Abstract
Skin is the largest body organ forming a metabolically active barrier between external and internal environments. The metabolic barrier is composed of cytochromes P450 (CYPs) that regulate its homeostasis through activation or inactivation of biologically relevant molecules. In this review we focus our attention on local steroidogenic and secosteroidogenic systems in relation to skin cancer, e.g., prevention, attenuation of tumor progression and therapy. The local steroidogenic system is composed of locally expressed CYPs involved in local production of androgens, estrogens, gluco- and mineralo-corticosteroids from cholesterol (initiated by CYP11A1) or from steroid precursors delivered to the skin, and of their metabolism and/or inactivation. Cutaneous 7-hydroxylases (CYP7A1, CYP7B1 and CYP39) potentially can produce 7-hydroxy/oxy-steroids/sterols with modifying effects on local tumorigenesis. CYP11A1 also transforms 7-dehydrocholesterol (7DHC)→22(OH)7DHC→20,22(OH)2-7DHC→7-dehydropregnenolone, which can be further metabolized to other 5,7-steroidal dienes. These 5,7-dienal intermediates are converted by ultraviolet radiation B (UVB) into secosteroids which show pro-differentiation and anti-cancer properties. Finally, the skin is the site of activation of vitamin D3 through two alternative pathways. The classical one involves sequential hydroxylation at positions 25 and 1 to produce active 1,25(OH)2D3, which is further inactivated through hydroxylation at C24. The novel pathway is initiated by CYP11A1 with predominant production of 20(OH)D3 which is further metabolized to biologically active but non-calcemic D3-hydroxyderivatives. Classical and non-classical (novel) vitamin D analogs show pro-differentiation, anti-proliferative and anticancer properties. In addition, melatonin is metabolized by local CYPs. In conclusion cutaneously expressed CYPs have significant effects on skin physiology and pathology trough regulation of its chemical milieu.
Keywords: CYP, steroids, secosteroids, vitamin D, skin cancer, melatonin
1. SKIN AS AN ENVIRONMENTALLY REGULATED ORGAN - IMPLICATIONS FOR CANCER: AN OVERVIEW
Skin, consisting of epidermal, dermal and subcutaneous layers, is the most important barrier between the environment and internal milieu. All layers fulfill distinct functions in order to protect the organism. Of those layers, the epidermis is mostly affected by external stimuli of different kinds. The proliferating keratinocytes form several layers that ultimately form a permeability barrier made of cross-linked proteins (cornified cell envelope) and lipids (cornified lipid envelope) [1]. Keratohyaline granules and lamellar bodies are early morphological expressions of protein and lipid complexes, respectively. Differentiating keratinocytes express different intermediate filaments (cytokeratins) and accessory proteins (involucrin, loricrin, cystatin, envoplakin, periplakin, fillagrin and others) in progressing layers to build the epidermal barrier. Cross-linking enzymes, most importantly transglutaminase 1 form lysine isopeptide bonds between proteins of the cornified cells’ envelope. Terminal differentiation is affected by the calcium gradient, Notch signaling and p63 [2–4]. Lamellar bodies contain various lipids (e.g phospholipids), enzymes (e.g. beta glucocerebrosidase, cathepsin), enzyme inhibitors (e.g. elafin) and antimicrobial peptides (e.g. beta-defensins). Ultimately, the lipid envelope is made of cholesterol, ceramides and free fatty acids present between protein blocks [4–6]. Keratinocytes though do not simply provide the structural scaffolding, but also actively produce several substances (cytokines, neurotransmitters, hormones) [7–10]. In addition to traditionally recognized external stimuli such as pressure, pain, temperature and UV light (melanocytes, keratinocytes), skin is recently recognized to respond to xenobiotics. Melanin, produced by neural crest derived melanocytes, serves as a scavenger of xenobiotics, reactive oxygen species and certainly light of a broad spectrum [11, 12]. Of note, xenobiotics are also processed by cytochrome P450 enzymes that are thus present not only in the liver, as commonly known, but also in other cells of the body, including keratinocytes [13, 14].
Most common skin neoplasms are derived from keratinocytes and are known as basal and squamous cell carcinoma (Fig. (1)). The etiologic factors for squamous cell carcinoma are multiple with UV light being recognized as the most important one. The chemical agents such as petroleum oils, coal tar, soot and arsenic are also causative. Response to physical injuries in the form of chronic scarring and chronic inflammation play a role the development of squamous cell carcinomas. P53 mutations have a major role in the development of both basal and squamous cell carcinomas. Squamous cell carcinomas are characterized by aneuploidy and deletions of several chromosomes including 3p, 9q, 9p, 13q, 17p and 17q [2]. PATCH has a role primarily in the development of basal cell carcinomas. The skin neoplasm derived from melanocytes is known as melanoma (Fig. (2)). Again, UV light (in particular UVB) is recognized as the most prominent etiologic agent, while P16, BRAF and NRAS are the main genes involved in its formation [15–19]. There are multiple types of vascular and pseudovascular lesions (Fig. (3)) including lobular capillary hemangiomas (Fig. (3A)), angiokeratomas (Fig. (3B)) and hemorrhagic dermatofibromas (Fig. (3C)). Estrogen receptors have been detected in some types of hemangioma and a role for steroid hormones in their development has been proposed [20]. Juvenile hemangiomas are characterized by rapid development and involution [2]. Female hormones, in particular estradiol, have been proposed to be associated with their development [21, 22]. These hormones might affect expression of angiopoietin-2, jagged-1, notch-4, neuropilin-2, plexindomain containing receptor 1 and ephrin receptor B3 that are overexpressed in the proliferating phase of growth of juvenile hemangiomas [23]. Malignant neoplasms derived from vasculature are known as angiosarcomas. They develop usually either in the setting of chronic sun damage or immunosuppression. An example of the latter is Kaposi’s sarcoma (Fig. (3D)), which is linked to HHV-8 infection [24]. Dermis is composed of type I collagen fibers, elastic tissue and various cells with fibroblasts being most prominent. There are many types of soft tissue neoplasms including soft tissue tumor, fibrosarcoma (Fig. (4)) and dermatofibrosarcoma protuberans. Dermatofibrosarcomaprotuberans has been linked to local trauma and immunosuppresion. It is characterized by translocation t(17;22) and supernumerary ring chromosomes containing sequences from chromosomes 17 and 22 [25, 26].
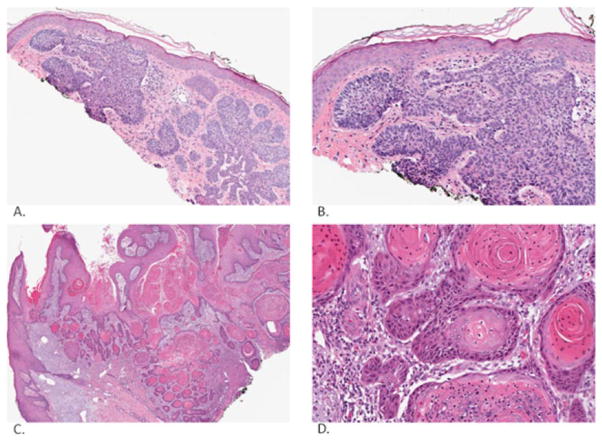
Fig. (1).
Basal cell carcinoma (low magnification: A, high magnification: B) and squamous cell carcinoma (low magnification: C, high magnification: D). Basal cell carcinoma is characterized by basaloid islands with prominent cleft artifact and abnormal surrounding stroma. Squamous cell carcinoma is characterized by keratin pearls, atypia of keratinocytes and infiltrative features. Pictures were taken and processed with an Aperio Imaging System.
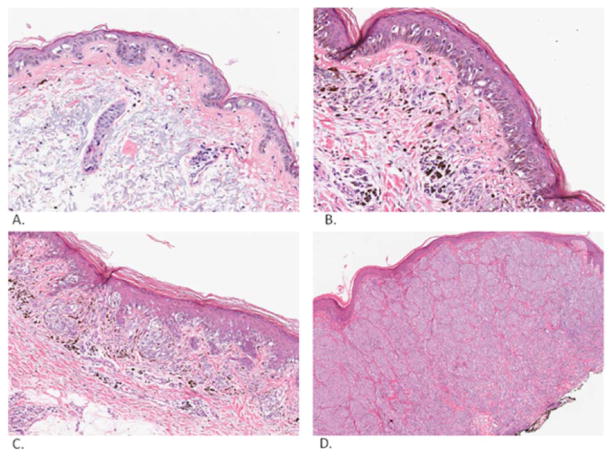
Fig. (2).
Melanoma. Melanoma in situ (A). This is a proliferation of atypical melanocytes along dermal-epidermal junction in sun damaged skin. Melanoma in radial and vertical phases of growth (superficial spreading type; high magnification: B, low magnification: C). This is a proliferation of nested atypical melanocytes with dusty melanin centered primarily along the dermal-epidermal junction with some melanocytes extending to the papillary dermis. Melanoma in the vertical phase of growth (nodular type, D). This is a dermal proliferation of nested atypical melanocytes. Pictures were taken and processed with an Aperio Imaging System.
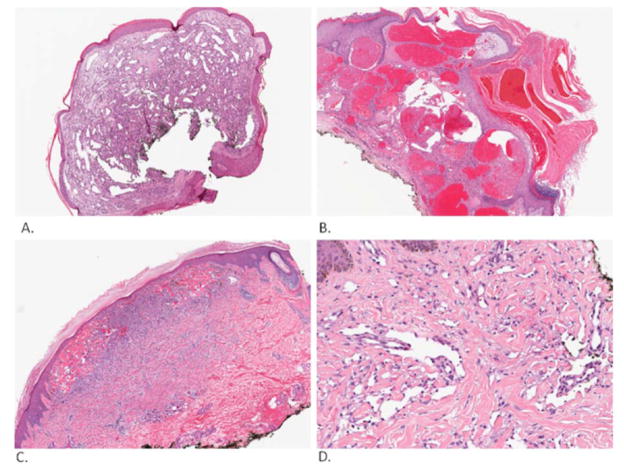
Fig. (3).
Vascular and pseudovascular lesions. Lobular capillary hemangioma (A). This lesion is composed of vessels in benign stroma surrounded by epidermal collarette. Angiokeratoma (B). This lesion has prominent acanthosis and hyperkeratosis. Hemorrhagic dermatofibroma (C). This lesion is composed primarily of dermal dendrocytes and has some extravasated blood within its boundaries. Kaposi’s sarcoma (D). This tumor is characterized by vessels lined by atypical endothelial cells dissecting collagen fibers. Pictures were taken and processed with an Aperio Imaging System.
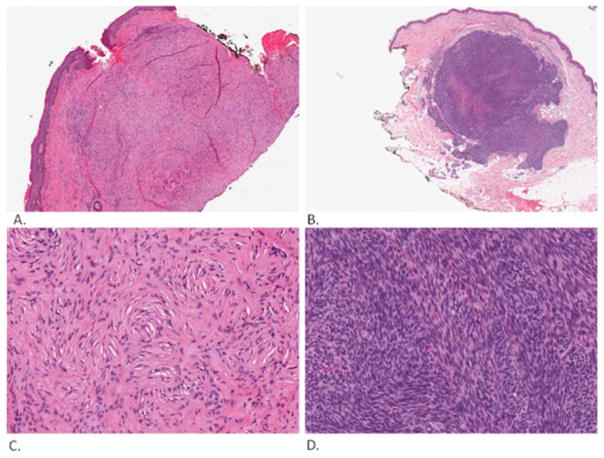
Fig. (4).
Soft tissue lesions. Solitary fibrous tumor (low magnification: A, high magnification: C). This is relatively well delineated tumor that consists of spindle cells in “patternless” pattern. Fibrosarcoma (low magnification: B, high magnification: D). This is also a relatively well delineated tumor that consists of spindle cells in herringbone pattern. Pictures were taken and processed with an Aperio Imaging System.
