Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a persistent environmental pollutant and teratogen that produces cardiac toxicity in the developing zebrafish. Here we adopted a label free quantitative proteomic approach based on normalized spectral abundance factor (NSAF) to investigate the disturbance of the cardiac proteome induced by TCDD in the adult zebrafish heart. The protein expression level changes between heart samples from TCDD treated and control zebrafish were systematically evaluated by a large scale MudPIT analysis which incorporated triplicate analyses for both control and TCDD exposed heart proteomic samples to overcome the data dependant variation in shotgun proteomic experiments and obtain a statistically significant protein dataset with improved quantification confidence. A total of 519 and 443 proteins were identified in hearts collected from control and TCDD treated zebrafish, respectively, among which 106 proteins showed statistically significant expression changes. After correcting for the experimental variation between replicate analyses by statistical evaluation, 55 proteins exhibited NSAF ratio above 2 and 43 proteins displayed NSAF ratio smaller than 0.5, with statistical significance by t-test (p < 0.05). The proteins identified as altered by TCDD encompass a wide range of biological functions including calcium handling, myocardium cell architecture, energy production and metabolism, mitochondrial homeostasis, and stress response. Collectively, our results indicate that TCDD exposure alters the adult zebrafish heart in a way that could result in cardiac hypertrophy and heart failure, and suggests a potential mechanism for the diastolic dysfunction observed in TCDD exposed embryos.
Keywords: TCDD, cardiac toxicity, zebrafish, heart proteomics, mass spectrometry, spectral counting, quantitation
Introduction
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is the most potent dioxin congener and the prototype of a class of ubiquitously existing environmental pollutants known as polyhalogenated aromatic hydrocarbons 1, 2. It originates from common municipal waste processing and is also a byproduct of industrial manufacturing processes 3. The chemical stability and hydrophobicity of TCDD has contributed to its widespread distribution in the environment and accumulation within the food chain, which poses health hazards to multiple animal species including man. TCDD causes a wide spectrum of toxic effects in experimental animals and human beings including fetotoxicity 4, 5, hepatotoxicity 6, 7, immunotoxicity 8, 9, teratogenicity 10, 11 and carcinogenicity 12–15. TCDD is known to cause disruption of cardiovascular development in vertebrates 16–19. The zebrafish (Danio rerio) has been used as an important vertebrate model to study the cardiovascular toxicity of TCDD, and a spectrum of developmental defects have been reported such as smaller ventricle size, reduced cardiac myocyte numbers, altered heart morphology, pericardial edema, hemorrhage and circulatory failure 18, 20, 21. The observed heart defects are associated with altered gene expression by TCDD as revealed by microarray analysis 20. These studies revealed a very rapid induction of putative aryl hydrocarboan receptor (AHR) target genes within 1- 4 hours of TCDD exposure, followed by a striking down-regulation of a large set of genes involved in cell cycle progression 22. The altered expression of these cell cycle progression genes correlated with a halt in heart growth in the developing zebrafish 22. The alteration in zebrafish cardiac gene expression by TCDD was explored previously 20. An important group of cardiac sarcomere components have shown transcriptional changes, including cardiac troponin T2 and multiple myosin isoforms, which is consistent with the hypothesis that TCDD causes dilated cardiomyopathy 20. A battery of other TCDD responsive genes have also been identified which are functionally involved in fatty acid and steroid metabolism, ribosomal activity as well as signal transduction.
Recent advances in MS based proteomics has facilitated large scale quantitative analysis of complex proteomes present in various biological matrices with high throughput and accuracy, thus allowing complementary perspectives for analysis of underlying disease mechanisms at the protein level 23. Among various quantitative proteomic approaches developed 24–30, label free quantification based on spectral count has recently drawn increasing interest in the application of quantitative proteomics 25, 31, 32. This method uses the number of total MS/MS spectra for each identified protein (spectral count) to evaluate the protein abundance in a sample and compare protein expression changes between different samples and biological states. Despite several limitations including compromised accuracy for low-abundance proteins with small changes and the requirement for more technical replicates 32, the spectral counting approach provides a viable quantitation method with excellent accuracy, sensitivity and dynamic range for both simple protein samples as well as complex proteomic systems 25. Compared with various isotopic labeling methods, spectral counting directly uses the inherent quantitative information embedded in the results of shotgun proteomic experiments and does not need complicated sample treatment procedures involved in various labeling schemes. Therefore this method can avoid drawbacks associated with various labeling schemes such as increased time and complexity of sample preparation as well as artifact involved in the labeling reactions and subsequent data interpretation. Compared with other label free quantification methods based on chromatographic or MS peak intensities, spectral counting method does not need specialized software to read out peak intensities and therefore is more straightforward to implement 33. Recent development of normalized spectral abundance factor (NSAF) by Washburn and coworkers has implemented normalization procedures to the spectral counting method 31. This normalized spectral counting method corrects for the influence of the protein size and the overall experiment variation, therefore allowing more accurate quantification of both protein actual abundances in a sample and expression level changes between different samples and experiments. The NSAF approach has been successfully applied to quantitative proteomic analysis of the expression changes of membrane proteins in S. cerevisiae 31 and the human transcriptional regulatory complex 34. It has also been demonstrated that the NSAF approach generates datasets that have a high degree of statistical similarity to the transcriptomic datasets 35.
In this work, we applied the spectral counting method to the quantification of proteomic expression changes in the adult zebrafish heart induced by TCDD exposure. To improve the confidence and accuracy of measured protein expression changes by the shotgun proteomic approach, we implemented cross sample comparisons which incorporated triplicate analyses of both the control and TCDD exposed heart samples to assess the repeatability and reproducibility associated with shotgun proteomic experiments. Through such rigorous comparison and statistical analysis, we were able to identify 106 proteins that showed statistically significant expression changes, out of a total of 692 proteins identified from both TCDD treated and control zebrafish hearts. This smaller pool of candidate proteins identified with improved quantification confidence was then annotated in detail to provide mechanistic insights into the acute effect of TCDD on the adult zebrafish heart.
Materials and Methods
Zebrafish Heart Treatment by TCDD
Zebrafish (AB wild type) were bred and raised in the School of Pharmacy Animal Facility according to the procedures described by Westerfield 36. All animal procedures were performed according to established animal care protocols as approved by the Research Animal Resources Center at the University of Wisconsin-Madison. The adult zebrafish were raised side by side in two groups (each group contains three separate tanks containing 10 fish each tank). Both groups were raised in DMSO media before one group was treated by a sublethal TCDD exposure for cardiac toxicity evaluation. TCDD dose at sublethal level has been shown to cause severe cardiac morphological and functional disorder in the developing zebrafish.18, 21 The concentration of TCDD exposure was 1 ng/ml in waterborne conditions, and the exposure period is 1 hour.37 (TCDD is a highly toxic chemical and proper precaution and disposal procedures should follow approved safety protocol) After TCDD exposure, the fish were rinsed with clean water and placed in aquaria. After one day both groups were anesthetized with tricane and sacrificed. The hearts were surgically removed and snap frozen in liquid nitrogen. The extracted hearts were stored at −80 °C until protein extraction and analysis.
Sample Preparation and Protein Digestion
Sample preparation was performed for the triplicate groups of zebrafish hearts treated by TCDD and DMSO media side by side following the same protocol. Briefly, proteins were extracted from the surgically removed zebrafsh hearts using the Dounce homogenizer in 1 ml protein extraction buffer (125 mM Tris-HCl, pH 8.2, 5 mM EDTA, 5 mM tributylphosphine) for 5 minutes at 4 °C. The homogenate were centrifuged at 16,000 rpm at 4 °C for 15 minutes to remove cellular debris and unsolubilized material. Approximately 30 µg protein was extracted from each replicate sample based on the Bradford protein assay. The protein extracts in the supernatant was denatured by adding 8 M urea, reduced with 20 mM DTT for 30 min and alkylated by iodoacetic acid (50 mM) prior to tryptic digestion by 3 µg trypsin (Promega, Madison, WI). Digested peptide mixture solution was acidified with 1% formic acid and centrifuged at 16,000 rpm prior to LC MS/MS analysis.
2D HPLC MS/MS Analysis of Zebrafish Proteomic Samples
Online fractionation and LC-MS/MS were performed on Eksigent HPLC coupled to Thermo LTQ mass spectrometer. The samples were run in an alternating order between the control and TCDD-exposed group to minimize the error associated with variations in instrumental conditions. Each sample was loaded onto a PolySulfoethyl A column (100 mm × 150 µm, 5 µm, 300 Å; Column Technology Inc., Fremont, CA) at a flow rate of 0.2 ml/min with mobile phase A (5 mM ammonium formate, 5% acetonitrile, pH 2.5). Fractions were eluted with a 10 step gradient (10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 99% mobile phase B (1 M ammonium formate, 5% acetonitrile, pH 6.8)). Each fraction was further separated on a Microtech C18 RP column (150 mm × 75 µm inner diameter, 300 Å, 5 µm; Microtech Inc., Fremont, CA) at a flow rate of 300 µl/min using a 120 min gradient (7–35% solvent B). Reversed phase mobile phases are A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile). The eluted peptides are injected into a Thermo Fisher linear ion trap (LTQ) mass spectrometer (Thermo Fisher, San Jose, CA) using electrospray ionization (ESI). The spray voltage was set at 3.5 kV and the temperature of the heated capillary was 180 °C. Nitrogen was used as the sheath (75 p.s.i.) and auxiliary (10 units) gas. The mass spectrometer was operated in a data-dependent mode in which a survey scan (m/z 400–2000) was followed by MS/MS scans of the top five most abundant ions with a collision energy of 25% normalized CID energy. Dynamic exclusion was used with the following settings: exclusion list size, 250; repeat count, 2; repeat duration, 30 s; exclusion time, 180 s; exclusion window, ± 0.75 Da. Target values were set at 5 × 105 and 104 for the survey and tandem MS scans, respectively, and the maximum ion accumulation times were set at 200 ms in both cases. Regular scans were used both for the precursor and tandem MS with no averaging. The precursor isolation window was set at 2 m/z with monoisotopic peak selection.
