Abstract
Among the EGFRs, HER2 is a major heterodimer partner and also has important implications in the formation of particular tumors. Interaction of HER2 protein with other EGFR proteins can be modulated by small molecule ligands and, hence, these protein-protein interactions play a key role in biochemical reactions related to control of cell growth. A peptidomimetic (compound 5-1) that binds to HER2 protein extracellular domain and inhibits protein-protein interactions of EGFRs was conjugated with BODIPY (4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene). Conjugation of BODIPY to the peptidomimetic was investigated by different approaches. The conjugate was characterized for its ability to bind to HER2 overexpressing SKBR-3 and BT-474 cells. Furthermore, cellular uptake of conjugate of BODIPY was studied in the presence of membrane tracker and Lyso tracker using confocal microscopy. Our results suggested that fluorescently labeled compound 5-7 binds to the extracellular domain and stays in the membrane for nearly 24 h. After 24 h there is an indication of internalization of the conjugate. Inhibition of protein-protein interaction and downstream signaling effect of compound 5-1 was also studied by proximity ligation assay and western blot analysis. Results suggested that compound 5-1 inhibits protein-protein interactions of HER2-HER3 and phosphorylation of HER2 in a time-dependent manner.
Keywords: BODIPY, Breast cancer, EGFR, Protein-Protein interactions, HER2, peptidomimetic, Phosphorylation
1. Introduction
Human epidermal growth factor receptors (EGFRs) are important molecules in signal transduction processes in cells and provide the signal for cell growth, differentiation, and motility. There are four well known EGFRs, namely, EGFR/HER1/ERBB1, HER2/ERBB2, HER3/ERBB3, and HER4/ERBB4. HER1 and HER2 have been associated with various cancers through their overexpression on the cell surfaces of tumors [1–3]. Each receptor exists as a monomer, consisting of an extracellular domain (containing four domains (I–IV)), a transmembrane domain, a cytosolic tyrosine-kinase-containing domain, and the regulatory domain, through which cell signaling to the nucleus occurs [3–7]. EGFRs interact with one another upon ligand binding to their extracellular domains, and form homo- and heterodimers [3, 8, 9]. Formation of these heterodimers is important in generating signals for cell growth and differentiation. Other possible dimers such as EGFR-HER2, HER2-HER3, and HER2-HER4 have been proposed in the literature [10]. Blockade of protein-protein interactions (PPI) of the extracellular domains of EGFRs ultimately leads to control of cell growth [11–14]. Among the EGFRs, HER2 is a major heterodimer partner and also has important implications in the formation of particular tumors. Interaction of HER2 protein with other EGFR proteins can be modulated by small molecule ligands and, hence, these protein-protein interactions play a key role in biochemical reactions related to control of cell growth [13, 15–18]. Antibodies and affibody molecules targeting HER2 protein are investigated as probes or as imaging agents with radioactive probes, quantum dots, and fluorescent probes [19, 20]. Peptides designed to target the extracellular domain (ECD) of HER2 have been reported to interfere with the dimer interface of HER2 with EGFR [21–23]. Other small molecules have been reported to interfere with the EGFR extracellular domain [24]. These molecules were designed for therapeutic or imaging purposes. Some of these molecules bind to HER2 protein with high affinity, but do not interfere with PPI [23]. Our approach is to use peptidomimetics and their conjugates to study hot spots in PPI in the ECD of EGFRs. Among the ECD of EGFRs, domain II is well characterized. Domain IV is close to the membrane environment and dynamic in nature; it is not well characterized because in the crystal structure the electron density of domain IV is not well defined [25, 26]. Domains IV of HER2 and EGFR are known to interact with each other. Thus, we chose to study domain IV PPI interaction. In our preliminary study, we have shown that a peptidomimetic, namely, compound 5-1 (Scheme 1), modulates dimerization of EGFR-HER2/HER2-HER3 [27–30]. This work focuses on conjugation of the fluorescent probe 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene (BODIPY) to compound 5-1 to modulate PPI interactions of EGFRs [31–34]. Furthermore, we characterized the binding and cellular uptake of BODIPY conjugate of 5-1 (compound 5-7) in HER2-overexpressing breast cancer cell lines. Inhibition of protein-protein interaction of the compound 5-1 was also studied by proximity ligation assay (PLA). Our results suggested that fluorescently labeled compound 5-7 binds to the extracellular domain and stays in the membrane for nearly 24 h. After 24 h there is an indication of internalization of the conjugate.
Scheme 1.
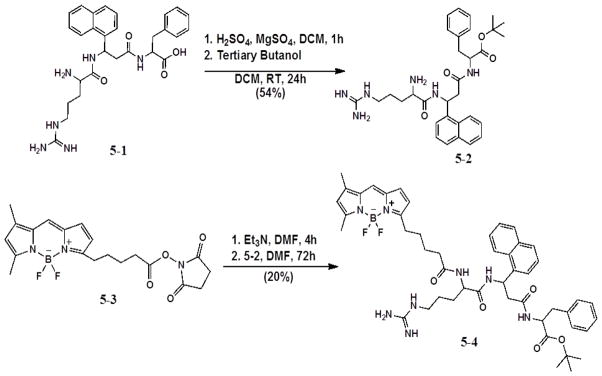
Synthesis of 5-2 and 5-4 (tert-Butyl ester terminated conjugate).
2. Results
2.1 Synthesis of BODIPY-compound 5-1 conjugates
The synthesis of BODIPY-compound 5-1 conjugate was performed using two methods: 1) solution-phase conjugation of peptomimetics 5-1 and 5-2 to 5-3 (Scheme 1) and 2) solid-phase conjugation using 5-6 (Scheme 2).
Scheme 2.
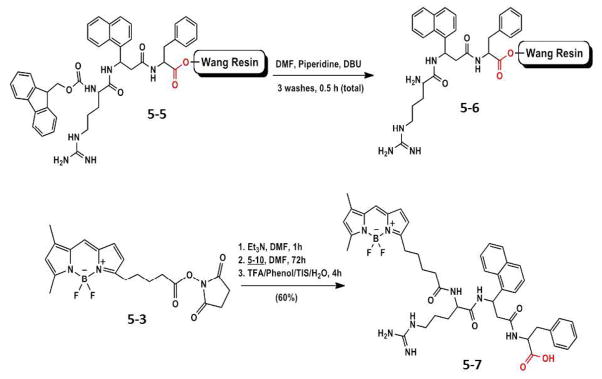
Synthesis of compound 5-7 using Wang resin.
The direct conjugation of peptidomimetic 5-1 to commercially available compound 5-3 under basic conditions (Et3N) gave low yields of conjugate due to the competing polymerization reaction of peptomimetic 5-1. Therefore, a tert-butyl protecting group was introduced at the C-terminus of 5-1, which is stable under the basic conditions needed for conjugation. The tert-butyl ester can be formed by several methods, including strong acidic conditions. Mechanistically, the reaction is acid catalyzed with sulfuric acid, then tertiary butanol is added to complete the esterification. To prevent water from forming and causing hydrolysis of the ester, anhydrous magnesium sulfate was added. Compound 5-2 was obtained in 54% yield. Solution-phase conjugation of 5-2 to compound 5-3 in DMF for 72 h gave 20% of tert-butyl protected compound 5-4. Due to the low overall yield of this method, solid-phase conjugation was then investigated.
