Abstract
Purpose
Concurrent inhibition of multiple oncogenic signaling pathways might improve the efficacy of anticancer agents and abrogate resistance mechanisms. This phase I study evaluated temsirolimus in combination with sunitinib in patients with advanced RCC.
Patients and Methods
Eligibility included advanced RCC and ≤ 2 previous systemic regimens. At the starting dose, temsirolimus 15 mg was administered by intravenous (I.V.) infusion once weekly, and sunitinib 25 mg was administered orally once daily for 4 weeks, followed by a 2-week rest period.
Results
In the first cohort, dose-limiting toxicities (grade 3 treatment-related toxicities that lasted ≥ 7 days) were observed in 2 of 3 patients. One patient experienced grade 3 rash during week 3, which led to treatment discontinuation. A second patient had grade 3 thrombocytopenia (platelet count, 48,000/μL), cellulitis, and gout during week 3 and was hospitalized; platelets recovered to 109,000/μL 4 days after discontinuation of protocol therapy. A third patient experienced rash, asthenia, diarrhea, stomatitis, constipation, fever, and rectal hemorrhage, all of which were mild in severity. The study was terminated because of dose-limiting toxicity observed at low starting doses of both agents.
Conclusion
Concomitant use of I.V. temsirolimus 15 mg weekly and oral sunitinib 25 mg daily (4 weeks on, 2 weeks off) is not recommended.
Keywords: Hypertriglyceridemia, Kidney cancer, Mammalian target of rapamycin, Mucositis, Targeted therapy
Introduction
The use of targeted therapies has substantially improved outcomes for patients with advanced renal cell carcinoma (RCC).1 One strategy to provide further clinical benefit is to combine active agents that target different oncogenic pathways and do not display overlapping toxicities.2,3 It is thought that concurrent inhibition of several oncogenic signaling pathways might improve the efficacy of anticancer agents and abrogate resistance mechanisms.
Treatment of advanced RCC with either temsirolimus or sunitinib has led to beneficial clinical outcomes relative to interferon (IFN)–α. Temsirolimus targets the mammalian target of rapamycin pathway, an important intracellular mediator of multiple growth signals.4-7 Treatment with temsirolimus significantly prolongs overall survival (P = .008) and progression-free survival (PFS; P < .001) compared with IFN in patients with previously untreated advanced RCC and poor-risk features.8
Sunitinib malate inhibits several receptor tyrosine kinases at the surface of tumor cells, including the vascular endothelial growth factor (VEGF) receptor, which is important for tumor angiogenesis.3,9 In patients with previously untreated metastatic RCC, treatment with sunitinib significantly prolongs PFS compared with IFN (P < .001).10
Based on the distinct targeted mechanisms and acceptable toxicity profiles of these 2 agents, a dual-dose escalation study of temsirolimus and sunitinib was conducted to evaluate the safety of this combination treatment in patients with advanced RCC. We found that there was substantial toxicity at low starting doses of both agents, and the study was terminated. This report describes the clinical course and toxicities experienced by patients who received concomitant treatment with temsirolimus plus sunitinib.
Patients and Methods
This phase I study was conducted to determine the maximum tolerated dose of temsirolimus and sunitinib when given in combination to patients with advanced RCC. The study population consisted of patients aged ≥ 18 years with histologically confirmed advanced RCC, unidimensionally measurable disease, and ≤ 2 previous systemic treatment regimens. Other inclusion criteria included Eastern Cooperative Oncology Group performance status of 0 or 1; adequate organ function defined as absolute neutrophil count ≥ 1500 cells/μL, hemoglobin ≥ 8.0 g/dL without transfusion within 2 weeks of starting treatment, serum calcium ≤ 12 mg/dL, serum creatinine ≤ 1.5 × the upper limit of normal (ULN), total serum bilirubin ≤ 1.5 × ULN, and serum transaminase levels ≤ 3 × ULN (≤ 5 × ULN if hepatic metastases are present); metabolic, thyroid, and pancreatic function within laboratory limits of normal; adequate cardiac function defined by the absence of all of the following for the 12 months before treatment: severe/unstable angina, myocardial infarction, coronary bypass graft and symptomatic congestive heart failure; ≥ 4 weeks since previous treatment with immunotherapy or other systemic therapy regimens with resolution of all toxic effects of previous therapy to grade ≤ 1 severity (National Cancer Institute Common Terminology Criteria for Adverse Events [NCI CTCAE], version 3.0); and signed informed consent. Patient exclusion criteria included active central nervous system primary or metastatic malignancy; major surgery, open biopsy, or traumatic injury within 4 weeks of screening or a nonhealing wound or ulcer; grade ≥ 3 hemorrhage within the past month; known pulmonary hypertension or pneumonitis; patients receiving concomitant strong CYP3A4 inhibitors and/or inducers, warfarin, or immunosuppressive agents; and chronic or active viral/bacterial/fungal illnesses such as HIV.