MS/MS Data Processing
Raw mass spectra were converted to DTA peak lists using BioWorks Software 3.2 (Thermo Fisher, San Jose, CA) with the following parameter settings: peptide mass range, 300–2000 Da; threshold, 10; precursor mass, ±1.8 Da; group scan, 1; minimum group count, 1; minimum ion count, 15. Protein database search was conducted using MASCOT search engine (Version 2.1.0) against a database containing both forward and reverse sequences of proteins contained in the NCBI protein database under the taxonomy Danio rerio (version 20091106, 26709 sequences). Enzyme specificity was specified as trypsin and a maximum of two missed cleavage sites was allowed with cysteine carbamidomethylation specified as possible modifications. Peptide mass tolerance was set as ±2.5 Da and fragment ion tolerance ±0.8 Da. Proteins were identified according to the following criteria: 1) proteins were identified based on significant peptide identifications above the Mascot identity threshold (95 % confidence); 2) at least one top hit peptide with score above 41; 3) identified peptide had at least 7 amino acids in length. The protein false discovery rate was below 2 % based on the above criteria and the reverse database search. Proteins with overlapped peptide identifications (shared peptides) were examined manually and further processed according to the following procedures: 1) For proteins with significant sequence overlaps (as determined by ClustalW2 sequence alignment (http://www.ebi.ac.uk/Tools/msa/clustalw2/, >95%), the full length protein was reported and redundant protein entries (especially unannotated and hypothetical proteins) were removed; 2) For proteins with noticeable but not significant overlap (<95% homology), protein entries were reported separately and their quantification was further evaluated by weighted spectral counting procedure described below. The search results were exported into MS Excel format and the spectral counting analyses were performed by a lab developed script.
Spectral Counting and Protein Expression Analysis
The raw protein spectral count was normalized according to the procedures reported by Washburn and coworkers. Briefly, the raw spectral counts were normalized both to the protein size and the overall protein spectral count in an SCX-RP LC MS/MS experiments to give the normalized spectral abundance factor (NSAF)
For shared peptides among multiple protein identifications, the spectral count of the shared peptide was adjusted by the following weighing factor and assigned to each protein accordingly
where αi is the adjusting factor according to the total spectral count of N sharing proteins and has the value between 0 and 1 for individual sharing proteins, similar to a procedure described by Florens and Nesvizhskii groups separately38,39 A “pseudo spectral count” of 0.1 was added to correct for the zero spectral count to avoid division by zero values when calculating the NSAF ratios for expression analysis and taking logarithm on zero’s when performing statistical test. This fractional count was selected on the basis of widely used approaches for statistical analysis, in order to maintain the normal distribution of the NSAF values for statistical analysis, while not to change the total spectral count sum significantly31. Proteins were considered significantly upor down-regulated if 1) they passed the t-test in three replicates at 95 % confidence (two tailed unpaired t-test was performed to determine the statistical significance of the change between the two groups) and 2) they were consistently up- or down-regulated in all biological replicates. Proteins were considered to exhibit significant expression changes with NSAFTCDD/DMSO ≥ 2 (p < 0.05) (over-expressed in TCDD treated hearts) and ≤ 0.5 (p < 0.05) (under expressed in TCDD treated hearts).
Results
Protein Identification from Control and TCDD Exposed Zebrafish Hearts
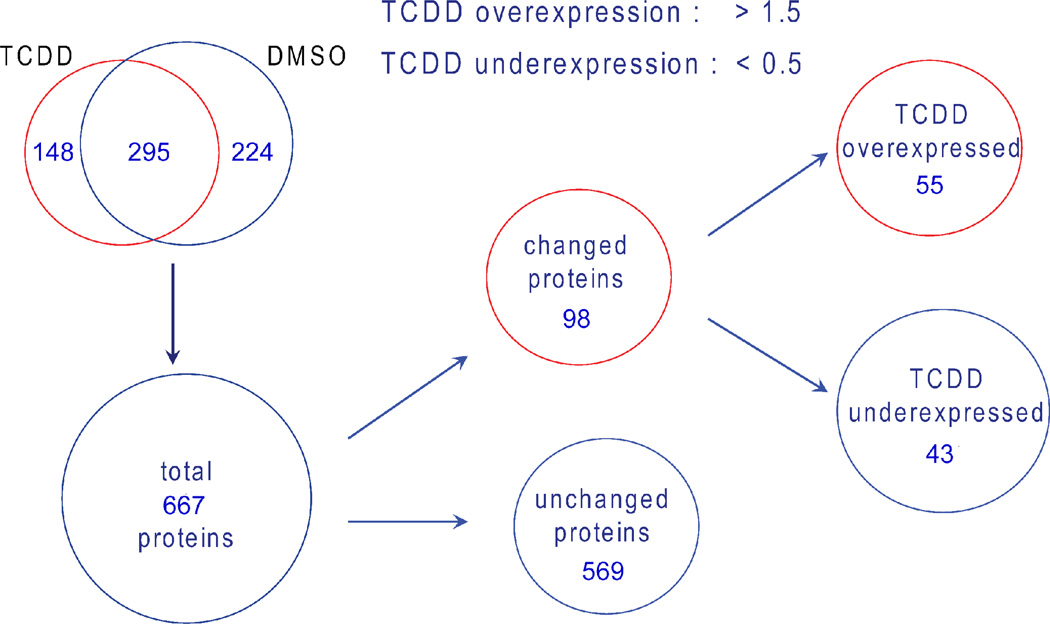
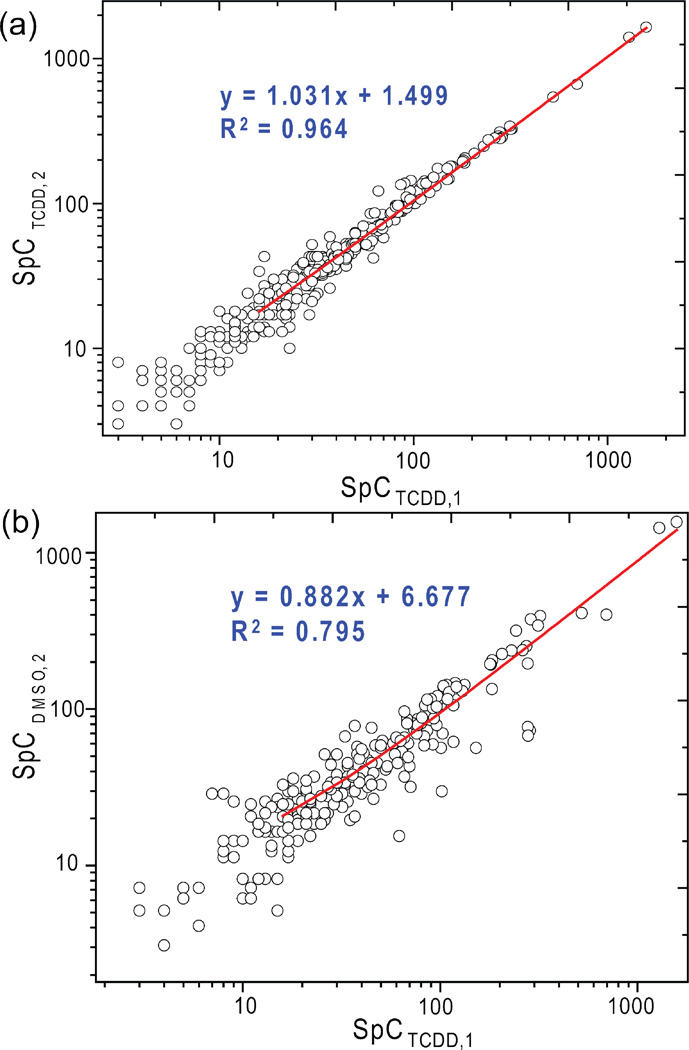
The protein digest samples from control and TCDD exposed zebrafish hearts were analyzed by the LC MS/MS method described in the experimental section (Figure 1). From the triplicate samples of DMSO group, 361, 369 and 378 proteins were identified, whereas 330, 338 and 352 proteins were identified from TCDD exposed group (Supplemental Table 1. MS/MS data for proteins with single significant peptide identifications were included in Supplemental Table 2). After consolidating proteins from the replicate samples from the each group, a total of 519 and 443 proteins were identified from DMSO and TCDD group, respectively (Figure 2). Comparison of proteins identified between replicate experiments within each group showed an average overlap of 76% between DMSO replicates and 79% overlap between TCDD replicates. Whereas common proteins identified between DMSO and TCDD groups (295 proteins) only account for 44% of the total 667 proteins identified. The higher protein identification overlap between replicate samples showed high technical reproducibility between samples of the same origin and experimental procedures, whereas samples from two different groups showed larger variability due to significant protein expression differences. The technical reproducibility is also apparent through the comparison between spectral counts of replicate samples as opposed to different samples. Figure 3 shows the raw spectral count comparison for common proteins identified between replicate TCDD samples (Figure 3 (a)) as well as such comparison between TCDD and DMSO samples (Figure 3 (b)). The higher spectral count consistency between replicate TCDD samples demonstrated high experimental reproducibility, whereas the biological variability is clearly shown by the wider spread of spectral counts between DMSO and TCDD treated heart samples.