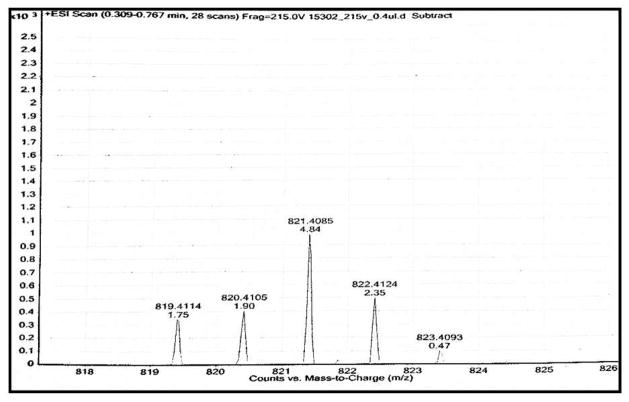
For the solid-phase conjugations, compound 5-1 was prepared by SPPS on two different resins, the Rink amide resin and the Wang resin. The Rink amide resin provided the final conjugate with a C-terminal amide functionality whereas the Wang resin gave product 5-7 with a carboxyl group at the C-terminal (similar to active compound 5-1) that was used in the biological studies. Conjugation of BODIPY 5-3 to compound 5-6 in Et3N and DMF proceeded for a total of 72 h. The progress of the reaction was monitored by mass spectrometry. Once the mass of the conjugation was confirmed, the entire conjugate was cleaved from the resin using cleavage cocktail, dried, and washed with cold ether to remove scavangers. Conjugation of 5-3 to compound 5-6 in Et3N and DMF gave the target conjugate 5-7 in 45% yield after purification by preparative TLC (10% MeOH/90% DCM) (Fig. 1)
Fig. 1.
High resolution mass spectrum (HR-MS) of compound 5-7.
2.2 PLA assay to study protein-protein interaction and its inhibition by compound 5-1
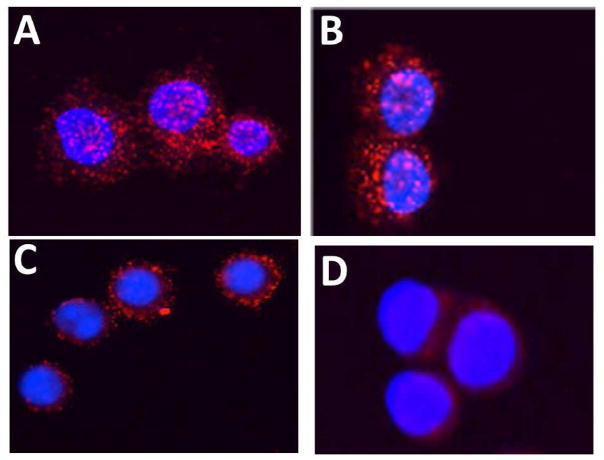
Any protein-protein interaction can be detected by PLA probes if the two proteins are in proximity of less than 16 nm [35]. One of our objectives is to design compounds to inhibit protein-protein interactions of EGFRs, in particular, EGFR-HER2 and HER2-HER3. PLA assay is used to study protein-protein interactions in native cellular environment [35, 36], and thus can be used to study HER2-HER3 interaction and its disruption by compound 5-1. This method provides direct evidence of modulation of HER2-HER3 interaction by peptides we have designed. PLA is an antibody-based method in which two proteins (or antigens) are immunolabeled first with two primary antibodies and then with different species-specific secondary antibodies conjugated to complementary oligonucleotides [36]. When two antibody molecules are in close proximity (<16 nm), the complementary DNA strands can be ligated, amplified, and visualized with a fluorescent probe as distinct fluorescent spots. Each fluorescent spot corresponds to a heterodimer on a breast cancer cell. Using PLA, we could observe HER2-HER3 heterodimers in SKBR3 cells by the probes, seen as red spots. If compound 5-1 inhibits HER2 heterodimerization, there should be no red spots or relatively fewer spots compared to the control. SKBR-3 cells were treated with compounds 5-1 and a control peptide for 24 h. Confocal images of the cells without any compound 5-1 treatment clearly show many HER2-HER3 heterodimers on SKBR-3 cells (Fig. 2A). Treatment with a control peptide did not inhibit HER2-HER3 heterodimers (Fig. 2B). A sub-optimum dose of compound 5-1 did not inhibit HER2-HER3 heterodimerization to a significant extent (Fig. 2C) whereas optimum doses of compound 5-1 at 0.8 μM inhibited HER2-HER3 heterodimerization significantly (Fig. 2D). This clearly suggests that compound 5-1 designed is a potent inhibitor of HER2 heterodimerization with HER3.
Fig. 2.
Inhibition of HER2-HER3 interaction evaluated by PLA assay (Duolink II). A) SKBR-3 cells without any treatment. Red dots indicate heterodimerization of HER2-HER3 proteins. Notice the overexpression and dimerization of HER2 with HER3. B) SKBR-3 cells treated with a control peptide. Notice that HER2-HER3 dimerization is not inhibited. C) and D), 0.5 μM and 0.8 μM compound 5-1 treated SKBR-3 cells. Compound 5-1 inhibited HER2-HER3 dimerization indicated by the significant reduction in the number of red dots.
2.3 Time-dependent inhibition of phosphorylation of HER2 kinase
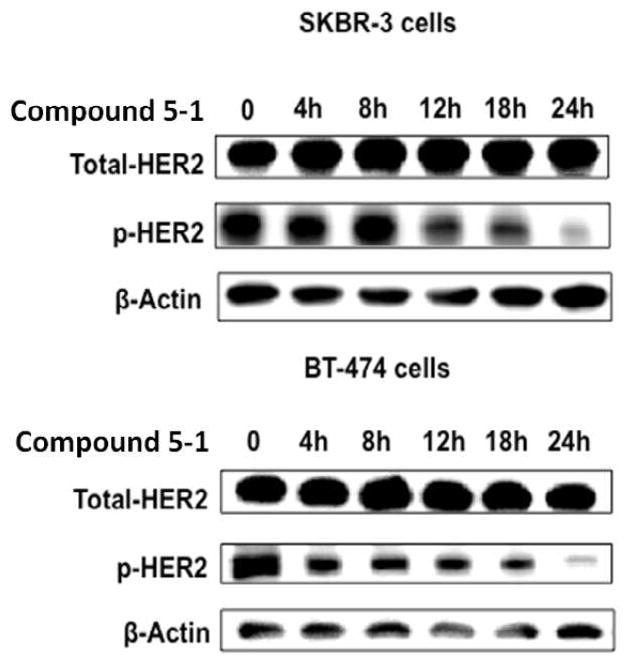
HER2 heterodimerization causes transphosphorylation and activation of the downstream signaling cascade, resulting in cell growth and proliferation [3, 5, 37]. Thus, inhibiting phosphorylation of HER2-mediated dimers plays a major role in inhibiting cell growth and proliferation. To evaluate the effect of compound 5-1 on the dimerization of full-length EGFRs in the native form in HER2-overexpressing breast cancer cell lines, the effect of compound 5-1 on the phosphorylation of HER2 kinase domain in BT-474, SKBR-3 and MCF-7 cells was investigated using Western blot. Inhibition of the receptor heterodimerization or association in cells by compound 5-1 should decrease the phosphorylation level of HER2 protein. Phosphorylation was detected using p-HER2, an antibody that specifically binds to the phosphorylated HER2 protein. Fig. 3 represents the Western blot analysis of the phosphorylation level in treated and untreated BT-474 and SKBR3 cells, respectively. Cells without compound 5-1 and AG-825 (0.38 μM, a kinase inhibitor, not shown) [37, 38] were used as a control. Comparison of the inhibition of phosphorylation and control suggested that compound 5-1 showed significant inhibition of phosphorylation of HER2 in BT-474 and SKBR-3 cells in a time-dependent manner. A significant inhibition of HER2 phosphorylation was observed after 18 h of treatment with compound 5-1. These results clearly suggest that binding of compound 5-1 to ECD affects the intracellular transphosphorylation and, hence, the signaling process. MCF-7 cells that do not show overexpression of HER2 protein were used as a control, and no phosphorylation of HER2 was detected in those cells.
