Abstract
A novel bilirubin oxidase (BOD), from the rice blast fungus Magnaporthe oryzae, has been identified and isolated. The 64-kDa protein containing four coppers was successfully overexpressed in Pichia pastoris and purified to homogeneity in one step. Protein yield is more than 100 mg for 2 L culture, twice that of Myrothecium verrucaria. The kcat/Km ratio for conjugated bilirubin (1,513 mM −1 s−1) is higher than that obtained for the BOD from M. verrucaria expressed in native fungus (980 mM−1 s−1), with the lowest Km measured for any BOD highly desirable for detection of bilirubin in medical samples. In addition, this protein exhibits a half-life for deactivation >300 min at 37 °C, high stability at pH 7, and high tolerance towards urea, making it an ideal candidate for the elaboration of biofuel cells, powering implantable medical devices. Finally, this new BOD is efficient in decolorizing textile dyes such as Remazol brilliant Blue R, making it useful for environmentally friendly industrial applications.
Keywords: Bilirubin oxidase, Pichia pastoris, Oxygen reduction, Biofuel cells, Magnaporthe oryzae, Decolorization of dyes, Bilirubin detection
Introduction
Multicopper oxidases (MCOs), one of only a few enzyme classes capable of reducing O2 to water, have been widely studied because of their utilization in biotechnological applications such as pulp bleaching, decolorization of dye, polymer synthesis, biofuel cells and biosensors, detoxification, and bioremediation (Barton et al. 2004; Mayer and Staples 2002; Xu 1996). Examples of MCOs include ascorbate oxidase (EC 1.10.3.3) (Hirose et al. 1994; Kawahara et al. 1985; Messerschmidt et al. 1989), ceruloplasmin (EC 1.16.3.1) (Zaitsev et al. 1999; Zaitseva et al. 1996), bilirubin oxidase (BOD) (EC 1.3.3.5) (Hiromi et al. 1992; Kataoka et al. 2005), and laccase (EC 1.10.3.2) (Giardina et al. 2010; Han et al. 2005), all employing a minimum of four Cu ions for catalytic activity. The four Cu’s are classified into three types, based on their optical and magnetic properties. Type I (T1) blue Cu shows an intense Cys to Cu (II) charge transfer band near 600 nm. This copper center accepts electrons from the electron-donating substrates including phenols, ascorbate, bil- irubin, and various metal ions and relays these to the O2 reduction site (Solomon et al. 2001). The latter is a trinuclear cluster (TNC), consisting of a paramagnetic type II (T2) copper ion and a type III (T3) pair of cupric ions with a characteristic 330-nm absorption shoulder.
Laccases, the most studied sub-group of MCOs, have been the enzyme of choice for industrial applications, primarily due to their broad substrate specificity, ease of cloning and expression, and high catalytic activity. More recently, BODs have attracted attention because, unlike laccases, these enzymes have high activity at neutral pH, high thermal tolerance, and are capable of oxidizing bilirubin.
In addition to more traditional laccase substrates like 2,2′-azinodi-[3-ethylbenzthiazoline-6-sulphonic acid] (ABTS) and syringaldazine (SGZ), BODs catalyze the oxidation of bilirubin to biliverdin (Eq. 1) and to a yet unidentified purple pigment (Koikeda et al. 1993; Murao and Tanaka 1981), hence the classification of BOD.
| (1) |
The detection of bilirubin is of particular importance in the medical field, making BODs attractive enzymes in that respect. Bilirubin is a bile pigment, a metabolic product of heme formed from the degradation of erythrocytes by reticuloendothelial cells. The typical biological form of bilirubin exits in serum either in free form, unconjugated bilirubin, or in conjugated form. The conjugated form results from the transformation of the unconjugated bilirubin in the liver by the hepatic enzyme glucuronyl transferase. Conjugated bilirubin is known to be a better indicator of liver function and is used in the diagnosis of jaundice and hyperbilirubinemia (Doumas et al. 1999; Kirihigashi et al. 2000; Kosaka et al. 1987; Kurosakaa et al. 1998). The detection of unconjugated bilirubin is more specifically used in newborns where the liver is not mature enough to convert the unconjugated bilirubin to conjugated bilirubin resulting in jaundice affecting ~60–80% of newborns (Kirk 2008).
In addition to medical applications, it was recently demonstrated that BODs, when immobilized on electrode surfaces (Mano et al. 2003, 2002), are more efficient than platinum for the four electron reduction of O2 to water, in physiological conditions (20 mM phosphate buffer, pH 7.2, 0,14 M NaCl, 37 °C), making these enzymes promising tools for the elaboration of oxygen cathodes in biofuel cells and implantable medical devices (Fernandez et al. 2004; Flexer and Mano 2010; Gao et al. 2007, 2010; Heller 2004; Rengaraj et al. 2011; Rincón et al. 2011; Wang et al. 2012; Zebda et al. 2011; Zloczewska et al. 2011).
Finally, BODs are promising candidates for applications in the degradation of textile effluents. Eight kilotons of synthetic dyes are produced per year and are extensively used in the textile, cosmetic, drug, and food industries. It has been reported that 10% to 20% of these dyes may be found in wastewater, most of them being toxic and carcinogenic (Fu and Viraraghavan 2001; Robinson et al. 2001; Weisburger 2002). Environmentally friendly technologies are therefore needed to remove them. Laccases have previously been used for this application. Most laccases, however, have an acidic pH optimum which restricts their applications to the decolorization of acidic effluents. A recent report by Liu et al. (2009) showed that BOD could potentially be used under mild pH conditions as an alternative for the decolorization of synthetic dyes.
Fungal BODs have been identified in Pleurotus ostreatus (Masuda-Nishimura et al. 1999), Trachyderma tsunodae (Hiromi et al. 1992), Myrothecium verrucaria (Guo et al. 1991), and Penicillium janthinellum (Seki et al. 1996). BOD activity has also been identified in bacteria such as CotA from Bacillus subtilis (Sakasegawa et al. 2006), Bacillus licheniformis (Yoshino et al. 1988), and Bacillus pumilus (Durand and Mano 2010).
Herein, we report the identification, expression in Pichia pastoris, purification, and biochemical characterization of a new MCO from the rice blast fungus Magnaporthe oryzae. Our biochemical and biophysical studies show that this enzyme can be classified as a BOD, exhibiting higher activity towards bilirubin than BOD from M. verrucaria (Guo et al. 1991). The facility of its production in large quantities in P. pastoris, the one-step purification protocol, the high catalytic activity at neutral pH, and its tolerance to urate make this new enzyme a promising biocatalyst for numerous industrial applications.
