SUMMARY
The posterior parietal cortex plays a central role in spatial functions, such as spatial attention and saccadic eye movements. However, recent work has increasingly focused on the role of parietal cortex in encoding nonspatial cognitive factors such as visual categories, learned stimulus associations, and task rules. The relationship between spatial encoding and nonspatial cognitive signals in parietal cortex, and whether cognitive signals are robustly encoded in the presence of strong spatial neuronal responses, is unknown. We directly compared nonspatial cognitive and spatial encoding in the lateral intraparietal (LIP) area by training monkeys to perform a visual categorization task during which they made saccades toward or away from LIP response fields (RFs). Here we show that strong saccade-related responses minimally influence robustly encoded category signals in LIP. This suggests that cognitive and spatial signals are encoded independently in LIP and underscores the role of parietal cortex in nonspatial cognitive functions.
INTRODUCTION
The ability to assign incoming sensory stimuli into behaviorally relevant categories is essential for recognizing the significance of sensory stimuli and for selecting appropriate behavioral responses. Flexible neuronal category or rule representations have been identified in prefrontal cortex (PFC) (Freedman et al., 2001, 2003; Wallis et al., 2001; Ferrera et al., 2009; Cromer et al., 2010; Roy et al., 2010; Goodwin et al., 2012) and posterior parietal cortex (PPC) (Stoet and Snyder, 2004; Freedman and Assad, 2006; Fitzgerald et al., 2011; Goodwin et al., 2012; Swaminathan and Freedman, 2012). However, a recent direct comparison of the lateral intraparietal (LIP) area and PFC during a visual motion categorization task found significantly stronger and shorter-latency category signals in LIP than PFC, as well as a stronger relationship between LIP activity and the animals’ category decisions (Swaminathan and Freedman, 2012). This study suggested that LIP is more directly involved than PFC in solving that categorization task. In contrast, neurons in the middle temporal (MT) area, which provides direct input to LIP (Lewis and Van Essen, 2000), showed strong direction selectivity but not category selectivity (Freedman and Assad, 2006). Together, these studies support the possibility that LIP plays a key role in transforming visual-feature selectivity in earlier sensory areas into abstract category signals during category-based decision making tasks, independent of LIP’s well-known role in spatial processing.
It is unclear whether such a direct role for LIP in nonspatial cognitive functions, such as categorization, is compatible with LIP’s well-known role in spatial attention (Colby et al., 1996; Herrington and Assad, 2010; Bisley and Goldberg, 2010) and saccadic eye movements (Snyder et al., 1997, 2000), which exert powerful influences over LIP activity. For example, a recent study employed pharmacological inactivation of LIP to assess its relative contribution to several spatial and nonspatial tasks (Balan and Gottlieb, 2009). That study found that parietal inactivation caused greater behavioral deficits for spatial compared to nonspatial aspects of the tasks, which lent support to the idea that LIP could play a greater role in spatial compared to nonspatial functions. Another study examined the spatial dependence of LIP category encoding using a motion-categorization task in which stimuli were presented either within or outside neurons’ response fields (RFs) (Freedman and Assad, 2009). That study revealed that the spatial position of stimuli exerted a stronger influence over neuronal firing rates than nonspatial category information, although category signals were still evident, though significantly weaker, when stimuli were placed outside neurons’ RFs. However, it was unclear whether the weaker category selectivity for stimuli shown outside the RF would be robust in the presence of potential interference from strong spatial responses or distractors. Furthermore, that study did not examine the interaction between distinct spatial and category signals, as the spatial and category stimuli were one and the same.
These prior studies raise the possibility that nonspatial encoding in LIP is a secondary function compared to spatial encoding. As such, during tasks with both spatial and nonspatial components, neuronal signals related to space (e.g., saccade, attentional, or bottom-up visual signals) might be expected to dominate, and perhaps interfere with, nonspatial signals. This view is at odds with our hypothesis that LIP plays a central role in encoding abstract category signals, independent of its role in spatial processing. To reconcile these two views, we trained monkeys on a novel behavioral paradigm that placed independent cognitive and spatial behavioral demands on the subjects. We assessed the strength and robustness of nonspatial category signals in the presence of strong neuronal responses related to visually cued saccadic eye movements that were directed either toward or away from LIP neurons’ RFs during the memory-delay period of a category-matching task. Here we show that category signals in LIP are encoded strongly and robustly even in the presence of strong saccade-related responses, lending support to the hypothesis that LIP is centrally involved in category-based decision making in parallel with its role in spatial functions.
RESULTS
Delayed Match-to-Category Task with Saccades
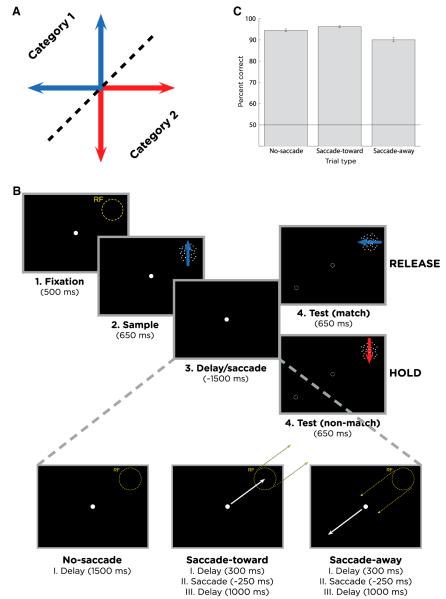
Monkeys were trained to perform a delayed match-to-category (DMC) task in which 360° of motion directions were divided into two categories by a learned category boundary (Figure 1A). Monkeys had to indicate whether a test stimulus was in the same category as a previously presented sample by releasing a manual lever (Figure 1B; see Experimental Procedures). Sample stimuli were presented within LIP neurons’ RFs, and test stimuli always were shown at the same location on the display as the sample. In the no-saccade condition, monkeys maintained their gaze on a central fixation spot that was stationary throughout the trial. In the saccade conditions, the central fixation spot was turned off (300 ms after sample offset) and immediately reappeared either within the neuron’s RF (saccade-toward condition) or at the same eccentricity directed 180° away from the RF (saccade-away condition). The monkeys were required to make an immediate (within 250 ms) saccade to the new fixation target, after which they maintained gaze at the new fixation location for the remainder of the trial. Saccade and no-saccade trials were randomly interleaved. Importantly, the saccades were not related to the monkeys’ category decisions, and the sample and test categories were chosen independently of saccade direction. The saccades are expected to produce large fluctuations in LIP firing rates (Snyder et al., 1997, 2000), allowing the impact of saccade-related neuronal responses on LIP category encoding to be assessed.