Fig. 3.
Western blot analysis of phosphorylated HER2. BT-474, SKBR3 cells that overexpress HER2 protein upon treatment with compound 5-1 (0.4 μM) showed decreased level of phosphorylated HER2 over time. Total HER2 protein is also shown. A time dependent inhibition of HER2 phosphorylation by compound 5-1 is observed in BT-474 and SKBR3.
2.4 Cellular binding and uptake studies
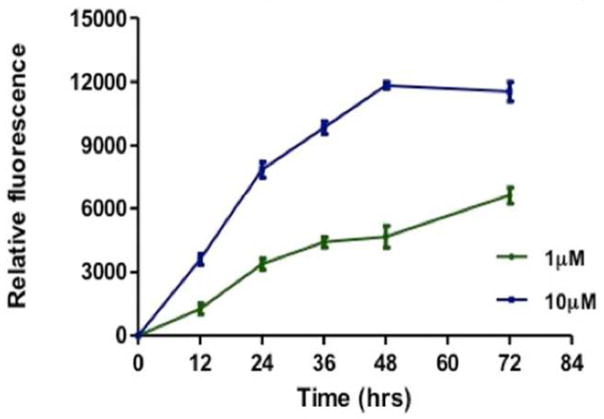
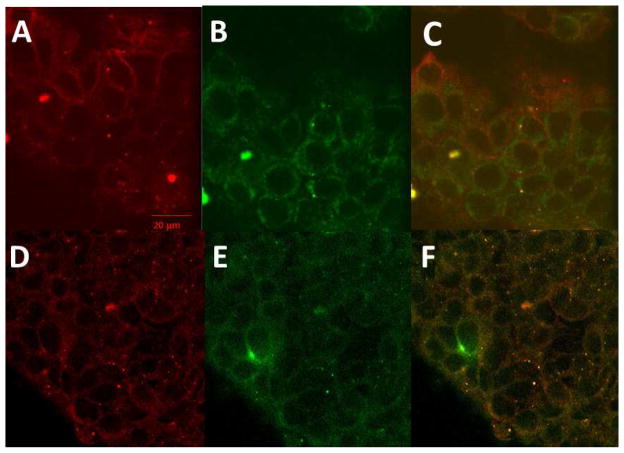
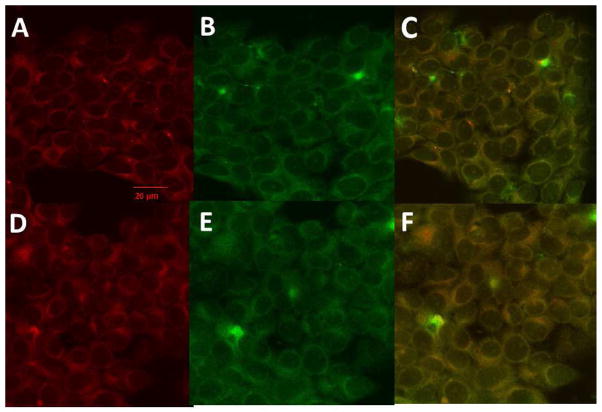
Compound 5-1 has been shown to bind to the HER2 extracellular domain on BT-474 and SKBR-3 cells that overexpress HER2 protein. Once compound 5-1 binds, inhibition of EGFR-HER2 and HER2-HER3 dimerization takes place and, hence, disrupts the phosphorylation. However, the fate of compound 5-1 after the inhibition of PPI of EGFRs is not known. In our earlier report we have shown that compound 5-1 with free carboxy terminal exhibits antiproliferative activity against HER2 overexpressing breast cancer cells [27]. Thus, we investigated the cellular binding and uptake of compound 5-1 by conjugating it with BODIPY (compound 5-7). Time-dependent uptake was monitored by fluorescence emitted from the SKBR-3 cells at two different concentrations of compound 5-7 (1 and 10 μM). At 1 μM concentration of the conjugate, there was a steady increase in fluorescence with respect to time up to 72 h. At 10 μM concentration of the conjugate, saturation was observed after 48 h. (Fig. 4). These data correlate with the time-dependent inhibition of HER2 phosphorylation by compound 5-1 (Fig. 3). To further investigate the importance of the increase in fluorescence and localization of the compound 5-7, cellular uptake was monitored by confocal microscopy using membrane and Lyso trackers. Confocal images suggested that, even after 24 h, the majority of compound 5-7 is on the surface or membrane of SKBR-3 cells (Fig. 5). Images of cells after 48 h of compound 5-7 incubation using Lyso tracker marker show clearly that part of the conjugate is taken up into the cell as indicated in Fig. 6. These data suggest that the compound 5-7 binds to the extracellular domain of HER2 protein on the surface of SKBR-3 cells and possibly is internalized after 48 h. The exact mechanism of internalization is not clear at this stage.
Fig. 4.
Time-dependent cellular binding and uptake of compound 5-7 at two concentrations.
Fig. 5.
Cellular localization of compound 5-7 monitored by membrane tracker. A) Membrane tracker, B) compound 5-7, 24 hr, C) overlap 24 hrs. D) membrane tracker 48 hrs, compound 5-7, 48 hrs, F) Overlap.
Fig. 6.
Cellular localization of compound 5-7 monitored by Lyso tracker. A) Lyso tracker, B) compound 5-7, 24 hr, C) overlap 24 hrs. D) Lyso tracker 48 hrs, compound 5-7, 48 hrs, F) Overlap.