2. ROLE OF CYTOCHROMES P450 IN PROTECTION AGAINST CARCINOGENS AND OXIDATIVE STRESS
Skin is exposed, both acutely and chronically, to a variety of physical-chemical factors including ultraviolet radiation (UVR), topically applied drugs and cosmetics, as well as environmental pollutants such as industrial chemicals and pesticides. The xenobiotics undergo degradation or activation processes in skin which may result in skin sensitization or even carcinogenesis. The CYPs (cytochromes P450) are the most important among the xenobiotic-metabolizing enzymes in the skin. In general, CYP-mediated reactions are beneficial in that they help the skin cells to eliminate hazardous foreign compounds. In many instances, however, these reactions could produce more harmful products than the parent compounds. UVR - mediated induction of CYP in skin may result in enhanced metabolic activation of xenobiotics to which humans are exposed, making the skin more susceptible to UVR or xenobiotic-induced skin cancers as well as allergic and irritant contact dermatitis.
Several studies demonstrate the expression in skin cells of various CYPs responsible for the metabolism of a large number of xenobiotics [13, 27, 28]. By different techniques (RT-PCR, immunoblot, immunohistochemistry, catalytic activity) constitutive expression of CYP1A1, CYP1B1, CYP2B6, CYP2E1, and CYP3A5 is detected in human keratinocytes [27]. It was found that a novel dioxin-inducible cytochrome P450, CYP2S1, is also expressed in skin [28]. According to immunocytochemical analyses, CYP1A1, CYP2B6, CYP2E1, and CYP3A, are expressed predominantly in keratinocytes as compared to fibroblasts [27].
The constitutive levels of some CYP enzymes in skin are too low to be measured without exposure to exogenous inducers. The expression of CYP1A, CYP2B, CYP2E, and CYP3A was enhanced at the mRNA level after induction with dexamethasone, a widely used topical agent in dermatologic practice [27, 29]. The exposure to polycyclic aromatic hydrocarbons, β-naphthoflavone, and glucocorticoid resulted in CYP1A1 induction in human and rodent skin, as well as human hair follicles [30–33]. Cutaneous expression of CYP2S1 was induced by ultraviolet radiation, coal tar, and all-trans retinoic acid [28].
The regulation of cutaneous CYPs content and activity apparently plays a critical role in cancerogenesis because several xenobiotics that induce skin CYPs are also initiators of skin tumors. CYP1A1 and CYP1B1 participate in the activation of polycyclic aromatic hydrocarbons to epoxide intermediates, which are converted with the aid of epoxide hydrolase to the ultimate carcinogens, diol-epoxides. CYP2E1 plays a major role in the bioactivation of a number of procarcinogens, including nitrosamines [34], benzene, vinyl chloride, trichloroethylene, and acrylonitrile [35].
UVR is an additional factor for regulation of expression of cutaneous CYPs. UVR exposure to solar-ultraviolet-protected human skin resulted in an UVB dose- and time-dependent induction in the epidermis of CYP1A1 and CYP1B1 both at mRNA and protein levels [36].
Combined action of UVR and xenobiotics can profoundly alter the activity and the inducibility of cutaneous CYPs. Exposure of the neonatal rats to UVB and topical application of crude coal tar separately caused a dose-dependent increase in aryl hydrocarbon hydroxylase, 7-ethoxyresorufin O-deethylase and 7-ethoxycoumarin O-deethylase activities in the skin. The treatment of animals with coal tar followed by UVB exposure resulted in additive effects on cytochrome P450-dependent activities in the skin [37].
Extensive and chronic exposure of the skin to UVR, associated with abundant ROS/RNS generation, leads to peroxidation of fatty acids with formation of corresponding hydroperoxides. The latter could be used by some cutaneous CYPs as co-substrates in the oxidation of polycyclic aromatic hydrocarbons. CYP2S1 is involved in fatty acid peroxide-dependent oxidation of benzo[a]pyrene-trans-7,8-dihydrodiol into the highly mutagenic and carcinogenic benzo [a]pyrene-r-7,t,t-8-dihydrodiol-t-9,10-epoxide [38]. Epoxidation probably proceeds by a free radical mechanism via peroxidative and peroxygenative reactions [39]. In addition CYP2S1 can oxidize several substrates using cumenehy droperoxide and hydrogen peroxide. CYP1A1, CYP1A2, CYP1B1, and CYP3A4 are also able to efficiently epoxidize benzo[a]pyrene-trans-7,8-dihydrodiol using various fatty acid hydroperoxides although at slower rates than CYP2S1 [38]. Thus, CYP2S1 and other CYPs to a less degree could contribute to the metabolism of carcinogens that come in contact with the skin via an NADPH independent activity.
CYP activity itself contributes to intracellular reactive oxygen species (ROS) generation and lipid peroxidation. The NADPH-dependent reduction of O2 to superoxide anion radical in the presence and absence of substrate is well known for cytochrome P450 [40, 41]. The superoxide anion radical can spontaneously dismutate and generate hydrogen peroxide, and, if transition metals are present, hydroxyl radicals will be generated. The high propensity to generate ROS is a general phenomenon for xenobiotics metabolizing CYPs: CYP2E1, CYP2B1, CYP1A1, CYP1A2 and CYP3A [42, 43]. It has been shown that hydroxyl radical generation by constitutive CYPs contributes only to a minor degree to total hydroxyl radical generation in vivo [44]. At the same time, induction of CYPs by several xenobiotics is associated with intensified production of hydroxyl radicals [44]. Phenobarbital, which is a tumor promoter and a typical inducer of CYP2B1 and CYP3A2, induces production of hydroxyl radicals as well as 8-hydroxy-2′-deoxyguanosine, a biomarker of DNA oxidation [45]. Moreover the carcinogenic action of 2,3,7,8-tetrachlorodibenzo-p-dioxin, a carcinogen showing a lack of direct genotoxicity, has been suggested to be the result of enhanced ROS generation due to the induction of CYP1A1/2 [46–48]. Thus, in many instances CYP-mediated metabolic pathways of xenobiotic metabolism lead to the formation of free radicals that cause oxidative DNA damage and cutaneous CYPs may facilitate carcinogenesis by two distinct mechanisms, namely, by oxidative activation of xenobiotics and by ROS generation.
The function of cutaneous xenobiotics-metabolizing CYPs is not confined to metabolism of exogenous chemicals. They have also a crucial role in biosynthesis and catabolism of a variety of endogenous substances in the skin. For example, they could participate in controlling cutaneous steady-state concentrations of melatonin, a neurohormone involved in biochemical regulation of the circadian rhythms and other biological functions throughout the body [49]. Metabolism of melatonin to 6-hydroxymelatonin is mediated by CYP1A1 [50, 51] and CYP1B1 [52]. It has also been shown that in rats melatonin undergoes CYP1A, CYP2E, and CYP3A-mediated reactions of O-demethylation and hydroxylation at two different positions: 2 and 6 [53]. The existence of cross-linking enzymes creates a strong basis for reciprocal regulation of biological effects caused by melatonin and xenobiotics. Cutaneous metabolism of endogenous or exogenous melatonin may modulate skin cancerogenesis via regulation of the catalytic activity of CYPs, which activate procarcinogens. Administration of melatonin to rodents decreases the incidence of tumorigenesis initiated by benzo[a]pyrene or 7,12-dimethylbenz[a]anthracene, which requires metabolic activation by CYP1A1, CYP1A2 and CYP1B1, to convert procarcinogens to carcinogenic metabolites [54, 55]. Melatonin can directly inhibit 7-ethoxyresorufin O-dealkylation activity of CYP1A1, CYP1A2 and CYP1B1, as determined with recombinant proteins, and does not affect basal or benzo[a]pyrene-inducible CYP1A1 or CYP1B1 gene expression in human mammary epithelial cells [54]. Thus, the mechanism of the chemopreventive effect of melatonin in relation to benzo[a]pyrene is most likely a direct inhibition of the CYPs. Interestingly, melatonin acts as a mixed inhibitor of CYP1A1, CYP1A2 and CYP1B1. Thus it is capable of binding to both the free enzyme and to the enzyme-substrate complex thereby preventing procarcinogen activation regardless of whether the xenobiotic is bound to the enzyme.
Melatonin and its metabolites, that are formed in enzymatic or non-enzymatic oxidation reactions, may also prevent skin cancerogenesis acting as a strong radical scavenger directed especially against hydroxyl radicals, which are thought to be the most damaging effectors produced during UVR or xenobiotics metabolism [49, 56–59].
3. STEROIDOGENESIS AND ITS RELATIONSHIP WITH THE SKIN
A. Steroid Synthesis in Humans
The first enzymatic step in steroid synthesis occurs in the mitochondrion and is catalyzed by CYP11A1, also known as cytochrome P450scc. This enzyme catalyzes three oxidative reactions on the side chain of cholesterol resulting in cleavage occurring between C20 and C22, producing pregnenolone and isocaproic aldehyde [60]. The reaction occurs by initial hydroxylation at C22, subsequent repositioning of the side chain in the active site resulting in a second hydroxylation at C20, then oxidative cleavage of the C20–C22 bond [61], as illustrated in Fig. (5). Electrons for these reactions, required for the activation of oxygen bound to the heme group of the CYP11A1, are provided by NADPH via a short electron transport chain comprising adrenodoxin reductase and adrenodoxin [60, 62]. Once produced, pregnenolone can leave the mitochondria and be converted to the various steroid hormones by cell and gland specific pathways, described later. The products of these pathways reflect the particular combination of steroidogenic enzymes expressed in the specific cell type or tissue.
Fig. (5).
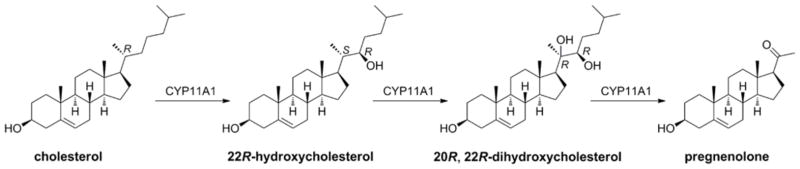
CYP11A1 mediated metabolism of cholesterol 22R-hydroxylation changes stereochemical descriptors at C-20 (see recommendations of IUPAC-IUBHy on vitamin D nomenclature [256]).
In three of the most active steroidogenic tissues, the adrenal cortex, corpus luteum and testis, steroid synthesis is acutely regulated by the action of the Steroidogenic Acute Regulatory (StAR) protein which controls cholesterol transport to the inner mitochondrial membrane site of CYP11A1 action [63–66]. Thus CYP11A1 activity is substrate limited and increased rates of cholesterol transport cause a corresponding increase in the rate of pregnenolone synthesis. For this acute regulation, the activity of the StAR protein is increased by both phosphorylation and increased synthesis, in response to ACTH (or angiotensin II for the zonaglomerulosa) binding to its receptor in the case of the adrenal gland, and LH binding to its receptor in the case of the gonads, and is mediated via cAMP [63].
Subsequent metabolism of pregnenolone to the various steroid hormones occurs by cell- and tissue-specific pathways involving a combination of CYPs and steroid dehydrogenases. Two of these CYPs, CYP11B1 and CYP11B2 are located in the inner mitochondrial membrane and use adrenodoxin reductase and adrenodoxin as electron transport partners, as for CYP11A1. The other steroidogenic CYPs, CYP17A1, CYP19A1 and CYP21A2 are located in the endoplasmic reticulum and use cytochrome P450 reductase as their redox partner [64]. Long term regulation of steroid synthesis by the tropic hormones ACTH and LH is achieved by stimulation of the transcription of genes encoding these steroidogenic enzymes, especially CYP11A1 [64].
There are different pathways and hence different steroid hormone products in the three zones of the adrenal cortex. Aldosterone is produced in the zona glomerulosa where pregnenolone is converted to progesterone by 3β-hydroxysteroid dehydrogenase (3β-HSD), progesterone is converted to 11-deoxycorticosterone by 21-hydroxylase (CYP21A2), 11-deoxycorticosterone is converted to corticosterone by the 11β-hydroxylase activity of aldosterone synthase (CYP11B2) and corticosterone is converted to aldosterone also by aldosterone synthase [64]. The zona fasciculata produces primarily cortisol. The pregnenolone is converted to progesterone by 3β-HSD, progesterone is converted to 17α-hydroxyprogesterone by the 17α-hydroxylase activity of CYP17A1, 17α-hydroxyprogesterone is converted to deoxycortisol by CYP21A2 and deoxycortisol is converted into cortisol by CYP11B1 [64].