Figure 1. Behavioral Task.
(A) Monkeys grouped four motion directions into two categories (the red and blue arrows) separated by a category boundary (the dashed black line).
(B) Delayed match-to-category task. A sample stimulus (650 ms) was followed by a delay (1,500 ms) and a test stimulus (650 ms). If the sample and test were in the same category, monkeys were required to release a lever before the test disappeared. If the test was a nonmatch, there was a second delay (150 ms) followed by a match (which required a lever release). In some trials, a saccade was required during the early delay period (300 ms after the start of the delay), directed either toward or away from the neuron’s RF. After the saccade, the monkey maintained gaze at the new fixation location for the remainder of the trial. The fixation point is indicated by the white spot in each panel, and the dotted outline spots in the test period panels (indicating the three possible fixation locations in the test period depending on the saccade condition).
(C) Monkeys’ average DMC task performance across all recording sessions was ~90% or better for all three saccade conditions. Error bars indicate the SEM.
The monkeys performed the DMC task at ~90% correct on both the no-saccade condition and saccade conditions (Figure 1C). Small but statistically significant differences in behavioral performance were observed across the three conditions according to a one-way ANOVA (p = 2 × 10−8) and are probably explained by the eccentricity of test stimulus presentation and the monkeys’ reduced acuity in the peripheral compared to central visual field. The best performance was observed in the saccade-toward condition (foveal presentation of test stimulus) and worst performance in the saccade-away condition (test stimulus presented in the farther periphery compared to the no-saccade condition). Note that all main analyses of neuronal data are focused on time epochs prior to onset of the test stimulus.
Category and Spatial Encoding in LIP during the DMC Task
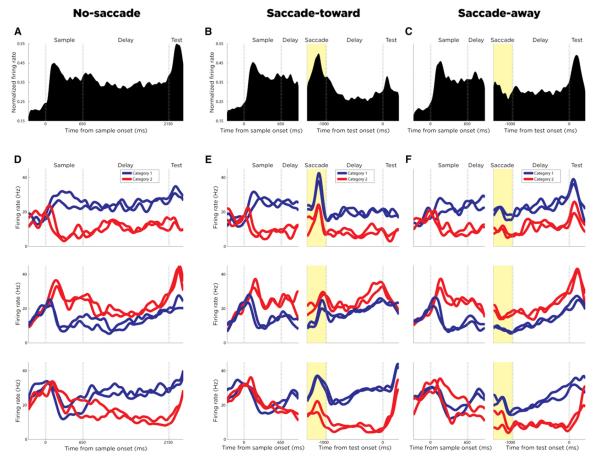
We recorded from 64 LIP neurons in two monkeys (monkey J: n = 35; monkey M: n = 29) during DMC task performance. Most analyses presented here focus on a subpopulation of 53 neurons (monkey J: n = 27; monkey M: n = 26) that differentiated between the two categories during the early delay (prior to the saccade cue), saccade, or late delay (after the saccade) periods of the DMC task in any task condition (Wilcoxon rank-sum test, p < 0.01; see Table 1). The populations of LIP neurons that were category selective did not show obvious differences in their spatial selectivity, delay activity, or presaccadic responses during the memory delayed saccade task when compared with neurons that were not category selective (see Figure S1 available online). During the DMC task, the LIP population showed a strong response to the sample (which was always presented in neurons’ RFs) and elevated activity during the delay in all three conditions (Figures 2A–2C). In the no-saccade condition, elevated LIP activity (compared to fixation) was maintained across the delay period and reached peak firing after test stimulus presentation (also within the RF). In the saccade-toward condition, the LIP population responded strongly around the time of the saccade—in fact, it reached a greater level of activity than during the response to the prior sample stimulus. In the saccade-away condition, average activity was slightly diminished around the time of the saccade.
Table 1.
Sample Category Selectivity by Epoch and Spatial Condition
| Sample | Early Delay (Presaccade) |
Saccade | Late Delay (Postsaccade) |
|
|---|---|---|---|---|
| No-saccade | 30 (47%) | 22 (34%) | 22 (34%) | 35 (55%) |
| Saccade-toward | 33 (52%) | 24 (38%) | 18 (28%) | 27 (42%) |
| Saccade-away | 27 (42%) | 21 (33%) | 18 (28%) | 29 (45%) |
The neuron count and percentage of the LIP population (n = 64) selective in various epochs and spatial conditions are shown. The early delay, saccade, and late delay periods in the no-saccade condition used the same window length and timing relative to sample/test onset as the epochs in the saccade conditions. Significance was determined by a Wilcoxon rank-sum test (p < 0.01, see Experimental Procedures).
Figure 2. Population Activity and Single Neuron Examples.
The yellow-shaded areas in saccade condition plots indicate the saccade periods.
(A–C) Normalized average population activity (across each neuron’s preferred category) in the no-saccade (A), saccade-toward (B), and saccade-away (C) condition is shown. In the no-saccade condition (A), the three vertical dotted lines indicate the sample onset, sample offset, and test onset. In the saccade conditions (B and C), the left panel is aligned on sample onset, while the right panel is aligned on test onset. The two vertical dotted lines in the left panel indicate sample onset and saccade cue. The two vertical dotted lines in the right panel indicate stable fixation after the saccade and test onset.
(D) Average activity to the four sample directions during the no-saccade condition for three example LIP neurons is shown. The blue and red traces correspond to the category membership of each direction.
(E and F) Average activity to the four sample directions during the saccade-toward and saccade-away conditions for the same three LIP neurons is shown.
In both the single neuron and population PSTHs, neuronal responses to test stimuli were of a similar strength in the no-saccade and saccade-away conditions, despite the test stimulus being presented at twice the eccentricity in the saccade-away condition. This is probably explained by the shape of some LIP RFs, which can be noncircular and/or extend far into the periphery (Ben Hamed et al., 2001). Thus, the test stimulus cannot be assumed to be outside the RF in the saccade conditions—particularly in the saccade-away condition. However, this is not a concern for this study as all main analyses are focused on neuronal responses prior to test onset.
As observed in previous studies, many LIP neurons showed activity that differed between the two categories during the sample and/or delay periods in the no-saccade condition of the DMC task (e.g., the three single neuron examples in Figure 2D). Interestingly, many LIP neurons also showed strong category selectivity during the saccade and delay periods of the saccade conditions despite the potential interference of strong saccade-related responses. Even for neurons that showed very strong responses during the saccade in the saccade-toward condition (e.g., Figure 2E), strong category selectivity was often maintained throughout the saccade itself and the subsequent memory delay. Likewise, many LIP neurons also showed strong category selectivity during the saccade and delay periods in the saccade-away condition (e.g., Figure 2F). Thus, the saccade and categorization processes both appeared to exert strong influences on the firing rates of such LIP neurons, and neuronal category signals were often robustly encoded even in the presence of strong spatial signals.