Materials and methods
Chemicals and materials
ABTS, unconjugated bilirubin, and Syringaldazine (SGZ) were purchased from Fluka (Sigma-Aldrich, Saint Louis, MO, USA). Conjugated bilirubin was purchased from Frontier scientific. All other chemicals were of analytical grade or higher and were purchased from Sigma (Sigma-Aldrich, Saint Louis, MO, USA). Restriction enzymes were from New England Biolabs (Ipswich, MA, USA), and zeocin was from Invitrogen. All solutions were made with deionized water passed through an AQ 10 Milli-Q purification system from Millipore (Molsheim, France). AKTA purifier 10 UV-900, prepacked HiLoad 26/10 Phenyl Sepharose HP (53 mL) column, and PD 10 desalting columns containing Sephadex G-25 medium were from GE Healthcare BioSciences AB (Uppsala, Sweden). Amicon Ultra ultrafiltration columns were from Millipore (Molsheim, France). Spectroscopic UV–visible measurements were performed on a Cary 100 system from Varian, Inc. (Palo Alto, CA, USA), equipped with a peltier thermostable multicell holder. Polymerase chain reactions (PCR) were carried out in an automated thermal cycler (VWR). DNA was introduced into Escherichia coli and P. pastoris GS115 by electroporation using an Eppendorf electroporator.
Strains, plasmid, and culture conditions
E. coli DH5α strain, P. pastoris GS115 strain, and pPICZα vector were purchased from Invitrogen. LB (Luria-Bertani) medium, YPD (yeast extract dextrose) medium, and MMH (minimal methanol+histidine) used for E. coli and P. pastoris growth were prepared following the user manual of the Pichia Expression Kit (Invitrogen).
Cloning of the BOD cDNA in the expression vector and integration in P. pastoris
Coding sequence of the hypothetical gene of BOD (Genbank accession EDJ95889.1) from M. oryzae (ATCC MYA-4617) was synthesized by Genecust (Luxembourg) without the 73 first nucleotides in 3′ of the gene, which presents a high percentage of homology with the signal peptide of the BOD from M. verrucaria. The specific sense primer (ACTGCTAGCAAGGACTGGATCAGCCCCCTGTAC) and the anti sense primer (ATTGCGGCCGCTCATGCGGCCGCTCACTGACGCGGCAT) were used to amplify the cDNA of M. oryzae coding for the protein deleted from its first 24 amino acids. PCR was carried out with Phusion polymerase (Finzymes). The sequences underlined correspond to the restriction sites of NheI (sense primer) and NotI (antisense primer) used to insert the gene sequence into the pPICZα vector. After electroporation, E. coli DH5α strain was used to isolate positive clones on LB+zeocin 25 μg/ml, and sequence analysis of a positive construct was performed to ensure that the open reading frame of the gene was in frame with the alpha factor. The pPICZα-BOD Magna was then linearized with PmeI, transformed in P. pastoris GS115 strain by electroporation, and positives clones were selected on YPD+zeocin at 300 μg/mL. A positive transformant was cultivated overnight in 200 ml of YPD+100 μg/ml zeocin at 30 °C, and then the culture was centrifuged at 4,000 rpm for 15 min, and the cell pellet was washed two times in sterile and cold water. After a third centrifugation, the pellet was resuspended in 5 mL of water and was used to inoculate 2 L of MMH medium in a 5-L flask at 25 °C supplemented with 1 mM CuSO4. Protein induction was performed with 0.5% of methanol (v/v) added in the culture, and the procedure was repeated daily for 5 days. Additional copper was added to the culture after the third day to increase the final concentration of CuSO4 to 2 mM, which was enough to insure the presence of four coppers per enzyme molecule in the secreted protein. The culture was finally centrifuged at 6,000×g for 20 min to remove yeast cells, and culture supernatant was filtered (0.5 μM) before BOD purification step.
Purification of the protein
The BOD from M. oryzae produced by P. pastoris was purified in one step by hydrophobic interaction chromatography using an AKTA purifier system. The culture media of P. pastoris containing the enzyme was concentrated by ultrafiltration with a 10-kDa YM10 membrane in a stirred cell. Five milliliters of this concentrated protein solution was equilibrated in a 50-mM potassium phosphate, 1.7 M (NH4)2SO4 pH 6, and then injected on the HiLoad 26/10 Phenyl Sepharose HP equilibrated in the same buffer. BOD was eluted from the column at a flow rate of 2.5 ml min−1 with a linear gradient of 1.7 to 0 M (NH4)2SO4 (in a 50-mM potassium phosphate buffer, pH 6). Protein elution was continuously monitored at 280 nm and also at 600 nm to discriminate Apo and Holo proteins. Active fractions were pooled, concentrated using an Amicon Ultra 15 (cut-off, 10 kDa) membrane, and desalted on a PD 10 column with the 50-mM potassium phosphate buffer, pH 6. At this step, the BOD can be stocked at −80 °C.
Amino acid analysis on a protein sample was performed to determine the specific molecular extinction coefficient at 280 and 600 nm of this new BOD at the Pasteur Institute (Paris). The total enzyme concentration was determined by the Bradford assay using BSA as a standard (Bradford 1976) or with the epsilon at 280 nm. Twelve percent polyacrylamide SDS-PAGE gels were performed to check the homogeneity of the purified enzyme using a Bio-Rad Mini Protean system followed by Coomassie blue staining. To estimate the molecular weight of the purified enzyme, prestained precision standards (19–118 kDa; Euromedex) were also used.
Enzyme assay and kinetic measurements
The enzyme activity was determined spectrophotometrically at 37 °C by following the oxidation of 1 mM ABTS at 420 nm (ε420 nm=36 mM −1 cm−1) in a Mcllvaine’s citrate–phosphate buffer, pH 3.2. Syringaldazine at 22 μM (SGZ, ε530 nm=64 mM −1 cm−1) was used to measure the protein activity at pH 6.2 in Mcllvaine’s citrate–phosphate buffers. Unconjugated bilirubin and conjugated bilirubin were used to assay the bilirubin activity at 37 °C and were respectively measured spectrophotometrically at 450 and 440 nm (ε450 nm =32 mM −1 cm−1 and ε440 nm=25 mM −1 cm−1) in a sodium phosphate 50-mM, pH 7.2, and in a Mcllvaine’s citrate–phosphate 0.1-M buffer, pH 4.8. One unit was defined as the amount of enzyme that oxidized 1 μmol of either unconjugated bilirubin or conjugated bilirubin per minute. Km and kcat were determined in the 2.5 μM–4 mM concentration range for ABTS, 1–80 μM for SGZ, and 1–100 μM for conjugated bilirubin by fitting the data with the simple Michaelis–Menten model by non-linear regression according to the following equation:
Michaelis–Menten’s model
Mcllvaine’s citrate–phosphate 0.1-M buffers at pH 3–7 and Tris-H2SO4 50-mM buffers at pH above 7 were used to study the influence of pH on the oxidation of the substrates by the enzyme. Mcllvaine’s citrate–phosphate 0.1-M buffer at pH 4 was used to study the influence of the temperature on the oxidation of ABTS by the enzyme. The pH stability was studied by incubating the purified enzyme in the absence of substrate at different pH for almost 8 days at 4 °C, and residual activities were determined under the standard assay conditions, with 1 mM ABTS, at 37 °C in a Mcllvaine’s citrate/phosphate 0.1-M buffer, pH 4. The thermostability of the enzyme was investigated by incubating the enzyme for different times in the absence of substrate in a Mcllvaine’s citrate/phosphate 0.1-M buffer, pH 7, at 37 °C and 60 °C. Residual activities were determined with 50 μM of conjugated bilirubin at 37 °C in a Mcllvaine’s citrate/phosphate 0.1-M buffer, pH 3.8, at 440 nm. The effect of urea on the enzyme activity was studied at 37 °C and 25 °C in the presence of either 1 mM ABTS in a Mcllvaine’s citrate–phosphate 0.1-M buffer, pH 4, or 50-μM conjugated bilirubin in a Mcllvaine’s citrate–phosphate 0.1-M buffer, pH 3.8. The effect of NaCl on the enzymatic activity was also studied with either 0.1 mM SGZ in a Mcllvaine’s citrate–phosphate 0.1-M buffer, pH 7, or with 0.075 mM conjugated bilirubin in a Mcllvaine’s citrate–phosphate 0.1-M buffer, pH 3.8. Decolorization of Remazol brilliant blue R at 80 μg ml−1 by the new BOD was measured in the presence and absence of 10 μM ABTS in a Mcllvaine’s citrate–phosphate 0.1-M buffer, pH 7. Each experiment was performed in duplicate or triplicate.