The zona reticularis produces large amounts of the inactive C19ar androgen, dehydroepiandrosterone (DHEA), much of which is sulfated [67]. It should be noted that CYP17A1 possesses both 17α-hydroxylase activity and C17-C20 lyase activity. Human CYP17A1 displays high lyase activity towards 17α-hydroxypregnenolone but very low lyase activity towards 17α-hydroxyprogesterone. The reticularis zone is relatively deficient in 3β-HSD activity so there is little conversion of pregnenolone to progesterone or of 17α-hydroxypregnenolone to 17α-hydroxyprogesterone. Thus CYP17A1 is able to exert its lyase activity on the abundant 17α-hydroxypregnenolone, producing the DHEA. The lyase activity of CYP17A1 is also enhanced by the binding of cytochrome b5, which is highly expressed in the zona reticularis [64, 67].
The corpus luteum produces large amounts of progesterone and also some estradiol. Progesterone is formed by the action of 3β-HSD on pregnenolone. 3β-HSD displays both dehydrogenase and isomerase activity with the dehydrogenase converting the 3β-hydroxyl group of pregnenolone to a ketone group accompanied by the reduction of NAD to NADH, and the isomerase shifting the double bond from C5-C6 to the C4-C5 position to produce progesterone [64]. Some pregnenolone is acted on by CYP17A1 which displays both 17α-hydroxylase and C17-20 lyase activities in the gonads producing DHEA, which is converted to androstendione by 3β-HSD. The androstendione is converted to testosterone by 17α-hydroxysteroid dehydrogenase, with most of the testosterone being converted to estradiol by the aromatase enzyme, CYP19A1 [66]. The Leydig cells of the testis are the site of testosterone synthesis in the male, via a pathway similar to that described for the corpus luteum, largely terminating at testosterone since there is only low expression of CYP19A1 [64, 66]. In the ovarian follicle, pathways for androgen and estrogen synthesis are separated but complementary. The granulosa cells produce pregnenolone form cholesterol by the action of CYP11A1. The granulosa cells lack CYP17A1 and the pregnenolone produced by the granulosa cells diffuses into the thecal cells where CYP17A1 is expressed and is converted to androgens, primarily androstendione. The thecal cells do not express CYP19A1 and hence the androgens must diffuse back into the granulosa cells for aromatisation to estrone and estradiol by CYP19A1 [64].
The human placenta produces large amounts of both progesterone and the three estrogens, estrone, estradiol and estriol. Progesterone is produced by the actions of CYP11A1 producing pregnenolone from cholesterol and 3β-HSD converting the pregnenolone into progesterone [60]. The human placenta does not express CYP17A1 and hence cannot make the androgen precursors of the estrogens from pregnenolone. Large amounts of DHEA sulfate are produced by the fetal adrenal [67] and this is taken up by the placenta for conversion to estrogens, primarily estradiol, by the actions of 3β-HSD converting the DHEA to androstendione, 17β-hydroxysteroid dehydrogenase converting this to testosterone and CYP19 carrying out the final conversion to estradiol. Some of the DHEA is hydroxylated in the 16α-position in the fetal liver by CYP3A7 before reaching the placenta and the resulting 16α-hydroxy-DHEA is converted to estriol by an analogous pathway [64]. An important difference between the placenta compared to the adrenal cortex, corpus luteum and testis is that the human placenta does not express the StAR protein and does not display acute regulation. Rather it appears that the placenta expresses MLN64, a protein related to StAR, which transports the cholesterol to the CYP11A1 in the inner mitochondrial membrane in a process that does not limit CYP11A1 activity [60, 63]. Current evidence indicates that the concentration of the electron transport protein, adrenodoxin reductase, limits progesterone synthesis in the placenta [60].
A number of other tissues express CYP11A1 and therefore can be considered to be steroidogenic tissues making a range of steroid hormones. These steroids are most likely involved in regulation at the autocrine or paracrine levels. Although such data is limited for the human brain [68], it is likely to produce neurosteroids from cholesterol as it has been well detailed in the rodent brain. Steroids produced include pregnenolone, pregnenolone sulfate, DHEA sulfate, progesterone and 3β and 5α reduced derivatives of progesterone [64]. The human gut expresses CYP11A1, CYP17A1 and CYP11B1, and can produce cortisol [69, 70]. Lymphocytes have also been reported to produce glucocorticoids. CYP11A1 is expressed in heart and there is some evidence for the production of aldosterone under particular conditions [69]. Bone expresses CYP11A1, but the predominant form has an N-terminal truncation that is only 30 kDa in size and has a non-mitochondrial localization, and is therefore unlikely to be catalytically active [71]. The skin also expresses CYP11A1 including its alternatively spliced isoform producing a shorter transcript, and a number of other steroidogenic enzymes [72, 73], to be discussed later.
Cholesterol is considered as the starting molecule for steroid synthesis. However, it is apparent that CYP11A1 can act on a range of cholesterol-like molecules. A small component of the cholesterol in cells is sulfated and CYP11A1 can carry out side chain cleavage of this producing pregnenolone sulfate, although the Km for the reaction is higher than that observed for cholesterol [74]. CYP11A1 can also cleave the side chain of short chain fatty acid esters of cholesterol, although with low efficiency [74]. The plant sterols β-sitosterol and campesterol which differ from cholesterol in their side chain can efficiently undergo the side-chain cleavage reaction by CYP11A1, producing pregnenolone [75]. CYP11A1 can also act on ergosterol, the membrane sterol of fungi and the vitamin D2 precursor [76, 77], which differs from cholesterol in that it has additional double bonds between C7-C8 and C22-C23 as well as a methyl group at C24. In this case no cleavage of the side chain occurs and the major products are 20-hydroxy-22,23-epoxy-22,23-dihydroergosterol and 22-keto-23-hydroxy-22,23-dihydroergosterol [77]. CYP11A1 can also act on the vitamin D3 precursor, 7-dehydrocholesterol (7DHC). The pathway for side-chain cleavage is analogous to that for cholesterol with the product being 7-dehydropregnenolone (7DHP) [73, 78, 79] (Fig. (6)). Endogenous production of 7DHP and its subsequent metabolism by other steroidogenic enzymes is illustrated by the finding of a number of 7-dehydro steroids in the urine of Smith-Lemli-Opitz syndrome patients which have elevated 7-dehydrocholesterol levels due to a partial deficiency in the enzyme that converts this sterol to cholesterol [80]. Finally, CYP11A1, as demonstrated for both the bovine [79, 81–83] and human enzymes [61, 84], can act on vitamin D3 catalyzing multiple hydroxylation reactions without any cleavage of the side chain. The major product is 20-hydroxyvitamin D3, with 20,22-dihydroxyvitamin D3, 20,23-dihydroxyvitamin D3, 17,20-dihydroxyvitamin D3 and 17,20,23-trihydroxyvitamin D3 also being produced. Similarly, CYP11A1 can metabolize vitamin D2 with the major products being 20-hydroxyvitamin D2, 17,20-dihydroxyvitamin D2 and 17,20,24-trihydroxyvitamin D2 [85, 86].
Fig. (6).
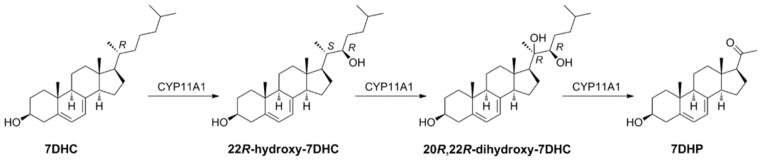
CYP11A1 mediated metabolism of 7-dehydrocholesterol. 22R-Hydroxylation changes stereochemical descriptors at C-20 (see recommendations of IUPAC-IUB [256]).
B. Local Steroidogenesis in the Skin with its Potential Implication for Carcinogenesis
Sex Hormones
Skin can be defined as the largest steroidogenic organ in term of mass, with a level of steroidogenic activity dependent on the nature, amount and spatial and cellular locations of final products [7, 10]. It produces sex hormones from dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEA-S) or androstenedione, which are either of systemic origin or synthesized locally [7, 87–91]. This process involves transformation of DHEA into 4-androstenedione and 5-androstene-3β,17β-diol, and of their further transformation with production of testosterone and 5α-dihydrotestosterone (DHT) [87, 91–95]. Furthermore, cutaneous fibrocytes/fibroblasts and adipocytes convert testosterone into estradiol, while keratinocytes can transform reversibly 17-estradiol into estrone. Both estrogens and androgens that are produced locally have a profound effect on the phenotype and function of the skin and its adnexal structures [87–89, 91, 95–100]. They also can regulate the skin pigmentary system [11, 101, 102]. It must be noted that skin with its subcutaneous fat represents a significant source of estrogens for systemic use, which is extremely important after menopause, and which is an example of the endocrine activity of this organ [7, 87, 88, 97].
There are indications for a role of locally produced/activated sex hormones in cutaneous carcinogenesis including non-melanoma [103–105], and melanocytic tumors [106–114]. However, the exact mechanism of estrogens or androgens involvement is largely unknown. This forms an exciting area of research to precisely define a role for locally produced androgens and estrogens, or their intermediates or products of local modification, in carcinogenesis and/or tumor suppression in a cell-type dependent manner. Filling this gap in our knowledge would lead to the educated preclinical testing of specific androgen and estrogen receptor(s) agonists or antagonists in therapy of local or disseminating non-melanoma and/or melanoma skin tumors.
Corticosteroids
The skin is also a site of corticosteroids synthesis [72, 115]. We were the first to demonstrate that skin cells express crucial genes of the corticosteroidogenic pathway CYP11A1, CYP17 and CYP21A as well as the MC2-R gene encoding the ACTH receptor [116] (Fig. (7)), (Table 1) and demonstrated functional activity of these and other steroidogenic enzymes in the skin or skin cells [73, 98, 117–119]. Cutaneous steroidogenesis can be initiated in the skin from cholesterol by the action of locally expressed CYP11A1 [73, 116, 120]. Pregnenolone can further be metabolized by cutaneous 3β-HSD [87, 90, 91] with subsequent steps involving the metabolism of progesterone to deoxycorticosterone (DOC) and 18-hydroxy-DOC, with final production of corticosterone [98, 117, 121, 122]. Human hair follicles [123, 124] and cultured normal epidermal melanocytes [122] and dermal fibroblasts [121, 125] have the capability to produce cortisol, of which the final identification was provided by liquid chromatography-mass spectrometry (LC/MS) analysis [122, 125]. The above discovery of cutaneous cortisol production through a local steroidogenic pathway was most recently confirmed by several other authors in skin cells in vitro [126–128] and by us in skin biopsies maintained ex vivo [129]. Cutaneous production of cortisol and the activity of the steroidogenic apparatus can be regulated by CRH, ACTH and cAMP [122, 123], IL-1 and the wound response [126], and ultraviolet B and C radiation [129]. In addition, skin cortisol can either be produced from 11-deoxycortisol by CYP11B1 or from cortisone by 11β-HSD1 [72, 126–128, 130]. Experiments performed on an immortalized line of epidermal human keratinocytes, HaCaT cells, demonstrated a rapid metabolism of progesterone and DOC with production of several major steroidal products with corticosterone, aldosterone and cortisol being below detectability[118], These cells exhibited a high catalytic rate for the metabolism of progesterone with production of several major products of which only DOC was identified [118], Interestingly they also transformed DOC to 5α-dihydroDOC as well as to a number of additional species including tetrahydroDOC (THDOC), 6-hydroxyTHDOC, 3α,21-dihydroxy-5-pregnen-20-one, 3β,21-dihydroxy-5-pregnen-20-one, 3α,21-dihydroxy-4-pregnen-20-one and 6-hydroxydihydroDOC [118]. The biological role of these compounds remains unclear.
Fig. (7).