3. Discussion
HER2, also known as ERBB2, is found to overexpress in many epithelial malignancies. It is well established that HER2-mediated heterodimerization plays key roles in the progression of such cancers [1–10, 17]. It has been reported that EGFR-HER2 and HER2-HER3 heterodimers are major contributors to mediating the pathological effects of this overexpression in HER2-overexpressing breast cancer [9, 10, 17]. Inhibition of HER2 heterodimerization contributes significantly to the treatment of HER2-positive breast cancers. Compound 5-1 that we have designed has been shown to bind to the HER2 extracellular domain and inhibit protein-protein interactions. Conjugation of compound 5-1 to BODIPY was achieved using different synthetic strategies. Although conjugation of fluorescent molecules seems to be quite simple using amine coupling methods, conjugation to purified compound 5-1 posed a problem. Hence, compound 5-1 had to be conjugated to the peptide on the resin. The conjugate (compound 5-7) was characterized by mass spectrometry and UV-fluorescence spectroscopy. Cellular uptake studies suggested that the compound 5-7 is taken up by HER2-overexpressing cell lines in 48 h and, at a concentration of 10 μM, saturation in the uptake was observed. At present, the exact mechanism cannot be proposed for this observed result; multiple mechanisms could be possible for the time-dependent uptake of the compound 5-7. As the binding site of compound 5-1 is HER2-domain IV, which is close to the cell membrane, its conjugate, compound 5-7 might be trapped in the cell membrane after dissociation. Finally, after exerting its action, compound 5-7 might internalize into the organelles of the breast cancer cell. To determine the possibility of internalization, cells were incubated with the compound 5-7 for 24 and 48 h and co-localization of compound 5-7 with membrane and Lyso trackers was observed using confocal microscopy. The results clearly suggested that the conjugate stays on the cellular surface up to 24 h, indicating binding to ECD of HER 2 protein. However, after 48 h, there was an indication of internalization of the compound 5-7. EGFR receptors are known to undergo internalization after forming ligand-bound dimers. These dimers are known to be sorted in endosomes and recycled back to the surface and/or undergo endosomal degradation [39–41]. Our results suggest that compound 5-7 bound to HER2 protein is retained extracellularly for up to 24 h and, hence, may not undergo recycling with the receptor. Previous studies have reported that overexpression of HER2 and EGFR-HER2 heterodimerization completely abolishes the EGFRs degradation process [42]. However, recent literature suggested that HER2 overexpression and heterodimerization slows down EGFR internalization and degradation [39–41]. Results from our studies suggested that compound 5-7 that binds to the HER2 extracellular domain is retained extracellularly for 24 h, which correlates with a slowdown of the internalization process of EGFR heterodimers. To show that compound 5-1 inhibits protein-protein interaction, we carried out PLA assay. Those results clearly suggest that inhibition of dimerization of extracellular domains of HER2 and HER3 results in inhibition of PPI of HER2-HER3. Full-length EGFRs have several regions of contact for PPI when they form homo- and heterodimers [3, 5, 9]. Domain II, the dimerization arm, domain IV, the transmembrane domain, and the kinase domain all form contacts for PPI of EGFRs. Among these, domain II interactions are well understood in EGFR homodimers, and the antibody pertuzumab, which binds to domain II of HER2 protein, inhibits PPI of EGFRs [14]. The interaction of domain IV is not well understood. The literature suggests that domain IV may be involved in stabilizing the PPI of EGFRs. However, the contribution of domain IV to PPI is very weak. Studies also suggest that domain IV helps to align the transmembrane domains [43]. The crystal structures of homodimers of EGFR suggest that hydrophobic residues such as Leu582, Trp584, and Tyr602 participate in PPI of domain IV to stabilize the interaction of homodimers of EGFR near the transmembrane [26]. The results from our current and previous studies [28, 30] clearly suggest that a peptidomimetic that we designed targets domain IV and inhibits PPI in vitro as seen in the PLA assay. This inhibition in turn has the pharmacological action of inhibition of phosphorylation of the kinase domain and, hence, modulation of the signal for cell growth. Furthermore, inhibition of the extracellular domain of HER2-HER3 inhibits the phosphorylation of HER2 protein in a time-dependent manner. Western blot analysis of HER2 phosphorylation showed maximum inhibition of HER2 phosphorylation after 24 h of treatment. We have also shown that conjugation of a peptidomimetic that binds to domain IV of HER2 with a fluorescent label such as BODIPY helps us to study the molecular mechanism of the signal transduction process. These studies taken together suggest that a compound 5-7 could be used as a tool to study the binding and internalization of EGFR receptors and their heterodimerization. The data presented here provide a new tool to study the EGFRs PPI inhibition and EGFR trafficking.
4. Conclusions
A peptidomimetic (compound 5-1) that binds to HER2 protein extracellular domain and inhibits protein-protein interactions of EGFRs was conjugated with the fluorescent dye BODIPY. Synthesis was achieved with conjugation on solid-phase synthesis. PPI inhibition activity of the compound was evaluated by proximity ligation assay. The PLA assay suggested that the compound inhibits HER2-HER3 heterodimerization in lower micromolar concentrations effectively. The downstream signaling effect of PPI inhibition was evaluated by time-dependent phosphorylation by compound 5-1. Compound 5-1 inhibited phosphorylation significantly within 18 to 24 h. To evaluate the effect of compound on the PPI of EGFR and the fate of the compound after PPI inhibition, cellular uptake of the newly synthesized BODIPY conjugate of compound 5-1 (compound 5-7) was studied by fluorescence plate reader assay and confocal microscopy with organelle tracers. Compound 5-7 seems to reside in the extracellular region or in the membrane for up to 24 h; at 48 h, there was an indication of internalization. The internalization was viewed in terms of EGFR trafficking. However, more detailed studies of the kinetics of EGFR trafficking are necessary to understand the receptor internalization and recycling to the surface of cells using fluorescent conjugates. Thus, the conjugates designed here will be useful tools for studying EGFR trafficking and the effect of inhibition of HER2 heterodimerization on EGFR trafficking and ligand receptor interactions.