The strength of neuronal category selectivity was quantified using an ROC-based category tuning index (rCTI) that compared neuronal discrimination between pairs of directions in the same versus different categories (see Experimental Procedures). Index values of +0.5 indicate strong category selectivity—a large activity difference between directions in different categories and no difference between directions in the same category. Values of −0.5 indicate the opposite, while values of zero indicate no category selectivity. For each LIP neuron, the time course of category selectivity in the no-saccade and saccade conditions was examined by computing rCTI values in a sliding window (width = 200 ms, step size = 10 ms). In the no-saccade condition, LIP rCTI values increased sharply during the early sample period and were maintained across the sample and delay periods, indicating significant category selectivity across the LIP population (Figure 3A). As expected, rCTI values in the saccade conditions were indistinguishable from the no-saccade condition during the sample (one-way ANOVA, p = 0.85) and early delay (first 300 ms) (p = 0.97), because the three trial types were randomly interleaved and were identical during those time periods.
Figure 3. Category and Saccade Effects across the LIP Population.
(A) The time course of category selectivity measured by the rCTI in the no-saccade and both saccade conditions across the LIP population (n = 53). The yellow patch indicates the time at which the saccade occurs in the saccade conditions. The shaded patch around each solid trace indicates SEM.
(B) For each neuron in (A), the strength of late delay category selectivity (rCTI) in the no-saccade condition versus saccade-toward condition is shown.
(C) The strength of late delay category selectivity in the no-saccade condition versus saccade-away condition is shown.
Interestingly, elevated rCTI values were also observed during the saccade and late (postsaccadic) delay periods of the saccade-toward and saccade-away conditions (Figure 3A), and a similar time course of category selectivity was evident in all three conditions. During the saccade period itself, when LIP neurons showed strong saccade-related responses (Figures 2A–2C), rCTI values were statistically indistinguishable between the no-saccade and the two saccade conditions (one-way ANOVA comparing rCTI values in the three conditions, p = 0.97). Likewise, rCTI values in the late delay period of the saccade conditions were not significantly different than during the no-saccade condition (one-way ANOVA, p = 0.84). This indicates that the ability to read out sample category from trial-by-trial neuronal activity in LIP is minimally influenced by strong saccade-related neuronal responses and changes in eye position.
The previous analysis revealed that, on average across the population, both spatial and category signals were strongly and simultaneously encoded in LIP. We subsequently examined whether individual LIP neurons showed strong category selectivity in both the no-saccade and saccade conditions. Notwithstanding the example neurons in Figure 2, it is possible that neurons might show saccade-selective activity or category-selective activity, but not both. It is also possible that nonoverlapping populations of LIP neurons might show category signals in either the no-saccade or saccade conditions, but not both. This would indicate an interaction between category and spatial signals. Alternatively, individual neurons might show category encoding in both no-saccade and saccade conditions, which would suggest that category and spatial factors separately influence neurons’ firing rates. We examined neurons’ rCTI values in the no-saccade condition and each of the saccade conditions during the late delay and found that a majority of neurons exhibited positive rCTI values (indicating category selectivity) in both the no-saccade and saccade conditions (Figures 3B and 3C). While elevated rCTI values were typically observed in both the no-saccade and saccade conditions, we did not observe strong correlations between the rCTI values themselves across conditions. There was not a significant correlation betweenr CTI values in the no-saccade and saccade-toward condition (Pearson’s correlation coefficient, r = 0.18, p = 0.20), while the correlation coefficient just reached significance for the no-saccade versus saccade-away condition (r = 0.31, p = 0.02).
We employed a bootstrap analysis (see Experimental Procedures) to evaluate whether individual LIP neurons showed significantly elevated rCTI values in each of the three task conditions. Among neurons that showed significant category selectivity in the late delay epoch of the no-saccade condition (n = 26), a majority (n = 21 or 81%) also showed significant category selectivity during the late (postsaccadic) delay in one or both of the saccade conditions (Table 2). Similar results were observed during the saccade epoch of the saccade conditions and the corresponding epoch of the no-saccade condition (Table 2). This indicates that the robust category selectivity observed at the LIP population level during both the no-saccade and saccade conditions is also evident for a majority of individual LIP neurons, although there were small neuronal subpopulations that were selective for only one of the three spatial conditions.
Table 2.
Incidence and Overlap of Category Selectivity in No-Saccade and Saccade Conditions
| Saccade | Late Delay | |
|---|---|---|
| No saccade | n = 15 neurons | n = 26 neurons |
| No-saccade only | 6/15 (40%) | 5/26 (19%) |
| No-saccade and toward | 3/15 (20%) | 5/26 (19%) |
| No-saccade and away | 1/15 (7%) | 8/26 (31%) |
| All three conditions | 5/15 (33%) | 8/26 (31%) |
| No-saccade and at least one saccade condition |
9/15 (60%) | 21/26 (81%) |
| Saccade-toward only | 4/53 (8%) | 5/53 (9%) |
| Saccade-away only | 4/53 (8%) | 8/53 (15%) |
The numbers and percentages of neurons selective in no-saccade and saccade conditions are shown during the saccade epoch (and corresponding time period in the no-saccade condition) and late delay. Significance was determined by a bootstrap analysis (p < 0.01, see Experimental Procedures).
We also assessed the independence of category and saccade selectivity by asking whether neurons showed an interaction between category and saccade effects using a two-way ANOVA (p < 0.01) with sample category and saccade condition (no-saccade, saccade-toward, and saccade-away conditions) as factors (Table 3). During the saccade epoch, a minority of neurons that were category selective in that epoch (n = 9/30 or 30%) also showed a significant interaction with the saccade factor. During the late delay, just over half of the category-selective neurons in that epoch (n = 25/42 or 60%) showed an interaction between category and saccade factors. This suggests that category and spatial factors exert independent influences on the responses of a substantial fraction of individual LIP neurons, particularly during the time epoch right around the saccade. We examined the relative strength of selectivity for category and saccade factors by computing eta-squared values for each neuron using the sum of squares information provided by the same two-way ANOVA. The eta-squared value corresponds to the fraction of variability that can be accounted for by each factor in the ANOVA (e.g., category and saccade factors). Mean category and saccade eta-squared values (across all LIP neurons that showed any significant main effect in each epoch) along with the maximum individual value (across neurons) are shown for each task epoch in Table 3. Note that since the epochs are different sizes (e.g., the late delay is a greater duration than the saccade epoch), the incidence of neuronal selectivity and eta-squared values can be quantitatively compared within, but not between, epochs.
