28:218–223. doi: 10.1159/000110091. [DOI] [PubMed] [Google Scholar]
- 31.Kawamoto R, Kohara K, Tabara Y, et al. An association between decreased estimated glomerular filtration rate and arterial stiffness. Intern Med. 2008;47:593–598. doi: 10.2169/internalmedicine.47.0825. [DOI] [PubMed] [Google Scholar]
- 32.Matsuda N, Takei T, Fujiu A, Ogawa T, Nitta K. Arterial stiffness in patients with non-diabetic chronic kidney disease (CKD) J Atheroscler Thromb. 2009;16:57–62. doi: 10.5551/jat.e602. [DOI] [PubMed] [Google Scholar]
- 33.Dengo AL, Dennis EA, Orr JS, et al. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension. 2010;55:855–861. doi: 10.1161/HYPERTENSIONAHA.109.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rider OJ, Tayal U, Francis JM, et al. The effect of obesity and weight loss on aortic pulse wave velocity as assessed by magnetic resonance imaging. Obesity (Silver Spring) 2010;18:2311–2316. doi: 10.1038/oby.2010.64. [DOI] [PubMed] [Google Scholar]
- 35.Seifalian AM, Filippatos TD, Joshi J, Mikhailidis DP. Obesity and arterial compliance alterations. Curr Vasc Pharmacol. 2010;8:155–168. doi: 10.2174/157016110790886956. [DOI] [PubMed] [Google Scholar]
- 36.Berl T, Hunsicker LG, Lewis JB, et al. Collaborative Study Group. Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol. 2005;16:2170–2179. doi: 10.1681/ASN.2004090763. [DOI] [PubMed] [Google Scholar]
- 37.Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, Anderson JE. Association of low blood pressure with increased mortality in patients with moderate to severe chronic kidney disease. Nephrol Dial Transplant. 2006;21:1257–1262. doi: 10.1093/ndt/gfk057. [DOI] [PubMed] [Google Scholar]
- 38.Miller DC, Trulson MF, McCann MB, White PD, Stare FJ. Diet, blood lipids and health of Italian men in Boston. Ann Intern Med. 1958;49:1178–1200. doi: 10.7326/0003-4819-49-5-1178. [DOI] [PubMed] [Google Scholar]
- 39.Ridderstråle M, Gudbjörnsdottir S, Eliasson B, Nilsson PM, Cederholm J. Steering Committee of the Swedish National Diabetes Register (NDR). Obesity and cardiovascular risk factors in type 2 diabetes: results from the Swedish National Diabetes Register. J Intern Med. 2006;259:314–322. doi: 10.1111/j.1365-2796.2006.01617.x. [DOI] [PubMed] [Google Scholar]
- 40.Vierhapper H, Nardi A, Grösser P. Prevalence of paradoxically normal serum cholesterol in morbidly obese women. Metabolism. 2000;49:607–610. doi: 10.1016/s0026-0495(00)80035-9. [DOI] [PubMed] [Google Scholar]
- 41.Lo JC, Go AS, Chandra M, Fan D, Kaysen GA. eGFR, body mass index, and low high-density lipoprotein concentration in adults with and without CKD. Am J Kidney Dis. 2007;50:552–558. doi: 10.1053/j.ajkd.2007.07.011. [DOI] [PubMed] [Google Scholar]