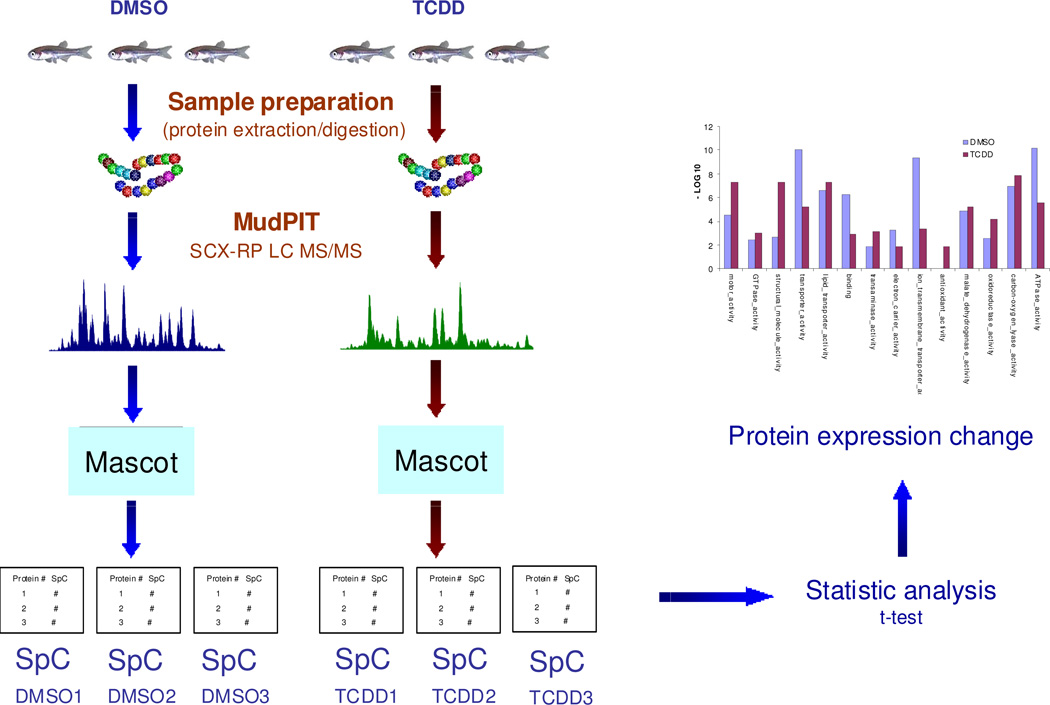
Figure 1.
Workflow of label free quantitative proteomic approach to TCDD cardiac toxicity in adult zebrafish heart. Adult zebrafish (3 month old) were treated in triplicates each by DMSO and TCDD, respectively. Proteins were extracted and digested following the same sample preparation protocol. The peptide samples were analyzed by parallel shotgun proteomic approach. The protein expression was analyzed by spectral count method supplemented by statistical test for significant protein expression change.
Figure 2.
Overall protein expression results by spectral count combined with statistical evaluation. 106 proteins were identified to be significantly changed by TCDD treatment.
Figure 3.
Experimental reproducibility and biological variability by quantitative proteomic spectral count method. (a) Spectral counts from two replicate analyses of TCDD treated heart samples show good experimental reproducibility of SpC method; (b) Spectral counts from parallel DMSO and TCDD samples revealed biological variability between two distinct samples, corresponding to potential protein differential expression induced by TCDD treatment.
Spectral Count Analysis of TCDD Induced Protein Expression Change
The raw spectral counts were normalized against the protein size and the overall experimental conditions according to the method developed by Washburn and coworkers 31. The average normalized spectral abundance factors (NSAFs) for each identified proteins were then compared between DMSO and TCDD sample groups to obtain the expression ratio between these two states (Supplemental Table 3). Among the total 667 identified proteins, 171 proteins (25.6 %) have average NSAF ratios (TCDD/DMSO) larger than 2.0 which is equivalent of greater than two fold up-regulation in TCDD treated hearts, if solely determined by the spectral count; whereas 254 proteins (38.1 %) have NSAF ratios smaller than 0.5, equivalent of more than 2 fold down-regulation in TCDD treated hearts by spectral count only. After correcting for the experimental variation between replicate analyses by statistical evaluation, 55 proteins (8.2 %) have NSAF ratios greater than 2 and 43 proteins (6.4 %) have NSAF ratio smaller than 0.5, with statistical significance by t-test (p < 0.05). It is clear that through replicate control and statistical evaluations, proteins with large variations in spectral counts and potentially false positives in quantification were excluded; only those proteins with consistent spectral counts between replicates were retained with statistical significance for protein expression analysis. For example, RNA-specific adenosine deaminase (accession ID gi|18858253), a protein responsible for RNA editing by site-specific deamination of adenosines, has an average NSAFTCDD/DMSO ratio of 0.006 implicating large down-regulation in TCDD treated samples. This protein was identified once in a DMSO replicate whereas not detected in two other DMSO replicates as well as three TCDD replicates (NSAFDMSO, 1.9 × 10−6, 7.9 × 10−4, 1.8 × 10−6; NSAFTCDD, 1.8 × 10−6, 1.8 × 10−6, 1.5 × 10−6), therefore the expression difference of this protein was considered statistically not significant (p = 0.42) due to the large variation of spectral count data. This single identification was not uncommon observation in shotgun proteomic experiments due to the low abundance of the proteins and the nature of data dependent analysis. However this type of identification was discarded as insignificant for the quantification purposes due to poor reproducibility. Similarly, aldehyde dehydrogenase 2 precursor (gi|41053732) was identified twice in TCDD replicates (NSAFTCDD, 1.1 × 10−3, 6.6 × 10−4, 4.1 × 10−6) while not detected in the DMSO triplicates (NSAFDMSO, 5.2 × 10−6, 4.7 × 10−4, 4.9 × 10−6). This protein was considered not significant either (p = 0.21) even though it has an apparent NSAFTCDD/DMSO ratio of 119.5. Through this rigorous reproducibility control, potential false positive protein expression changes were significantly reduced and a protein dataset of smaller size but improved quantification confidence was obtained for meaningful expression analysis.
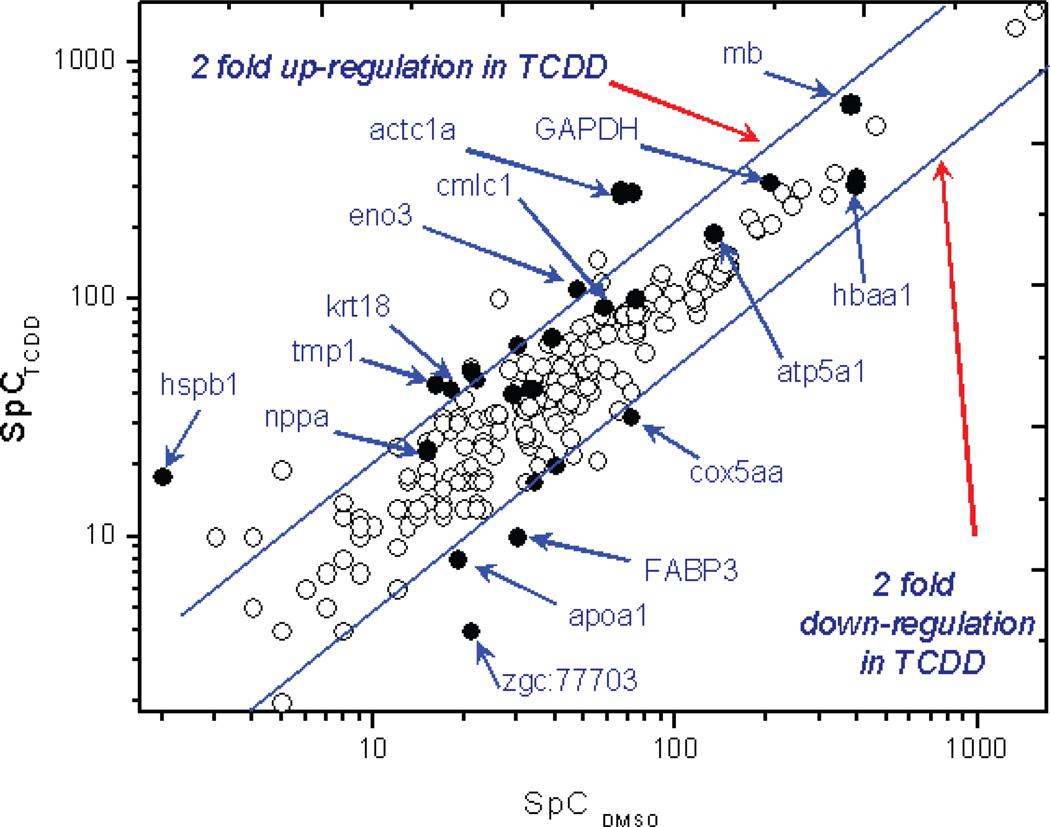
This statistically controlled NSAF approach also enabled detection of small but significant protein expression changes. Tropomyosin (isoform beta, gi|50344894), an important light chain regulatory protein in the cardiac muscle, showed a small degree of up-regulation in the TCDD treated heart samples (NSAFTCDD/DMSO = 1.52). However, replicate analyses indicated that this protein scored consistent spectral counts in both DMSO and TCDD treated heart samples (NSAFDMSO, 5.5 × 10−3, 5.0 × 10−3, 5.2 × 10−3; NSAFTCDD, 8.0 × 10−3, 7.8 × 10−3, 8.0 × 10−3). Therefore this small expression change is statistically highly significant (p = 0.003). This finding is consistent with several previous TCDD toxicological studies where very similar upregulations in various tropomyosin isoforms were observed at both protein 40, 41 and mRNA levels 42 in TCDD treated mouse liver and differentiating osteoblasts. Through the reproducibility control and statistically enhanced NSAF approach, such mild and significant protein expression changes can be effectively identified. Figure 4 shows the spectral count comparison between replicate analyses of TCDD and DMSO groups. Proteins with significant expression changes were distinguished from a large number of total proteins with similar fold changes yet statistically less reliable due to greater variability, which highlighted the strength of this statistically enhanced spectral counting method.
