Abstract
Purpose
We conducted a safety and efficacy evaluation of intraprostatic injection of PRX302, a modified pore forming protein (proaerolysin) activated by prostate specific antigen, as a highly targeted, localized approach to treat lower urinary tract symptoms due to benign prostatic hyperplasia.
Materials and Methods
A total of 92 patients with I-PSS (International Prostate Symptom Score) 15 or greater, peak urine flow 12 ml or less per second and prostate volume 30 to 100 ml were randomized 2:1 to a single ultrasound guided intraprostatic injection of PRX302 vs vehicle (placebo) in this phase IIb double-blind study. Injection was 20% of prostate volume and 0.6 μg PRX302 per gm prostate. Peak urine flow was determined by a blinded reviewer. Benign prostatic hyperplasia medications were prohibited. The primary data set of efficacy evaluable patients (73) was analyzed using last observation carried forward.
Results
PRX302 treatment resulted in an approximate 9-point reduction in I-PSS and 3 ml per second increase in peak urine flow that were statistically significant changes from baseline compared to vehicle. Efficacy was sustained for 12 months. Early withdrawal for other benign prostatic hyperplasia treatment was more common for patients in the vehicle group. Relative to vehicle, PRX302 apparent toxicity was mild, transient, and limited to local discomfort/pain and irritative urinary symptoms occurring in the first few days, with no effect on erectile function.
Conclusions
A single administration of PRX302 as a short, outpatient based procedure was well tolerated in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. PRX302 produced clinically meaningful and statistically significant improvement in patient subjective (I-PSS) and quantitative objective (peak urine flow) measures sustained for 12 months. The side effect profile is favorable with most effects attributed to the injection itself and not related to drug toxicity.
Keywords: aerolysin, PRX302, prostatic hyperplasia, lower urinary tract symptoms
Benign prostatic hyperplasia is the enlargement of the prostate gland commonly seen in older men1,2 that leads to lower urinary tract symptoms.3 Current medical treatment options for patients presenting with these symptoms include 5-ARIs4,5 and α-adrenergic blockers,6 both of which may cause drug related adverse events and to which the condition may become refractory.7,8 The reported side effects of oral therapies include sexual adverse effects (eg impotence, erectile dysfunction, retrograde ejaculation), decreased libido, gynecomastia, dizziness, somnolence and postural hypotension.9–11 Patients for whom medical therapies are ineffective or who present with complications such as refractory urinary retention are usually offered more invasive surgical options including prostatectomy via TURP, open surgery or other minimally invasive surgical therapies. Surgical approaches are associated with significant morbidity, including perioperative complications, urinary incontinence, bleeding requiring transfusions and sexual side effects, and re-treatment may be required within 3 years.10,12
PRX302 is a genetically modified form of proaerolysin in which the native furin activation site has been replaced with a sequence that is highly specific for enzymatically active PSA, present only in prostate tissue. PRX302 remains inactive in the absence of enzymatically active PSA in vitro and in vivo,13 and is not activated outside of the prostate by PSA in circulation due to inhibition by serum protease inhibitors. After activation, PRX302 spontaneously oligomerizes and forms a stable transmembrane heptameric pore that leads to cell death (fig. 1). After injection into the transition zone, PSA activation of PRX302 can lead to ablation of tissue that may alleviate LUTS. On this basis PRX302 is being clinically developed as a highly targeted, localized therapy for symptomatic BPH administered as a single, brief, clinic based, intraprostatic injection that is expected to avoid many of the side effects associated with oral and surgical therapies. The favorable safety and efficacy of PRX302 in earlier phase I and II open label clinical trials have been previously reported.14 To our knowledge the current trial was the first placebo (vehicle) controlled evaluation of the intraprostatic injection of PRX302 in subjects with moderate to severe LUTS secondary to BPH.
Figure 1.