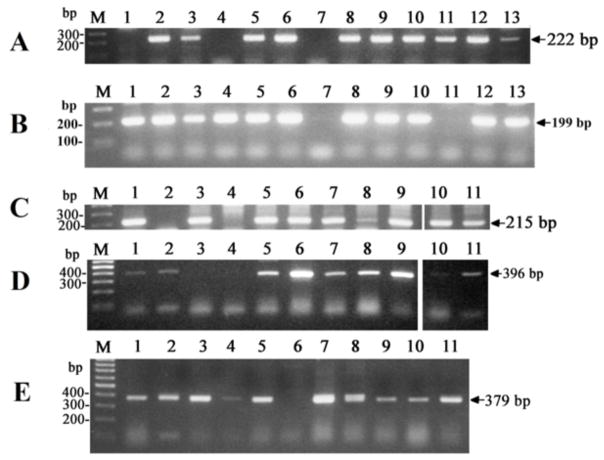
RT-PCR of the cytochrome gene products in human cell lines: A) CYP17A; B) CYP21A2 C) CYP7A1; D) CYP7B1; E) CYP39A1. RT-PCR was performed using primers listed in Table 1 following methodology described before [81, 257]. Briefly, the following program was used: initial heating at 95°C for 3 min, followed by 30 cycles of 94°C for 30s, 55°C for 40s, 72°C for 40s. Products of CYP7B1, CYP39A1 and CYP17A amplification were visualized by agarose gel electrophoresis. Products of CYP7A1 and CYP21A2 amplification were diluted 40 times with water and used as the templates for the nested round of amplification using same program and also visualized on anagarose gel. The products were sequenced and they showed 100% homology with the corresponding genes. Location of samples on gels A and B: DNA ladder (M), immortalized epidermal keratinocytes (HaCaT) (1), normal human epidermal keratinocytes (HeKa) (2), immortalized human epidermal melanocytes (Pig1) (3), primary human epidermal melanocytes (HeM) (4), dermal fibroblasts (5), squamous cell carcinoma (C1–4) (6), and SKMELl188 (7), SBCE2 (8), WM35 (9), WM98 (10), WM164 (11), WM1341 (12) melanoma lines, subcutaneous adipose tissue (13). Location of samples on the gels C, D and E: DNA ladder (M), HeM melanocytes (1), fibroblasts (2), C1–4 cells (3), SKEMEL188 (4), SBCE2 (5), WM35 (6), WM98 (7), WM164 (8), WM1341 (9), HaCaT keratinocytes (10), HeKa keratinocytes (11).
Table 1.
Primers used for the RT-PCR.
| Gene | Primer Name | Primer Sequence | Primer Location | Fragment Size, bp |
|---|---|---|---|---|
| CYP7A1 | P404 | first pair of primers CATACCTGGGCTGTGCTCTG |
exon 2 | |
| P548 | GTTTTCCATCATGCTTTCCGTG | exon 3 | ||
| CYP7A1 | P404 | nested primers CATACCTGGGCTGTGCTCTG |
exon 2 | 215 |
| P405 | GTGCCCAAATGCCTTCGCAG | exon 3 | ||
| CYP7B1 | P549 | ACGGCAGAACTGTATCCATTC | exon 2 | 396 |
| P550 | AAGATAATACATTGCCCAGAAC | exon 3 | ||
| CYP39A1 | P551 | ACAATGGACCTGAACAACTTAG | exon 3 | 379 |
| P552 | CAGGAACAGCATTAGACAGAG | exon 5 | ||
| CYP17 | P565 | first pair of primers CTCTAGACATCGCGTCCAAC |
exon 2 | |
| P566 | GAAGCAGATCAAGGAGATGAC | exon 3 | ||
| CYP17 | P587 | nested primers TCGCGTCCAACAACCGTAAG |
exon 2 | 222 |
| P688 | CATTGGTTACCGCCACGAAG | exon 3 | ||
| CYP21A2 | P396 | TGGAGGGACATGATGGACTAC | exon 7 | 199 |
| P397 | CCTGCAGTCGCTGCTGAATC | exon 8 |
Production of corticosterone and cortisol and of their precursors in the epidermis, dermis and adnexal structures implicate a role for this endogenous steroidogenic pathway in skin carcinogenesis. Specifically, cortisol and corticosterone will generate an immunosuppressive environment allowing or facilitating malignant trans- formation or tumor progression. This concept is supported by an increased risk of developing non-melanoma skin cancers including squamous cell carcinoma in patients taking oral glucocorticoids [131] or patients with rheumatoid arthritis treated with prednisone [132]. Similarly immunosuppression with cyclosporine A plus prednisolone in cesium-137-irradiated Ptch1+/− mice increased the incidence of basal cell carcinoma [133]. A phenomenon of increased incidence of epidermal skin cancers in immunosuppressed patients with organ transplants is well documented in the medical literature [134,–135]. In addition, UVB, a main inducer of basal and squamous cell carcinomas, stimulates cortisol production by human skin and co-cultured keratinocytes and melanocytes [129] with predictable implications in local cancerogenesis. In addition to immune cells, keratinocytes, melanocytes and fibroblasts express glucocorticoid and minelarocorticoid receptors (reviewed in [7, 10]) making these cells a direct target for regulation with implications on malignant transformation and tumor growth. For example, oral steroid use is associated with a nearly 6-fold elevated risk of squamous cell carcinoma among individuals with a common genetic variant in the steroid receptor (NR3C1) gene [136]. Concerning melanomas, it has to be noted that human melanoma cells showed progressive transformation of progesterone to DOC, 18-hydroxy-DOC and corticosterone, [117], in addition almost all melanomas tested by us express CYP11A1 with detectable production of pregnenolone [73, 137]. Thus an endogenous steroido- genic activity may facilitate melanoma growth and metastasis through generation of a local immunosuppressive environment. However, this process can be more complex since melanomas expressing glucocorticoid receptors are inhibited by exogenous glucocorticoids [138, 139]. Also, delivery of glucocorticoids in liposomes to mice bearing B16 melanoma inhibited the tumor growth [140, 141]. Thus, the role of glucocorticoids in melanoma growth can be dual, e.g., they can inhibit growth of tumors when pharmacological doses are applied, and they may facilitate tumor growth when endogenously produced by the tumor through modification of its environment. This calls for careful clinic-pathological analyses of skin specimens with cancer, melanocytic tumors and melanoma for changes in expression of glucocorticoid and minelarocorticoid receptors on one hand and steroidogenic enzymes on the other. In our assessment the interactions between steroids and skin tumors, melanoma in particular, would be nonlinear. For example, increased expression of proopiomelanocortin (POMC) with products of its processing ACTH, MSH and β-endorphin is seen in skin pathology [8, 142, 143], melanoma in particular [11, 144, 145], where there is a positive correlation between POMC expression and tumor progression [145, 146]. Of note, POMC derived α-MSH stimulates melanocyte proliferation, inhibits apoptosis and acts as a powerful immunosuppressor [147, 148], identifying it as a possible tumor progression promoting factor [11]. In this context it is worthy to mention that glucocorticoids inhibit POMC expression.
7-Hydroxylationhy
7-Hydroxylation is an ancient pathway of sterol metabolism which can be traced down to prokaryotic organisms [149]. In humans the reaction involves the enzymatic hydroxylation of the B ring of 3β-hydroxysterols including cholesterol, pregnenolone, dehydroepiandrosterone (DHEA), androstane-3β,17–β-diol, and testosterone [149–151]. The major enzyme responsible is 7α-hydroxylase (CYP7A1) which participates in a pathway that eventually leads to bile acid production. In the case of CYP7A1 deficiency, bile acids are produced by an alternative pathway by reactions catalyzed by CYP27A1, and the 7-hydroxylase activity of CYP7B1 [149, 152]. The third enzyme with 7α-hydroxylase activity is hepatic CYP39A1. Interestingly, 7-dehydrocholesterol (7DHC) might be also be a substrate for oxidation by CYP7A1 which leads to production of 7-ketocholesterol, which is a potent competitive inhibitor of CYP7A1, and toxic in liver cells and is a major oxysterol in human atherosclerotic plaques and photodamaged rat retina [153]. Thus, in addition to the malfunction of cholesterol synthesis, SLOS patients might also have their bile acid synthesis pathway impaired [154, 155]. Interestingly, non-enzymatic oxidation of the B-ring in sterols and steroids appears to be conserved and might represent one of the first stress-generated signaling molecules in evolution [149, 156]. CYP7B1 is also involved in 7α-steroid hydroxylation in the brain, liver, intestine, kidney and other organs [149, 157–160]. 7β-hydroxysteroids are converted to 7β-hydroxysteroids through the enzymatic action of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) [161–164], or possibly by other enzymes [165–167]. However, non-enzymatic conversions between 7α-hydroxy, 7-oxo-intermediates and 7β-hydroxy metabolites are also possible [149, 156]. In human tissues and body fluids 7α-hydroxy-, 7-oxo-, and 7β-hydroxysteroids are detected.
7-hydroxysterols/oxysterols 7-hydroxysteroids are involved in the regulation of apoptosis and immune functions, and they exert a variety of neural and endocrine effects [149, 168–174]. In the brain, 7α-hydroxylation is the major metabolic pathway for dehydroepiandrosterone (DHEA) inactivation. DHEA and pregnenolone were found to promote synaptic plasticity and memory function and their oxidation or hydroxylation stimulate these functions, because 7-oxoDHEA and 7-(OH)DHEA) show neuroprotective activities and promote brain functions [149]. In addition, 7-hydroxypregnenolone can act as a neural activator and modulator of dopaminergic activity [157, 175, 176].
Our molecular analysis of RNA isolated from human skin cells shows that they do express genes encoding 7-hydroxylases including CYP7A1, CYP7B1 and CYP39A1 (Fig. (7C–E)). This provides strong support for the hypothesis that 7-hydroxy/oxy- derivatives of steroids or sterols are produced in the skin locally in order to regulate cutaneous homeostasis. Concerning cutaneous carcinogenesis, 7-hydroxylations could regulate local concentrations of steroids by diverting steroidogenic metabolism into 7-hydroxyderivatives with modifying effects on local tumorogenesis. 7-hydroxy/oxy- derivatives of steroids could interact with classical steroidal receptors expressed on normal and malignant skin cells, or interact with specific receptors yet to be identified. They could also act in a receptor-independent manner through modification of intracellular redox status. Finally, 7-hydroxycholesterol is a ligand for the retinoic acid orphan receptor (ROR)α and γ [177], and both RORα [49, 178, 179] and γ (Slominski et al., unpublished) are widely expressed in the skin. The reciprocal interaction between 7-hydroxy/oxy- derivatives of sterols or steroids with RORα and γ represent an exciting area for exploration, because these receptors regulate expression of CYP7B1 and of sulfotransferases and they can act as “tumor suppressors” [180].
4. VITAMIN D3 ACTIVATION AND INACTIVATION IN THE SKIN
A. Enzymes Involved in Activation and Inactivation of Vitamin D3 in the Skin
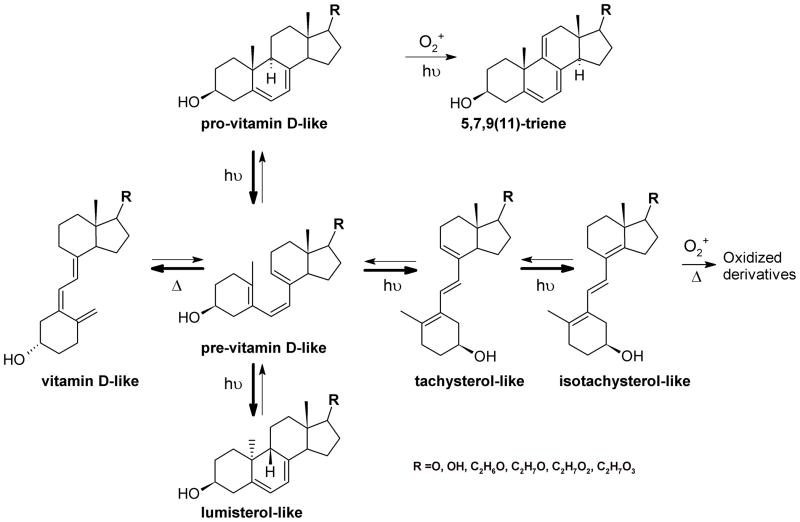
Under physiological conditions the skin is a natural source of at least 90% of the vitamin D in humans. 7-Dehydrocholesterol (cholesta-5,7-dien-3β-ol, 7-DHC) is a key substrate for production of both cholesterol and UVB-driven production of vitamin D3. Photolysis of the B-ring of 7DHC takes place in keratinocytes of the base layer of the epidermis. This nonenzymatic reaction is triggered by UV-B radiation (290 to 320 nm wavelength) and leads to formation of previtamin D3, which further isomerizes to vitamin D3, tachysterol3 (T3) and lumisterol3 (L3) [181]. From the skin, vitamin D3 (also known as cholecalciferol) is transported into the circulation by a mechanism for which the full nature still remains to be established [182]. It binds to the vitamin D binding protein for transport to multiple target organs. Vitamin D3 itself is not fully active and requires further enzymatic modification by hydroxylation at position 25 in the liver by vitamin D3 25-hydroxylases (primarily mitochondrial CYP27A1 or microsomal CYP2R1) and 1α-hydroxylation in kidneys by 25(OH)D3 1α-hydroxylase encoded by CYP27B1. To date six cytochrome P450 family members have been reported to catalyze the initial 25-hydroxylation. These are CYP27A1, CYP2R1, CYP2J2/3, CYP3A4, CYP2D25 and CYP2C11[183]). Interestingly, CYP3A4 has a higher affinity for 1α(OH)D3 and 1α(OH)D2 compared to parental vitamin D3 and D2. Moreover, this cytochrome also exhibits both vitamin D 25-hydroxylase and 24-hydroxylase activities under high concentration of substrate [184], which may cause fast clearance of therapeutic doses of vitamin D [183]. Unlike CYP27A1, it can 25-hydroxylate vitamin D2 as well as vitamin D3. CYP27A1 displays the highest maximum velocity for vitamin D3 metabolism of all the 25-hydroxylases characterized to date [185]. Finally, two bacterial enzymes have also been found capable of vitamin D 25- and 1α-hydroxylation, CYP105A1 and CYP107BR1 [186].