5. EXPERIMENTAL PROCEDURES
5.1 General information
All chemicals, biochemicals and solvents were purchased from commercial sources. All peptide synthesis reagents were purchased from Fisher Scientific or Sigma Aldrich as ACS grade or peptide synthesis grade solvents. Amino acids were purchased from AnaSpec (Fremont, CA) or Applied Biosystems (Carlsbad, CA). Beta-amino acid, Fmoc-3-amino-3-(1-naphthyl)-propionic acid]-OH, was purchased from Chem-Impex International (Wood Dale, IL). Analytical thin-layer chromatography (TLC) was carried out using polyester backed TLC plates 254 (precoated, 200 μm) from Sorbent Technologies. NMR spectra were recorded on an AV-400 LIQUID Bruker spectrometer (400 MHz for 1H). 2D TOCSY spectra of compounds 5-1 and 5-7 were recorded in 500 and 700 MHz Varian NMR spectrometers respectively. The chemical shifts are reported in δ ppm using the following deuterated solvents as internal references: acetone-d6 2.05 ppm (1H), DMF-d7 8.03 ppm (1H), H2O 90%/D2O 10%. HPLC analyses were carried out on a Dionex system equipped with a P680 pump and UVD340U detector. MALDI-TOF mass spectra were recorded on a Bruker ProFlex III mass spectrometer using dithranol as the matrix or Bruker UltrafleXtreme (MALDI-TOF/TOF) using 4-chloro-α-cyanocinnamic acid as the matrix; ESI mass spectra were obtained on an Agilent Technologies 6210 time-of-flight LC/MS with a quaternary gradient module pump, 2489 UV-visible detector, and fraction collector III. Analytical HPLC was carried out using a XBridge C18 300 Å, 5 μm, 4.6 mm × 250 mm column (Waters, USA) and a stepwise gradient. Semipreparative HPLC was carried out using a XBridge C18 300 Å, 5 μm, 10 mm × 250 mm column (Waters, USA) and a stepwise gradient. The solvent system for peptides consisted of Millipore water and HPLC-grade acetonitrile/methanol. Antibodies for Western blot analysis were obtained from Abcam, Inc. (Cambridge, MA). Antibodies and wash buffers A and B for PLA were obtained from Axxora, LLC (Farmingdale, NY) and nanoTools (San Diego, CA). Control peptide was custom synthesized by Polypeptide laboratories (San Diego, CA).
5.2 Chemistry
5.2.1 Synthesis of compound 5-1
The peptidomimetic (compound 5-1) was synthesized by different methods to determine the efficiency of conjugation. Conjugation of BODIPY to compound 5-1 was attempted using different methods: 1) conjugation to derivative compound 5-2 in solution phase (Scheme 1), 2) solid-phase conjugation using on Wang resin and on Rink amide resin. Since biological activity of compound 5-7 sythesized using Wang resin was evaluated. Details of synthesis of Rink amide resin based conjugate is provided elsewhere (Ph.D dissertation, Alecia McCall, Louisiana State University April 2012).
Peptidomimetic compound 5-1 was synthesized by manual solid-phase synthesis using standard procedures[44]. The peptide was synthesized on Wang resin on a 0.2 mmol scale using the Fmoc strategy of solid-phase peptide synthesis. A four fold excess of the L-Fmoc-protected amino acids was coupled using PyAOP/HOBT or HATU as the coupling and activating agents. Resins were allowed to swell in DMF for 1 h, and the Fmoc-protecting group was removed from the resin. For coupling Arg to the growing peptide chain, double coupling was performed. The Fmoc-protecting group was cleaved with 93% DMF, 5% piperidine, and 2% DBU once for 3 min, once for 5 min, and once for 10 min. This was followed by washing the peptide several times with DMF after which the next amino acid was coupled. The Fmoc group from phenylalanine was not cleaved until the peptide was ready for use. The peptide was then washed with DMF (3 × 30 s) and DCM (3 × 30 s), respectively, and dried under high vacuum for 24 h. The peptide was then cleaved using a cleavage cocktail consisting of TFA/liquefied phenol/water/Tris (88:5:5:2) for 5 h. This cocktail was then precipitated into anhydrous ethyl ether (−80 °C) and centrifuged. The precipite was washed three times with anhydrous ethyl ether (−80 °C) and then dissolved in water and acetonitrile and lyophilized. Reversed-phase HPLC analysis, mass spectrometry, and NMR studies were performed to evaluate the purity of compound 5-1. High resolution mass spectrum and 1H 2D NMR of compound 5-1 with assignments are provided as supporting information.
5.2.2 Synthesis of tert-butyl Peptomimetic (5-2)
In a small round bottom flask, anhydrous magnesium sulfate (0.232 mg, 0.00193 mmol) in 3 mL of dry DCM was stirred vigorously for 10 min. Excess concentrated sulfuric acid (92.00 mg, 0.9380 mmol) was added to the flask. The mixture was stirred for 1 h. Compound 5-1 (0.25 mg, 4.82×10−4 mmol) was then added to the mixture (Scheme 1). The flask was sonicated to ensure solubility and stirred for an additional 5 min before adding tertiary butanol (0.228 mL, 0.00241 mmol). The flask was stoppered and stirred for 24 h at 25 °C under N2 gas. The reaction was quenched with saturated sodium bicarbonate (3 mL) by adding this to the reaction flask until all of the magnesium sulfate had dissolved. The organic phase was separated, washed with brine, dried with Na2SO4, and purified by preparative TLC to afford the t-butyl protected compound (5-2) in 54% yield (0.15 mg). 1H NMR (400 MHz, acetone-d6) δ ppm 1.28 (br, 9H), 1.51–1.62 (m, 2H), 1.79 (m, 2H), 3.30 (m, 2H), 4.50–4.63 (m, 1H), 5.34 ( 1H), 6.24–6.31 (m, 1H), 7.00–7.10 (m 5H), 7.15 (1H), 7.32 (1H) 7.31 (d, J=4.26 Hz 1H), 7.42–7.80 (m 7H), 7.93 (d, J=7.31 Hz 1H), 8.15 (br, 1H), 8.22 (m, 1H). Arg side chain guanidine group protons (4H) were broad, and phenyl alanine beta protons (2H) were under the solvent peak around 3 ppm, and hence could not be identified. MS (MALDI-TOF): m/z 597.428. Calcd. for C32H42N6O4: 597.316 [M+Na].
5.2.3 Synthesis of BODIPY succinimidyl ester tert-butyl Arg-[3-amino-3-(1-napthyl)-propionic acid]-Phe (5-4) (Scheme 1)
Compound 5-3 was purchased from Life Technologies (Carlsbad, CA). To a small round bottom flask was added 5-3 (0.826 mg, 0.00198 mmol) in peptide synthesis grade DMF (1 mL). Compound 5-3 was sonicated to ensure solubility. Six (6) equivalents of triethylamine (1.65 μL, 0.011 mmol) were added to the flask. Compound 5-3 was activated for 4.5 h under inert conditions (N2 gas) before compound 5-2 (2.3 mg, 0.00198 mmol) in 0.5 mL peptide synthesis grade DMF was added to the flask. Conjugation took place for 72 h under nitrogen. Confirmation of mass was observed by MS (ESI-MS): m/z 820.737. Calcd. for C44H51BF2N8O5 820.734 [M-tBu].