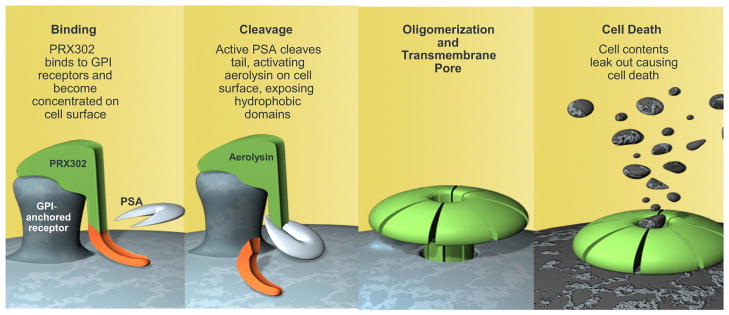
PRX302 binding to cell membrane, cleavage and activation, and formation of transmembrane pore. GPI, glycosylphosphatidyl inositol.
MATERIALS AND METHODS
Subject Selection
Entry criteria specified men age 40 to 80 years with moderate to severe LUTS, I-PSS 15 or greater, PV 30 to 100 ml, Qmax 12 ml or less per second, PVR less than 200 ml, ability to void 150 ml urine or greater and serum PSA less than 4 ng/ml or 4 to 10 ng/ml if prostate cancer was ruled out. Subjects were untreated with, intolerant of, or had symptoms refractory to α-blockers and/or 5-ARIs. In addition, they were off treatment with α-blockers for at least 4 weeks and off 5-ARIs for at least 6 months.
Randomization and Study Drug Administration
This was a prospective, randomized, double-blind, vehicle controlled study of a single transperineal intraprostatic injection of PRX302 under TRUS guidance conducted under Good Clinical Practices after institutional review board approval. Subjects were randomly assigned in a ratio of 2:1 (PRX302-to-vehicle), stratified by baseline PV (45 ml or less vs greater than 45 ml) and I-PSS total score (19 or less vs 20 or greater). PRX302 was administered at a volume equivalent to 20% of PV and at a fixed concentration of 3.0 μg/ml for a fixed dose of 0.6 μg/gm prostate. Vehicle treatment consisted of injection of only the diluent (2% human serum albumin) at the same 20% of PV. Administration consisted of 1 needle stick in the transition zone of each lobe of the prostate (left and right), with generally 3 deposits per lobe along the needle track as the needle was retracted.
Assessments
Assessments were at screening; study drug treatment (day 0); and days 3 to 5 and 14; and months 1, 3, 6, 9 and 12 after treatment. The primary efficacy end point was I-PSS total score change from baseline. Additional efficacy end points included I-PSS subscores, disease specific QoL (I-PSS question 8), Qmax, voided volume, percent voided volume, PVR, PV, and I-PSS and Qmax responders. Safety assessments included AEs, physical examinations, vital signs, laboratory values, electrocardiograms, IIEF (International Index of Erectile Function) and antibodies to PRX302. The primary analysis was at month 3 after injection and subjects were to be followed for a total of 12 months.
Statistical Methods
The protocol defined primary analysis population was the EE population of subjects, defined as those who received the full treatment, completed the month 3 assessments and had no major protocol violation as determined by a blinded, independent review panel of urology experts. The ITT and safety populations consisted of all subjects who received any study drug. Efficacy analyses used the last observation carried forward method to impute missing post-baseline data. The ANCOVA model (for continuous variables) and logistic regression model (for responder analyses) included treatment group, study site, age, baseline I-PSS total score, baseline PV and all major variables having significant imbalances (p <0.10) at baseline as factors. All statistical testing was 2-sided and performed at the 0.05 significance level, with no statistical adjustments for multiple testing.
RESULTS
Subject Baseline Characteristics and Disposition
A total of 92 subjects were randomized and treated (ITT population), consisting of 61 treated with PRX302 and 31 treated with vehicle across 9 study sites in Canada from February to September 2009. The study completion rate was 90.3% (28 of 31) for vehicle vs 98.3% (60 of 61) for PRX302 for month 3 and 67.7% (21 of 31) vs 85.7% (54 of 61), respectively, for the entire 12 months. The most common reason for premature withdrawal was incomplete symptom resolution with need for further BPH treatment and occurred in 16.1% (5 of 31) of vehicle subjects vs 3.2% (2 of 61) of PRX302 subjects. Other reasons for withdrawal were subject request (9.7% vehicle vs 6.6% PRX302), lost to followup (6.5% vehicle vs 0% PRX302) and nondrug related AE (0% vehicle vs 1.6% PRX302).