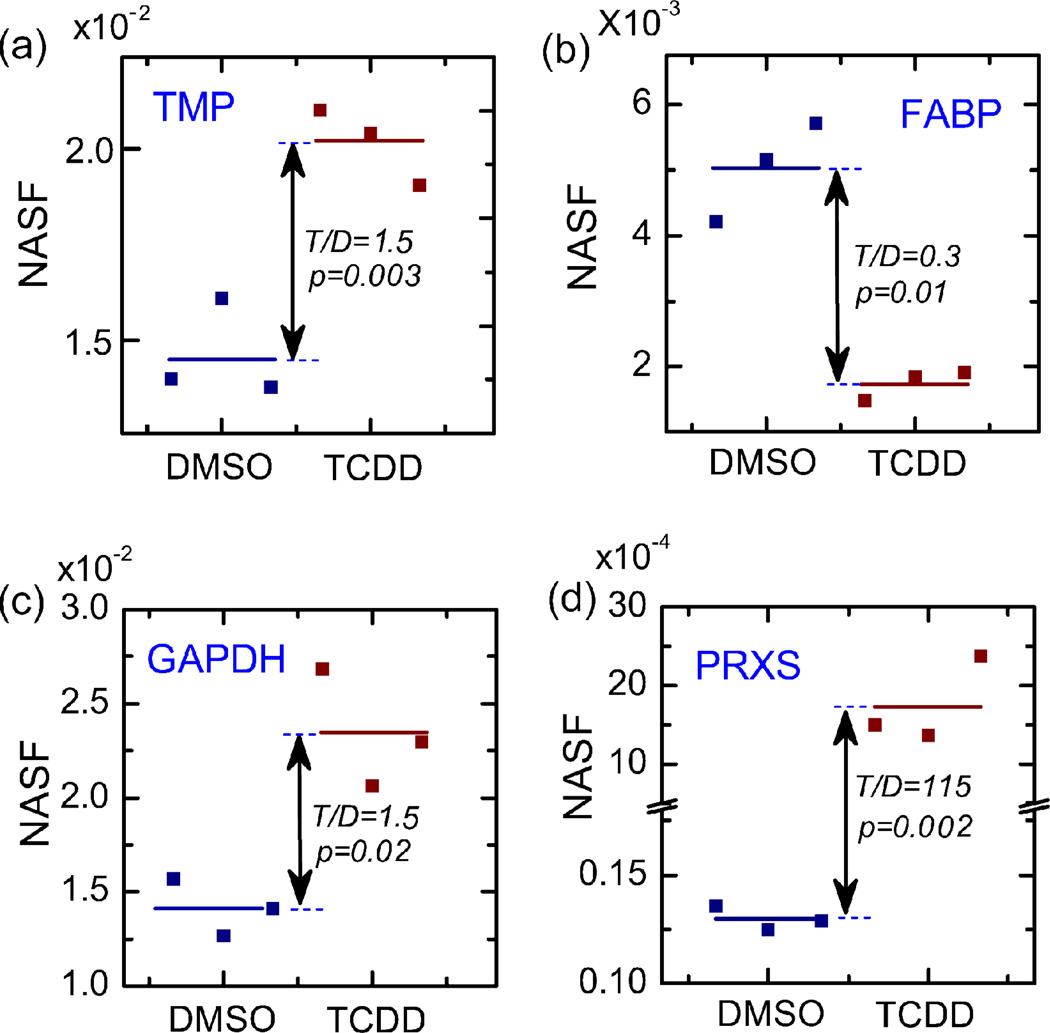
Figure 4.
Significantly changed protein expressions by spectral count and statistical analysis. The two blue lines indicated 2 fold up- and down-regulation of proteins in TCDD treated samples. Dark dots represent proteins with statistically significant expression changes. Proteins are labeled by their encoding gene names.
Table 1 summarized the identified proteins with significant expression changes according to their biological functions. These proteins are involved in a wide range of biological functional categories, including both myocardium sarcomere proteins which perform the essential buildup and regulatory functions in the heart muscle such as several important isoforms of tropomyosin, as well as enzymes of lower abundance which are involved in cardiac energy metabolisms, oxidative stress, protein degradation and apoptosis and membrane transfer. The detailed spectral counts for proteins from several highlighted functional categories are shown in Figure 5.
Table 1.
| Protein Name and Functional Category | NCBI Accession Number |
Mr (kDa) | Subcellular location |
Protein function | NSAF tcdd/dmso |
p-value |
|---|---|---|---|---|---|---|
| Cardiac muscle structural proteins | ||||||
| tropomyosin 2 (beta) [Danio rerio] | gi|50344894 | 32 | cytosol | muscle contraction | 1.52 | 0.003 |
| plectin 1 isoform 1 [Danio rerio] | gi|189529437 | 518 | cytosol | cytoskeleton | + | 0.001 |
| cardiac myosin light chain-1 [Danio rerio] | gi|55926111 | 22 | nucleus | muscle structure | 1.57 | 0.04 |
| radixin isoform 1 [Danio rerio] | gi|51972166 | 68 | cytosol | cytoskeleton | + | 0.001 |
| WD repeat domain 1 [Danio rerio] | gi|41054770 | 66 | cytosol | cytoskeleton | + | 0.005 |
| si:dkey-151c10.1 [Danio rerio] | gi|153792369 | 522 | cytosol | cytoskeleton | + | 0.005 |
| keratin 8 [Danio rerio] | gi|125849603 | 50 | cytoplasma | cytoskeleton | 144 | 0.011 |
| Oxygen transport | ||||||
| myoglobin [Danio rerio] | gi|41053652 | 16 | cytosol | oxygen transport | 1.69 | 0.003 |
| erythrocyte membrane protein band 4.1 (elliptocytosis 1, RH-linked) [Danio rerio] | gi|28201964 | 180 | cytosol | oxygen transport | + | 0.002 |
| Protein degradation | ||||||
| calpastatin [Danio rerio] | gi|189533701 | 85 | nucleus | protease inhibitor | 0.002 | 0.001 |
| si:ch73-252g14.4 [Danio rerio] | gi|153791419 | 42 | cytosol | protease inhibitor | 0.006 | 0.011 |
| Apoptosis/Stress | ||||||
| annexin A5 [Danio rerio] | gi|40254661 | 35 | cytosol | apoptosis | 0.007 | 0.010 |
| annexin A2a [Danio rerio] | gi|32308153 | 38 | cytosol | apoptosis | 0.004 | 0.017 |
| polymerase (RNA) III (DNA directed) polypeptide B [Danio rerio] | gi|125843259 | 127 | cytosol | apoptosis | + | 0.004 |
| G2/M-phase specific E3 ubiquitin ligase [Danio rerio] | gi|189526852 | 84 | cytoplasm | apoptosis | + | 3 × 10−4 |
| Molecular chaperons | ||||||
| heat shock 70kDa protein 5 [Danio rerio] | gi|47085775 | 72 | ER | chaperons | 0.003 | 0.001 |
| PREDICTED: similar to heat shock protein 8 [Danio rerio] | gi|189517055 | 77 | mitochondrial | chaperons | 0.003 | 0.005 |
| heat shock protein, alpha-crystallin-related, 1 [hspb1] | gi|56693302 | 22 | nucleus | chaperons | 2.47 | 0.034 |
| Oxidative stress | ||||||
| peroxiredoxin 2 [Danio rerio] | gi|50539996 | 22 | cytosol | antioxidation | 115 | 0.002 |
| aldehyde dehydrogenase 4 family, member A1 [Danio rerio] |
gi|41055855 | 61 | cytoplasma | oxidation | 3.63 | 0.025 |
| aflatoxin B1 aldehyde reductase member 2 [Danio rerio] | gi|50539806 | 36 | cytoplasma | antioxidation | 70 | 0.012 |
| cytochrome c oxidase subunit Vaa [Danio rerio] [cox5aa] | gi|66773138 | 16 | mitochondria | antioxidation | 0.42 | 0.012 |
| cytochrome c oxidase subunit VIa polypeptide 1 [Danio rerio] |
gi|53933242 | 12 | mitochondria | antioxidation | 0.01 | 0.028 |
| alcohol dehydrogenase 8b [Danio rerio] | gi|44917595 | 41 | cytoplasm | oxidation | + | 0.007 |
| Membrane transfer | ||||||
| monocarboxylate transporter 4 [Danio rerio] | gi|47086623 | 55 | membrane | membrane transfer | + | 0.015 |
| mitochondrial carrier homolog 2 [Danio rerio] | gi|18859045 | 33 | membrane | membrane transfer | 155 | 0.006 |
| Mitochondrial energy metabolism | ||||||
| ATP synthase, H+ transporting, mitochondrial F1 complex, delta subunit [Danio rerio] |
gi|41152365 | 17 | mitochondria | energy metabolism | 0.01 | 0.015 |
| Glycolysis | ||||||
| glyceraldehyde-3-phosphate dehydrogenase [Danio rerio] [GAPDH] |
gi|169403947 | 36 | cytosol | glycolysis | 1.52 | 0.019 |
| phosphorylase, glycogen; brain [Danio rerio] | gi|136256027 | 97 | cytosol | glycolysis | + | 0.028 |
| pyruvate kinase, muscle, b [Danio rerio] | gi|51011067 | 58 | cytosol | glycolysis | + | 0.004 |
| branched chain keto acid dehydrogenase E1, alpha polypeptide [Danio rerio] |
gi|66773104 | 51 | cytosol | glycolysis | 0.01 | 0.002 |
| glyceraldehyde-3-phosphate dehydrogenase, spermatogenic [Danio rerio] |
gi|47085833 | 36 | cytosol | glycolysis | 0.11 | 0.021 |
| Histones | ||||||
| PREDICTED: similar to Histone H1.2 (H1 VAR.1) (H1c) [Danio rerio] |
gi|189522065 | 20 | nucleus | DNA binding | 0.002 | 0.008 |
| Protein synthesis and processing | ||||||
| ribosomal protein L19 [Danio rerio] | gi|47086009 | 23 | cytosol | protein synthesis | 0.01 | 0.001 |
| PREDICTED: similar to ribophorin I [Danio rerio] | gi|189516116 | 58 | plasmic | protein glycosylation | 101 | 0.005 |
| ribosomal protein S7 [Danio rerio] | gi|41152175 | 22 | cytosol | protein synthesis | 79 | 0.008 |
| Lipid metabolism | ||||||
| hydroxysteroid dehydrogenase like 2 [Danio rerio] | gi|41054573 | 44 | cytosol | lipid metabolism | 0.005 | 0.001 |
| apolipoprotein A-IV [Danio rerio] | gi|119943123 | 29 | extracellular | lipid metabolism | 0.01 | 0.012 |
| apolipoprotein A-I [Danio rerio] [apoa1] | gi|18858281 | 30 | extracellular | lipid metabolism | 0.38 | 0.015 |
| fatty acid binding protein 3, muscle and heart [Danio rerio] [FABP3] | gi|23308625 | 15 | cytosol | lipid metabolism | 0.36 | 0.007 |
| acyl-Coenzyme A dehydrogenase, C-4 to C-12 straight chain | gi|47085823 | 46 | mitochondria | lipid metabolism | 0.003 | 3 × 10−4 |
| Transport | ||||||
| tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, epsilon polypeptide [Danio rerio] |
gi|47086819 | 29 | cytosol | transport | 130 | 0.006 |
| solute carrier family 8 (Na+/Ca+ exchanger), member 1a [Danio rerio] | gi|80751141 | 108 | plasmic | transport | 295 | 0.009 |
| ATPase, Na+/K+ transporting, alpha 2a polypeptide [Danio rerio] | gi|18858305 | 112 | membrane/pla smic |
transport | 0.003 | 3 × 10−4 |
| ATPase, Na+/K+ transporting, alpha 1 subunit [Danio rerio] | gi|18858295 | 113 | membrane/pla smic |
transport | 0.002 | 0.003 |
| Catalytic/transferase activity | ||||||
| branched chain aminotransferase 2, mitochondrial [Danio rerio] | gi|50540420 | 46 | plasmic | 249 | 0.023 | |
| Hydroxyacylglutathione hydrolase [Danio rerio] | gi|41053309 | 29 | cytosol | 0.01 | 0.020 | |
| lactate dehydrogenase A4 [Danio rerio] | gi|18858959 | 36 | cytosol | 0.21 | 0.045 |
+ sign indicates the protein is only found in TCDD treated heart samples; Protein subcellular localization was determined by the WoLF PSORT algorithm (http://wolfpsort.org/). When multiple cellular regions were predicted for a protein sequence, the one with highest score was used.