Results
Identification, cloning, and overexpression of the recombinant BOD
To identify amino acid sequences corresponding to MCOs with BOD activity, we performed blast searches in the ExPASy proteomics server. The amino acid sequence of BOD from M. verrucaria (UniProt accession no. Q12737) was used as a template. Multiple sequence alignments with different bacterial and fungal proteomes revealed a potential candidate protein from the rice blast fungus M. oryzae (A4QV27), which shows 58% identity with M. verrucaria. This sequence includes the four histidinerich, copper-binding domains (Fig. 1) characteristic of MCOs.
Fig. 1.
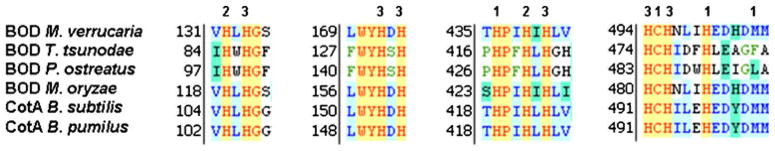
Amino acid sequence alignment of potential copper coordination sites of different BOD proteins: CotA from Bacillus subtilis (accession P07788), BOD from Myrothecium verrucaria (accession Q12737), CotA from Bacillus pumilus (accession A8FAG9), BOD from Trachyderma tsunodae (accession O61263), and BOD from Pleurotus ostreatus (accession Q9UVY4). The numbers 1, 2, and 3 correspond to the coordination sites for the types 1, 2, and 3 coppers. The amino acids presumed to be involved in the copper binding are represented in red
The corresponding cDNA, constituting 1,785 nucleotides, was synthesized to facilitate the production of the protein encoded by this gene. As the BOD from M. verrucaria is cleaved at the N-terminal to eliminate the peptide signal generating the mature protein, we decided to remove a similar sequence at the N-terminal of the protein from M. oryzae. By sequence analogy, the DNA sequence coding for the first 24 amino acids of the protein was removed from the final construct, and the pPICZα bearing this truncated DNA sequence was linearized and transformed in the P. pastoris GS115 strain by electroporation. The cassette containing the leader sequence of alpha factor from Saccharomyces cerevisiae, the hypothetical protein gene sequence, and the terminator under control of the AOX1 promoter allowed the protein secretion in P. pastoris by induction with methanol. Protein overexpression was done without further optimization since a large production of protein was obtained after 5 days from 2-L flask cultures with daily 0.5% methanol induction at 25 °C. Protein activity against ABTS was clearly observed in the culture media and not in yeast cells confirming the protein secretion.
Purification and spectroscopic characterization of the secreted protein
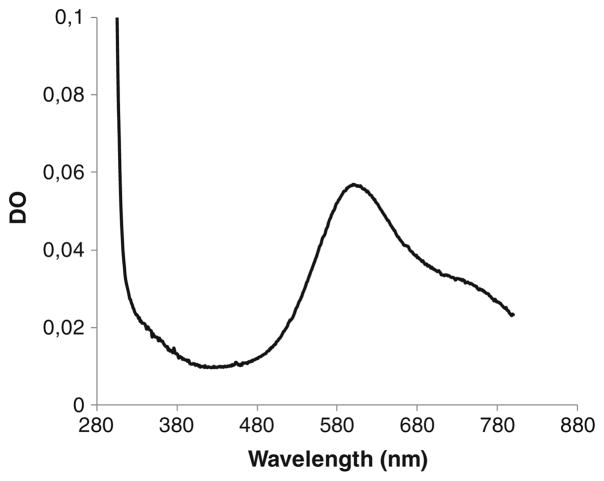
Figure 2 shows the purified protein in SDS-PAGE migrating to slightly above its theoretical weight, the difference ascribed to glycosylation. In addition, a band at ~49 kDa is observed, similar to observations for previously purified BODs (Kataoka et al. 2005). The origin of this band has yet to be determined. Further purification on a Superdex 200 (50 mM sodium phosphate, 1 ml/min) did not result in increase in specific activity; instead, the protein yield decreased by 44%. The resulting mature protein, after N-terminal cleavage, is composed of 571 amino acids with a theoretical molecular weight of 63,975 kDa. The isoelectric point and molecular extinction coefficient (ε280) were experimentally determined to 5.78 and 1,300 mM−1 cm−1, respectively. Cu loading, determined by the biquinoline method (Felsenfeld 1960) and by atomic absorption spectroscopy from a solution of BOD in neutral pH, was 3.8 Cu/enzyme, consistent with close to full occupancy of all Cu sites. As isolated, BOD from M. oryzae has the typical deep blue color of MCOs, corresponding to a UV–visible absorption band at 530–650 nm, with maximum intensity at 596 nm (ε596=6.0 mM −1 cm−1), originating from the T1 copper (Fig. 3). Upon complete reduction, followed by reoxidation by O2, a shoulder at 330 nm is also observed in the UV–visible spectrum, corresponding to the antiferromagnetically coupled T3 Cu(II)’s. A detailed evaluation of this unusual behavior will be published (Kjaergaard, submitted). The absorbance ratio between 596 and 278 nm is 21 and constant for different purification batches.
Fig. 2.
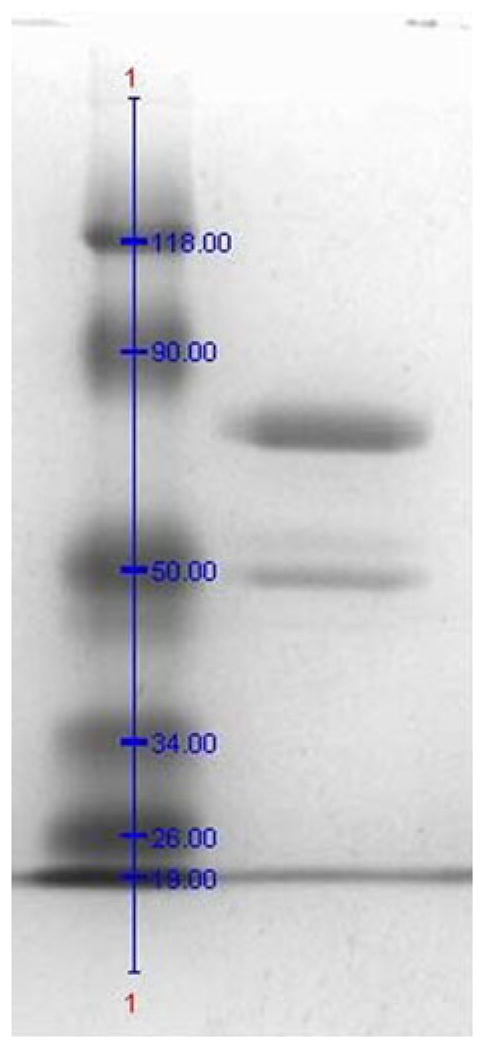
Blue Coomassie-stained SDS-PAGE gel (12%) of purified recombinant BOD from Magnaporthe oryzae
Fig. 3.