Expression of vitamin D-metabolizing cytochromes P450 is not restricted to classical organs (kidneys and liver) and other organs including skin also express the fully functional machineries for vitamin D activation [187, 188]. This is exemplified by human keratinocytes and sebocytes where expression of CYP27A1 and CYP27B1 has been detected [189–192] and by the efficient conversion of vitamin D3 to 1,25(OH)2D3 (calcitriol) demonstrated in keratinocytes [190, 193, 194]. It has to be noted that one study using CYP27B1 null mice reported that the renal cortex may be the sole source of 1,25(OH)2D3 synthesis in mice under physiological conditions, because the promoter of CYP27B1 fused with β-galactosidase gene was inactive in the skin and other extrarenal tissues [195]. This might be due to differential regulation of the expression of CYP27B1 outside kidneys. Expression of the 25-hydroxylase, CYP2R1, was detected in a dermal fibroblast cell line (BJ strain) along with efficient conversion of 1(OH)D3 to 1,25(OH)2D3 [196]. Different skin cell populations such as keratinocytes, melanocytes and sebocytes were found to express several variants of CYP27B1. Expression and alternative splicing of CYP27B1 in HaCaT keratinocytes was found to be regulated by UV, cell density and calcium with differentiation signals inhibiting expression of CYP27B1, and UV inducing alternative splicing in a dose dependent manner [197]. The expression of CYP27B1 was not detected in three of five basal cell carcinoma cell lines (BCC), but the pattern of splicing variants was not affected in the remaining two cell lines in comparison to normal skin [198]. It was suggested that expression of CYP27B1 decreases in BCC. On the other hand quantitative analyses revealed that RNA levels for VDR, CYP27A1, CYP27B1, and CYP24A1 were significantly elevated in cutaneous squamous cell carcinoma (SCC) as compared to healthy skin [199]. Alternative splicing of CYP27B1was also investigated in melanoma cells [197, 200–202]. The level of CYP27A1 and CYP27B1 was not elevated in melanoma cell lines studied but 1,25(OH)2D3 treatment did result in a change in the expressional pattern of CYP27B1 in SkMel28 melanoma [202]. Moreover, constitutive overexpression of CYP27B1 in melanoma was not regulated by 1,25(OH)2D3 or 25(OH)D3 in contrast to renal 1α-hydroxylase [200]. Similarly, our testing for the expression of genes encoding 25-hydroxylases (CYP27A1 and CYP2R1), 1α-hydroxylase (CYP27B1), 24-hydroxylase (CYP24A1), P450scc (CYP11A1) and VDR showed considerable variation between the different melanoma lines without a specific pattern [137, 203]. Interestingly, recent studies have revealed a positive correlation between a decrease in CYP27B1 expression and melanoma progression to more advanced stages as well as increased mortality [203].
The final product of vitamin D3 activation, 1,25(OH)2D3 is the fully active form of vitamin D3, but is not stable in the serum, with a half-life less than 18 hours. Its concentration is tightly controlled by 24-hydroxylase (CYP24A1). The 24-hydroxylase inactivates not only 1,25(OH)2D3 but also its precursor 25(OH)D3 and potentially other secosteroids. In the skin, 1,25(OH)2D3 negatively regulates its own level but in contrast to the kidney it is mainly achieved by stimulation of CYP24A1and not by inhibition of CYP27B1, which may explain why the 1,25(OH)2D3 produced in the skin does not enter the circulation efficiently. The expression of CYP24A1 was found to be tightly regulated by 1,25(OH)2D3 in melanomas sensitive to secosteroids in contrast to resistant ones. Because CYP24A1 is a major target of VDR activation, this phenomenon was explained by a disturbance of VDR signaling in secosteroid-resistant melanomas [200]
B. Potential Role of Enzymes Involved in Metabolism of Vitamin D3 in Skin Cancerogenesis
Vitamin D plays an essential role in cancer prevention, which is achieved both by adequate synthesis of vitamin D in the skin or proper oral supplementation, and its metabolism by 25-, 1α- and 24-hydroxylases belonging to the cytochrome P450 family [182, 187, 204, 205] (Fig. (8)). Thus, single nucleotide polymorphism (SNP) and other genetic or epigenetic factors related to the expression or activity of this enzymatic machinery may influence development and/or subsequent resistance of skin cancers. So far, the best documented is the correlation between the vitamin D receptor (VDR) and cancer development including skin cancer [204, 206]. Both the expression and multiple SNPs in VDR modulate cancer susceptibility and this subject was reviewed recently in [204, 207].
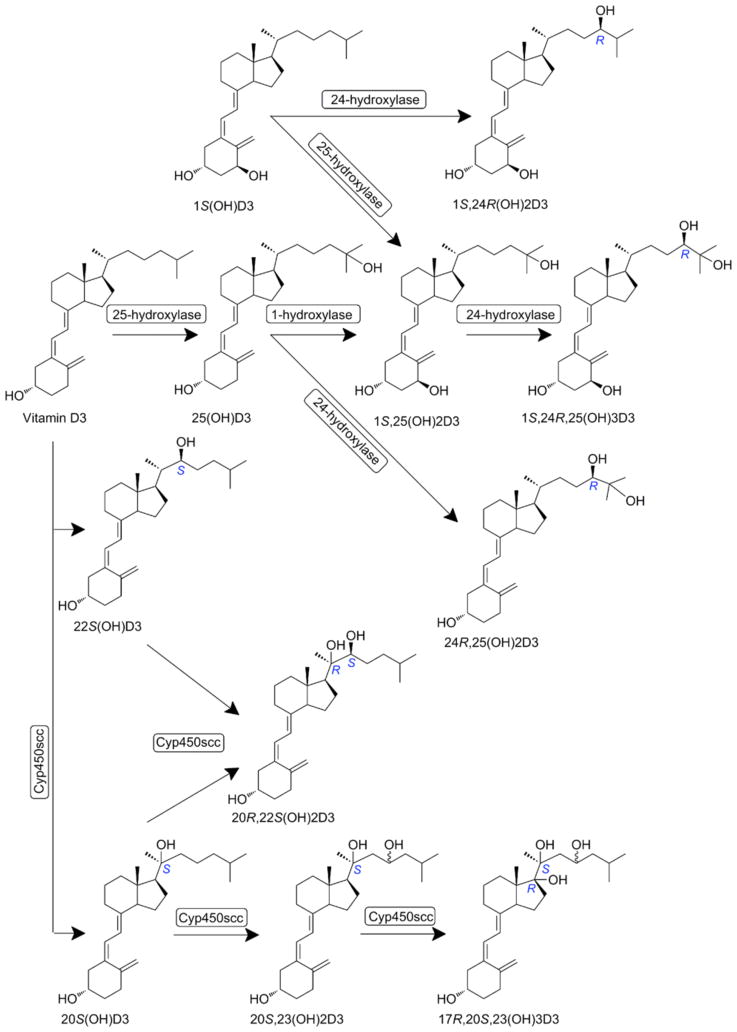
Fig. (8).
Classical (upper portion) and non-classical (CYP11A1-initiated) pathways of vitamin D activation and metabolism.
Although VDR appears to be a master regulator of the expression of CYPs involved in vitamin D metabolism at the systemic level, in the skin expression of CYP27A1, CYP27B1 and CYP24A1 can be regulated differentially. Also the VDR SNPs in skin cancer development including melanoma and none-melanoma skin cancers have been evaluated by several groups and the relationship between specific SNPs and melanoma was confirmed (for recent review see [207]). The best studied are rs10735810 (T>A at FokI), rs1544410 (G>A at BsmI) and rs731236 (T>C at TaqI). For instance, rs10735810 A allele (FokIf allele) [208, 209] and rs1544410 A allele (BsmIb allele) [210] act as the risk alleles, while rs731236 C allele (TaqIt allele) has been identified as the protective allele [211] (see [207] for further discussion). A similar correlation was found between FokI, BsmI, TaqI and ApaI SNPs of VDR and the occurrence of BCC [212]. On the other hand, it has to be noted that other studies did not find statistically significant correlations of selected SNPs of VDR (rs757343, rs731236, rs2107301, rs7975232) in melanoma [213]. There is a shortage of information on involvement of SNPs in other genes encoding cytochrome P450 enzymes in development of skin cancer. A significant decrease in oral cancer susceptibility was found in individuals with a heterozygous genotype of the CYP24A1 gene (rs2296241) in comparison with the homozygote [214]. On the other hand, a recent study [213] on a group of 305 patients with histopathologically confirmed melanoma found no correlation between SNPs in CYP27B1 (rs4646536), CYP24A1 (rs927650), vitamin D binding protein VDBP (rs1155563, rs7041) and melanoma risk [213]. Other studies indicated an association of CYP24A1 SNP (rs2248137) with lower vitamin D levels, and the CYP2R1 SNP (rs10766197) was linked to asthma [215]. Another study confirmed association between three SNPs in CYP27B1 and multiple sclerosis [216]. The presence of specific SNPs in VDR and CYP27A1 were associated with the risk of lethal prostate cancer [217]. Others have demonstrated that one particular SNP in CYP27B1 was correlated with overall colon cancer occurrence and three CYP24A1 polymorphisms with the risk of distal colon cancer [218, 219]. It has to be underlined that detailed studies on the influence of SNPs in CYP450s expression on vitamin D status in skin cancer remain to be performed.
Alternatives splicing is the powerful mechanism of posttranslational regulation which may generate multiple isoforms of proteins with unique functions. In recent years several splicing variants of CYP27B1 have been described in normal keratinocytes [197], and cell lines derived from BCC [198], SCC [199] and melanoma [200, 201, 220] patients. Nevertheless, the pattern of isoforms did not change when normal cells and malignant cells were investigated; however, quantitative analysis for each isoform was not performed.
Although, the presence of VDR and CYP27B1 was found to be essential for the production and release of 1,25(OH)2D3 into the circulation and regulation of calcium homeostasis, mice lacking CYP27B1 do not develop alopecia and are not prone to UV induced skin tumorigenesis as they do for VDR null mice [195, 204]. This discrepancy is either due to regulatory actions of VDR independent on the presence of the ligand [204, 221], or the action of previously unrecognized but locally produced ligands. Examples of the latter could be the novel vitamin D3 derivatives produced by the action of CYP11A1 [73, 81, 82], and which are endogenously produced in keratinocytes [84].