Table 3.
Incidence of Category and Saccade Selectivity across Task Epochs According to Two-Way ANOVA
| Sample | Early Delay | Saccade Late | Delay | |
|---|---|---|---|---|
| Category only | 39/53 (74%) | 36/53 (68%) | 10/53 (19%) | 5/53 (9%) |
| Saccade only | 0/53 (0%) | 0/53 (0%) | 12/53 (23%) | 9/53 (17%) |
| Category and saccade | 3/53 (6%) | 1/53 (2%) | 20/53 (38%) | 37/53 (70%) |
| Nonselective | 11/53 (21%) | 16/53 (30%) | 11/53 (21%) | 2/53 (4%) |
| Category eta-squared (mean) | 0.20 (max: 0.69) | 0.16 (max: 0.50) | 0.10 (max: 0.38) | 0.14 (max: 0.59) |
| Saccade eta-equared (mean) | 0.01 (max: 0.06) | 0.01 (max: 0.04) | 0.13 (max: 0.39) | 0.20 (max: 0.78) |
The numbers and percentages of neurons selective for category and saccade factors in each task epoch are shown. Significance was determined by a two-way ANOVA with category and saccade condition as factors (p < 0.01). Eta-squared values (which indicate the fraction of variability explained by each factor) were computed for each neuron. Mean eta-squared values across the population (excluding nonselective neurons in each epoch) are reported along with the maximum value (in parentheses) observed across neurons in each task epoch.
Relationship between Spatial and Category Signals in the LIP Population
A key question is whether there is a relationship between the strength of spatial selectivity and category selectivity across the LIP population. For example, LIP neurons that are strongly category selective might also tend to show strong spatial selectivity (a positive correlation). Alternatively, distinct subpopulations of LIP neurons could encode either spatial or category factors (a negative correlation). Spatial selectivity during the saccade epoch of the DMC task was quantified with a spatial tuning index (STI, Experimental Procedures). Values of the STI could vary from 1.0 to −1.0, where positive values indicate greater activity in the saccade-toward than saccade-away condition, and negative values indicate the opposite. STI values across the LIP population were, as expected, significantly shifted toward positive values (mean = 0.26; t test, p = 7 × 10−9; Figures 4A and 4B). Interestingly, there was no observed relationship between the saccade-period STI values and late delay rCTI values (during the no-saccade condition) across the LIP population (Figure 4A; Pearson’s correlation coefficient, r = 0.08, p = 0.57). Similarly, no relationship was observed between the saccade-period STI values and rCTI values from the portion of the delay period of the no-saccade condition that corresponds to the saccade epoch in the saccade conditions (Figure 4B; Pearson’s correlation coefficient, r = 0.08, p = 0.55). This suggests that the strengths of neuronal spatial and category selectivity are not correlated and further argues for independence of spatial and nonspatial information in LIP.
Figure 4. Spatial versus Category Encoding across the LIP Population.

For each neuron (n = 53), late delay period category selectivity (rCTI) in the no-saccade condition versus spatial selectivity (STI) in the DMC task is shown in (A). The same plot, using rCTI values from the saccade period, is shown in (B). The least-squares linear regression fit is indicated by the dotted line in each plot.
The results above suggest that population-level category signals in LIP are not significantly degraded by spatial signals related to saccadic eye movements and that category and saccade signals exert independent influences on LIP activity. However, an aim of this study is to better understand the rules governing how spatial and category signals are combined by individual neurons. The largely independent nature of category and saccade signals described above could indicate that these two signals are combined additively, in a manner in which each signal exerts a distinct influence on neuronal firing rates. An alternative is that the two signals could be combined in a multiplicative fashion, equivalent to a gain change in neuronal responsiveness. Previous studies have described both additive and multiplicative interactions between cognitive and sensory signals, particularly in studies examining the influence of spatial and object-based attention on visual feature selectivity throughout visual cortex (McAdams and Maunsell, 1999; Treue and Martínez Trujillo, 1999; Treue, 2001; Thiele et al., 2009). However, it is not known whether similar principles would apply to the interaction between category and spatial signals in parietal cortex.
To better understand how spatial and category signals are combined by LIP neurons, we examined both the influence of the saccade on neuronal category selectivity and the influence of neuronal category signal on the saccade response. Among all neurons that were category selective during the saccade epoch in the no-saccade or saccade-toward conditions (Wilcoxon rank-sum test, p < 0.01, n = 38), we compared the absolute difference in firing rate between the two categories in both the saccade-toward and no-saccade conditions. This revealed that the magnitude of category selectivity did not significantly differ in the saccade-toward (5.83 Hz) and no-saccade (6.41 Hz) conditions (paired t test, p = 0.57; Figure 5A), which would be more consistent with an additive than multiplicative interaction. To examine how the category signal influenced the saccade response, we computed the strength of the saccade signal (i.e., the difference in firing rate between the saccade-toward and no-saccade conditions) during the saccade epoch according to the sample category (preferred or nonpreferred) on that trial. This revealed that saccade responses were significantly weaker when sample stimuli had been in the preferred category (3.87 Hz) than nonpreferred category (6.85 Hz) according to a paired t test (p = 0.04; Figure 5B; see Figure S2 showing population PSTHs separately across neurons’ preferred and nonpreferred categories). This is inconsistent with a multiplicative interaction with a gain factor greater than 1.0 and could be consistent with an additive-like interaction if, for example, neuronal activity was near saturation in the preferred-category condition.
Figure 5. Interaction between Category and Spatial Signals in LIP.

(A) The absolute difference in firing rate between the two categories is shown during the no-saccade condition (early delay epoch) and saccade-toward condition (saccade epoch in saccade condition and corresponding early delay in no-saccade condition). Note that the average difference in firing rate between categories was similar in the saccade-toward and no-saccade conditions.
(B) The difference in firing rate, in the saccade epoch, between the saccade-toward and no-saccade conditions is shown separately for trials in which the sample had been in the nonpreferred (x axis) and preferred (y axis) categories. Note that saccade responses were weaker (on average) for the preferred than nonpreferred category. In both plots, the diagonal dotted line indicates the unity line, and the mean along each axis is indicated by the star symbol. The coefficient and p values of a linear correlation are shown in the upper left of each plot.