UV–visible spectrum of purified BOD from Magnaporthe oryzae (0.5955 mg/ml)
Biochemical properties of BOD from M. oryzae
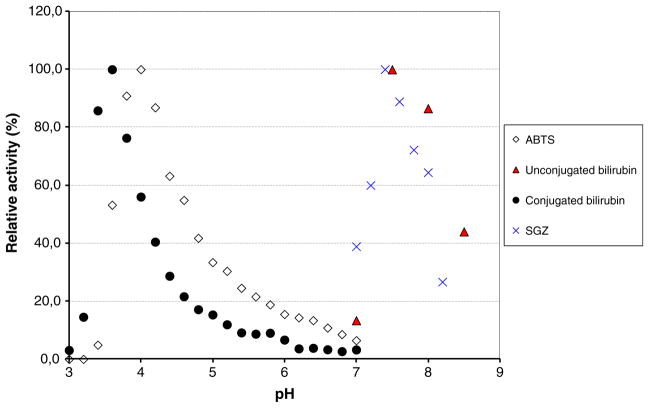
Enzyme activity towards nonphenolic (ABTS) and phenolic (SGZ) laccase substrates was determined. For each substrate, the optimal pH was determined in a Mcllvaine’s citrate–phosphate 0.1 M buffer with pH ranging from 2.6 to 7.5 and in Tris-H2SO4 50 mM buffer for pH above 7.2 (Fig. 4). Optimal pH’s for ABTS and SGZ activity were 4 and 7.4, respectively. Michaelis–Menten parameters were determined for ABTS and SGZ at their optimal pH and 37 °C. kcat and Km were 664 s −1 and 429 μM for ABTS, and 14.1 s−1 and 26.3 μM for SGZ. To assess whether the new enzyme is a BOD, bilirubin activity was investigated. Kinetic parameters for conjugated bilirubin were kcat=29 s−1 and Km =18.5 μM at 37 °C at the optimal pH of 3.6. Oxidation of unconjugated bilirubin did not show Michaelis–Menten behavior, as reported for other BODs (Reiss et al. 2011), instead, a maximal specific activity of 32 U/mg with 70 μM unconjugated bilirubin was determined at pH 7.5 and 37 °C.
Fig. 4.
Dependence of the enzyme activity on the pH for the purified BOD from Magnaporthe oryzae. The activity was measured at 37 °C using 1 mM ABTS (diamonds), 22 μM SGZ (crosses), 25 μM conjugated bilirubin (circles), and 30 μM unconjugated bilirubin (triangles) in 0.1-M phosphate–citrate buffer for pH between 3 and 7.2 and Tris- H2SO4 for pH above 7.2
With respect to temperature dependence of the enzyme, turnover studies were conducted with ABTS that showed maximal activity at ~60 °C (Fig. 5). Furthermore, thermal deactivation was investigated by pre-incubation of the enzyme at 37 °C and 60 °C, followed by activity measurements. As seen in Fig. 6, the half-life for deactivation was ~70 min at 60 °C and more than 300 min at 37 °C.
Fig. 5.
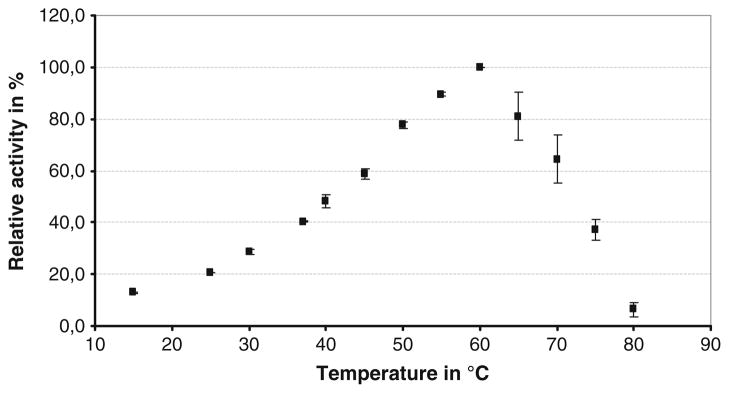
Dependence of the enzyme activity on the temperature for the purified BOD from Magnaporthe oryzae. The activity was measured in a 0.1-M phosphate–citrate buffer, pH 4, in presence of 1 mM ABTS
Fig. 6.
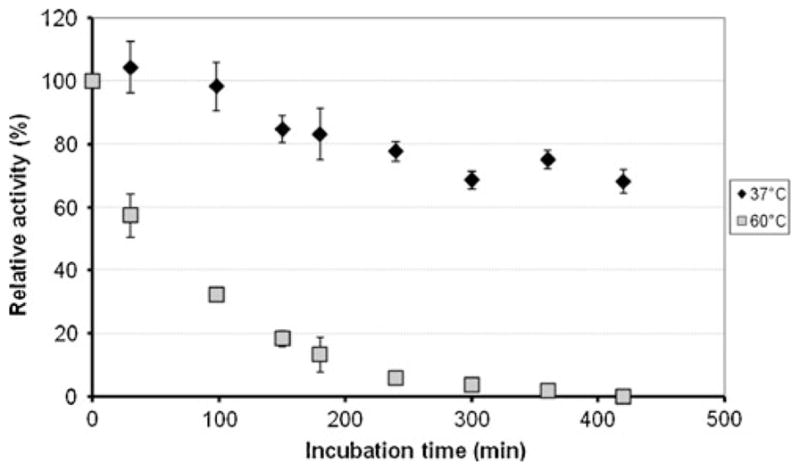
Thermostability of the purified BOD from Magnaporthe oryzae incubated either at 37 °C or 60 °C in a 0.1-M citrate–phosphate buffer, pH 7. The enzyme activity was measured at 37 °C in a 0.1-M citrate–phosphate buffer, pH 3.8, in presence of 50-μM conjugated bilirubin
Figure 7 shows the pH dependence on deactivation of enzyme activity in the pH 3–9 range. During incubation, the protein was stored at 4 °C to avoid any thermal deactivation. After 8 days, the enzyme activity had decreased by 15% in the pH 4–7 range, 20% in the pH 8–9 range, and 80% at pH 3.
Fig. 7.