Of the ITT subjects 10 PRX302 and 9 vehicle were excluded from the EE population for lacking month 3 assessment (4 subjects) or for a major protocol violation (15 subjects) such as receiving a medication potentially affecting I-PSS score, undergoing cystoscopy, having prostatitis and having difficulty understanding the I-PSS questionnaire. Baseline subject characteristics are shown in table 1. The treatment groups were reasonably well balanced for baseline characteristics.
Table 1.
Baseline subject characteristics of EE population
| Vehicle | PRX302 | p Value (t test) | |
|---|---|---|---|
| No. pts | 21 | 52 | |
| Mean ± SD age | 64.5 ± 7.1 | 63.7 ± 8.8 | 0.72 |
| No. race (%): | |||
| Caucasian | 21 (100) | 49 (94.2) | 1.00* |
| Asian | 0 | 1 (1.9) | |
| Other | 0 | 2 (3.8) | |
| Mean ± SD I-PSS total score (possible range 0–35) | 22.5 ± 4.3 | 23.9 ± 4.9 | 0.24 |
| Mean ± SD gm PV | 49.9 ± 16.0 | 48.6 ± 15.2 | 0.74 |
| Mean ± SD ml/sec Qmax | 8.9 ± 1.2 | 8.4 ± 2.3 | 0.34 |
| Mean ± SD QoL score (possible range 0–6) | 4.3 ± 1.0 | 4.4 ± 0.9 | 0.70 |
| Mean ± SD ml % voided vol | 68.8 ± 11.1 | 75.7 ± 17.2 | 0.09 |
| Mean ± SD ml PVR | 105 ± 43 | 73 ± 60 | 0.03 |
| Mean ± SD (ng/ml) serum PSA | 3.1 ± 2.6 | 3.2 ± 2.3 | 0.89 |
| Mean ± SD IIEF total score (possible range 5–75) | 45.9 ± 21.7 | 38.0 ± 23.1 | 0.19 |
Fisher’s exact test.
International Prostate Symptom Score
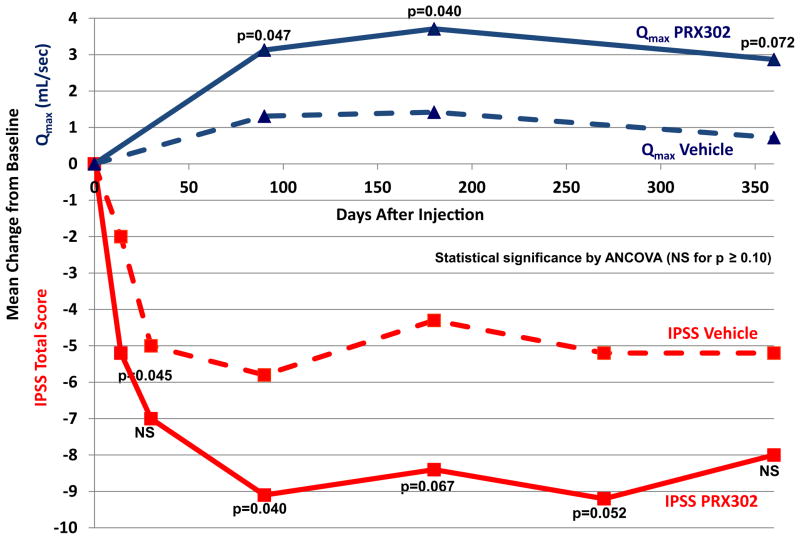
PRX302 treatment resulted in an approximate 9-point average reduction of I-PSS that was sustained for 12 months. This improvement in I-PSS total score was clinically meaningful (3-point or greater reduction) and superior to vehicle, and was apparent from the first post-baseline assessment and sustained during the 12 months of observation (fig. 2). Mean change from baseline treatment effect (PRX302 minus vehicle) was 3.3 points at month 3 (ANCOVA p = 0.040), and 4.1 (p = 0.067), 4.0 (p = 0.052) and 2.8 points (p>0.05) at months 6, 9 and 12, respectively.