Patients were enrolled from March 2007 to April 2007 after approval of the protocol by the Memorial Sloan-Kettering Cancer Center Institutional Review Board. At the starting dose, temsirolimus 15 mg was administered by intravenous (I.V.) infusion over a 30-minute period once weekly, and sunitinib 25 mg was administered orally once daily for 4 weeks, followed by a 2-week rest period. All patients were premedicated with I.V. diphenhydramine 25 mg to 50 mg 30 minutes before each temsirolimus infusion.
Dose levels were planned to be escalated in successive cohorts of 3 to 6 patients. In a given cohort, all patients were to be treated through day 42 (cycle 1) before enrollment of the next cohort. Dose-limiting toxicities (DLTs) were defined as treatment-related grade 3/4 toxicities that lasted ≥ 7 days or toxicities that required dose suspension and occurred during cycle 1 of treatment. Toxicities were graded according to the NCI CTCAE, version 3.0.
Results
The study was terminated for safety concerns after 3 patients (2 men, 1 woman) were enrolled and were treated at the starting dose levels. All 3 patients received 3 weekly doses of I.V. temsirolimus 15 mg and once-daily sunitinib 25 mg capsules for 19 to 21 days. Adverse events (AEs; Table 1) were mostly mild in severity (grade 1/2) and almost all were considered to be related to treatment. Rash occurred in all 3 patients; asthenia, constipation, and diarrhea were reported in 2 patients each.
Table 1.
Number of Patients with Treatment-Emergent Adverse Events
| Adverse Event | Temsirolimus 15 mg/Sunitinib 25 mg (N = 3) | |
|---|---|---|
| Any Grade* | Grade 3* | |
| Any | 3 | 2 |
| Rash | 3 | 1 |
| Asthenia | 2 | 0 |
| Constipation | 2 | 0 |
| Diarrhea | 2 | 0 |
| Cellulitis | 1 | 1 |
| Fever | 1 | 0 |
| Dyspepsia | 1 | 0 |
| Rectal Hemorrhage | 1 | 0 |
| Stomatitis | 1 | 0 |
| Ecchymosis | 1 | 0 |
| Thrombocytopenia | 1 | 1 |
| Gout | 1 | 1 |
| Hypertriglyceridemia | 1 | 0 |
| Epistaxis | 1 | 0 |
Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.
Two patients had treatment-related DLTs that led to discontinuation (Table 2): grade 3 erythematous acneiform rash involving the groin and axilla (patient 1) and grade 3 cellulitis (patient 2). One patient (patient 3) had more moderate toxicities and discontinued treatment when the study was terminated.
Table 2.
Reasons for Discontinuation of Combined Treatment with Intravenous Temsirolimus 15 mg Weekly and Oral Sunitinib 25 mg Daily (N = 3)
| Patient Age, Years | Sex | Treatment Duration (Days) | Primary Reason for Discontinuation |
|---|---|---|---|
| 64 | Male | 21 | Adverse event (grade 3* groin and axillary erythematous rash) |
| 66 | Male | 19 | Adverse event (grade 3* cellulitis requiring hospitalization) |
| 33 | Female | 22 | Study termination |
Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.
Patient 1
A man aged 64 years was diagnosed with metastatic RCC (papillary subtype) in October 2006 and underwent nephrectomy; subsequent evaluation of the extent of disease revealed growing retroperitoneal lymphadenopathy. He had received no systemic therapy for metastatic RCC before initiating treatment with temsirolimus plus sunitinib on March 15, 2007. The patient was treated for a period of 21 days, during which time he reported grade 2 dyspepsia, grade 1 asthenia, grade 1 ecchymosis (considered not related to treatment), and grade 1 hypertriglyceridemia. He also had a rash (erythematous, groin) that was initially grade 2 but escalated to grade 3 (erythematous, acneiform groin, and axillary rash) during week 3 (Figure 1). This AE was a DLT and led to discontinuation of treatment; the rash resolved gradually over 1 to 2 weeks.
Figure 1. Grade 3 Rash Observed in Patient 1 During Week 3 of Treatment with Temsirolimus/Sunitinib.