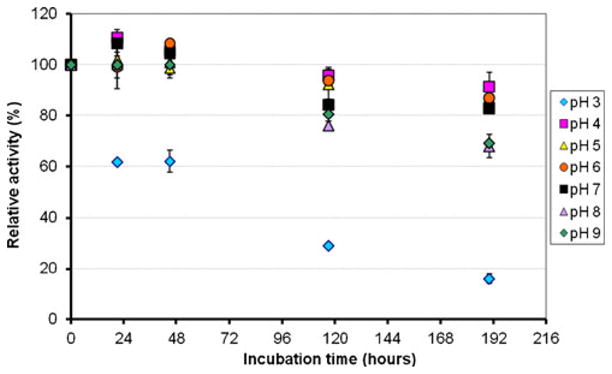
Dependence of the enzyme stability on the pH for the purified BOD from Magnaporthe oryzae. The residual enzyme activity was measured at 37 °C in a 0.1-M phosphate–citrate buffer, pH 4, in presence of 1 mM ABTS
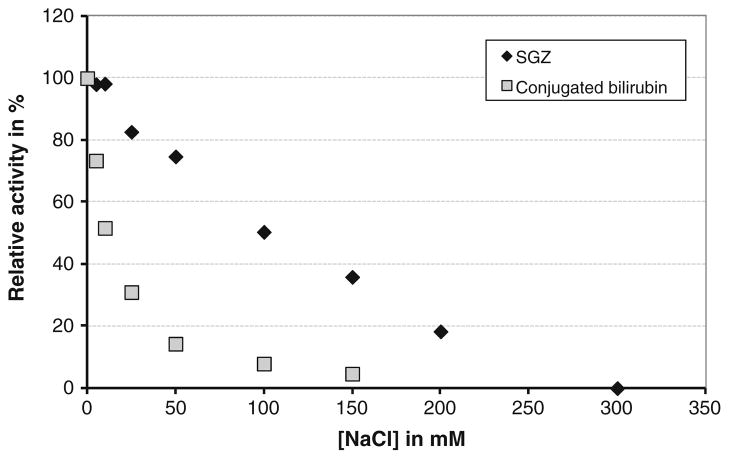
Inhibition of MCOs by urea and/or chloride is often observed. Therefore, enzyme activity, of the BOD, in the presence of these potential inhibitors was analyzed. Figure 8 shows the activity dependence on urea concentrations in the 0–5-M range at 25 °C and 37 °C, measured in the presence of either 1 mM ABTS in a Mcllvaine’s citrate–phosphate 0.1-M buffer, pH 4, or 50-μM conjugated bilirubin in a Mcllvaine’s citrate–phosphate 0.1-M buffer, pH 3.6. The influence of urea on the enzymatic activity appears to be substrate-dependent. In the presence of ABTS and upon increasing urea concentrations, the relative activity decreases continuously to less than 50% with 5 M urea at 37 °C or 25 °C. With conjugated bilirubin as substrate, the enzyme activity increases with urea concentration until it reaches a plateau at 1.5 M at 25 °C and 0.5 M at 37 °C followed by a slow decrease with higher concentrations. The dependence of enzyme activity on the NaCl concentration was measured in the presence of 0.1 mM SGZ or 75 μM conjugated bilirubin (Fig. 9). At 150 mM NaCl, the enzymatic activity decreased by ~60% for SGZ and ~85% for bilirubin.
Fig. 8.
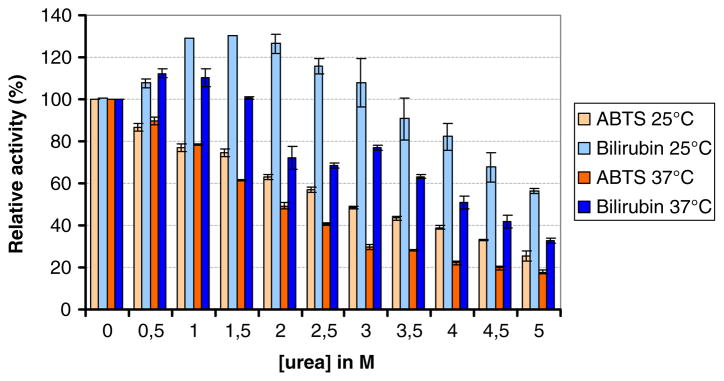
Dependence of the enzyme activity on the urea concentration for the purified BOD from Magnaporthe oryzae at 25 °C and 37 °C in presence of 1 mM ABTS or 50 μM conjugated bilirubin. The enzyme activity was measured in a 0.1-M phosphate–citrate buffer, pH 4, for ABTS or pH 3.8 for conjugated bilirubin
Fig. 9.
Dependence of the enzyme activity on the NaCl concentration for the purified BOD from Magnaporthe oryzae in presence of either 0.1 mM SGZ in a Mcllvaine’s citrate–phosphate 0.1-M buffer, pH 7, or with 75 μM conjugated bilirubin in a Mcllvaine’s citrate–phosphate 0.1-M buffer, pH 3.8
Because BOD from M. verrucaria is able to decolorize a large variety of synthetic dyes (Brissos et al. 2009; Pereira et al. 2009; Liu et al. 2009), we performed activity experiments with Remazol brilliant blue R as substrate. As shown in Fig. 10, the decolorization in the presence of ABTS is high, with >95% efficiency after 20 min at 37 °C with 10 μM ABTS. Without the mediator, decolorization is sluggish.
Fig. 10.
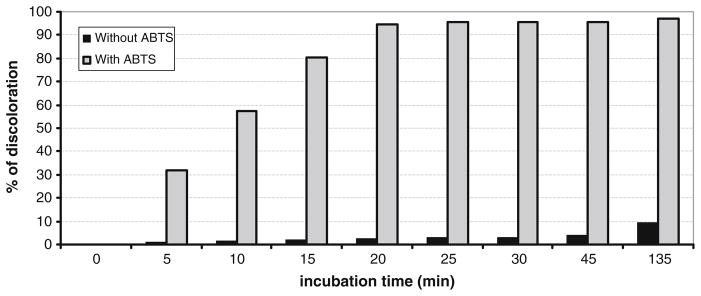
Decolorization of 80 μg ml−1 Remazol brilliant blue R by the purified BOD from Magnaporthe oryzae in presence or absence of 10 μM of ABTS in a Mcllvaine’s citrate–phosphate 0.1-M buffer, pH 7
Discussion
BODs, a sub-group of MCOs, have attracted significant interest due to their ability to oxidize bilirubin to biliverdin, which is utilized in the determination of bilirubin levels in serum. Furthermore, BODs are attractive candidates for enzyme cathodes in biofuel cells and biosensors because unlike laccase, they are stable under physiological conditions.
Here, we identified and characterized a new BOD from M. oryzae with a molecular weight of 63.7 kDa and large homology with BOD from M. verrucaria. Amino acid sequence alignments showed that this putative protein exhibits all the consensus sequences of a MCO. Molecular cloning was performed on the coding sequence of the corresponding predicted protein to overexpress the recombinant enzyme in P. pastoris. By amino acid sequence alignments, we isolated a hypothetical peptide signal that was removed from the final protein. The pPICZα vector was then used to clone the resulting sequence in-frame with the α-peptide signal to allow for the enzyme secretion in the culture media. The yeast P. pastoris has already been used for the production of large number of proteins since a highly efficient secretion system is available by regulation of the alcohol oxidase promoter of this fungus (Cereghino and Cregg 2000; Higgins 2001). Moreover, in control conditions, P. pastoris only secretes a few proteins into the culture media, which facilitates the purification of the recombinant protein. It is then possible to produce this enzyme at high yield, ~50 mg/L, twice that of M. verrucaria (Kataoka et al. 2005) in only one step by hydrophobic interaction chromatography, while it needs two steps for other BODs (Kataoka et al. 2005; Shimizu et al. 1999). Detailed spectroscopic features of the new BOD will be described in a subsequent paper (Kjaergaard, submitted) but generally resemble those of MCOs.