Local metabolism of vitamin D and its analogs in the skin may modulate skin pathology, cancerogenesis and responses of cancer cells to the treatment. The level of expression of vitamin D metabolizing cytochromes P450 might be a prognostic factor which would help to establish potential sensitivity of cancer cells before treatment with vitamin D and its analogs. For example, expression of CYP27B1 decreases during progression of melanocytic lesions [203]. Although such a trend was not seen in the case of CYP24A1 [220], it is likely that vitamin D related cytochromes P450 represent realistic targets for anticancer therapy. For instance, while 1,25(OH)2D3 was found to inhibit growth of several cancer cell lines including melanomas (for recent review see [207]), the beneficial effects could be attenuated by high levels of CYP24A1 [222]. Thus, application of selective CYP24A1 inhibitors [223, 224] may substantially increase and extend the effects of treatment with vitamin D or its analogs. Moreover, CYP3A4, well known as a detoxifying enzyme, possess 24/25-hydroxylase activity and may also inactive vitamin D analogs, especially when therapeutic concentration are used [225]. Thus, use of specific CYP3A4 inhibitors [226] may enhance the efficiency of treatment with vitamin D and its analogs. It has to be noted that some short side-chain vitamin D analogs have been successfully tested against melanoma [207, 227–229] and their biological activity should not be modified by CYP24A1 nor CYP3A4.
Novel very interesting frontiers in vitamin D research might concern microRNA. Recent studies revealed that the amount of CYP24A1 mRNA in cancer cells might be regulated post-transcriptionally by miR-125b as a decrease in miR-125b levels in breast cancer resulted in overexpression of CYP24A1. This phenomenon could be exploited in potential melanoma therapy by vitamin D3 or its analogs, by using antisense oligonucleotides for miR-125b to decrease the level of CYP24A1 [230, 231]. Another microRNA, miR-21, was found to target the vitamin D-dependent antimicrobial peptides (CAMP and DEFB4A) in leprosy and this was mediated by direct downregulation of Toll-like dependent expression of CYP27B1 and IL1B [232]. Because, overexpression of miR-21 was detected in many human tumors and was found to facilitate metastasis, it is likely that this microRNA might represent an additional target in melanoma prevention and treatment with vitamin D analogs, which are activated by CYP27B1. Of note, inhibition of miR-21 was found to sensitize cells to vitamin D induced apoptosis [233, 234].
Summarizing, vitamin D hydroxylases which belongs to the cytochrome P450 family are essential elements responsible for activation and inactivation of vitamin D and it analogs. Their expression is altered in many cancers including melanoma where levels of CYP27A1 and CYP27B1 decrease while CYP24A1 increases. The potential mechanisms may involve occurrence of specific SNPs or modulation of expression by microRNA or other epigenetic factors which result in a depleted pool of endogenous 1,25(OH)2D3 and decrease the efficiency of therapeutic use of 1,25(OH)2D3 and its analogs. Thus, expressional analyses of selected cytochromes P450 may help in development of melanoma therapy based on vitamin D analogs. microRNAs are also new, but very promising targets for melanoma therapy in combination with the use of vitamin D analogs.
C. Novel P450scc Activated Secosteroidogenic Pathway and it Putative Role in Cancerogenesis
As already mentioned above, CYP11A1 hydroxylates vitamin D3 in a sequential manner at positions C20, C23 and/or C22 and C17 (Fig. 8)) to produce several hydroxyderivatives with 20(OH)D3 acting as the first and main metabolite that can detach from the catalytic site of the enzyme [79, 81–83]. Additional important metabolites are 20,23(OH)2D3, 20,22(OH)2D3, 22(OH)D3, and 17,20,23(OH)3D3, with minor products represented by 17(OH)D3, 23(OH)D3 and 17,20(OH)2D3 [82, 83]. Some of these products can be hydroxylated by CYP27B1 to produce 1α-hydroxyderivatives [235, 236] while 20(OH)D3 can also be hydroxylated by CYP27A1 to produce 20,25(OH)2D3 and 20,26(OH)2D3 [185]. Also 1(OH)D3 is transformed by CYP11A1 to 1,20(OH)D3 [237]. We also found that ex vivo vitamin D3 can be metabolized to 20(OH)D3, 22(OH)D3, 20,23(OH)2D3, 20,22(OH)2D3, 1,20(OH)2D3, 1,20,23(OH)3D3 and 17,20,23(OH)3D3 in the placenta, adrenal glands and keratinocytes, and we detected the predominant metabolite [20(OH)D3] in human serum with a relative concentration about 1/20th that of 25(OH)D3 [238]. Concerning vitamin D2 metabolism we have found that CYP11A1 hydroxylates it sequentially producing 20(OH)D2, 17,20(OH)2D2 and 17,20,24(OH)3D2 [85, 86]. Importantly, we have established chemical routes for synthesis of some of these analogs [239, 240].
We also found that CYP11A1 in vitro and in placenta and adrenal glands ex vivo as well as in epidermal keratinocytes can metabolize 7DHC to 7DHP with 22(OH)7DHC and 20,22(OH)2 7DHC serving as intermediates [73, 78, 241]. 7DHP undergoes further metabolism with production of 7-dehydroprogesterone and hydroxy-7DHP and potentially to other 7Δ-hydroxysteroids through existing steroidogenic enzymes [73, 78, 241]. These, when exposed to UVB can be converted to androsta-calciferols (aD) and pregna-calciferols (pD), i.e. vitamin D compounds with a short or absent side chain [227, 229, 242] (Fig. (9)). In addition, 5,7-diene, 3β-hydroxyandrosta-5,7-diene-17β-carboxylic acid (17-COOH-7DA) was discovered during synthesis of 21(OH)7DHP and it showed high antiproliferative potency, without toxicity [243].
Fig. (9).
UVB-induced production of pregna- or androsta-secosteroids with short or absent side chain.
Novel vitamin D hydroxyderivatives and 7Δ-hydroxysteroid show potent biological activity. P450scc-derived vitamin D hydroxyderivatives show anti-proliferative and pro-differentiation activities in a cell-type restricted fashion that is dependent on the length of the side chain [78, 83, 137, 239, 240, 244–254]. These compounds also express anti-cancer activities including on malignant melanoma. In the epidermis, 20(OH)D3 and 20,23(OH)2D3 stimulate the keratinocyte differentiation program and inhibit cell proliferation and NF-κB activity, being as potent as 1,25(OH)2D3. They act through the VDR [254], however, as partial receptor agonists, since unlike 1,25(OH)2D3, they only weakly stimulate CYP24 expression [78, 239, 244, 245].
As tested by Drs Chen and Holick, 20(OH)D3 at a doses as high as 3.0 μg/kg had no calcemic activity in rats whereas 1,25(OH)2D3 at lower doses raised calcium to 16.0 ± 1.2 mg/dL [228]. Importantly, addition of a 1α-hydroxyl group to 20(OH)D3 induced calcemic activity [228]. We repeated this testing and administered 20(OH)D3 doses as high as 30 μg/kg to C57BL/6 mice daily for 14 days and found no significant differences in sera Ca+2 levels compared to control mice and a lack of toxicity determined by serum chemistry and histological analyses of heart, liver, spleen and kidney [253]. Furthermore, 17,20(OH)2pD [251] and 20,23(OH)2D3 [255] are non-calcemic least up to 3 μg/kg. This lack of toxicity and relatively high biological activity of novel vitamin D derivatives make them excellent candidates for therapy or adjuvant therapy of different cancers including melanoma. Interestingly, 5.7-dienal precursors of secosteroids also show anti-proliferative and anti-melanoma activities [78, 227, 228, 241].
5. CONCLUDING REMARKS AND FUTURE PERSPECTIVE
Skin cancers represent a significant clinical problem affecting a large segment of the population. Squamous and basal cell carcinomas represent the most frequent environmentally induced cancers, while malignant melanoma, a deadly skin disease, is the sixth most common cancer in the USA. The current lifetime risk in the USA for developing invasive melanoma is 1 per 57 individuals. There are also other lethal skin cancers including Merkel cell carcinoma and sarcomas of which the incidence is rising. Recent advances in skin biochemistry show that this organ can produce and metabolize steroids, secosteroids and melatonin. Normal and malignant skin cells also express the corresponding receptors which mediate the regulatory activities of sex hormones, corticosteroids, secosteroids, melatonin, hydroxycholesterols and potentially 7-hydroxy-steroids/cholesterol. Therefore, the activities of cytochrome P450 enzymes involved in the production, activation or inactivation of the above endogenous products define the local chemical environment which may facilitate or suppress cutaneous carcinogenesis (Fig. (10)). Accordingly, the expression levels of these P450s may represent markers of skin pathology. Furthermore, such enzymes represent a realistic target for pharmacological modification trough topical application of small molecules to produce local anti-tumorigenic factors. The preferred targets are CYP11A1, CYPs involved in the metabolism of vitamin D3 and CYPs regulating steroidogenic activity. Novel and promising molecules for prevention or therapy of skin cancer are the recently discovered non-calcemic secosteroids derived from the action of CYP11A1, and phylogenetically old molecules such as melatonin and its metabolites including AFMK. The challenge is to define their interaction with their classical receptors (VDR and MT) or with other receptors yet to be identified.
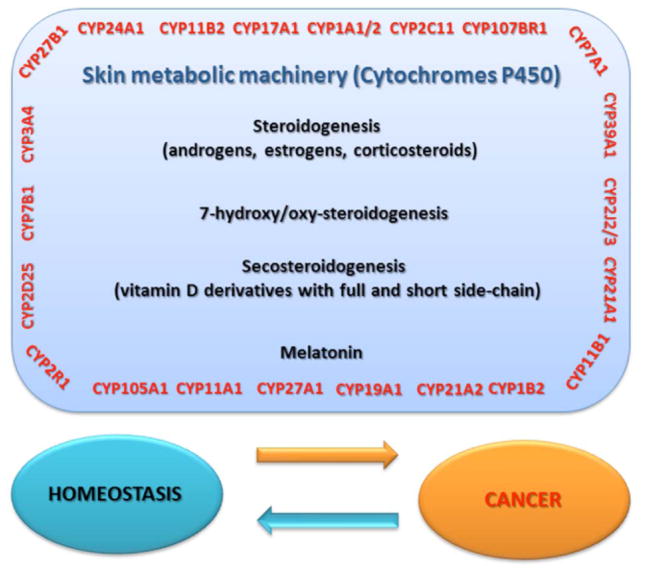
Fig. (10).
Cytochromes P450 are the guardians of the key metabolic pathways, including: steroidogenesis, secosteroidogenesis, melatonin synthesis. The loss of their proper function by genetic and epigenetic factors results in destabilisation of internal homeostasis that eventually leads to cancer progression.