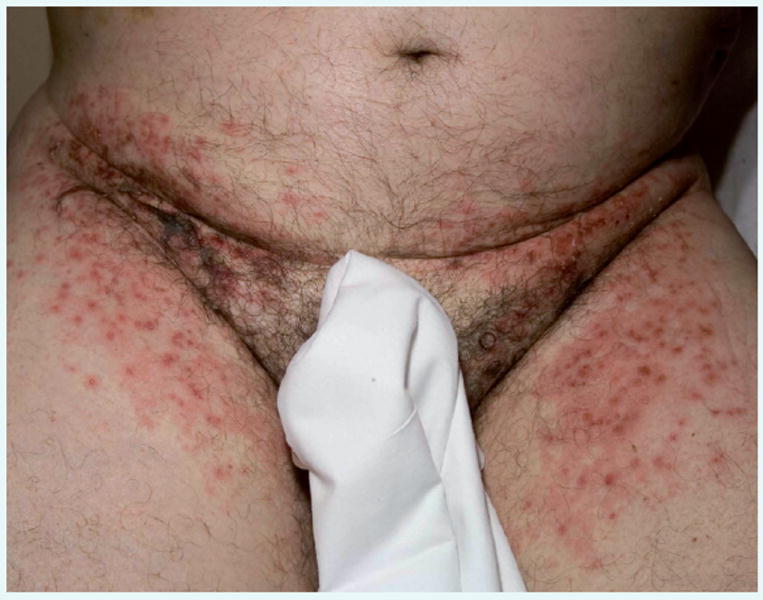
Assessed by Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.
Patient 2
A 66-year-old man was diagnosed in July 2003 with poorly differentiated clear-cell metastatic RCC and underwent nephrectomy. On subsequent imaging examinations, slowly growing pulmonary nodules were detected. He began treatment with temsirolimus and sunitinib on April 23, 2007. On day 15, the patient reported grade 1 constipation and grade 2 thrombocytopenia, which escalated to grade 3 (platelet count, 48,000/μL) on day 20. Treatment was discontinued on day 19, when he developed grade 3 gout and cellulitis and was hospitalized. These AEs resolved with corticosteroids and I.V. antibiotics. The patient was discharged after 3 days, at which time his platelets recovered to 109,000/μL. He reported grade 1 epistaxis, which resolved within 15 days without any therapeutic intervention, as well as grade 1 diarrhea and rash (redness in the face). Grade 1 mucositis and palmar-plantar erythrodysesthesia were also reported on day 36, 17 days after treatment was discontinued. The hospitalization for cellulitis and discontinuation of treatment constituted a DLT.
Patient 3
A 33-year-old woman with RCC (unclassified subtype) meta-static to the retroperitoneum was diagnosed in November 2005 and initially underwent nephrectomy. Subsequent computed tomography scans showed enlarging pulmonary nodules, and the patient began treatment in a clinical trial with a sunitinib-plus-bevacizumab combination regimen. After 6 cycles, the disease progressed, and she subsequently started treatment with temsirolimus plus sunitinib on April 5, 2007, and continued for 22 days. This patient experienced an acne-like rash on her back, along with diarrhea, stomatitis, constipation, fever (not deemed related to treatment), and rectal hemorrhage, all of which were mild (grade 1), and grade 2 asthenia. She discontinued treatment upon termination of the study.
Discussion
For patients with advanced RCC, the recommended clinical dose of temsirolimus is 25 mg I.V. weekly and, for sunitinib, it is 50 mg orally once daily for 4 weeks, followed by 2 weeks off.11,12 Despite using low doses of both agents at the starting dose level (I.V. temsirolimus 15 mg weekly and oral sunitinib 25 mg daily), combination treatment resulted in DLTs in 2 of the 3 patients (severe acneiform erythematous rash and gout/cellulitis requiring hospitalization). These DLTs led to termination of the study rather than continuing at even lower dose levels, owing to efficacy concerns about lower doses of both agents. Because target saturation for temsirolimus (FKBP-12) occurs at a dose of approximately 12.5 mg,13 dose reduction below 15 mg was not pursued in this study.11
The 15-mg dose level of temsirolimus, 40% lower than the clinical dose, was previously studied in combination with IFN and was found to exhibit acceptable tolerability.8,14 Although only 3 patients were treated in our study, the fact that 2 had DLTs indicates that the combination of temsirolimus and sunitinib showed excessive toxicity. Reasons for the excessive toxicity are not known. Whereas rash occurs in patients receiving either of these agents alone, it is typically mild to moderate in severity and is manageable.8,10 Thus, it was not surprising that all 3 patients who were treated with the combination of temsirolimus and sunitinib developed rash. The severe rash that developed in 1 patient might have been a synergistic effect. Additionally, peripheral edema occurs in patients treated with either agent alone, but cellulitis is infrequently observed with temsirolimus and is not reported as being associated with sunitinib.11,12