Figure 2.
Change from baseline for I-PSS total score and Qmax
Peak Urine Flow
PRX302 treatment resulted in an approximate 3 ml per second average increase in Qmax that was sustained for 12 months. The improvement in Qmax for PRX302 was clinically meaningful and superior to vehicle, apparent from the first post-baseline assessment and sustained for the 12 months of observation (fig. 2). Mean change from baseline treatment effect (PRX302 minus vehicle) was 1.8 ml per second at month 3 (ANCOVA p = 0.047), 2.3 ml per second at month 6 (p = 0.040) and 2.2 ml per second at month 12 (p = 0.072).
Other Efficacy End Points
The changes from baseline to month 3 and the end of study for other secondary efficacy end points are presented in table 2. There were more I-PSS and Qmax responders for PRX302 than for vehicle at each post-baseline point. Changes in other end points, including PV as measured by TRUS, were generally small, and without clinically meaningful differences between PRX302 and vehicle.
Table 2.
Changes from baseline of other efficacy end points for EE population
| Mean ± SD Vehicle |
Mean ± SD PRX302 |
p Value* (ANCOVA) | |
|---|---|---|---|
| QoL score (possible range 0–6): | |||
| Mo 3 | −1.0 ± 1.4 | −1.4 ± 1.4 | 0.392 |
| Mo 12 | −1.0 ± 1.6 | −1.2 ± 1.3 | 0.271 |
| % Voided vol (ml): | |||
| Mo 3 | 5.4 ± 15.8 | 0.8 ± 16.0 | 0.827 |
| Mo 12 | 2.0 ± 19.5 | −0.7 ± 16.0 | 0.729 |
| PVR (ml): | |||
| Mo 3 | −0.7 ± 86.7 | 3.6 ± 62.9 | 0.424 |
| Mo 12 | 5.3 ± 87.4 | 12.2 ± 60.6 | 0.443 |
| PV (gm): | |||
| Mo 3 | −9.5 ± 9.6 | −6.1 ± 8.4 | 0.298 |
| Mo 6 (no mo 12 assessment) | −4.4 ± 10.0 | −2.2 ± 9.4 | 0.440 |
Serum PSA
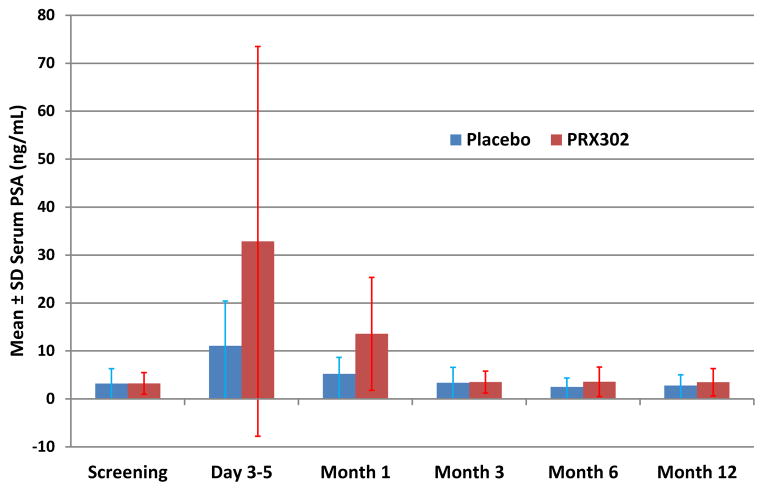
At 3 to 5 days after injection, serum PSA increased from baseline tenfold for PRX302 vs fourfold for vehicle (fig. 3), and then gradually trended toward baseline without a meaningful change from baseline for either treatment group.
Figure 3.