Figure 5.
Representative proteins with significant SpC changes. (a)–(d) Representative proteins from significantly changed functional categories.
Gene Ontology Annotation of Significantly Changed Proteins
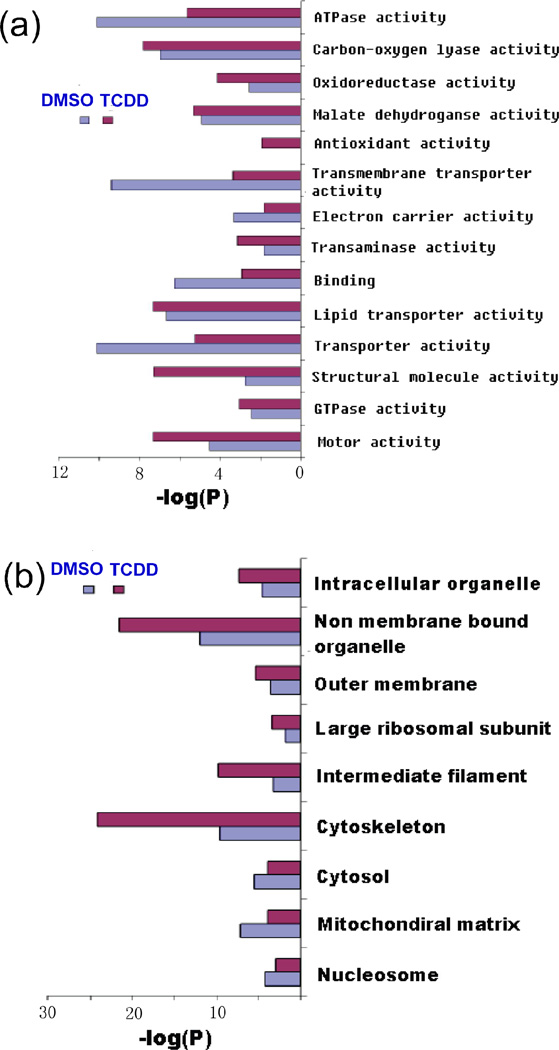
The proteins with significant expression changes in TCDD treated hearts were annotated by gene ontology (GO) analysis for significantly enriched GO terms in biological function and cellular localization (Figure 6). Consistent with proteins categorized in Table 1, the TCDD over-expressed proteins showed significant enrichment in several key GO terms including structural molecule activity, antioxidant activity, oxidoreductase activity and motor activity. Subcellular localization of the TCDD over-expressed proteins also indicated these proteins had significant enrichment in GO terms related to cellular architecture and structural filament.
Figure 6.
Gene ontology annotation of significantly changed proteins detected by spectral count method. Significantly changed proteins were analyzed for their (a) biological function and (b) cellular localization by the program GoMiner. The significantly enriched GO categories were listed to show comparison between TCDD and DMSO samples.
Discussion
Reproducibility of MudPIT Approach Based on Spectral Counting for Proteomic Quantification
Protein identification reproducibility in shotgun proteomics experiments is a long standing yet still actively discussed topic in MS based proteomics 25, 43, 44. The reproducibility of such large-scale experiments is often compromised by the complexity in the current shotgun proteomic platform which involves multiple complicated procedures including sample preparation, 2D HPLC separation, MS/MS identification and subsequent MS data processing and interpretation. Variations in any of these procedures as well as instrumental conditions can cause corresponding difference in the final protein discovery results. The complexity of proteomic samples and the nature of data dependent analysis are also major contributing factors in the observed variation in proteomic experiments, since the simplest proteomic digest samples could generate more peptide species than the most advanced LC MS/MS platforms can handle. Even under strictly controlled parallel conditions, a significant number of novel proteins and peptides could be identified from replicate analyses of the same sample 25. Previous studies by Yates and coworkers estimated that approximately 76% protein identification overlap could be observed between replicate analyses of the same yeast proteomic digest sample in 9 LC/LC/MS/MS runs, whereas 24% of the total protein identifications were found only in one single replicate experiment in data dependent analysis mode 25. Recent studies by Tabb et al. reported similar findings from large scale interlaboratory comparisons of 144 LC MS/MS datasets acquired on Thermo LTQ and Orbitrap instruments 43. Although such variability is inherent in shotgun proteomic experiments and could be useful for protein identification purpose, this variation may reduce reliability in quantitative proteomics and result in misinterpretation of the protein expression data. A large number of quantitative proteomic studies thus far are based on the simple comparison between two large scale proteomic datasets, while our studies described here suggest that quantification based on a single proteomic experiment could be unreliable and misrepresentative of the actual protein levels. Therefore, the reproducibility of LC MS/MS experiments should be effectively controlled and evaluated to provide a measure of confidence for meaningful and reliable quantification, similar to the role of false positive control in the meaningful identification of proteins. A simple and effective approach to ensure reliability is to increase technical replicates and analyze them under the same experimental conditions. Our results show that proteins identified from a single experiment do not provide significant statistical confidence for quantification due to low reproducibility, although these proteins can be identified confidently according to widely accepted protein identification criteria. Therefore expression information from these proteins is regarded as less reliable and not included as a meaningful protein expression change. On the other hand, several cardiac muscle proteins, such as tropomyosin, showed moderate expression changes below two fold. This slight increase in the detected protein levels would be difficult to distinguish from experimental variations without solid evidence regarding experimental reproducibility. In this study, the comparison between TCDD and DMSO replicate groups showed that these detected protein level changes were highly consistent between replicate experiments, indicating that these expression changes were valid and statistically significant. Therefore, the statistically controlled NSAF approach provides two fold benefits, first by controlling “false positives” in protein expression changes and secondly by increasing sensitivity enabling the detection of small but significant changes in expression level.
TCDD Exposure Alters Proteins Associated with Calcium Handling in the Adult Zebrafish Heart
Of particular interest among our findings were decreases in the levels of Na+/K+ ATPase alpha subunits and an increase in the levels of Slc8a1, the Na+/Ca2+ exchanger (NCX1). The Na+/K+ ATPase is composed of two subunits, alpha and beta. The alpha subunit is the catalytic portion of the pump and binds ATP, Na+, and K+ while the beta subunit is required for membrane localization and is necessary for alpha subunit function. The pump uses ATP to drive Na+ ions out of the cell in exchange for the import of K+ ions. NCX1 exchanges Na+ for Ca2+ and can facilitate intracellular calcium removal when acting in the forward direction, or increase intracellular calcium when acting in the reverse mode. In cardiac myocytes the Na+/K+ ATPase acts in close association with NCX1 to modulate intracellular calcium levels. Inhibition of the Na+/K+ ATPase by cardiac glycosides increases intracellular Na+ levels and drives Na+ export through NCX1, which in turn elevates intracellular calcium level and increases the force of cardiac contraction, an effect that might be phenocopied by down regulation of the Na+/K+ ATPase alpha subunits. Decreased Na+/K+ ATPase activity has previously been reported in rat liver surface membranes following TCDD exposure 45.
Up-regulation of NCX1 can have variable effects on intracellular calcium. In some studies over-expression of NCX1 resulted in calcium depletion and loss of calcium from sarcoplasmic reticulum stores 46. Others have reported the opposite effect, specifically calcium overload, following NCX1 over-expression 47. The outcome of over-expression is dependent upon the directionality of the NCX1 transporter, which is affected by intracellular sodium levels. Of particular relevance to our findings is a study that over expressed NCX1 in rabbit cardiomyocytes while simultaneously inhibiting the Na+/K+ ATPase with oubain 48. This combination produced impaired relaxation in cardiomyocytes, suggestive of diastolic calcium overload. Notably, TCDD exposure increases calcium levels in the chick embryo heart 49, and reduces cardiac end diastolic volume in the zebrafish embryo 22.