The optimal pHs for oxidation of ABTS and SGZ are similar to those of bacterial (Durao et al. 2008; Koschorreck et al. 2008) and fungal BODs (Baldrian 2006; Pakhadnia et al. 2009). Compared to these, the kcat is one of the highest reported, with ABTS as substrate. In contrast, the Km values are higher, and the ratio of kcat/Km suggests that this protein is not as efficient in the oxidation of phenolic substrates such as SGZ.
Catalytic activity towards conjugated and unconjugated bilirubin confirmed that this enzyme should be classified as a BOD, the only sub-class of MCOs showing this reactivity. The kcat/Km ratio for the new enzyme, with conjugated bilirubin (1,513 mM−1 s−1) as substrate, is higher than that obtained for BOD from M. verrucaria expressed in native fungus (980 mM−1 s−1), but lower than M. verrucaria expressed in P. pastoris (2,000 mM−1 s−1) and for BOD from B. subtilis (3,400 mM−1 s−1). The new BOD, however, showed the lowest Km (18.5 μM) reported so far for conjugated bilirubin, and also shows high activity towards un-conjugated bilirubin with a specific activity of 32 U/mg. This, in combination with high urea tolerance, makes it an ideal candidate for the determination of serum bilirubin levels in clinical samples (Kurosakaa et al. 1998) and in newborns (Newman and Maisels 1992). It was shown previously that among serum components, urate may coordinate to Cu centers of T. tsunodae BOD, resulting in loss of activity (Kang et al. 2006). As for BOD from B. pumilus (Durand and Mano 2010), this new BOD showed activity increase with increasing urea concentrations, with conjugated bilirubin as the substrate. At 25 °C and 37 °C, the enzyme activity increased by 20% in the presence of 1.5 M urea. In the case of B. pumilus, the activity increases by 20% in the presence of 3 M urea. A similar effect has been reported for other proteins such as a dihydrofolate reductase or adenylate kinase that were activated 5 and 1.6 times in the presence of 4 or 1 M urea, respectively (Fan et al. 1996; Zhang et al. 1997). In both cases, the higher enzyme activity was attributed to an increased flexibility of the active site conformation upon addition of urea. This may also be the case for this BOD.
In addition to stability in the presence of urea, to be used as enzyme cathode in biofuel cells, the new enzyme must be stable at 37 °C, under neutral pH, and in the presence of NaCl. The highest activity of the enzyme is reached at 60 °C, 20 °C higher than that of the fungal M. verrucaria but 20 °C lower than the temperature for CotA BOD. Thermostability experiments (Fig. 6) showed that the new BOD is one of the most stable of the fungal BODs with a half life >300 min at 37 °C and >70 min at 60 °C. For comparison, among reported fungal BODs, the half-life of the native M. verrucaria enzyme is only 15 min at 60 °C and is increased to 90 min for the recombinant protein produced in P. pastoris (Kataoka et al. 2005), while the BOD from T. tsunodae loses 40% of its activity after 30 min at 40 °C (Sakasegawa et al. 2006). However, fungal BODs are generally less stable than bacterial BODs which display the highest thermostability among all MCOs with half-lives of >100 min at 80 °C (Hilden et al. 2009). The pH dependence of the new BOD demonstrated that this enzyme is highly stable after 8 days, which makes it an ideal candidate for the elaboration of biosensors and biofuel cells. For comparison, after 8 days, native M. verrucaria loses 50% of its activity, while the new BOD only loses 15%.
Laccases are known to be inhibited by anions, including Cl−, at low concentrations. For this BOD, Cl− inhibition is observed with two different substrates in solution, although to different extents. Preliminary electrochemical experiments, however, indicate that when immobilized in a redox polymer, the BOD is highly insensitive to Cl− (data not shown).
Finally, there is a great desire to develop effective ways to degrade textile dyes in wastewater at neutral pH—economically viable and with minimal environmental impact. BOD, from M. oryzae, efficiently decolorizes Remazol Brilliant Blue R at pH 7, especially with ABTS as a mediator (Fig. 10). To our knowledge, this is only the second time this activity has been demonstrated by a BOD. This may lead to industrial applications for this new BOD, not obtainable with laccases.
In summary, due to the high yield after overexpression, one-step purification, good thermal stability, high activity at pH 7, stability in the presence of urea, and low Km for bilirubin, BOD from M. oryzae is an excellent candidate for the elaboration of highly efficient biofuel cells (Gao et al. 2010), bilirubin biosensors operating in serum, and numerous industrial processes including the decolorization of synthetic dyes.
Acknowledgments
This work was supported by a European Young Investigator Award (EURYI), la Région Aquitaine. EIS thanks NIH for support (grant no. DK-31450).
Contributor Information
Fabien Durand, CNRS, CRPP, UPR 8641, Université Bordeaux, 33600, Pessac, France.
Sébastien Gounel, CNRS, CRPP, UPR 8641, Université Bordeaux, 33600, Pessac, France.
Christian H. Kjaergaard, Department of Chemistry, Stanford University, Stanford, CA 94305, USA
Edward I. Solomon, Department of Chemistry, Stanford University, Stanford, CA 94305, USA
Nicolas Mano, Email: mano@crpp-bordeaux.cnrs.fr, CNRS, CRPP, UPR 8641, Université Bordeaux, 33600, Pessac, France.
References
- Baldrian P. Fungal laccases—occurrence and properties. FEMS Microbiol Rev. 2006;30:212–242. doi: 10.1111/j.1574-4976.2005.00010.x. [DOI] [PubMed] [Google Scholar]
- Barton SC, Gallaway J, Atanassov P. Enzymatic biofuel cells for implantable and microscale devices. Chem Rev. 2004;104:4867–4886. doi: 10.1021/cr020719k. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brissos V, Pereira L, Munteanu FD, Cavaco-Paulo A, Martins LO. Expression system of CotA-laccase for directed evolution and high-throughput screenings for the oxidation of highredox potential dyes. Biotechnol J. 2009;4(4):558–563. doi: 10.1002/biot.200800248. [DOI] [PubMed] [Google Scholar]
- Cereghino JL, Cregg JM. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol Rev. 2000;24 (1):45–66. doi: 10.1111/j.1574-6976.2000.tb00532.x. [DOI] [PubMed] [Google Scholar]
- Doumas BT, Yein F, Perry B, Jendrzejczak B, Kessner A. Determination of the sum of bilirubin sugar conjugates in plasma by bilirubin oxidase. Clin Chem. 1999;45(8):1255–1260. [PubMed] [Google Scholar]
- Durand F, Mano N. Nouvelle Bilirubine Oxydase de Bacillus pumilus. WO 2011117839 Centre National de la Recherche Scientifique, Fr, France. 2010 FR2010–1167, 03/24/10.