Change from baseline for serum PSA
Safety
There were no deaths and no serious or grade 3 or greater AEs that were drug related. One subject who received PRX302 experienced 4 nondrug related serious AEs (multiple myeloma, renal calculus, stent pain and postoperative respiratory insufficiency) beginning nearly 1 year after injection. The most common AEs are presented in table 3. Based on AE incidence for PRX302 compared to vehicle, the most likely drug related AEs appear to be dysuria, pollakiuria, micturition urgency, perineal pain and malaise. In the PRX302 group these AEs were nearly exclusively mild or moderate, had onset primarily on the day of injection and rarely more than 4 days later, and all had a median duration of less than 2 days. The data do not suggest any drug related impairment of sexual function based on AE reporting and IIEF. No hypersensitivity reactions were observed.
Table 3.
Adverse events occurring in 5% or more of subjects in either treatment group (safety population)
| No. Vehicle (%)* | No. PRX302 (%)* | p Value (Fisher’s exact test) | |
|---|---|---|---|
| Hematuria | 11 (35.5) | 18 (29.5) | 0.64 |
| Dysuria | 2 (6.5) | 17 (27.9) | 0.027 |
| Pollakiuria | 5 (16.1) | 14 (23.0) | 0.59 |
| Micturition urgency | 3 (9.7) | 13 (21.3) | 0.25 |
| Perineal pain | 0 (0.0) | 7 (11.5) | 0.091 |
| Vertigo | 2 (6.5) | 4 (6.6) | 1.00 |
| Malaise | 0 (0.0) | 4 (6.6) | 0.30 |
| Insomnia | 3 (9.7) | 2 (3.3) | 0.33 |
| Arthralgia | 2 (6.5) | 2 (3.3) | 0.60 |
| Hematospermia | 2 (6.5) | 2 (3.3) | 0.60 |
| Nocturia | 2 (6.5) | 2 (3.3) | 0.60 |
| Prostatic pain | 3 (9.7) | 1 (1.6) | 0.11 |
| Headache | 2 (6.5) | 1 (1.6) | 0.26 |
| Urinary retention | 3 (9.7) | 0 (0.0) | 0.036 |
| Anxiety | 2 (6.5) | 0 (0.0) | 0.11 |
| Diarrhea | 2 (6.5) | 0 (0.0) | 0.11 |
MedDRA (Medical Dictionary for Regulatory Activities) coded preferred terms.
Number of patients with 1 or more events of that nature.
Anti-PRX302 Antibodies
After injection 73% (43 of 59) of PRX302 subjects vs 0% (0 of 28) of vehicle subjects had new or increased titers of APA, which were highest at month 3 and did not exceed a titer of 1:2,560. APA titers decreased from months 3 to 6 and decreased further from months 6 to 12. At exit from the study all but 1 subject had titer 1:320 or less. In 80% (16 of 20) of subjects APA neutralized PRX302 cytolytic activity in vitro.
DISCUSSION
LUTS secondary to BPH are highly prevalent, with 50% of men older than age 50 years and 70% of those older than age 70 years complaining of some form of these symptoms. According to treatment guidelines for bothersome LUTS provided by all relevant associations, treatment should start with an α-blocker and/or 5-ARI. Patients presenting with more severe symptoms or those with progression on oral therapy are traditionally treated with TURP. These patients are usually older, have many comorbid factors, are frequently anticoagulated and often considered high risk for surgery. Many alternative minimally invasive treatment options continue to emerge. Most of the options that aim to create a TURP-like cavity within the prostate usually require anesthesia and postoperative catheterization, which limit access for some patients and add to the cost. Such procedures are also associated with a high rate of reoperation.
There remains an obvious need for a safe and effective injectable agent that would target prostatic cells specifically, with minimal or no collateral damage to normal tissues. Such a designer drug would be an ideal approach for treating BPH and focal prostate cancer since the prostate produces high levels of the protease PSA, which is uniquely active only in the prostate and enzymatically inactivated upon entering the bloodstream. Nonclinical studies have demonstrated that PRX302 effectively kills target tissue without damaging adjacent or distant normal tissues, and is selectively activated by the PSA present in the prostate.
Patients frequently stop oral medical therapy for lack of efficacy, side effects or noncompliance with the requirement of taking 1 or more pills daily. These patients eventually need an alternative. PRX302 provides an attractive choice compared to daily oral medications by virtue of being a single administration with potentially fewer side effects and a greater reduction in LUTS. It has advantages compared to surgery and minimally invasive surgical therapies by virtue of being less invasive with fewer side effects and complications.