Acknowledgments
This work was supported by NIH grants R01AR052190 and 1R01AR056666-01A2 to AS, R01CA148706, 1S10RR026377-01 and 1S10OD010678-01 to WL, Polish Ministry of Science and Higher Education, grant N405 623238 to MAZ, by the University of Western Australia and by the College of Pharmacy at the University of Tennessee Health Science Center. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
References
- 1.Botchkarev VA, Gdula MR, Mardaryev AN, Sharov AA, Fessing MY. Epigenetic Regulation of Gene Expression in Keratinocytes. J Invest Dermatol. 2012;132(11):2505–21. doi: 10.1038/jid.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolognia JL, Jarizzo JJ, Rapini RP. Dermatology. Mosby Elsevier; St. Louis: 2008. [Google Scholar]
- 3.Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen KF. Dermatology in General Medicine. McGraw Hill; New York: 1993. [Google Scholar]
- 4.Feingold KR. Lamellar bodies: The key to cutaneous barrier function. J Invest Dermatol. 2012;132(8):1951–1953. doi: 10.1038/jid.2012.177. [DOI] [PubMed] [Google Scholar]
- 5.Elias PM, Choi EH. Interactions among stratum corneum defensive functions. Exp Dermatol. 2005;14(10):719–726. doi: 10.1111/j.1600-0625.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 6.Feingold KR. The importance of lipids in cutaneous function. J Lipid Res. 2007;48(12):2529–2530. doi: 10.1194/jlr.E700004-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21(5):457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 8.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80(3):979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 9.Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J, Tobin DJ. Corticotropin releasing hormone and the skin. Front Biosci. 2006;11:2230–2248. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat, Embryol Cell Biol. 2012;212:1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 12.Slominski A, Zmijewski MA, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25(1):14–27. doi: 10.1111/j.1755-148X.2011.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad N, Mukhtar H. Cytochrome p450: A target for drug development for skin diseases. J Invest Dermatol. 2004;123(3):417–425. doi: 10.1111/j.0022-202X.2004.23307.x. [DOI] [PubMed] [Google Scholar]
- 14.Sutter CH, Yin H, Li Y, Mammen JS, Bodreddigari S, Stevens G, Cole JA, Sutter TR. EGF receptor signaling blocks aryl hydrocarbon receptor-mediated transcription and cell differentiation in human epidermal keratinocytes. ProcNatl Acad Sci USA. 2009;106(11):4266–4271. doi: 10.1073/pnas.0900874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zbytek B, Carlson JA, Granese J, Ross J, Mihm MC, Jr, Slominski A. Current concepts of metastasis in melanoma. Expert Rev Dermatol. 2008;3(5):569–585. doi: 10.1586/17469872.3.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flaherty KT. Targeting metastatic melanoma. Annu Rev Med. 2012;63:171–183. doi: 10.1146/annurev-med-050410-105655. [DOI] [PubMed] [Google Scholar]
- 17.Flaherty KT, Hodi FS, Fisher DE. From genes to drugs: Targeted strategies for melanoma. Nat Rev Cancer. 2012;12(5):349–361. doi: 10.1038/nrc3218. [DOI] [PubMed] [Google Scholar]
- 18.Slominski A, Wortsman J, Carlson AJ, Matsuoka LY, Balch CM, Mihm MC. Malignant melanoma. Arch Pathol Lab Med. 2001;125(10):1295–1306. doi: 10.5858/2001-125-1295-MM. [DOI] [PubMed] [Google Scholar]
- 19.Linos K, Slominski A, Ross JS, Carlson JA. Melanoma update: Diagnostic and prognostic factors that can effectively shape and personalize management. Biomark Med. 2011;5(3):333–360. doi: 10.2217/bmm.11.39. [DOI] [PubMed] [Google Scholar]
- 20.Reggiani Bonetti L, Boselli F, Lupi M, Bettelli S, Schirosi L, Bigiani N, Sartori G, Rivasi F. Expression of estrogen receptor in hemangioma of the uterine cervix: Reports of three cases and review of the literature. Arch Gynecol Obstet. 2009;280(3):469–472. doi: 10.1007/s00404-009-0928-0. [DOI] [PubMed] [Google Scholar]
- 21.Sun ZY, Yang L, Yi CG, Zhao H, Han DL, Yang T, Wang L, Nie CL, Zhang GY, Yin GQ, Wang G, Teng XP, Fei DM, Wang J, Zhou WK, Li Y, Liu B, Liu Y, Zhang MJ, Wu SM, Zhang X, Pan H, Xiao B, Zhao KF, Liu D, Guo SZ. Possibilities and potential roles of estrogen in the pathogenesis of proliferation hemangiomas formation. Med Hypotheses. 2008;71(2):286–292. doi: 10.1016/j.mehy.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Yang XJ, Jiang YH, Zheng JW, Hong L, Zhou Q, Qin ZP. The role of serum basic fibroblast growth factor, estradiol and urine basic fibroblast growth factor in differentiating infantile haemangiomas from vascular malformations. Phlebology. 2012;26(5):191–196. doi: 10.1258/phleb.2010.010020. [DOI] [PubMed] [Google Scholar]
- 23.Calicchio ML, Collins T, Kozakewich HP. Identification of signaling systems in proliferating and involuting phase infantile hemangiomas by genome-wide transcriptional profiling. Am J Pathol. 2009;174(5):1638–1649. doi: 10.2353/ajpath.2009.080517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy JA. A new human herpesvirus: KSHV or HHV8? Lancet. 1995;346(8978):786. doi: 10.1016/s0140-6736(95)91611-3. [DOI] [PubMed] [Google Scholar]
- 25.Reddy KK, Hanke CW, Tierney EP. Malignancy arising within cutaneous tattoos: case of dermatofibrosarcoma protuberans and review of literature. J Drugs Dermatol. 2012;10(8):837–842. [PubMed] [Google Scholar]
- 26.Slominski A, Wortsman J, Carlson A, Mihm M, Nickoloff B, McClatchey K. Molecular pathology of soft tissue and bone tumors. A review. Arch Path Lab Med. 1999;123(12):1246–1259. doi: 10.5858/1999-123-1246-MPOSTA. [DOI] [PubMed] [Google Scholar]
- 27.Baron JM, Holler D, Schiffer R, Frankenberg S, Neis M, Merk HF, Jugert FK. Expression of multiple cytochrome p450 enzymes and multidrug resistance-associated transport proteins in human skin keratinocytes. J Invest Dermatol. 2001;116(4):541–548. doi: 10.1046/j.1523-1747.2001.01298.x. [DOI] [PubMed] [Google Scholar]
- 28.Smith G, Wolf CR, Deeni YY, Dawe RS, Evans AT, Comrie MM, Ferguson J, Ibbotson SH. Cutaneous expression of cytochrome P450 CYP2S1: Individuality in regulation by therapeutic agents for psoriasis and other skin diseases. Lancet. 2003;361(9366):1336–1343. doi: 10.1016/S0140-6736(03)13081-4. [DOI] [PubMed] [Google Scholar]
- 29.Jugert FK, Agarwal R, Kuhn A, Bickers DR, Merk HF, Mukhtar H. Multiple cytochrome P450 isozymes in murine skin: Induction of P450 1A, 2B, 2E, and 3A by dexamethasone. J Invest Dermatol. 1994;102(6):970–975. doi: 10.1111/1523-1747.ep12384210. [DOI] [PubMed] [Google Scholar]
- 30.Mukhtar H, Bickers DR. Age-related changes in benzo(a)pyrene metabolism and epoxide-metabolizing enzyme activities in rat skin. Drug Metab Dispos. 1983;11(6):562–567. [PubMed] [Google Scholar]
- 31.Finnen MJ, Herdman ML, Shuster S. Distribution and sub-cellular localization of drug metabolizing enzymes in the skin. Brit J Dermatol. 1985;113(6):713–721. doi: 10.1111/j.1365-2133.1985.tb02407.x. [DOI] [PubMed] [Google Scholar]
- 32.Merk HF, Mukhtar H, Kaufmann I, Das M, Bickers DR. Human hair follicle benzo[a]pyrene and benzo[a]pyrene 7,8-diol metabolism: Effect of exposure to a coal tar-containing shampoo. J Invest Dermatol. 1987;88(1):71–76. doi: 10.1111/1523-1747.ep12465053. [DOI] [PubMed] [Google Scholar]
- 33.Whitlock JP, Jr, Chichester CH, Bedgood RM, Okino ST, Ko HP, Ma Q, Dong L, Li H, Clarke-Katzenberg R. Induction of drug-metabolizing enzymes by dioxin. Drug Metab Rev. 1997;29(4):1107–1127. doi: 10.3109/03602539709002245. [DOI] [PubMed] [Google Scholar]
- 34.Yamazaki H, Inui Y, Yun CH, Guengerich FP, Shimada T. Cytochrome P450 2E1 and 2A6 enzymes as major catalysts for metabolic activation of N-nitrosodialkylamines and tobacco-related nitrosamines in human liver microsomes. Carcinogenesis. 1992;13(10):1789–1794. doi: 10.1093/carcin/13.10.1789. [DOI] [PubMed] [Google Scholar]
- 35.Guengerich FP, Kim DH, Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991;4(2):168–179. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- 36.Katiyar SK, Matsui MS, Mukhtar H. Ultraviolet-B exposure of human skin induces cytochromes P450 1A1 and 1B1. J Invest Dermatol. 2000;114(2):328–333. doi: 10.1046/j.1523-1747.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- 37.Mukhtar H, DelTito BJ, Jr, Matgouranis PM, Das M, Asokan P, Bickers DR. Additive effects of ultraviolet B and crude coal tar on cutaneous carcinogen metabolism: Possible relevance to the tumorigenicity of the Goeckerman regimen. J Invest Dermatol. 1986;87(3):348–353. doi: 10.1111/1523-1747.ep12524446. [DOI] [PubMed] [Google Scholar]
- 38.Bui PH, Hsu EL, Hankinson O. Fatty acid hydroperoxides support cytochrome P450 2S1-mediated bioactivation of benzo[a]pyrene-7,8-dihydrodiol. Mol Pharmacol. 2009;76(5):1044–1052. doi: 10.1124/mol.109.057760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bui PH, Hankinson O. Functional characterization of human cytochrome P450 2S1 using a synthetic gene-expressed protein in Escherichia coli. Mol Pharmacol. 2009;76(5):1031–1043. doi: 10.1124/mol.109.057752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koop DR. Oxidative and reductive metabolism by cytochrome P450 2E1. FASEB J. 1992;6(2):724–730. doi: 10.1096/fasebj.6.2.1537462. [DOI] [PubMed] [Google Scholar]
- 41.Wu D, Cederbaum AI. Oxidative stress mediated toxicity exerted by ethanol-inducible CYP2E1. Toxicol Appl Pharmacol. 2005;207(2 Suppl):70–76. doi: 10.1016/j.taap.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 42.Puntarulo S, Cederbaum AI. Production of reactive oxygen species by microsomes enriched in specific human cytochrome P450 enzymes. Free Radical Biol Med. 1998;24(7–8):1324–1330. doi: 10.1016/s0891-5849(97)00463-2. [DOI] [PubMed] [Google Scholar]
- 43.Paolini M, Antelli A, Pozzetti L, Spetlova D, Perocco P, Valgimigli L, Pedulli GF, Cantelli-Forti G. Induction of cytochrome P450 enzymes and over-generation of oxygen radicals in beta-carotene supplemented rats. Carcinogenesis. 2001;22(9):1483–1495. doi: 10.1093/carcin/22.9.1483. [DOI] [PubMed] [Google Scholar]
- 44.Strolin-Benedetti M, Brogin G, Bani M, Oesch F, Hengstler JG. Association of cytochrome P450 induction with oxidative stress in vivo as evidenced by 3-hydroxylation of salicylate. Xenobiotica; the fate of foreign compounds in biological systems. 1999;29(11):1171–1180. doi: 10.1080/004982599238038. [DOI] [PubMed] [Google Scholar]
- 45.Imaoka S, Osada M, Minamiyama Y, Yukimura T, Toyokuni S, Takemura S, Hiroi T, Funae Y. Role of phenobarbital-inducible cytochrome P450s as a source of active oxygen species in DNA-oxidation. Cancer Lett. 2004;203(2):117–125. doi: 10.1016/j.canlet.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Park JY, Shigenaga MK, Ames BN. Induction of cytochrome P4501A1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin or indolo(3,2-b)carbazole is associated with oxidative DNA damage. Proc Natl Acad Sci USA. 1996;93(6):2322–2327. doi: 10.1073/pnas.93.6.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shertzer HG, Nebert DW, Puga A, Ary M, Sonntag D, Dixon K, Robinson LJ, Cianciolo E, Dalton TP. Dioxin causes a sustained oxidative stress response in the mouse. Biochem Biophys Res Commun. 1998;253(1):44–48. doi: 10.1006/bbrc.1998.9753. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida R, Ogawa Y. Oxidative stress induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin: an application of oxidative stress markers to cancer risk assessment of dioxins. Ind Health. 2000;38(1):5–14. doi: 10.2486/indhealth.38.5. [DOI] [PubMed] [Google Scholar]