- Durao P, Chen Z, Fernandes AT, Hildebrandt P, Murgida DH, Todorovic S, Pereira MM, Melo EP, Martins LO. Copper incorporation into recombinant CotA laccase from Bacillus subtilis: characterization of fully copper loaded enzymes. J Biol Inorg Chem. 2008;13(2):183–193. doi: 10.1007/s00775-007-0312-0. [DOI] [PubMed] [Google Scholar]
- Fan YX, Ju M, Zhou JM, Tsou CL. Activation of chicken liver dihydrofolate reductase by urea and guanidine hydrochloride is accompanied by conformational change at the active site. Biochem J. 1996;315(Pt 1):97–102. doi: 10.1042/bj3150097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld G. The determination of cuprous ion in copper proteins. Arch Biochem Biophys. 1960;87:247–251. doi: 10.1016/0003-9861(60)90168-5. [DOI] [PubMed] [Google Scholar]
- Fernandez JL, Mano N, Heller A, Bard AJ. Optimization of “wired” enzyme O2-electroreduction catalyst composition by scanning electrochemical microscopy. Angew Chem Int Ed. 2004;43:6355–6357. doi: 10.1002/anie.200461528. [DOI] [PubMed] [Google Scholar]
- Flexer V, Mano N. From dynamic measurements of photosynthesis in a living plant to sunlight transformation into electricity. Anal Chem. 2010;82(4):1444–1449. doi: 10.1021/ac902537h. [DOI] [PubMed] [Google Scholar]
- Fu Y, Viraraghavan T. Fungal decolorization of dye wastewaters: a review. Bioresour Technol. 2001;79:251–262. doi: 10.1016/s0960-8524(01)00028-1. [DOI] [PubMed] [Google Scholar]
- Gao F, Yan Y, Su L, Wang L, Mao L. An enzymatic glucose/O2 biofuel cell: preparation, characterization and performance in serum. Electrochem Comm. 2007;9:989–996. [Google Scholar]
- Gao F, Viry L, Maugey M, Poulin P, Mano N. Engineering hybrid nanotube wires for high-power biofuel cells. Nat Commun. 2010;1(1):1–7. doi: 10.1038/ncomms1000. [DOI] [PubMed] [Google Scholar]
- Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G. Laccases: a never-ending story. Cell Mol Life Sci. 2010;67 (3):369–385. doi: 10.1007/s00018-009-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Liang XX, Mo PS, Li GX. Purification and properties of bilirubin oxidase from Myrothecium verrucaria. Appl Biochem Biotechnol. 1991;31(2):135–143. doi: 10.1007/BF02921784. [DOI] [PubMed] [Google Scholar]
- Han MJ, Choi HT, Song HG. Purification and characterization of laccase from the white rot fungus Trametes versicolor. J Microbiol. 2005;43(6):555–560. [PubMed] [Google Scholar]
- Heller A. Miniature biofuel cells. Phys Chem Chem Phys. 2004;6:209–216. [Google Scholar]
- Higgins DR. Overview of protein expression in Pichia pastoris. Curr Protoc Protein Sci. 2001;Chapter 5(Unit 5.7) doi: 10.1002/0471140864.ps0507s02. [DOI] [PubMed] [Google Scholar]
- Hilden K, Hakala TK, Lundell T. Thermotolerant and thermostable laccases. Biotechnol Lett. 2009;31(8):1117–1128. doi: 10.1007/s10529-009-9998-0. [DOI] [PubMed] [Google Scholar]
- Hiromi K, Yamaguchi S, Sugiura Y, Iwamoto H, Hirose J. Bilirubin oxidase from Trachyderma tsunodae K-2593, a multi-copper enzyme. Biosci Biotech Biochem. 1992;56:1349–1350. [Google Scholar]
- Hirose J, Sakurai T, Imamura K, Watanabe H, Iwamoto H, Hiromi K, Itoh H, Shin T, Murao S. Characterization of ascorbate oxidase from Acremonium sp. HI-25. J Biochem. 1994;115(5):811–813. doi: 10.1093/oxfordjournals.jbchem.a124420. [DOI] [PubMed] [Google Scholar]
- Kang C, Shin H, Heller A. On the satbility of the wired bilirubin oxidase oxygen cathode in serum. Bioelectrochemistry. 2006;68:22–26. doi: 10.1016/j.bioelechem.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Kataoka K, Tanaka K, Sakai Y, Sakurai T. High-level expression of Myrothecium verrucaria bilirubin oxidase in Pichia pastoris, and its facile purification and characterization. Protein Expr Purif. 2005;41(1):77–83. doi: 10.1016/j.pep.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Kawahara K, Suzuki S, Sakurai T, Nakahara A. Characterization of cucumber ascorbate oxidase and its reaction with hexacyanoferrate (II) Arch Biochem Biophys. 1985;241(1):179–186. doi: 10.1016/0003-9861(85)90374-1. [DOI] [PubMed] [Google Scholar]
- Kirihigashi K, Tatsumi N, Hino M, Yamane T, Ohta K. Basic and clinical evaluation of a newly-developed enzymatic bilirubin assay. Osaka City Med J. 2000;46(1):55–70. [PubMed] [Google Scholar]
- Kirk JM. Neonatal jaundice: a critical review of the role and practice of bilirubin analysis. Ann Clin Biochem. 2008;45:452–462. doi: 10.1258/acb.2008.008076. [DOI] [PubMed] [Google Scholar]
- Koikeda S, Ando K, Kaji H, Inoue T, Murao S, Takeuchi K, Samejima T. Molecular cloning of the gene for bilirubin oxidase from Myrothecium verrucaria and its expression in yeast. J Biol Chem. 1993;268(25):18801–18809. [PubMed] [Google Scholar]
- Kosaka A, Yamamoto A, Morishita Y, Nakane K. Enzymatic determination of bilirubin fractions in serum. Clin Biochem. 1987;20:451–458. doi: 10.1016/0009-9120(87)90014-2. [DOI] [PubMed] [Google Scholar]
- Koschorreck K, Richter SM, Ene AB, Roduner E, Schmid RD, Urlacher VB. Cloning and characterization of a new laccase from Bacillus licheniformis catalyzing dimerization of phenolic acids. Appl Microbiol Biotechnol. 2008;79(2):217–224. doi: 10.1007/s00253-008-1417-2. [DOI] [PubMed] [Google Scholar]
- Kurosakaa K, Senbaa S, Tsubotab T, Kondoa H. A new enzymatic assay for selectively measuring conjugated bilirubin concentration in serum with use of bilirubin oxidase. Clin Chim Acta. 1998;269:125–136. doi: 10.1016/s0009-8981(97)00194-0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang J, Zhang X. Decolorization and biodegradation of remazol brilliant blue R by bilirubin oxidase. J Biosci Bioeng. 2009;108 (6):496–500. doi: 10.1016/j.jbiosc.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Mano N, Kim H-H, Zhang Y, Heller A. An oxygen cathode operating in a physiological solution. J Am Chem Soc. 2002;124:6480–6486. doi: 10.1021/ja025874v. [DOI] [PubMed] [Google Scholar]