In phase I–IIa studies of PRX302 no dose limiting toxicities were reported and the maximum tolerable dose was not reached.14 Based on the definition of 30% improvement in I-PSS score as a responder, 64% and 67% of subjects in phase I and IIa, respectively, were responders at 1 year.14 Patients in these studies had 9 to 12-point reductions in I-PSS that were sustained for 1 year of followup. In this phase IIb study a fixed dose concentration with a PV adjusted injection volume (20%) was administered vs vehicle. Patients treated with PRX302 had an average approximate 9-point reduction in I-PSS that was sustained during 1 year of followup. It is well-known, primarily based on the subjective outcomes for BPH treatment, that treatment options for BPH are associated with a high placebo effect. This placebo effect must be factored into the trial design to determine if the treatment is truly producing benefit. For the primary efficacy outcome in this study of I-PSS total score (fig. 2), there was a treatment effect (PRX302 minus vehicle) mean improvement of approximately 3 to 4 points that was fairly well sustained during the 12 months of followup. The approximately 9-point reduction in I-PSS is highly encouraging given that a change in I-PSS total score by 3 or more points is usually recognized by the patient as a significant change in symptoms.15 Regarding Qmax, PRX302 treatment produced an average of approximately 3 ml per second improvement in Qmax (fig. 2) and the treatment effect (PRX302 minus vehicle) mean improvement was 1.8 to 2.3 ml per second sustained during the 12 months of followup.
Although the PRX302 treatment effect was obvious for I-PSS and Qmax, less effect was shown for other outcome parameters (table 2). There was no significant change in total prostate size as measured by TRUS in either treatment group, consistent with published data showing a lack of correlation between prostatic volume reduction and degree of symptom relief.16
Based on the comparison to vehicle group (table 3), most AEs most likely attributable to PRX302 can be grouped into the 2 main categories of 1) irritative urinary symptoms characteristic of prostate inflammation (AEs of dysuria, pollakiuria, and micturition urgency) and 2) pain/discomfort localized to the injection area (AEs of perineal pain and suprapubic pain). The median duration for each of these most common AEs attributable to PRX302 was less than 2 days. In general these AEs were mild, transient, began within the first few days after treatment, primarily on the same day as the study drug injection, and resolved without sequelae. There were no reports of bacteremia, sepsis or hypersensitivity, and no serious or grade 3 or greater AEs that were drug related.
In summary, the current trial demonstrated a clinically meaningful and statistically significant PRX302 treatment effect as measured by I-PSS and Qmax with an excellent safety profile. A study is ongoing to assess the safety and efficacy of the transrectal administration of PRX302. Future phase III studies are planned to confirm the efficacy and safety of PRX302 for the treatment of LUTS due to BPH, and also assess the safety of repeat administration.
In terms of study limitations, the sample size was small. Safety and efficacy need to be verified for the transrectal approach, which is the preferred route for urologists. The relatively high rate of patient dropout from the vehicle group was most frequently due to lack of efficacy and the need for alternative therapy. As with all BPH studies, the placebo effect is high and needs to be considered.
CONCLUSIONS
A single transperineal intraprostatic administration of PRX302 as a short, outpatient based procedure was well tolerated in patients with LUTS due to BPH. PRX302 produced clinically meaningful and statistically significant improvement in patient subjective (I-PSS) and quantitative objective (Qmax) measures sustained during the 12 months of followup in an EE patient population. The safety profile is favorable, with most effects attributed to the injection itself. Adverse effects attributable to PRX302 were generally mild and transient, beginning within the first few days after treatment and resolving without sequelae. Further evaluation is warranted to evaluate longer term effects in larger, phase III studies.
Acknowledgments
Supported by Sophiris Bio Corp. (formerly Pro-tox Therapeutics).
Abbreviations and Acronyms
- AE
adverse event
- APA
anti-PRX302 antibody
- 5-ARI
5α-reductase inhibitor
- BPH
benign prostatic hyperplasia
- EE
efficacy evaluable
- ITT
intent to treat
- LUTS
lower urinary tract symptoms
- PSA
prostate specific antigen
- PV
prostate volume
- PVR
post-void residual
- Qmax
peak urine flow
- QoL
quality of life
- TRUS
transrectal ultrasound
- TURP
transurethral resection of the prostate
Footnotes
Study received institutional review board approval.