Weaker saccade responses for the preferred category and stronger saccade responses for the nonpreferred category during the saccade-toward condition could result in reduced category selectivity (compared to the no-saccade condition), as it would diminish the firing rate difference between the two categories in that epoch. However, the ROC-based rCTI measure of category selectivity showed that the strength of category selectivity during the saccade epoch was indistinguishable between the no-saccade and saccade-toward conditions (Figure 3A). To better understand this discrepancy, we examined the between category discrimination (BCD) and within category discrimination (WCD) values that were used to compute the rCTI. The time course of BCD and WCD values during the saccade-toward condition (across the population of 38 saccade epoch category-selective neurons, as above) is shown in Figure S3. Note that baseline WCD and BCD values above 0.5 are expected since the raw ROC values range from 0.0 to 1.0 and are rectified about 0.5 (see Experimental Procedures). During the saccade epoch, neuronal discriminability between directions in the same (WCD) and different (BCD) categories were not strongly or significantly influenced by the saccade (paired t tests comparing either BCD or WCD values in the early delay versus saccade epochs; WCD, p = 0.36; BCD, p = 0.59). This suggests that saccade-related responses, which differed between the preferred and nonpreferred categories as described above, had little effect on the neuronal discriminability between directions in the same or different categories.
The increased neuronal response in the saccade-toward condition might be expected to add variability in neuronal spike counts (assuming Poisson-like spiking statistics), which could interfere with category selectivity. For example, the rCTI analysis relies on ROC to measure the overlap between the distributions of firing rates to pairs of directions in both the same and different categories. An increase in spike count variance could increase the overlap in firing rate distributions and result in a decrease in ROC values between directions in opposite categories in the case that the mean difference in firing rate between categories remained constant. However, we found that category selectivity was not significantly different in the no-saccade and saccade-toward conditions (Figure 3A), even during the saccade epoch itself (when saccade-related responses were largest). To examine this, we examined the spike count variance between the no-saccade and saccade-toward conditions for each of the 38 saccade epoch category-selective (during the no-saccade or saccade-toward conditions) neurons used above across all correct trials. This revealed that there was not a significant difference in mean spike count variance between the no-saccade (variance = 5.70) and saccade-toward (variance = 5.99) conditions (paired t test, p = 0.56), despite a 5.24 spikes/s greater mean spike rate in the saccade-toward condition. Similar results were found when the same analysis was applied separately to trials in which neurons’ preferred or nonpreferred sample category had been presented (paired t test, preferred category: p = 0.62; nonpreferred category: p = 0.15). This suggests that the strength of category selectivity during the saccade epoch is not diminished in the saccade-toward condition because the saccade-related neuronal response does not add to the spike count variance.
DISCUSSION
Together, these results indicate that saccade-related spatial signals and nonspatial category signals in LIP are distinct at both the single-neuron and population levels. Many individual neurons that were category selective in the no-saccade version of the task also showed strong category selectivity in the saccade conditions—both during the saccade period itself and in the subsequent memory delay epoch. It is notable that, across the LIP population, the strengths of category selectivity during the saccade and late (postsaccadic) delay periods were statistically indistinguishable from those during the no-saccade condition. This is particularly striking as visual-spatial processing and saccade planning are traditionally thought to be cardinal functions of LIP and produce large neuronal responses that might have been expected to interfere with nonspatial category signals. Finally, there was no obvious relationship between spatial and category signals across the LIP population, suggesting independent encoding of these factors.
An interpretation of these results is that LIP plays a more central role in nonspatial cognitive processing than is often assumed, especially during complex behavioral tasks that require abstraction, working memory, or flexible sensory-motor mappings. This is consistent with a number of studies that found encoding of nonspatial and/or cognitive factors in parietal cortex (Sereno and Maunsell, 1998; Toth and Assad, 2002; Nieder et al., 2006; Oristaglio et al., 2006; Gottlieb and Snyder, 2010; Rao et al., 2012), and recent work suggests that LIP could be a source of these cognitive signals to other brain areas. For example, we recently demonstrated that LIP shows a stronger-and shorter-latency encoding of motion categories than the lateral PFC (Swaminathan and Freedman, 2012) and that explicit category signals are not observed in MT (Freedman and Assad, 2006), a key motion-processing area (Born and Bradley, 2005) that provides input to LIP (Lewis and Van Essen, 2000). However, it was previously unknown whether category signals in LIP could persist in the presence of strong task-irrelevant spatial signals. The present study shows that LIP’s capacity to encode nonspatial cognitive signals occurs independently of spatial processes related to saccadic eye movements, which are understood to be a key function of LIP. In fact, some neurons in our population showed a stronger encoding of category signals than saccade-related spatial signals. From these results, we speculate that, in addition to its spatial functions, LIP could play a central role in transforming sensory signals in earlier sensory-processing areas (such as MT) into more flexible and abstract signals (Freedman and Assad, 2006; Ferrera and Grinband, 2006) for the purpose of solving complex behavioral tasks.
Throughout the manuscript, we refer to “spatial” and “nonspatial” neuronal responses in LIP. The primary nonspatial neuronal signal considered here is the selectivity for the motion-direction categories that developed as a result of the subjects’ prior training on the DMC task. In this study, spatial responses can refer to the following interrelated spatial factors: (1) spatially selective visual responses triggered by the presence of either the visual motion stimulus or the saccade target within a neuron’s RF (Ben Hamed et al., 2001; Freedman and Assad, 2009) and (2) preparatory motor and/or motor responses related to the planning and execution of a saccadic eye movement either toward or away from a neuron’s RF (Snyder et al., 1997, 2000). A third spatial factor to consider is the deployment of spatial attention either toward or away from a neuron’s RF (Colby et al., 1996; Herrington and Assad, 2010; Bisley and Goldberg, 2010). However, this study was not explicitly designed to dissociate spatially selective saccadic and attentional effects. Although in principal, strong spatial signals related to any of these spatial factors could interfere or otherwise influence nonspatial visual category signals.