We also detected a decrease in annexin A5 levels following TCDD exposure. Annexin A5 is a calcium binding protein that is localized to the sarcolemma and T-tubules in cardiac myocytes. It has been found to associate with the intracytoplasmic loop of NCX1 50, which is involved in NCX1 regulation, and has been detected in a complex with SERCA 2 in failing human hearts 51. The role of annexin A5 in these interactions is unknown, although an antiannexin A5 antibody inhibits SERCA 2 activity 51 suggesting that annexin A5 regulates SERCA 2 and may be required for optimal function. Taken together these results show that TCDD alters the levels of proteins involved in calcium handling in the adult heart in a way that could result in calcium overload, and furthermore suggests a mechanism for the diastolic dysfunction observed in embryonic hearts following TCDD exposure.
Detection of Early Indicators of Hypertrophy and Heart Failure
In addition to the alterations in calcium handling proteins, we also detected changes in proteins that indicate that the TCDD exposed heart is under stress, including metabolic and structural markers linked to cardiac hypertrophy and heart failure. One such indicator of cellular stress is peroxiredoxin, a protein with important roles in cellular antioxidation 52, 53, which is significantly overexpressed in TCDD exposed heart samples (Figure 5(d)), suggesting an increase in oxidative stress. Another protein, cytochrome c oxidase subunit Vaa, the orthologue of human cytochrome c oxidase subunit Va (COX5a) which transfers electrons from cytochrome c, was found to be decreased. Transcript levels of COX5a were also reportedly decreased in tissue samples obtained from infarcted hearts 52–54. This may appear to suggest a mixed effect on the oxidative stress of TCDD exposure. However this protein also has important energy metabolic roles 55 and its down-regulation is often associated with severe and fatal metabolic disorders 56.
Another group of proteins that show significant alterations following TCDD exposure are involved in energy metabolism, particularly lipid metabolism and glycolysis. Fatty acid binding protein 3 (FAB3), a 15 kDa non-enzymatic protein, belongs to a family of carrier proteins for fatty acids and other lipophilic substances such as eicosanoids and retinoids 57. These proteins transport fatty acids across extra- and intracellular membranes, and therefore have an important role in lipid metabolism58. FAB3 may play a general and fundamental role in fatty acid transportation in tissues exhibiting anabolic and catabolic lipid metabolism and is also believed to transport lipophilic molecules from outer cell membrane to certain intracellular receptors such as the peroxisome proliferator-activated receptor (PPAR). This protein has shown a downregulation by more than one fold in TCDD treated heart samples (Figure 5(b)), suggesting a corresponding decrease in lipid metabolism in TCDD treated zebrafish heart tissue. Other important enzymes involved in lipid metabolism, such as acyl-Coenzyme A dehydrogenase and hydroxyacyl-Coenzyme A dehydrogenase, also showed variable but consistent reductions in expression.
In contrast, proteins involved in glycolysis showed consistent up-regulation in the TCDD treated heart samples. Glyceraldehyde-3-phosphate dehydrogenase [GAPDH], a major enzyme involved in glycolysis and energy production in the cytosol of eukaryotic cells, was found to be upregulated in TCDD exposed heart samples (Figure 5(c)). We also found that monocarboxylate transporter 4 was overexpressed following TCDD exposure. Monocarboxylate transporters, or MCTs, are a family of proton-linked plasma membrane transporters that carry monocarboxylates, such as lactate and pyruvate, across biological membranes.59, 60 Monocarboxylates are produced in large amounts as end-products of glycolysis. Consistent upregulation of other proteins involved in glycolysis was also observed. Collectively, these results suggest that TCDD induces an increase in cardiac glycolysis, while concurrently decreasing lipid metabolism.
The observed concurrent expression changes in lipid metabolic and glycolytic proteins is a particularly interesting finding, given the widely reported observation that this energy substrate switch is a characteristic hallmark in the development of heart hypertrophy and heart failure 61–63. In vertebrate hearts with normal cardiac functions, metabolism of fatty acid and lipid constitutes the major energy source to maintain cardiac contraction and relaxation 61. However, during the development of cardiac hypertrophy and heart failure, the chief myocardial energy source was observed to switch from fatty acid beta-oxidation to glycolysis 64. From these studies key regulatory genes governing the energy metabolism during cardiac hypertrophic growth were identified. One of the key fatty acid beta-oxidation genes, acyl-Coenzyme A dehydrogenase (ACAD), was found to be down-regulated during the progression from cardiac hypertrophy to ventricular dysfunction 64. Its protein product, acyl-Coenzyme A dehydrogenase (gi|47085823), was also found to be significantly downregulated in the TCDD treated heart tissue samples, suggesting nascent remodeling that could lead to cardiac hypertrophy.
Lastly, a number of key proteins involved in myocardium structure and regulatory functions were increased in TCDD treated heart samples based on the spectral count analysis. These proteins include a tropomyosin beta isoform, in addition to radixin and plectin. Tropomyosin (TPM) is an important regulatory protein in the light chain of cardiac muscle 65, 66. Along with the troponin complex, TPM associates with actin in muscle fibers and regulates muscle contraction by regulating the binding of myosin upon extracellular Ca2+ signaling 67–70. In vertebrate four TPM genes (alpha, beta, delta and gamma) have been identified which encode the four alpha helical subunits coiling together to form the quaternary TPM structure 71–74. Among these Tpm genes, Tpm2 has been found to play an important role in cardiac muscle function and has been shown to associate with familial hypertrophic cardiomyopathy in human 75, 76. Alteration of other TPM genes has also been associated with various myopathy phenotypes 71, 77. In a few recent studies TCDD treatment has been found to cause up-regulation of various TPM isoforms in different tissues of the mouse and rat at both protein 40, 41 and mRNA levels 42, indicating that this may be a characteristic TCDD toxic response commonly observed in vertebrate and mammalian species. In our experiments, up-regulation was observed for three TPM subunits by approximately the same level as these previous studies. The over expression of TPM2 in the developing mouse heart produces severe cardiac abnormalities such as altered diastolic function, thrombus formation in both atria and the left ventricle, atrial enlargement and fibrosis, diffuse myocytolysis and severely impaired myocardial contraction and relaxation 78.
Conclusions
In this work, we developed a statistically enhanced spectral counting approach to quantitatively study the protein expression changes induced by TCDD exposure in adult zebrafish hearts. This statistically controlled label free quantitative proteomics approach incorporated large scale MudPIT analysis with statistical evaluation of experimental reproducibility to provide more reliable protein expression analysis in large scale proteomic experiments. This methodology allows for the identification of a smaller protein dataset with improved quantification confidence. Through this approach we have identified significant changes in the proteome of the adult zebrafish heart following TCDD exposure. These changes suggest that TCDD alters calcium handling, energy metabolism and the cellular redox state of the adult zebrafish heart. Collectively, the protein expression information provides molecular insight into potential mechanisms of cardiac toxicity following TCDD exposure that warrants further study in both embryonic and adult zebrafish models.
Supplementary Material
Acknowledgement
We thank University of Wisconsin Human Proteomics Program for access to the LTQ instrument. This project was supported by grant number T32 ES007015 from the National Institute of Environmental Health Sciences (NIEHS), NIH and the Wisconsin Alumni Research Foundation at the University of Wisconsin-Madison. L.L. acknowledges an H. I. Romnes Research Fellowship. We thank Huseyin Guner for assistance in the Excel macro scripts for spectral counting and data analysis.