5.2.4 Conjugation of BODIPY 5-3 to Arg-[3-amino-3-(1-napthyl)-propionic acid]-Phe on Wang Resin (5-7)
Synthesis of compound 5-7 (Scheme 2) was achieved by routine solid-phase peptide synthesis. To a reaction vessel compound 5-3 (0.0021 g, 0.0050 mmol) was added and solubilized in 0.9 mL DMF. Triethylamine (0.006 mL, 0.043 mmol) was added to the vessel and stirred to activate the ester (Scheme 2). The reaction continued for 1 h. This solution was added to the peptide synthesis chamber containing 5-6 (0.0050 g, 0.00479 mmol) (Scheme 2). The reaction proceeded with shaking for approximately 72 h under N2. The beads were then washed with DMF 4 times until the filtrate was colorless to remove residual compound 5-3. A cleavage cocktail consisting of 1 mL of TFA (88%), liquefied phenol (5%), triisopropylsilane (2%), and water (5%) was added to the resin beads for 4 h. The resin was filtered and washed with TFA (3 × 1 mL). Filtrates were combined, evaporated, and washed with cold anhydrous ether. Purification was conducted by preparative TLC (10% MeOH/90% DCM) and analytical HPLC (ACN/H2O) to yield 45%–65% of conjugate. 1H NMR (700 MHz, DMF-d7) δ ppm 8.75 (br, 1H), 8.14 (d, J=7.0Hz 1H), 7.99 (d, J= 7.52 1H), 7.88 (br, 1H), 7.60–7.80 (m, 4H), 7.31(br, 1H), 7.24(m, 3H), 6.80–7.00(m,6H), 6.41–6.62(br. 2H), 6.30(br, 1H), 6.00 (br, 1H), 4.45(m, 1H), 4.31 (m, 1H), 3.21–3.42(br, 6H), 2.80–2.84 (m, 3H), 2.42(m, 3H), 1.90–2.00 (m, 2H), 1.61–1.77 (m, 3H), 1.45–1.50 (m, 3H), 1.21–1.60 (m, 6H). C-terminal carboxylic acid proton were broad and were not observed. MS (ESI-TOF): m/z 821.408. Cald. for C44H51BF2N8O5 821.411 [M+H] (Fig. 1). UV-vis (DMSO) λmax (nm) (ε, L mol−1 cm−1): 485 nm (4.49E+05), 351 (5.90E+05).
5.3 Proximity ligation assay
Proximity ligation assays (PLA) were performed on breast cancer cells SKBR-3 that overexpress HER2 protein [35, 36]. PLA assay on breast cancer cells was performed by coating 5 × 104 cells onto each well in an 8-chambered plate. The plate was incubated for 24 h at 37 °C. Compound 5-1 was added at two different concentrations (0.5 and 0.8 μM). A control peptide (supporting information) was also added at 1 μM concentration. After 24 h, the medium was removed and the wells were washed 3 times with 500 μL of PBS. The cells were fixed by adding 500 μL of ice-cold methanol for 5–8 min and incubating the plate at −20 °C. The methanol was discarded and immediately 500 μL of PBS was added twice to the wells to prevent cell dehydration. The cells were then blocked with 200 μL of 5% BSA for 1 h in a humidity chamber. The blocking solution in the wells was discarded, and 200 μL of 1:400 dilutions of primary antibodies (both HER2 (anti-rabbit) and HER3 (anti-mouse)) in 2% BSA were added to the wells. The 8-chambered well plate was then incubated at 4 °C in a humidity chamber overnight. Primary antibodies were discarded and the plate was washed twice with wash buffer (from PLA kit) for 5 min. To the cells, two PLA probes (anti-mouse and anti-rabbit) at 1:5 dilution in 2% BSA were added and incubated for 1 h at 37 °C in a humidity chamber. Slides were washed with wash buffer for 5 min twice, and ligation stock at 1:5 dilution in high purity water was added and incubated for 30 min at 37 °C. The slides were washed again with wash buffer, amplification solution was added, and the slide was incubated for 2 h at 37 °C. Slides were then washed twice with wash buffer for 10 min. The slides were dried at room temperature, mounted with mounting medium (Duolink II), and stored at −20 °C until confocal images were taken. Cells were viewed with a confocal microscope (LSM 5 PASCAL). Images were taken at 40X as wells and at 60X under oil immersion.
5.4 Time-dependent inhibition of phosphorylation of HER2 kinase
BT-474 cells were maintained in RPMI-1640 supplemented with pen-strep 1 mL/100 mL, insulin 1 mg/100 mL, and 10% FBS. SKBR-3 cells were maintained in McCoy’s medium supplemented with penstrep 1 mL/100 mL, insulin 1 mg/100 mL, and 10% FBS. After confluency, cells were trypsinized and plated in T-75 flasks in triplicate. After 24 h, the cells were either treated with compound 5-1 (0.4 μM) or left untreated. Cells with compounds were incubated at 37 °C and 5% CO2. After the incubation period, cells were trypsinized, collected in 15 mL tubes, and centrifuged at 200 ×g. To the pellet, 200 μL of cell lysis buffer containing 20 μL of protease inhibitor and 20 μL of phosphatase inhibitor was added. Tubes were vortexed for 1 min and then kept on ice for 10 min. This process was repeated twice. Cells were subjected to centrifugation at high speed (450 ×g) and the supernatant was collected in separate tubes and stored at −80 °C until further use. Protein concentration in the tubes was analyzed using Bradford’s assay. 40 μg of protein from each sample was loaded on 10% SDS-polyacrylamide minigels. The gels were run at 30 mA, 125 V and then transferred at 30 V for 12–16 h at 4 °C onto a nitrocellulose membrane. After transfer, the membranes were blocked with 5% milk for 1 h. The nitrocellulose membranes were then probed with specific primary antibodies against p-HER2 diluted 1:3000 in 5% milk for 15 h at 4 °C. After incubation with primary antibody, the membranes were washed five times with PBS and then incubated with secondary antibody to p-HER2 (anti-rabbit secondary antibody) diluted 1:2000 in 5% milk with 0.001% Tween 20 for 1 h. The membranes were washed five times with PBS, and proteins were visualized using the Super Signal enhanced chemiluminescence kit (Pierce, Rockford, IL) [37]. The Kodak Gel Logic-1500 imaging system (Carestream Molecular Imaging, New Haven, CT) was used to visualize the luminescent proteins. All experiments were repeated at least three times. A representative Western blot image was used for the final representation. The visualization of β-actin was used to ensure equal sample loading in each lane. Total HER2 protein was detected using an antibody to the HER2 extracellular domain (Abcam, Cambridge, MA).
5.5 Binding and cellular uptake of compound 5-7
Peptidomimetic compound 5-1 was conjugated with BODIPY dye to study its interaction with HER2 protein. SKBR-3 cells were plated at 10,000 per well in a 96-well plate and allowed to grow for 24 h. Compound 5-7 stock solution was diluted to 1 and 10 μM in McCoy’s medium and added to the cells. Compound 5-7 was added to the 96-well plates coated with SKBR3, and the plates were incubated at 37 °C. After time points of 0, 12, 24, 36, 48 and 72 h, the medium with compound 5-7 in the plates was removed, and the cells were washed with PBS twice. The fluorescence of compound 5-7 was determined by reading its fluorescence emission at 490 nm/530 nm (excitation/emission) using a BioTek plate reader (Cary, NC). Similar experiments were conducted using chamber slides, and images of cells with fluorescence were obtained using a confocal microscope. To the 8-well plates coated with SKBR-3, compound 5-7 at a concentration of 0.4 μM was added, and the plates were incubated at 37 °C. After time points of 24 and 48 h, the medium with compound 5-7 in the plates was removed, and the cells were washed with PBS twice. The cells were then fixed by adding 500 μL of ice-cold methanol and incubating the plate at −20 °C for 5 min. The methanol was discarded and immediately 500 μL of PBS was added twice to the wells to prevent cell dehydration. Membrane and lyso trackers were diluted in serum-free medium and added to the wells treated with compound 5-7 as well as to the wells without any treatment. The plates were incubated for 5 min (membrane tracker) and 30 min (Lyso tracker) at 37 °C. After the incubation period, the wells were washed with PBS twice, dried, and mounted with mounting medium for imaging [45]. Cells were viewed with a confocal microscope (LSM 5 PASCAL). Images were taken at 40X under oil immersion with argon and HeNe lasers. Images were analyzed with ZEN 2009 software.