The limits of LIP’s involvement in mediating nonspatial and/or cognitive tasks remain to be determined. One recent study examined the relative contributions of LIP to spatial and nonspatial functions by employing reversible pharmacological inactivation of LIP during performance of several spatial tasks (e.g., a double-target saccade task) and nonspatial tasks (e.g., visual form discrimination) (Balan and Gottlieb, 2009). This study revealed significant behavioral deficits for the spatial tasks and for spatial aspects of the nonspatial tasks (i.e., spatially specific visual discrimination deficits). However, global nonspatial deficits were not observed. This was taken as evidence that LIP is primarily involved in spatial compared to nonspatial functions, and the authors suggested that LIP may play a relatively minor role in the computation of nonspatial signals (Balan and Gottlieb, 2009; Gottlieb and Snyder, 2010). However, a distinction with our current study is in the complexity and demands of the behavioral tasks in each study. For example, the tasks in the Balan and Gottlieb study had limited short-term memory demands and did not require subjects to classify stimuli according to a learned or abstract rule. A question to be tested in future studies is whether LIP is more involved in mediating nonspatial behavioral or perceptual tasks that involve abstraction, flexibility, or substantial working memory demands compared to simpler tasks such as visual discrimination. Inactivation of LIP during the motion categorization task would be expected to impair the subject’s ability to attend to the affected region of space and would produce spatially specific deficits in task performance similar to the Balan and Gottlieb study. Whether inactivation would also produce nonspatial categorization deficits, suggesting that LIP is causally involved in and necessary for the categorization process, remains to be determined.
The neuronal processing of spatial and object information is thought to rely on specialized and distinct processing stages in dorsal (parietal) and ventral (temporal) processing streams, respectively (Mishkin and Ungerleider, 1982). The current study, along with other recent work, suggests that LIP may play a role in integrating spatial information with nonspatial or cognitive stimulus attributes, a role that would be supported by LIP’s diverse interconnections with brain areas involved in sensory, motor, and cognitive processing (Lewis and Van Essen, 2000). However, the mechanisms by which multiple factors, such as category and saccade signals, are multiplexed in LIP and decoded by downstream areas remain unclear, as are the limits in the number of distinct signals that can be simultaneously encoded by the LIP population. Furthermore, the observation that orthogonal spatial and nonspatial cognitive factors exert independent influences on individual neurons’ firing rates places constraints on the mechanisms used for reading out such information.
An important related question to be examined in future work is to understand how neuronal category representations develop in the LIP population during the learning process. The finding that the LIP population can independently encode, or multiplex, category and spatial signals suggests that the same neuronal pool can be recruited in order to solve diverse behavioral tasks. One possibility is that native direction tuning in LIP observed prior to category training (Fanini and Assad, 2009) is transformed into abstract category representations via long-term training on the motion-direction category task. Recordings from LIP during the learning process itself could reveal how LIP neurons are recruited to represent the learned categories and to determine whether there is a relationship between neurons’ spatial or feature selectivity prior to learning and their patterns of category selectivity after learning.
While the current study points out that LIP is independently engaged by diverse spatial and nonspatial factors, the theoretical implications of this work regarding the overall function of LIP and relevance to existing models remains to be examined further. For example, an existing model of perceptual decision making in LIP employs a firing rate threshold bound that, when reached, triggers a saccade toward the RF in order to report the animal’s decision (Gold and Shadlen, 2007). The current study shows that both decision-related category information and saccade signals (which were not directly decision related in our study) can independently influence LIP activity, which implies a decoding mechanism that can take task-irrelevant spatial modulations into account during readout of a task-relevant perceptual signal (e.g., motion direction or category).
Category-, rule-, and decision-related representations in LIP are likely to be a relatively general phenomenon (Freedman and Assad, 2011) and not specific for visual-motion stimuli or the DMC task. For example, recent studies in parietal cortex found category-like encoding of learned shape associations (Fitzgerald et al., 2011), rule-related signals during a cognitive set-shifting task (Stoet and Snyder, 2004), and abstract decision signals during a perceptual decision task (Bennur and Gold, 2011). Despite strong neuronal correlates of cognitive functions such as categorization, abstract decision making, and our current finding that such cognitive signals are encoded independently of spatial signals, the hypothesis that LIP is causally involved and necessary for solving such cognitive tasks remains to be examined in future studies.
EXPERIMENTAL PROCEDURES
Behavioral Task
Monkeys were trained to perform a delayed match-to-category (DMC) task in which they indicated (by releasing a manual lever) whether a test stimulus was in the same category as a previously presented sample. On some trials, monkeys were cued to make a saccade during the early-delay period by relocating the fixation point to a new position on the display. Saccade and no-saccade trials were randomly interleaved. The range of saccade amplitudes (corresponding to the location of RF centers) tested during the DMC-task recording sessions was 8.9°–14.1°. In all conditions, monkeys had to release a manual touch bar if the test was a category match to the sample. Average reaction times on correct match trials were 307 ms (SD = 58 ms) and 350 ms (SD = 51 ms) for monkeys J and M, respectively.
Monkeys were required to fixate within 2° (monkey M) or 2°–3° (monkey J) of a 0.2° fixation spot throughout the trial, except during the saccade period. During no-saccade trials, the sample was followed by a 1,500 ms delay and one or two test stimuli (Figure 1B). On saccade trials, the sample was followed by a 300 ms delay, after which the fixation point was instantaneously extinguished and reappeared at a new location. The monkey had to reestablish fixation at the new location within 250 ms and maintain fixation for the remainder of the delay (1,000 ms) and test periods.
Visual Stimuli
Stimuli were circular patches (~5° diameter) of ~100 high-contrast square dots that moved with 100% coherence at a speed of 12°s−1. Monkeys were trained on the DMC task using a large number (n > 22) of unique motion directions. During recordings, four directions (0°, 90°, 180°, and 270°; Figure 1A) and six directions (15°, 75°, 135°, 195°, 255°, and 315°) were used as sample and test stimuli, respectively. Sample stimuli were presented as close to the center of neurons’ RFs as possible such that saccades of equal magnitude toward and away from the RF could be cued on the display. Sample and test stimuli were presented at the same location on the display. Up until the time that the fixation point was relocated (on saccade trials), the no-saccade and saccade conditions were identical.
RF-Mapping Procedures
Prior to running the DMC task, IPS neurons were tested with a memory-saccade task in order to identify area LIP and to determine the locations of neuronal RFs (which defined the position of stimuli and saccade targets during the DMC task). Most IPS neurons were also tested with a “flash-mapping” task that displayed a sparse noise stimulus using a 5 × 5 grid of locations centered in the contralateral hemifield during passive fixation (Ben Hamed et al., 2001). The range of saccade amplitudes tested during the memory-saccade task varied among neurons according to the average position of RFs encountered on that electrode penetration but was typically in a range of 6.0°–14.0°. Neurons were considered to be in LIP if they showed spatially selective visual responses, delay activity, and/or presaccadic responses during the memory-saccade task. The flash-mapping task was used to better identify RF position and shape for neurons that showed visual responses during that task. Neuronal data during both RF-mapping tasks was analyzed in real time during recordings by automated MATLAB scripts that generated plots that displayed neuronal spatial selectivity. Sample stimuli during the DMC task were always presented within LIP RFs.