Footnotes
Supporting Information. Additional information as noted in the text. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kociba RJ, Schwetz BA. Toxicity of 2, 3, 7, 8-tetrachlorodibenzo-para-dioxin (TCDD) Drug Metabolism Reviews. 1982;13(3):387–406. doi: 10.3109/03602538209029986. [DOI] [PubMed] [Google Scholar]
- 2.Lilienfeld DE, Gallo MA. 2,4-D, 2,4,5-T, and 2,3,7,8-TCDD - An Overview. Epidemiologic Reviews. 1989;11:28–58. doi: 10.1093/oxfordjournals.epirev.a036044. [DOI] [PubMed] [Google Scholar]
- 3.Skene SA, Dewhurst IC, Greenberg M. Polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans - the risks to human health - a review. Human Toxicology. 1989;8(3):173–203. doi: 10.1177/096032718900800301. [DOI] [PubMed] [Google Scholar]
- 4.McNulty WP. Fetotoxicity of 2,3,7,8-tetrachlorodibenzo-para-dioxin (TCDD) for Rhesus Macaques (Macaca-mulatta) American Journal of Primatology. 1984;6(1):41–47. doi: 10.1002/ajp.1350060105. [DOI] [PubMed] [Google Scholar]
- 5.Hassoun EA, Walter AC, Alsharif NZ, Stohs SJ. Modulation of TCDD-induced fetotoxicity and oxidative stress in embryonic and placental tissues of C57BL/6J mice by vitamin E succinate and ellagic acid. Toxicology. 1997;124(1):27–37. doi: 10.1016/s0300-483x(97)00127-3. [DOI] [PubMed] [Google Scholar]
- 6.Elizondo G, Mejia-Garcia A, Sanchez-Ocampo E, Reyes-Hernandez O, Shibayama M. TCDD Potentiate CCl4 Hepatotoxicity Effects by Increasing CYP2E1 Hepatic Levels. Role of Aryl Hydrocarbon Receptor. Drug Metabolism Reviews. 2009;41:86. [Google Scholar]
- 7.Seo SH, Ryu YM, Kim KN, Kim HW, Kim YR, Lee SH, Won NH, Kim MK. TCDD(2,3,7,8,-Tetrachlorodibenzo-p-dioxin)-induced hepatotoxicity. Molecular & Cellular Toxicology. 2007;3(4):52–52. [Google Scholar]
- 8.Sherr DH. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and long term immunologic memory. Toxicological Sciences. 2004;79(2):211–213. doi: 10.1093/toxsci/kfh136. [DOI] [PubMed] [Google Scholar]
- 9.Kerkvliet NI. Recent advances in understanding the mechanisms of TCDD immunotoxicity. International Immunopharmacology. 2002;2(2–3):277–291. doi: 10.1016/s1567-5769(01)00179-5. [DOI] [PubMed] [Google Scholar]
- 10.LaMont Bryant P, Schmid JE, Fenton SE, Buckalew AR, Abbott BD. Teratogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the expression of EGF and/or TGF-alpha. Toxicological Sciences. 2001;62(1):103–114. doi: 10.1093/toxsci/62.1.103. [DOI] [PubMed] [Google Scholar]
- 11.Yasuda M, Igarashi E, Datu AR, Igawa H. Teratogenicity of 2,3,7,8-tetracholorodibenzo-para-dioxin (TCDD) in JCL-ICR Mice. Teratology. 1986;34(3):454–455. [Google Scholar]
- 12.Johnson ES. Important aspects of the evidence for TCDD carcinogenicity in man. Environmental Health Perspectives. 1993;99:383–390. doi: 10.1289/ehp.9399383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huff J, Lucier G, Tritscher A. Carcinogenicity of TCDD - Experimental, Mechanistic, and Epidemiologic Evidence. Annual Review of Pharmacology and Toxicology. 1994;34:343–372. doi: 10.1146/annurev.pa.34.040194.002015. [DOI] [PubMed] [Google Scholar]
- 14.Johnson ES. Human exposure to 2,3,7,8-TCDD and risk of cancer. Crit Rev Toxicol. 1991;21(6):451–63. doi: 10.3109/10408449209089883. [DOI] [PubMed] [Google Scholar]
- 15.Sielken RL. Quantitative Cancer Risk Assessments for 2,3,7,8-Tetrachlorodibenzopara-dioxin (TCDD) Food and Chemical Toxicology. 1987;25(3):257–267. doi: 10.1016/0278-6915(87)90093-7. [DOI] [PubMed] [Google Scholar]
- 16.Cheung MO, Peterson RE, Gilbert EF. Cardiovascular Teratogenicity of “2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) in Chick-embryo. Teratology. 1980;21(2):A34–A34. doi: 10.1016/0041-008x(81)90409-9. [DOI] [PubMed] [Google Scholar]
- 17.Jokinen MP, Walkers NJ, Brix AE, Sells DM, Nyska A. Exacerbation of cardiovascular patholgies in female Sprague-Dawley rats following chronic treatment with 3,3,4,4,5-pentachlorobiphenyl (PCB126) or 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Toxicological Sciences. 2003;72:142. doi: 10.1385/CT:3:4:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta V, Peterson RE, Heideman W. 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure prevents cardiac valve formation in developing zebrafish. Toxicological Sciences. 2008;104(2):303–311. doi: 10.1093/toxsci/kfn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carney SA, Prasch AL, Heideman W, Peterson RE. Understanding dioxin developmental toxicity using the zebrafish model. Birth Defects Research Part a-Clinical and Molecular Teratology. 2006;76(1):7–18. doi: 10.1002/bdra.20216. [DOI] [PubMed] [Google Scholar]
- 20.Handley-Goldstone HM, Grow MW, Stegeman JJ. Cardiovascular gene expression profiles of dioxin exposure in zebrafish embryos. Toxicological Sciences. 2005;85(1):683–693. doi: 10.1093/toxsci/kfi116. [DOI] [PubMed] [Google Scholar]
- 21.Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicological Sciences. 2005;84(2):368–377. doi: 10.1093/toxsci/kfi073. [DOI] [PubMed] [Google Scholar]
- 22.Carney SA, Chen J, Burns CG, Xiong KM, Peterson RE, Heideman W. Aryl hydrocarbon receptor activation produces heart-specific transcriptional and toxic responses in developing zebrafish. Molecular Pharmacology. 2006;70(2):549–561. doi: 10.1124/mol.106.025304. [DOI] [PubMed] [Google Scholar]
- 23.Ong SE, Foster LJ, Mann M. Mass spectrometric-based approaches in quantitative proteomics. Methods. 2003;29(2):124–130. doi: 10.1016/s1046-2023(02)00303-1. [DOI] [PubMed] [Google Scholar]
- 24.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Molecular & Cellular Proteomics. 2002;1(5):376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Sadygov RG, Yates JR. A Model for Random Sampling and Estimation of Relative Protein Abundance in Shotgun Proteomics. Analytical Chemistry. 2004;76(14):4193–4201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 26.Fenselau C, Yao X. 18O2-Labeling in Quantitative Proteomic Strategies: A Status Report. Journal of Proteome Research. 2009;8(5):2140–2143. doi: 10.1021/pr8009879. [DOI] [PubMed] [Google Scholar]
- 27.Yao XD, Afonso C, Fenselau C. Dissection of proteolytic O-18 labeling: Endoprotease-catalyzed O-16-to-O-18 exchange of truncated peptide substrates. Journal of Proteome Research. 2003;2(2):147–152. doi: 10.1021/pr025572s. [DOI] [PubMed] [Google Scholar]
- 28.Dong X, Xiong L, Jiang X, Wang Y. Quantitative Proteomic Analysis Reveals the Perturbation of Multiple Cellular Pathways in Jurkat-T Cells Induced by Doxorubicin. Journal of Proteome Research. 2010;9(11):5943–5951. doi: 10.1021/pr1007043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang P, Lo A, Young JB, Song JH, Lai R, Kneteman NM, Hao C, Li L. Targeted Quantitative Mass Spectrometric Identification of Differentially Expressed Proteins between Bax-Expressing and Deficient Colorectal Carcinoma Cells. Journal of Proteome Research. 2009;8(7):3403–3414. doi: 10.1021/pr9000477. [DOI] [PubMed] [Google Scholar]
- 30.De Souza AG, MacCormack TJ, Wang N, Li L, Goss GG. Large-Scale Proteome Profile of the Zebrafish (Danio rerio) Gill for Physiological and Biomarker Discovery Studies. Zebrafish. 2009;6(3):229–238. doi: 10.1089/zeb.2009.0591. [DOI] [PubMed] [Google Scholar]
- 31.Zybailov B, Mosley AL, Sardiu ME, Coleman MK, Florens L, Washburn MP. Statistical Analysis of Membrane Proteome Expression Changes in Saccharomyces cerevisiae . Journal of Proteome Research. 2006;5(9):2339–2347. doi: 10.1021/pr060161n. [DOI] [PubMed] [Google Scholar]
- 32.Lundgren DH, Hwang S-I, Wu L, Han DK. Role of spectral counting in quantitative proteomics. Expert Review of Proteomics. 2010;7(1):39–53. doi: 10.1586/epr.09.69. [DOI] [PubMed] [Google Scholar]
- 33.Zybailov B, Coleman MK, Florens L, Washburn MP. Correlation of Relative Abundance Ratios Derived from Peptide Ion Chromatograms and Spectrum Counting for Quantitative Proteomic Analysis Using Stable Isotope Labeling. Analytical Chemistry. 2005;77(19):6218–6224. doi: 10.1021/ac050846r. [DOI] [PubMed] [Google Scholar]
- 34.Paoletti AC, Parmely TJ, Tomomori-Sato C, Sato S, Zhu DX, Conaway RC, Conaway JW, Florens L, Washburn MP. Quantitative proteomic analysis of distinct mammalian Mediator complexes using normalized spectral abundance factors. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(50):18928–18933. doi: 10.1073/pnas.0606379103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavelka N, Fournier ML, Swanson SK, Pelizzola M, Ricciardi-Castagnoli P, Florens L, Washburn MP. Statistical Similarities between Transcriptomics and Quantitative Shotgun Proteomics Data. Molecular & Cellular Proteomics. 2008;7(4):631–644. doi: 10.1074/mcp.M700240-MCP200. [DOI] [PubMed] [Google Scholar]
- 36.Westerfield M. The Zebrafish Book; A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio) 2nd Ed. Eugene, OR: University of Oregon Press; 1993. [Google Scholar]
- 37.Lanham KA, Peterson RE, Heideman W. Sensitivity to Dioxin Decreases as Zebrafish Mature. Toxicological Sciences. 2012;127(2):360–370. doi: 10.1093/toxsci/kfs103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Wen Z, Washburn MP, Florens L. Refinements to Label Free Proteome Quantitation: How to Deal with Peptides Shared by Multiple Proteins. Analytical Chemistry. 2010;82(6):2272–2281. doi: 10.1021/ac9023999. [DOI] [PubMed] [Google Scholar]
- 39.Fermin D, Basrur V, Yocum AK, Nesvizhskii AI. Abacus: A computational tool for extracting and pre-processing spectral count data for label-free quantitative proteomic analysis. Proteomics. 2011;11(7):1340–1345. doi: 10.1002/pmic.201000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishimura R, Ohsako S, Kawakami T, Sakaue M, Aoki Y, Tohyama C. Altered Protein Profile and Possible Hypoxia in the Placenta of 2,3,7,8-Tetrachlorodibenzo-p-dioxin-Exposed Rats. Toxicology and Applied Pharmacology. 2002;185(3):197–206. doi: 10.1006/taap.2002.9539. [DOI] [PubMed] [Google Scholar]