Supplementary Material
Highlights.
A peptidomimetic targeting HER2 protein was conjugated with fluorescent label BODIPY.
Cellular uptake of the conjugate was studied by confocal microscopy
Inhibition of protein-protein interaction was studied by proximity ligation assay
Designed compound inhibited HER2-HER3 interaction and inhibited phosphorylation
Fluorescent conjugate designed is useful to study EGFR trafficking
Acknowledgments
Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103424 and Louisiana Board of Regents (NSF(2010)-PFUND-219) (SJ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The authors thank Dr. Karen Briski, Chair, Department of Basic Pharmaceutical Sciences, College of Pharmacy at ULM for the use of confocal microscopy facility. Department of Chemistry, Louisiana State University, Baton Rouge, LA, is acknowledged for mass and NMR spectra of compounds. Authors would like to thank Ms. Nancy Harmony for help with editing the manuscript.
ABBREVIATIONS USED
- ACN
acetonitrile
- BODIPY
4,4-difluoro-4-bora-3a,4a-diaza-s-indacene
- BSA
bovine serum albumin
- ECD
extracellular domain
- 6-Cl-HOBt
6-chloro-1-hydroxy-1H-benzotriazole
- DMF
dimethylformamide
- DCM
dichloromethane
- DBU
1,8-diazabicyclo[5.4.0]undec-7-ene
- EGFR
Epidermal growth factor receptor
- Et3N
triethylamine
- HATU
O-(7-Azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate
- HER
human epidermal growth factor receptor
- HRMS (ESI)
high-resolution mass spectrometry (electro-spray ionization)
- LC/MS
liquid chromatography-mass spectrometry
- MALDI-TOF
matrix-assisted laser desorption/ionization-time of flight
- MeOH
methanol
- PBS
phosphate-buffered saline
- p-HER2
phosphorylated HER2
- PLA
proximity ligation assay
- PPI
protein-protein interactions
- PyAOP
7-azabenzotriazol-1-yloxy) tripyrrolidinophosphonium hexafluorophosphate
- SDS
sodium dodecyl sulfate
- SPSS
solid-phase peptide synthesis
- TFA
trifluoroacetic acid
Footnotes
Supporting Information Available: Schematic diagram of compound 5-1, 5-5, control peptide, elemental analysis, mass spectra, HPLC of compounds, 1H 2D TOCSY NMR spectra and fragmentation data from mass spectral analysis of the compounds.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nature reviews Cancer. 2005;5:341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 2.Dougherty U, Sehdev A, Cerda S, Mustafi R, Little N, Yuan W, Jagadeeswaran S, Chumsangsri A, Delgado J, Tretiakova M. Epidermal growth factor receptor controls flat dysplastic aberrant crypt foci development and colon cancer progression in the rat azoxymethane model. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:2253–62. doi: 10.1158/1078-0432.CCR-07-4926. [DOI] [PubMed] [Google Scholar]
- 3.Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, Lemmon MA, Sliwkowski MX, Ward CW, Yokoyama S. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Molecular cell. 2003;12:541–52. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson KM. Active and inactive conformations of the epidermal growth factor receptor. Biochemical Society transactions. 2004;32:742–5. doi: 10.1042/BST0320742. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson KM. Structure-based view of epidermal growth factor receptor regulation. Annual review of biophysics. 2008;37:353–73. doi: 10.1146/annurev.biophys.37.032807.125829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson JP, Berger MB, Lin CC, Schlessinger J, Lemmon MA, Ferguson KM. Epidermal growth factor receptor dimerization and activation require ligand-induced conformational changes in the dimer interface. Molecular and cellular biology. 2005;25:7734–42. doi: 10.1128/MCB.25.17.7734-7742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landgraf R. HER2 therapy. HER2 (ERBB2): functional diversity from structurally conserved building blocks. Breast cancer research : BCR. 2007;9:202. doi: 10.1186/bcr1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao RH, I, Maruyama N. All EGF(ErbB) receptors have preformed homo- and heterodimeric structures in living cells. Journal of cell science. 2008;121:3207–17. doi: 10.1242/jcs.033399. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nature reviews Cancer. 2009;9:463–75. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 10.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, Sliwkowski MX, Stern HM. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer research. 2008;68:5878–87. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 11.Ekerljung L, Lindborg M, Gedda L, Frejd FY, Carlsson J, Lennartsson J. Dimeric HER2-specific affibody molecules inhibit proliferation of the SKBR-3 breast cancer cell line. Biochemical and biophysical research communications. 2008;377:489–94. doi: 10.1016/j.bbrc.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 12.Nahta R, Esteva FJ. HER-2-targeted therapy: lessons learned and future directions. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:5078–84. [PubMed] [Google Scholar]
- 13.Chang JC. HER2 inhibition: from discovery to clinical practice. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:1–3. doi: 10.1158/1078-0432.CCR-06-2405. [DOI] [PubMed] [Google Scholar]
- 14.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer cell. 2004;5:317–28. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 15.Kumagai T, Katsumata M, Hasegawa A, Furuuchi K, Funakoshi T, Kawase I, Greene MI. Role of extracellular subdomains of p185c-neu and the epidermal growth factor receptor in ligand-independent association and transactivation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9220–5. doi: 10.1073/pnas.1633546100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nahta R, Esteva FJ. Herceptin: mechanisms of action and resistance. Cancer letters. 2006;232:123–38. doi: 10.1016/j.canlet.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 17.Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nature reviews Clinical oncology. 2012;9:16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 18.Menard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003;22:6570–8. doi: 10.1038/sj.onc.1206779. [DOI] [PubMed] [Google Scholar]
- 19.Lyakhov I, Zielinski R, Kuban M, Kramer-Marek G, Fisher R, Chertov O, Bindu L, Capala J. HER2- and EGFR-specific affiprobes: novel recombinant optical probes for cell imaging. Chembiochem : a European journal of chemical biology. 2010;11:345–50. doi: 10.1002/cbic.200900532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C, Peng J, Xia HS, Yang GF, Wu QS, Chen LD, Zeng LB, Zhang ZL, Pang DW, Li Y. Quantum dots-based immunofluorescence technology for the quantitative determination of HER2 expression in breast cancer. Biomaterials. 2009;30:2912–8. doi: 10.1016/j.biomaterials.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Berezov A, Chen J, Liu Q, Zhang HT, Greene MI, Murali R. Disabling receptor ensembles with rationally designed interface peptidomimetics. The Journal of biological chemistry. 2002;277:28330–9. doi: 10.1074/jbc.M202880200. [DOI] [PubMed] [Google Scholar]