Because these 2 agents have different targeted mechanisms, concomitant treatment might result in additive or synergistic effects on overlapping toxicities, as was suggested in the case of rash, or might inhibit compensatory mechanisms that interfere with the development of specific drug toxicities that are not seen clinically with the single agents. Preliminary results from a phase I trial of temsirolimus and the anti-VEGF monoclonal antibody bevacizumab showed that this combination could be administered at full doses.15 Dose-limiting toxicities were grade 3 hypertriglyceridemia and mucositis, each occurring in 1 of 12 patients. A phase III trial is currently studying temsirolimus/bevacizumab versus bevacizumab/IFN-α in a larger patient population. The combination of sunitinib and bevacizumab was also explored in a phase I study but, while active, exhibited unacceptable toxicity at the doses tested.16
Conclusion
Because of excessive toxicity, the combination of I.V. temsirolimus 15 mg weekly and oral sunitinib 25 mg daily (4 weeks on, 2 weeks off) should not be used clinically. Ongoing and planned studies are investigating each of these agents in combination with other targeted agents in patients with advanced RCC. In addition, alternate dosing strategies for the combination of temsirolimus and sunitinib could be considered within the context of clinical trials. The results of this study underscore the need for careful assessment of overlapping toxicities when combining active agents in clinical trials and for considering the possibility of unanticipated toxicity at low starting-dose levels in the design of future studies.
Acknowledgments
The authors wish to thank Patricia Fischer for her expert nursing assistance and Drs Gary Hudes, Michael Carducci, David McDermott, Greg Lubiniecki, and Mark Gelder for their critical review and contributions to the protocol. We also thank Peloton Advantage for assisting with manuscript preparation.
References
- 1.Motzer RJ, Basch E. Targeted drugs for metastatic renal cell carcinoma. Lancet. 2007;370:2071–3. doi: 10.1016/S0140-6736(07)61874-1. [DOI] [PubMed] [Google Scholar]
- 2.Sosman JA, Puzanov I, Atkins MB. Opportunities and obstacles to combination targeted therapy in renal cell cancer. Clin Cancer Res. 2007;13:764s–9s. doi: 10.1158/1078-0432.CCR-06-1975. [DOI] [PubMed] [Google Scholar]
- 3.Patel PH, Chadalavada RS, Chaganti RS, et al. Targeting von Hippel-Lindau pathway in renal cell carcinoma. Clin Cancer Res. 2006;12:7215–20. doi: 10.1158/1078-0432.CCR-06-2254. [DOI] [PubMed] [Google Scholar]
- 4.Abraham RT, Gibbons JJ. The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res. 2007;13:3109–14. doi: 10.1158/1078-0432.CCR-06-2798. [DOI] [PubMed] [Google Scholar]
- 5.Fingar DC, Richardson CJ, Tee AR, et al. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24:200–16. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Ma WW, Jimeno A. Temsirolimus. Drugs Today (Barc) 2007;43:659–69. doi: 10.1358/dot.2007.43.10.1148059. [DOI] [PubMed] [Google Scholar]
- 8.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 9.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884–96. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in meta-static renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 11.Torisel [package insert] Philadelphia, PA: Wyeth Pharmaceuticals, Inc; 2007. [Google Scholar]
- 12.Sutent [package insert] New York: Pfizer Inc; 2007. [Google Scholar]
- 13.Boni J, Burns J, Hug B, et al. mTOR inhibition following a single intravenous infusion of temsirolimus in healthy individuals. Presented at: AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics; San Francisco, CA. October 22-27, 2007. [Google Scholar]
- 14.Motzer RJ, Hudes GR, Curti BD, et al. Temsirolimus plus interferon alfa phase I trial in patients with advanced renal cell carcinoma. J Clin Oncol. 2007;25:3958–64. doi: 10.1200/JCO.2006.10.5916. [DOI] [PubMed] [Google Scholar]
- 15.Merchan JR, Liu G, Fitch T, et al. Phase I/II trial of CCI-779 and bevacizumab in stage IV renal cell carcinoma: phase I safety and activity results. J Clin Oncol. 2007;25(18 suppl):243s. Abstract 5034. [Google Scholar]
- 16.Feldman DR, Ginsberg MS, Baum M, et al. Phase I trial of bevacizumab plus sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26(20 suppl):259s. doi: 10.1200/JCO.2008.19.0108. Abstract 5099. [DOI] [PMC free article] [PubMed] [Google Scholar]