References
- 1.Wei JT, Calhoun E, Jacobsen SJ. Benign prostatic hyperplasia. In: Litwin MS, Saigal CS, editors. Urologic Diseases in America. Washington, DC: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007. [Accessed October 26, 2012.]. pp. 45–69. NIH Publication No. 07-5512. Available at http://kidney.niddk.nih.gov/statistics/uda/ [Google Scholar]
- 2.Roehrborn CG, Marks L, Harkaway R. Enlarged prostate: a landmark national survey of its prevalence and impact on US men and their partners. Prostate Cancer Prostatic Dis. 2006;9:30. doi: 10.1038/sj.pcan.4500841. [DOI] [PubMed] [Google Scholar]
- 3.National Kidney and Urologic Diseases Information Clearinghouse (NKUDIC) Prostate Enlargement: Benign Prostatic Hyperplasia. Bethesda: National Institute of Diabetes and Digestive and Kidney Diseases; 2006. [Accessed October 26, 2012.]. NIH Publication No. 07-3012. Available at http://kidney.niddk.nih.gov/kudiseases/pubs/prostateenlargement/ [Google Scholar]
- 4.Roehrborn CG. The clinical benefits of dutasteride treatment for LUTS and BPH. Rev Urol, suppl. 2004;6:S22. [PMC free article] [PubMed] [Google Scholar]
- 5.Sandhu JS, Vaughan ED., Jr Combination therapy for the pharmacological management of benign prostatic hyperplasia: rationale and treatment options. Drugs Aging. 2005;22:901. doi: 10.2165/00002512-200522110-00002. [DOI] [PubMed] [Google Scholar]
- 6.Lepor H. The evolution of alpha-blockers for the treatment of benign prostatic hyperplasia. Rev Urol, suppl. 2006;8:S3. [PMC free article] [PubMed] [Google Scholar]
- 7.McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan SA. Factors in predicting failure with medical therapy for BPH. Rev Urol, suppl. 2005;7:S34. [PMC free article] [PubMed] [Google Scholar]
- 9.Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol, suppl. 2005;7:S3. [PMC free article] [PubMed] [Google Scholar]
- 10.Deliveliotis C, Liakouras C, Delis A, et al. Prostate operations: long-term effects on sexual and urinary function and quality of life. Comparison with an age-matched control population. Urol Res. 2004;32:283. doi: 10.1007/s00240-004-0411-0. [DOI] [PubMed] [Google Scholar]
- 11.Bouza C, Lopez T, Magro A, et al. Systemic review and meta-analysis of transurethral needle ablation in symptomatic benign prostatic hyperplasia. BMC Urol. 2006;6:14. doi: 10.1186/1471-2490-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leitao VA, Haleblian GE, Albala DM. Minimally invasive techniques for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: an update. Curr Bladder Dysfunct Rep. 2007;2:244. [Google Scholar]
- 13.Williams SA, Merchant RF, Garrett-Mayer E, et al. A prostate-specific antigen-activated channel-forming toxin as therapy for prostatic disease. J Natl Cancer Inst. 2007;99:376. doi: 10.1093/jnci/djk065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denmeade SR, Egerdie B, Steinhoff G, et al. Phase 1 and 2 studies demonstrate the safety and efficacy of intraprostatic injection of PRX302 for the targeted treatment of lower urinary tract symptoms secondary to prostatic hyperplasia. Eur Urol. 2011;59:747. doi: 10.1016/j.eururo.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index is perceptible to patients? J Urol. 1995;154:1770. doi: 10.1016/s0022-5347(01)66780-6. [DOI] [PubMed] [Google Scholar]
- 16.Chuang YC, Chiang PH, Yoshimura N, et al. Efficacy and length of symptom improvement after botulinum toxin type A injection in BPH patients not correlated with change in prostate volume. J Urol, suppl. 2007;177:610, abstract 1837. [Google Scholar]