- 22.Dakappagari NK, Lute KD, Rawale S, Steele JT, Allen SD, Phillips G, Reilly RT, Kaumaya PT. Conformational HER-2/neu B-cell epitope peptide vaccine designed to incorporate two native disulfide bonds enhances tumor cell binding and antitumor activities. The Journal of biological chemistry. 2005;280:54–63. doi: 10.1074/jbc.M411020200. [DOI] [PubMed] [Google Scholar]
- 23.Ren G, Zhang R, Liu Z, Webster JM, Miao Z, Gambhir SS, Syud FA, Cheng Z. A 2-helix small protein labeled with 68Ga for PET imaging of HER2 expression. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009;50:1492–9. doi: 10.2967/jnumed.109.064287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang RY, Yang KS, Pike LJ, Marshall GR. Targeting the dimerization of epidermal growth factor receptors with small-molecule inhibitors. Chemical biology & drug design. 2010;76:1–9. doi: 10.1111/j.1747-0285.2010.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH, Saito K, Sakamoto A, Inoue M, Shirouzu M. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110:775–87. doi: 10.1016/s0092-8674(02)00963-7. [DOI] [PubMed] [Google Scholar]
- 26.Lu C, Mi LZ, Grey MJ, Zhu J, Graef E, Yokoyama S, Springer TA. Structural evidence for loose linkage between ligand binding and kinase activation in the epidermal growth factor receptor. Molecular and cellular biology. 2010;30:5432–43. doi: 10.1128/MCB.00742-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satyanarayanajois S, Villalba S, Jianchao L, Lin GM. Design, synthesis, and docking studies of peptidomimetics based on HER2-herceptin binding site with potential antiproliferative activity against breast cancer cell lines. Chemical biology & drug design. 2009;74:246–57. doi: 10.1111/j.1747-0285.2009.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banappagari S, Ronald S, Satyanarayanajois SD. A conformationally constrained peptidomimetic binds to the extracellular region of HER2 protein. Journal of biomolecular structure & dynamics. 2010;28:289–308. doi: 10.1080/07391102.2010.10507360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banappagari S, Ronald S, Satyanarayanajois SD. Structure-activity relationship of conformationally constrained peptidomimetics for antiproliferative activity in HER2-overexpressing breast cancer cell lines. MedChemComm. 2011;2:752–759. doi: 10.1039/C1MD00126D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banappagari S, Corti M, Pincus S, Satyanarayanajois S. Inhibition of protein-protein interaction of HER2-EGFR and HER2-HER3 by a rationally designed peptidomimetic. Journal of biomolecular structure & dynamics. 2012;30:594–606. doi: 10.1080/07391102.2012.687525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loudet A, Burgess K. BODIPY dyes and their derivatives: syntheses and spectroscopic properties. Chemical reviews. 2007;107:4891–932. doi: 10.1021/cr078381n. [DOI] [PubMed] [Google Scholar]
- 32.Bergstrom F, Mikhalyov I, Hagglof P, Wortmann R, Ny T, Johansson LB. Dimers of dipyrrometheneboron difluoride (BODIPY) with light spectroscopic applications in chemistry and biology. Journal of the American Chemical Society. 2002;124:196–204. doi: 10.1021/ja010983f. [DOI] [PubMed] [Google Scholar]
- 33.Ulrich G, Ziessel R, Harriman A. The chemistry of fluorescent bodipy dyes: versatility unsurpassed. Angewandte Chemie. 2008;47:1184–201. doi: 10.1002/anie.200702070. [DOI] [PubMed] [Google Scholar]
- 34.Gresser R, Hummert M, Hartmann H, Leo K, Riede M. Synthesis and characterization of near-infrared absorbing benzannulated aza-BODIPY dyes. Chemistry. 2011;17:2939–47. doi: 10.1002/chem.201002941. [DOI] [PubMed] [Google Scholar]
- 35.Trifilieff P, Rives ML, Urizar E, Piskorowski RA, Vishwasrao HD, Castrillon J, Schmauss C, Slattman M, Gullberg M, Javitch JA. Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. BioTechniques. 2011;51:111–8. doi: 10.2144/000113719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fredriksson S, Gullberg M, Jarvius J, Olsson C, Pietras K, Gustafsdottir SM, Ostman A, Landegren U. Protein detection using proximity-dependent DNA ligation assays. Nature biotechnology. 2002;20:473–7. doi: 10.1038/nbt0502-473. [DOI] [PubMed] [Google Scholar]
- 37.Gijsen M, King P, Perera T, Parker PJ, Harris AL, Larijani B, Kong A. HER2 phosphorylation is maintained by a PKB negative feedback loop in response to anti-HER2 herceptin in breast cancer. PLoS biology. 2010;8:e1000563. doi: 10.1371/journal.pbio.1000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–8. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 39.Hendriks BS, Opresko LK, Wiley HS, Lauffenburger D. Quantitative analysis of HER2-mediated effects on HER2 and epidermal growth factor receptor endocytosis: distribution of homo- and heterodimers depends on relative HER2 levels. The Journal of biological chemistry. 2003;278:23343–51. doi: 10.1074/jbc.M300477200. [DOI] [PubMed] [Google Scholar]
- 40.Hendriks BS, Opresko LK, Wiley HS, Lauffenburger D. Coregulation of epidermal growth factor receptor/human epidermal growth factor receptor 2 (HER2) levels and locations: quantitative analysis of HER2 overexpression effects. Cancer research. 2003;63:1130–7. [PubMed] [Google Scholar]
- 41.Hendriks BS, Wiley HS, Lauffenburger D. HER2-mediated effects on EGFR endosomal sorting: analysis of biophysical mechanisms. Biophysical journal. 2003;85:2732–45. doi: 10.1016/s0006-3495(03)74696-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Worthylake R, Opresko LK, Wiley HS. ErbB-2 amplification inhibits down-regulation and induces constitutive activation of both ErbB-2 and epidermal growth factor receptors. The Journal of biological chemistry. 1999;274:8865–74. doi: 10.1074/jbc.274.13.8865. [DOI] [PubMed] [Google Scholar]
- 43.Saxon ML, Lee DC. Mutagenesis reveals a role for epidermal growth factor receptor extracellular subdomain IV in ligand binding. The Journal of biological chemistry. 1999;274:28356–62. doi: 10.1074/jbc.274.40.28356. [DOI] [PubMed] [Google Scholar]
- 44.Coin I, Beyermann M, Bienert M. Solid-phase peptide synthesis: from standard procedures to the synthesis of difficult sequences. Nature protocols. 2007;2:3247–56. doi: 10.1038/nprot.2007.454. [DOI] [PubMed] [Google Scholar]
- 45.Ongarora BG, Fontenot KR, Hu X, Sehgal I, Satyanarayana-Jois SD, Vicente MG. Phthalocyanine-peptide conjugates for epidermal growth factor receptor targeting. Journal of medicinal chemistry. 2012;55:3725–38. doi: 10.1021/jm201544y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.