Monitoring Eye Position
Gaze positions were measured using an EyeLink 1000 optical eye tracker (SR Research) at a sampling rate of 1.0 kHz and stored for offline analysis. A MATLAB-based library of routines (Monkeylogic, http://www.monkeylogic.net) was used to control task events, stimuli, and reward and to monitor and store behavioral events (Asaad and Eskandar, 2008; Asaad et al., 2013). Stimuli were displayed on a 21 inch color CRT monitor (1,280 × 1,024 resolution, 75 Hz, 57 cm viewing distance).
Physiological Techniques
All surgical and experimental procedures followed the University of Chicago’s Animal Care and Use Committee and US National Institutes of Health guidelines. Two male rhesus monkeys (Macaca mulatta, weighing ~8–10 kg) were implanted with a head post and recording chamber. Recording chambers were implanted over the intraparietal sulcus (IPS) according to coordinates (~3.0 mm posterior to the intra-aural line) determined by MRI scans obtained prior to implantation of the head post and recording chamber.
LIP recordings were conducted using single 75 μm tungsten microelectrodes (FHC), a dura piercing guide tube, and microdrive system (NAN Instruments). Neurophysiological signals were amplified, digitized, and stored for offline spike sorting (Plexon) to verify the quality and stability of neuronal isolations. Because anatomical MRI images were obtained prior to chamber implantation, no claims are made with respect to the anatomical subdivisions of LIP from which our neurophysiological recordings were made.
Data Analysis
All analyses were conducted across correct trials. The pattern of behavioral and neuronal results was similar, and all main effects were observed in both monkeys. Thus, the two data sets were combined for all population analyses.
Neuronal selectivity between pairs of motion directions was evaluated using a receiver operating characteristic (ROC) analysis (Green and Swets, 1966; Tolhurst et al., 1983) applied to the trial-by-trial firing rates to each motion direction. The ROC analysis was applied to the distributions of trial-by-trial firing rates for the four pairs of directions that were 90° apart. This analysis, applied separately to each analysis epoch, quantified each neuron’s ability to discriminate between pairs of directions on a scale of 0.5 (no discrimination) to 1.0 (perfect discrimination). Average ROC values for each neuron were computed for the two pairs containing directions in different categories (BCD) and the two pairs containing directions in the same categories (WCD).
A category selectivity index (rCTI) was computed for each neuron by subtracting the WCD from the BCD. Values of the rCTI could vary from +0.5 (strong binary-like differences in activity to directions in the two categories) to −0.5 (large activity differences between directions in the same category, no difference between categories). An rCTI value of 0.0 indicates the same difference in ROC value between and within categories. The significance of rCTI values was determined for each neuron using a bootstrap analysis in which sample direction labels were repeatedly permuted. The rCTI value from the actual order of sample presentation was then compared to a distribution of 100,000 permutations and using a p < 0.01 significance threshold. Very similar results were obtained using an ROC that compared firing rates on all trials for each category and a CTI applied to average firing rates for each direction (Swaminathan and Freedman, 2012). The rCTI has the advantage of taking into account neuronal tuning with respect to the category boundary and the reliability of category selectivity from a readout perspective.
We verified that the rCTI was not inherently biased (i.e., shifted toward positive or negative values) by computing the index for sets of 60 randomly generated values (simulating the firing rates to 15 trials of the 4 sample directions) that could vary from 0 to 100. We repeated this process one million times to generate a distribution of rCTI values and found that the mean index value (0.000022) was not significantly different from zero (t test, p = 0.65).
Spatial selectivity during the DMC task was evaluated using a spatial tuning index (STI) computed during the saccade epoch of the DMC task. The STI was defined (during the saccade epoch) as the firing rate difference between saccades toward and away from the neuron’s RF divided by the maximum firing rate observed to those two saccades. Values of the STI could vary from +1.0 (high activity for toward-RF saccades and no activity during saccades away from the RF) and values of −1.0 indicate the opposite. An STI of 0.0 indicates the same activity for saccades toward and away from the RF. The RF location for each neuron (which defined the position of the saccade targets during the DMC task) was determined by RF-mapping tasks (memory-saccade and flash-mapping tasks) prior to running the main DMC task. Thus, neurons with near-zero or negative STI values indicate neurons that showed weaker or different patterns of spatial selectivity during the DMC task compared to RF-mapping tasks, or saccade responses that varied between categories.
Epoch-based analyses were conducted in time windows corresponding to phases of the task: fixation, sample, early (presaccadic) delay, saccade, and late (postsaccadic) delay. The fixation epoch was a 500 ms window ending at sample onset. The sample epoch was a 650 ms window that began 80 ms after sample onset (to account for neuronal response latencies). For no-saccade trials, early delay (300 ms, beginning 80 ms after sample offset) and late delay (1,080 ms, beginning 1,000 ms prior to test onset) epochs were used. Saccade trials had a 300 ms early (presaccadic) delay epoch beginning 80 ms after sample offset, followed by a saccade epoch (250 ms, ending 80 ms after the monkey fixated the new fixation target), and a 1,080 ms late (postsaccadic delay) epoch (beginning after fixation had been maintained for 150 ms at the target location). Similar results were obtained with a variety of window widths and starting points.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Jonathan Hodnefield, Adam Stevenson, Steve McClellan, Navaneethan Santhanam, and Stephanie Thomas for technical assistance and the staff of The University of Chicago Animal Resources Center for expert veterinary assistance. We also thank the following for helpful discussions and/or comments on this manuscript: John Assad, Jamie Fitzgerald, Jared Clemens, Nicolas Masse, Doug Ruff, Sruthi Swaminathan, and Jonathan Wallis. This work was supported by NIH R01 EY019041. Additional support was provided by an NSF CAREER award, a McKnight Scholar award, the Alfred P. Sloan Foundation, and The Brain Research Foundation. D.J.F. and C.A.R. designed the experiments and wrote the manuscript. C.A.R. trained the animals and performed all neurophysiological recordings and data analysis. D.J.F. assisted in animal training, neurophysiological recordings, and data analysis. G.H. assisted in animal training, neurophysiological recordings, behavioral data analysis, and edited the manuscript.
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information includes three figures and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2013.01.007.