- Mano N, Fernandez JL, Kim Y, Shin W, Bard AJ, Heller A. Oxygen Is electroreduced to water on a “wired” enzyme electrode at a lesser overpotential than on platinum. J Am Chem Soc. 2003;125:15290–15291. doi: 10.1021/ja038285d. [DOI] [PubMed] [Google Scholar]
- Masuda-Nishimura I, Ichikawa K, Hatamoto O, Abe K, Koyama Y. cDNA cloning of bilirubin oxidase from Pleurotus ostreatus strain Shinshu and its expression in Aspergillus sojae: an efficient screening of transformants, using the laccase activity of bilirubin oxidase. J Gen Appl Microbiol. 1999;45:93–97. doi: 10.2323/jgam.45.93. [DOI] [PubMed] [Google Scholar]
- Mayer AM, Staples RC. Laccase: new functions for an old enzyme. Phytochemistry. 2002;60(6):551–565. doi: 10.1016/s0031-9422(02)00171-1. [DOI] [PubMed] [Google Scholar]
- Messerschmidt A, Rossi A, Ladenstein R, Huber R, Bolognesi M, Gatti G, Marchesini A, Petruzzelli R, Finazzi-Agro A. X-ray crystal structure of the blue oxidase ascorbate oxidase from zucchini. Analysis of the polypeptide fold and a model of the copper sites and ligands. J Mol Biol. 1989;206(3):513–529. doi: 10.1016/0022-2836(89)90498-1. [DOI] [PubMed] [Google Scholar]
- Murao S, Tanaka N. A new enzyme “bilirubin oxidase” produced by Myrothecium verrucaria MT-1. Agric Biol Chem. 1981;45:2383–2384. [Google Scholar]
- Newman TB, Maisels MJ. Evaluation and treatment of jaundice in the term newborn: a kinder, gentler approach. Pediatrics. 1992;89:809–818. [PubMed] [Google Scholar]
- Pakhadnia YG, Malinouski NI, Lapko AG. Purification and characteristics of an enzyme with both bilirubin oxidase and laccase activities from mycelium of the basidiomycete Pleurotus ostreatus. Biochemistry (Mosc) 2009;74(9):1027–1034. doi: 10.1134/s0006297909090119. [DOI] [PubMed] [Google Scholar]
- Pereira L, Coelho AV, Viegas CA, Santos MM, Robalo MP, Martins LO. Enzymatic biotransformation of the azo dye Sudan Orange G with bacterial CotA-laccase. J Biotechnol. 2009;139(1):68–77. doi: 10.1016/j.jbiotec.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Reiss R, Ihssen J, Thony-Meyer L. Bacillus pumilus laccase: a heat stable enzyme with a wide substrate spectrum. BMC Biotechnol. 2011;11(1):9. doi: 10.1186/1472-6750-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengaraj S, Mani V, Kavanagh P, Rusling J, Leech D. A membrane-less enzymatic fuel cell with layer-by-layer assembly of redox polymer and enzyme over graphite electrodes. Chem Commun. 2011;47:11861–11863. doi: 10.1039/c1cc15002b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón RC, Lau C, Luckarift HR, Garcia KE, Adkins E, Johnson GR, Atanassov P. Enzymatic fuel cells: Integrating flow-through anode and air-breathing cathode into a membrane-less biofuel cell design. Biosens Bioelectron. 2011;27:132–136. doi: 10.1016/j.bios.2011.06.029. [DOI] [PubMed] [Google Scholar]
- Robinson T, McMullan G, Marchant R, Nigam P. Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol. 2001;77:247–255. doi: 10.1016/s0960-8524(00)00080-8. [DOI] [PubMed] [Google Scholar]
- Sakasegawa S, Ishikawa H, Imamura S, Sakuraba H, Goda S, Ohshima T. Bilirubin oxidase activity of Bacillus subtilis CotA. Appl Environ Microbiol. 2006;72(1):972–975. doi: 10.1128/AEM.72.1.972-975.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki Y, Takeguchi M, Okura I. Purification and properties of bilirubin oxidase from Penicillium janthinellum. J Biotechnol. 1996;46:145–151. [Google Scholar]
- Shimizu A, Kwon JH, Sasaki T, Satoh T, Sakurai N, Sakurai T, Yamaguchi S, Samejima T. Myrothecium verrucaria bilirubin oxidase and its mutants for potential copper ligands. Biochemistry. 1999;38:3034–3042. doi: 10.1021/bi9819531. [DOI] [PubMed] [Google Scholar]
- Solomon EI, Chen P, Metz M, Lee SK, Palmer AE. Oxygen binding, activation, and reduction to water by copper proteins. Angew Chem Int Ed Engl. 2001;40(24):4570–4590. doi: 10.1002/1521-3773(20011217)40:24<4570::aid-anie4570>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Wang X, Falk MC, Ortiz R, Matsumura H, Boback J, Ludwig R, Bergelin M, Gorton L, Shleev S. Mediatorless sugar/oxygen enzymatic fuel cells based on gold nanoparticle-modified electrodes. Biosens Bioelectron. 2012;31:219–225. doi: 10.1016/j.bios.2011.10.020. [DOI] [PubMed] [Google Scholar]
- Weisburger JH. Comments on the history and importance of aromatic and heterocyclic amines in public health. Mutat Res. 2002;506:9–20. doi: 10.1016/s0027-5107(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Xu F. Oxidation of phenols, anilines, and benzenethiols by fungal laccases: correlation between activity and redox potentials as well as halide inhibition. Biochemistry. 1996;35:7608–7614. doi: 10.1021/bi952971a. [DOI] [PubMed] [Google Scholar]
- Yoshino EM, Imamura SS, Matsuura KS, Misaki HS. Thermostable bilirubin oxidase and production process thereof. Toyo Jozo Co., Ltd; Shizuoka: 1988. [Google Scholar]
- Zaitsev VN, Zaitseva I, Papiz M, Lindley PF. An X-ray crystallographic study of the binding sites of the azide inhibitor and organic substrates to ceruloplasmin, a multi-copper oxidase in the plasma. J Biol Inorg Chem. 1999;4(5):579–587. doi: 10.1007/s007750050380. [DOI] [PubMed] [Google Scholar]
- Zaitseva I, Zaitsev V, Card G, Moshkov K, Bax B, Ralph A, Lindley P. The X-ray structure of human serum ceruloplasmin at 3.1 Å: nature of the copper centres. J Biol Inorg Chem. 1996;1(1):15–23. [Google Scholar]
- Zebda A, Gondran C, Le Goff A, Holzinger M, Cosnier S. Mediatorless high-power glucose biofuel cells based on compressed carbon nanotube-enzyme electrodes. Nat Commun. 2011;2:370. doi: 10.1038/ncomms1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HJ, Sheng XR, Pan XM, Zhou JM. Activation of adenylate kinase by denaturants is due to the increasing conformational flexibility at its active sites. Biochem Biophys Res Commun. 1997;238 (2):382–386. doi: 10.1006/bbrc.1997.7301. [DOI] [PubMed] [Google Scholar]
- Zloczewska A, Jonsson-Niedziolka M, Rogalski J, Opallo M. Vertically aligned carbon nanotube film electrodes for bioelectrocatalytic dioxygen reduction. Electrochim Acta. 2011;56:3947–3953. [Google Scholar]