- 41.Carpi D, Korkalainen M, Airoldi L, Fanelli R, Hakansson H, Muhonen V, Tuukkanen J, Viluksela M, Pastorelli R. Dioxin-Sensitive Proteins in Differentiating Osteoblasts: Effects on Bone Formation In Vitro. Toxicol. Sci. 2009;108(2):330–343. doi: 10.1093/toxsci/kfp021. [DOI] [PubMed] [Google Scholar]
- 42.Fletcher N, Wahlstrom D, Lundberg R, Nilsson CB, Nilsson KC, Stockling K, Hellmold H, Hakansson H. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) alters the mRNA expression of critical genes associated with cholesterol metabolism, bile acid biosynthesis, and bile transport in rat liver: A microarray study. Toxicology and Applied Pharmacology. 2005;207(1):1–24. doi: 10.1016/j.taap.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Tabb DL, Vega-Montoto L, Rudnick PA, Variyath AM, Ham A-JL, Bunk DM, Kilpatrick LE, Billheimer DD, Blackman RK, Cardasis HL, Carr SA, Clauser KR, Jaffe JD, Kowalski KA, Neubert TA, Regnier FE, Schilling B, Tegeler TJ, Wang M, Wang P, Whiteaker JR, Zimmerman LJ, Fisher SJ, Gibson BW, Kinsinger CR, Mesri M, Rodriguez H, Stein SE, Tempst P, Paulovich AG, Liebler DC, Spiegelman C. Repeatability and Reproducibility in Proteomic Identifications by Liquid Chromatography Tandem Mass Spectrometry. Journal of Proteome Research. 2009;9(2):761–776. doi: 10.1021/pr9006365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Chang-Wong T, Tang H-Y, Speicher DW. Comparison of Extensive Protein Fractionation and Repetitive LC-MS/MS Analyses on Depth of Analysis for Complex Proteomes. Journal of Proteome Research. 2009;9(2):1032–1040. doi: 10.1021/pr900927y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterson RE, Madhukar BV, Yang KH, Matsumura F. Depression Of Adenosine-Triphosphatase Activities In Isolated Liver Surface-Membranes Of 2,3,7,8-Tetrachlorodibenzo-para-Dioxin-Treated Rats - Correlation With Effects On Ouabain Biliary-Excretion And Bile-Flow. Journal of Pharmacology and Experimental Therapeutics. 1979;210(2):275–282. [PubMed] [Google Scholar]
- 46.Schmidt-Schweda SH, Linnemuller S, Hasenfuss G, Prestle J, Pieske B, Goebel J, Hellige N. Adenovirus mediated overexpression of Na+/Ca2+-exchanger in rabbit cardiomyocytes impairs force-frequency behavior and Ca2+-cycling. European Heart Journal. 2001;22:539–539. [Google Scholar]
- 47.Terracciano CMN, De Souza AI, Philipson KD, MacLeod KT. Na+-Ca2+ exchange and sarcoplasmic reticular Ca2+ regulation in ventricular myocytes from transgenic mice overexpressing the Na+-Ca2+ exchanger. Journal of Physiology-London. 1998;512(3):651–667. doi: 10.1111/j.1469-7793.1998.651bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schillinger W, Ohler A, Emami S, Muller F, Christians C, Janssen PML, Kogler H, Teucher N, Pieske B, Seidler T, Hasenfuss G. The functional effect of adenoviral Na+/Ca2+ exchanger overexpression in rabbit myocytes depends on the activity of the Na+/K+-ATPase. Cardiovascular Research. 2003;57(4):996–1003. doi: 10.1016/s0008-6363(02)00829-5. [DOI] [PubMed] [Google Scholar]
- 49.Canga L, Paroli L, Blanck TJJ, Silver RB, Rifkind AB. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Increases Cardiac Myocyte Intracellular Calcium And Progressively Impairs Ventricular Contractile Responses To Isoproterenol And To Calcium In Chick-Embryo Hearts. Molecular Pharmacology. 1993;44(6):1142–1151. [PubMed] [Google Scholar]
- 50.Camors E, Charue D, Trouve P, Monceau V, Loyer X, Russo-Marie F, Charlemagne D. Association of annexin A5 with Na+/Ca2+ exchanger and caveolin-3 in nonfailing and failing human heart. Journal of Molecular and Cellular Cardiology. 2006;40(1):47–55. doi: 10.1016/j.yjmcc.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 51.Moraru II, Syrbu S, Zecevic N, Hager WD, Watras J, Messineo F. An antibody to annexin V blocks Ca2+-dependent ATPase activity of sarcoplasmic reticulum in human failing hearts. Cardiac Sarcoplasmic Reticulum Function and Regulation of Contractility. 1998;853:333–337. doi: 10.1111/j.1749-6632.1998.tb08293.x. [DOI] [PubMed] [Google Scholar]
- 52.Wood ZA, Schr 鰀 er E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends in Biochemical Sciences. 2003;28(1):32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 53.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radical Biology and Medicine. 2005;38(12):1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 54.Wood ZA, Schroder E, Harris JR, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends in Biochemical Sciences. 2003;28(1):32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 55.Fontanesi F, Soto IC, Horn D, Barrientos A. Assembly of mitochondrial cytochrome c-oxidase, a complicated and highly regulated cellular process. American Journal of Physiology - Cell Physiology. 2006;291(6):C1129–C1147. doi: 10.1152/ajpcell.00233.2006. [DOI] [PubMed] [Google Scholar]
- 56.Pecina P, Houstkova H, Hansikova H, Zeman J, Houstek J. Genetic defects of cytochrome C oxidase assembly. Physiological Research. 2004;53:S213–S223. [PubMed] [Google Scholar]
- 57.Chmurzynska A. The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism. Journal of Applied Genetics. 2006;47(1):39–48. doi: 10.1007/BF03194597. [DOI] [PubMed] [Google Scholar]
- 58.Weisiger RA. Cytosolic fatty acid binding proteins catalyze two distinct steps in intracellular transport of their ligands. Molecular and Cellular Biochemistry. 2002;239(1–2):35–43. [PubMed] [Google Scholar]
- 59.Halestrap A, Meredith D. The SLC16 gene family—from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Archiv European Journal of Physiology. 2004;447(5):619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 60.Wang Q, Morris ME. The role of monocarboxylate transporter 2 and 4 in the transport of gamma-hydroxybutyric acid in mammalian cells. Drug Metabolism and Disposition. 2007;35(8):1393–1399. doi: 10.1124/dmd.107.014852. [DOI] [PubMed] [Google Scholar]
- 61.Sack MN, Kelly DP. The energy substrate switch during development of heart failure: Gene regulatory mechanisms (Review) International Journal of Molecular Medicine. 1998;1(1):17–24. doi: 10.3892/ijmm.1.1.17. [DOI] [PubMed] [Google Scholar]
- 62.Sack MN, Rader TA, Kelly DP. Characterization of a gene regulatory pathway involved in the energy substrate switch during development of cardiac hypertrophy in transgenic mice. Journal of Investigative Medicine. 1996;44(7):A366–A366. [Google Scholar]
- 63.Sack MN, Rader TA, Schaffer JE, Rockman HA, Kelly DP. Expression of genes involved in myocardial fatty acid utilization and glycolysis is regulated in parallel with the energy substrate switch in the pressure overloaded ventricle. Circulation. 1996;94(8):1791–1791. [Google Scholar]
- 64.Barger PM, Kelly DP. Fatty acid utilization in the hypertrophied and failing heart: Molecular regulatory mechanisms. American Journal of the Medical Sciences. 1999;318(1):36–42. doi: 10.1097/00000441-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 65.Gunning P, O'Neill G, Hardeman E. Tropomyosin-based regulation of the actin cytoskeleton in time and space. Physiological Reviews. 2008;88(1):1–35. doi: 10.1152/physrev.00001.2007. [DOI] [PubMed] [Google Scholar]
- 66.Wolska BM, Wieczorek DF. The role of tropomyosin in the regulation of myocardial contraction and relaxation. Pflugers Archiv-European Journal of Physiology. 2003;446(1):1–8. doi: 10.1007/s00424-002-0900-3. [DOI] [PubMed] [Google Scholar]
- 67.Gordon AM, Regnier M, Homsher E. Skeletal and cardiac muscle contractile activation: Tropomyosin “rocks and rolls”. News in Physiological Sciences. 2001;16:49–55. [PubMed] [Google Scholar]
- 68.Elsaleh SC, Warber KD, Potter JD. The Role of Tropomyosin Troponin in the Regulation of Skeletal-muscle Contraction. Journal of Muscle Research and Cell Motility. 1986;7(5):387–404. doi: 10.1007/BF01753582. [DOI] [PubMed] [Google Scholar]
- 69.Solaro RJ, Rarick HM. Troponin and tropomyosin - Proteins that switch on and tune in the activity of cardiac myofilaments. Circulation Research. 1998;83(5):471–480. doi: 10.1161/01.res.83.5.471. [DOI] [PubMed] [Google Scholar]
- 70.Zot AS, Potter JD. Structural Aspects of Troponin-Tropomyosin Regulation of Skeletal-muscle Contraction. Annual Review of Biophysics and Biophysical Chemistry. 1987;16:535–559. doi: 10.1146/annurev.bb.16.060187.002535. [DOI] [PubMed] [Google Scholar]
- 71.Perry SV. Vertebrate tropomyosin: distribution, properties and function. Journal of Muscle Research and Cell Motility. 2001;22(1):5–49. doi: 10.1023/a:1010303732441. [DOI] [PubMed] [Google Scholar]
- 72.Gunning PW, Schevzov G, Kee AJ, Hardeman EC. Tropomyosin isoforms: divining rods for actin cytoskeleton function. Trends in Cell Biology. 2005;15(6):333–341. doi: 10.1016/j.tcb.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 73.Leesmiller JP, Helfman DM. The Molecular-basis for Tropomyosin Isoform Diversity. Bioessays. 1991;13(9):429–437. doi: 10.1002/bies.950130902. [DOI] [PubMed] [Google Scholar]
- 74.Moraczewska J. [Role of tropomyosin isoforms in diversification of actin filaments functions] Postepy Biochem. 2009;55(2):201–6. [PubMed] [Google Scholar]
- 75.Tardiff JC. Sarcomeric proteins and familial hypertrophic cardiomyopathy: Linking mutations in structural proteins to complex cardiovascular phenotypes. Heart Failure Reviews. 2005;10(3):237–248. doi: 10.1007/s10741-005-5253-5. [DOI] [PubMed] [Google Scholar]
- 76.Alcalai R, Seidman JG, Seidman CE. Genetic basis of hypertrophic cardiomyopathy: From bench to the clinics. Journal of Cardiovascular Electrophysiology. 2008;19(1):104–110. doi: 10.1111/j.1540-8167.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 77.Laing NG. Congenital myopathies. Current Opinion in Neurology. 2007;20(5):583–589. doi: 10.1097/WCO.0b013e3282ef6e69. [DOI] [PubMed] [Google Scholar]
- 78.Muthuchamy M, Boivin GP, Grupp IL, Wieczorek DF. [beta]-Tropomyosin Overexpression Induces Severe Cardiac Abnormalities. Journal of Molecular and Cellular Cardiology. 1998;30(8):1545–1557. doi: 10.1006/jmcc.1998.0720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.