REFERENCES
- Asaad WF, Eskandar EN. A flexible software tool for temporally-precise behavioral control in Matlab. J. Neurosci. Methods. 2008;174:245–258. doi: 10.1016/j.jneumeth.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaad WF, Santhanam N, McClellan S, Freedman DJ. High-performance execution of psychophysical tasks with complex visual stimuli in MATLAB. J. Neurophysiol. 2013;109:249–260. doi: 10.1152/jn.00527.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan PF, Gottlieb J. Functional significance of nonspatial information in monkey lateral intraparietal area. J. Neurosci. 2009;29:8166–8176. doi: 10.1523/JNEUROSCI.0243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Hamed S, Duhamel JR, Bremmer F, Graf W. Representation of the visual field in the lateral intraparietal area of macaque monkeys: a quantitative receptive field analysis. Exp. Brain Res. 2001;140:127–144. doi: 10.1007/s002210100785. [DOI] [PubMed] [Google Scholar]
- Bennur S, Gold JI. Distinct representations of a perceptual decision and the associated oculomotor plan in the monkey lateral intraparietal area. J. Neurosci. 2011;31:913–921. doi: 10.1523/JNEUROSCI.4417-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu. Rev. Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born RT, Bradley DC. Structure and function of visual area MT. Annu. Rev. Neurosci. 2005;28:157–189. doi: 10.1146/annurev.neuro.26.041002.131052. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J. Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- Cromer JA, Roy JE, Miller EK. Representation of multiple, independent categories in the primate prefrontal cortex. Neuron. 2010;66:796–807. doi: 10.1016/j.neuron.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanini A, Assad JA. Direction selectivity of neurons in the macaque lateral intraparietal area. J. Neurophysiol. 2009;101:289–305. doi: 10.1152/jn.00400.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrera VP, Grinband J. Walk the line: parietal neurons respect category boundaries. Nat. Neurosci. 2006;9:1207–1208. doi: 10.1038/nn1006-1207. [DOI] [PubMed] [Google Scholar]
- Ferrera VP, Yanike M, Cassanello C. Frontal eye field neurons signal changes in decision criteria. Nat. Neurosci. 2009;12:1458–1462. doi: 10.1038/nn.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JK, Freedman DJ, Assad JA. Generalized associative representations in parietal cortex. Nat. Neurosci. 2011;14:1075–1079. doi: 10.1038/nn.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DJ, Assad JA. Experience-dependent representation of visual categories in parietal cortex. Nature. 2006;443:85–88. doi: 10.1038/nature05078. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Assad JA. Distinct encoding of spatial and nonspatial visual information in parietal cortex. J. Neurosci. 2009;29:5671–5680. doi: 10.1523/JNEUROSCI.2878-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DJ, Assad JA. A proposed common neural mechanism for categorization and perceptual decisions. Nat. Neurosci. 2011;14:143–146. doi: 10.1038/nn.2740. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291:312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. A comparison of primate prefrontal and inferior temporal cortices during visual categorization. J. Neurosci. 2003;23:5235–5246. doi: 10.1523/JNEUROSCI.23-12-05235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu. Rev. Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Goodwin SJ, Blackman RK, Sakellaridi S, Chafee MV. Executive control over cognition: stronger and earlier rule-based modulation of spatial category signals in prefrontal cortex relative to parietal cortex. J. Neurosci. 2012;32:3499–3515. doi: 10.1523/JNEUROSCI.3585-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb J, Snyder LH. Spatial and non-spatial functions of the parietal cortex. Curr. Opin. Neurobiol. 2010;20:731–740. doi: 10.1016/j.conb.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. John Wiley & Sons; New York: 1966. [Google Scholar]
- Herrington TM, Assad JA. Temporal sequence of attentional modulation in the lateral intraparietal area and middle temporal area during rapid covert shifts of attention. J. Neurosci. 2010;30:3287–3296. doi: 10.1523/JNEUROSCI.6025-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J. Comp. Neurol. 2000;428:112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH. Effects of attention on orientationtuning functions of single neurons in macaque cortical area V4. J. Neurosci. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav. Brain Res. 1982;6:57–77. doi: 10.1016/0166-4328(82)90081-x. [DOI] [PubMed] [Google Scholar]
- Nieder A, Diester I, Tudusciuc O. Temporal and spatial enumeration processes in the primate parietal cortex. Science. 2006;313:1431–1435. doi: 10.1126/science.1130308. [DOI] [PubMed] [Google Scholar]
- Oristaglio J, Schneider DM, Balan PF, Gottlieb J. Integration of visuospatial and effector information during symbolically cued limb movements in monkey lateral intraparietal area. J. Neurosci. 2006;26:8310–8319. doi: 10.1523/JNEUROSCI.1779-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V, DeAngelis GC, Snyder LH. Neural correlates of prior expectations of motion in the lateral intraparietal and middle temporal areas. J. Neurosci. 2012;32:10063–10074. doi: 10.1523/JNEUROSCI.5948-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JE, Riesenhuber M, Poggio T, Miller EK. Prefrontal cortex activity during flexible categorization. J. Neurosci. 2010;30:8519–8528. doi: 10.1523/JNEUROSCI.4837-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno AB, Maunsell JH. Shape selectivity in primate lateral intraparietal cortex. Nature. 1998;395:500–503. doi: 10.1038/26752. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Intention-related activity in the posterior parietal cortex: a review. Vision Res. 2000;40:1433–1441. doi: 10.1016/s0042-6989(00)00052-3. [DOI] [PubMed] [Google Scholar]
- Stoet G, Snyder LH. Single neurons in posterior parietal cortex of monkeys encode cognitive set. Neuron. 2004;42:1003–1012. doi: 10.1016/j.neuron.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Swaminathan SK, Freedman DJ. Preferential encoding of visual categories in parietal cortex compared with prefrontal cortex. Nat. Neurosci. 2012;15:315–320. doi: 10.1038/nn.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele A, Pooresmaeili A, Delicato LS, Herrero JL, Roelfsema PR. Additive effects of attention and stimulus contrast in primary visual cortex. Cereb. Cortex. 2009;19:2970–2981. doi: 10.1093/cercor/bhp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst DJ, Movshon JA, Dean AF. The statistical reliability of signals in single neurons in cat and monkey visual cortex. Vision Res. 1983;23:775–785. doi: 10.1016/0042-6989(83)90200-6. [DOI] [PubMed] [Google Scholar]
- Toth LJ, Assad JA. Dynamic coding of behaviourally relevant stimuli in parietal cortex. Nature. 2002;415:165–168. doi: 10.1038/415165a. [DOI] [PubMed] [Google Scholar]
- Treue S. Neural correlates of attention in primate visual cortex. Trends Neurosci. 2001;24:295–300. doi: 10.1016/s0166-2236(00)01814-2. [DOI] [PubMed] [Google Scholar]
- Treue S, Martínez Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399:575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature. 2001;411:953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.