- 49.Slominski A, Fischer TW, Zmijewski MA, Wortsman J, Semak I, Zbytek B, Slominski RM, Tobin DJ. On the role of melatonin in skin physiology and pathology. Endocrine. 2005;27(2):137–148. doi: 10.1385/ENDO:27:2:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Facciola G, Hidestrand M, von Bahr C, Tybring G. Cytochrome P450 isoforms involved in melatonin metabolism in human liver microsomes. Eur J Clin Pharmacol. 2001;56(12):881–888. doi: 10.1007/s002280000245. [DOI] [PubMed] [Google Scholar]
- 51.Skene DJ, Papagiannidou E, Hashemi E, Snelling J, Lewis DF, Fernandez M, Ioannides C. Contribution of CYP1A2 in the hepatic metabolism of melatonin: Studies with isolated microsomal preparations and liver slices. J Pineal Res. 2001;31(4):333–342. doi: 10.1034/j.1600-079x.2001.310408.x. [DOI] [PubMed] [Google Scholar]
- 52.Ma X, Idle JR, Krausz KW, Gonzalez FJ. Metabolism of melatonin by human cytochromes p450. Drug Metabol Dispos. 2005;33(4):489–494. doi: 10.1124/dmd.104.002410. [DOI] [PubMed] [Google Scholar]
- 53.Semak I, Korik E, Antonova M, Wortsman J, Slominski A. Metabolism of melatonin by cytochrome P450s in rat liver mitochondria and microsomes. J Pineal Res. 2008;45(4):515–523. doi: 10.1111/j.1600-079X.2008.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang TK, Chen J, Yang G, Yeung EY. Inhibition of procarcinogen-bioactivating human CYP1A1, CYP1A2 and CYP1B1 enzymes by melatonin. J Pineal Res. 2010;48(1):55–64. doi: 10.1111/j.1600-079X.2009.00724.x. [DOI] [PubMed] [Google Scholar]
- 55.Kumar CA, Das UN. Effect of melatonin on two stage skin carcinogenesis in Swiss mice. Medical science monitor. Int Med J Exp Clin Res. 2000;6(3):471–475. [PubMed] [Google Scholar]
- 56.Semak I, Naumova M, Korik E, Terekhovich V, Wortsman J, Slominski A. A novel metabolic pathway of melatonin: Oxidation by cytochrome C. Biochem. 2005;44(26):9300–9307. doi: 10.1021/bi050202d. [DOI] [PubMed] [Google Scholar]
- 57.Fischer TW, Sweatman TW, Semak I, Sayre RM, Wortsman J, Slominski A. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 2006;20(9):1564–1566. doi: 10.1096/fj.05-5227fje. [DOI] [PubMed] [Google Scholar]
- 58.Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R. Melatonin in the skin: Synthesis, metabolism and functions. Trends Endocrin Metabol. 2008;19(1):17–24. doi: 10.1016/j.tem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Fischer TW, Slominski A, Zmijewski MA, Reiter RJ, Paus R. Melatonin as a major skin protectant: from free radical scavenging to DNA damage repair. Exp Dermatol. 2008;17(9):713–730. doi: 10.1111/j.1600-0625.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- 60.Tuckey RC. Progesterone synthesis by the human placenta. Placenta. 2005;26(4):273–281. doi: 10.1016/j.placenta.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Strushkevich N, MacKenzie F, Cherkesova T, Grabovec I, Usanov S, Park HW. Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system. Proc Natl Acad Sci USA. 2011;108(25):10139–10143. doi: 10.1073/pnas.1019441108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988;9(3):295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- 63.Miller WL, Bose HS. Early steps in steroidogenesis: Intracellular cholesterol trafficking. J Lipid Res. 2011;52(12):2111–2135. doi: 10.1194/jlr.R016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller WL. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporterBiochim. Biophys Acta. 2007;1771(6):663–676. doi: 10.1016/j.bbalip.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 66.Hu J, Zhang Z, Shen WJ, Azhar S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutrition Metab. 2010;7:47. doi: 10.1186/1743-7075-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrin Metab. 2002;13(6):234–239. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- 68.Watzka M, Bidlingmaier F, Schramm J, Klingmuller D, Stoffel-Wagner B. Sex- and age-specific differences in human brain CYP11A1 mRNA expression. J Neuroendocrinol. 1999;11(12):901–905. doi: 10.1046/j.1365-2826.1999.00407.x. [DOI] [PubMed] [Google Scholar]
- 69.Taves MD, Gomez-Sanchez CE, Soma KK. Extra-adrenal glucocorticoids and mineralocorticoids: Evidence for local synthesis, regulation, and function. AmJ Physiol Endocrinol Metab. 2011;301(1):E11–24. doi: 10.1152/ajpendo.00100.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fernandez-Marcos PJ, Auwerx J, Schoonjans K. Emerging actions of the nuclear receptor LRH-1 in the gut. Biochim Biophys Acta. 2011;1812(8):947–955. doi: 10.1016/j.bbadis.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teplyuk NM, Zhang Y, Lou Y, Hawse JR, Hassan MQ, Teplyuk VI, Pratap J, Galindo M, Stein JL, Stein GS, Lian JB, van Wijnen AJ. The osteogenic transcription factor runx2 controls genes involved in sterol/steroid metabolism, including CYP11A1 in osteoblasts. Mol Endocrinol. 2009;23(6):849–861. doi: 10.1210/me.2008-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007:265–266. 143–149. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, Tuckey RC. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271(21):4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tuckey RC, Lawrence J, Cameron KJ. Side-chain cleavage of cholesterol esters by human cytochrome P-450(scc) J Steroid Biochem Mol Biol. 1996;58(5–6):605–610. doi: 10.1016/0960-0760(96)00071-4. [DOI] [PubMed] [Google Scholar]
- 75.Tuckey RC, Cameron KJ. Side-chain specificities of human and bovine cytochromes P-450scc. Eur J Biochem. 1993;217(1):209–215. doi: 10.1111/j.1432-1033.1993.tb18235.x. [DOI] [PubMed] [Google Scholar]
- 76.Slominski A, Semak I, Zjawiony J, Wortsman J, Gandy MN, Li J, Zbytek B, Li W, Tuckey RC. Enzymatic metabolism of ergosterol by cytochrome p450scc to biologically active 17alpha,24-dihydroxyergosterol. Chem Biol. 2005;12(8):931–939. doi: 10.1016/j.chembiol.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 77.Tuckey RC, Nguyen MN, Chen J, Slominski AT, Baldisseri DM, Tieu EW, Zjawiony JK, Li W. Human cytochrome P450scc (CYP11A1) catalyzes epoxide formation with ergosterol. Drug Metab Dispos. 2012;40(3):436–444. doi: 10.1124/dmd.111.042515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Slominski AT, Zmijewski MA, Semak I, Sweatman T, Janjetovic Z, Li W, Zjawiony JK, Tuckey RC. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PloS One. 2009;4(2):e4309. doi: 10.1371/journal.pone.0004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: Unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc Natl Acad Sci USA. 2003;100(25):14754–14759. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shackleton C, Roitman E, Guo LW, Wilson WK, Porter FD. Identification of 7(8) and 8(9) unsaturated adrenal steroid metabolites produced by patients with 7-dehydrosterol-delta7-reductase deficiency (Smith-Lemli-Opitz syndrome) J Steroid Biochem Mol Biol. 2002;82(2–3):225–232. doi: 10.1016/s0960-0760(02)00155-3. [DOI] [PubMed] [Google Scholar]
- 81.Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, Tuckey RC. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. The FEBS J. 2005;272(16):4080–4090. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tuckey RC, Li W, Zjawiony JK, Zmijewski MA, Nguyen MN, Sweatman T, Miller D, Slominski A. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J. 2008;275(10):2585–2596. doi: 10.1111/j.1742-4658.2008.06406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tuckey RC, Li W, Shehabi HZ, Janjetovic Z, Nguyen MN, Kim TK, Chen J, Howell DE, Benson HA, Sweatman T, Baldisseri DM, Slominski A. Production of 22-hydroxy-metabolites of vitamin D3 by cytochrome P450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab Dispos. 2011;39(9):1577–88. doi: 10.1124/dmd.111.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EKY, Nguyen MN, Benson HAE, Korik E, Janjetovic Z, Chen J, Yates CR, Postlethwaite A, Li W, Tuckey RC. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26(9):3901–15. doi: 10.1096/fj.12-208975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Slominski A, Semak I, Wortsman J, Zjawiony J, Li W, Zbytek B, Tuckey RC. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J. 2006;273(13):2891–2901. doi: 10.1111/j.1742-4658.2006.05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nguyen MN, Slominski A, Li W, Ng YR, Tuckey RC. Metabolism of vitamin D2 to 17,20,24-trihydroxyvitamin D2 by cytochrome p450scc (CYP11A1) Drug Metab Dispos. 2009;37(4):761–767. doi: 10.1124/dmd.108.025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Labrie F, Luu-The V, Labrie C, Belanger A, Simard J, Lin SX, Pelletier G. Endocrine and intracrine sources of androgens in women: Inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003;24(2):152–182. doi: 10.1210/er.2001-0031. [DOI] [PubMed] [Google Scholar]
- 88.Zouboulis CC, Chen WC, Thornton MJ, Qin K, Rosenfield R. Sexual hormones in human skin. Horm Metabol Res. 2007;39(2):85–95. doi: 10.1055/s-2007-961807. [DOI] [PubMed] [Google Scholar]
- 89.Zouboulis CC, Degitz K. Androgen action on human skin -- from basic research to clinical significance. Exp Dermatol. 2004;13 (Suppl 4):5–10. doi: 10.1111/j.1600-0625.2004.00255.x. [DOI] [PubMed] [Google Scholar]
- 90.Milewich L, Shaw CB, Sontheimer RD. Steroid metabolism by epidermal keratinocytes. Ann New York Acad Sci. 1988;548:66–89. doi: 10.1111/j.1749-6632.1988.tb18793.x. [DOI] [PubMed] [Google Scholar]
- 91.Labrie F, Luu-The V, Labrie C, Pelletier G, El-Alfy M. Intracrinology and the skin. Horm Res. 2000;54(5–6):218–229. doi: 10.1159/000053264. [DOI] [PubMed] [Google Scholar]
- 92.Simard J, Couet J, Durocher F, Labrie Y, Sanchez R, Breton N, Turgeon C, Labrie F. Structure and tissue-specific expression of a novel member of the rat 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase (3 beta-HSD) family. The exclusive 3 beta-HSD gene expression in the skin. J Biol Chem. 1993;268(26):19659–19668. [PubMed] [Google Scholar]
- 93.Milewich L, Shaw CE, Doody KM, Rainey WE, Mason JI, Carr BR. 3 beta-Hydroxysteroid dehydrogenase activity in glandular and extraglandular human fetal tissues. J ClinEndocrin Metab. 1991;73(5):1134–1140. doi: 10.1210/jcem-73-5-1134. [DOI] [PubMed] [Google Scholar]
- 94.Zouboulis CC, Baron JM, Bohm M, Kippenberger S, Kurzen H, Reichrath J, Thielitz A. Frontiers in sebaceous gland biology and pathology. Exp Dermatol. 2008;17(6):542–551. doi: 10.1111/j.1600-0625.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 95.Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol. 2004;22(5):360–366. doi: 10.1016/j.clindermatol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 96.Randall VA, Thornton MJ, Messenger AG, Hibberts NA, Loudon AS, Brinklow BR. Hormones and hair growth: Variations in androgen receptor content of dermal papilla cells cultured from human and red deer (Cervus elaphus) hair follicles. J Invest Dermatol. 1993;101(1 Suppl):114S–120S. doi: 10.1111/1523-1747.ep12363039. [DOI] [PubMed] [Google Scholar]
- 97.Ohnemus U, Uenalan M, Inzunza J, Gustafsson JA, Paus R. The hair follicle as an estrogen target and source. Endocr Rev. 2006;27(6):677–706. doi: 10.1210/er.2006-0020. [DOI] [PubMed] [Google Scholar]
- 98.Slominski A, Gomez-Sanchez C, Foecking MF, Wortsman J. Active steroidogenesis in the normal rat skin. Biochim Biophys Acta. 2000;1474:1–4. doi: 10.1016/s0304-4165(99)00215-9. [DOI] [PubMed] [Google Scholar]
- 99.Ramot Y, Paus R, Tiede S, Zlotogorski A. Endocrine controls of keratin expression. Bio Essays. 2009;31(4):389–399. doi: 10.1002/bies.200800121. [DOI] [PubMed] [Google Scholar]
- 100.Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81(1):449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 101.Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol. 2005;124(1):13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Slominski A. Neuroendocrine activity of the melanocyte. Exp Dermatol. 2009;18(9):760–763. doi: 10.1111/j.1600-0625.2009.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mancuso M, Gallo D, Leonardi S, Pierdomenico M, Pasquali E, De Stefano I, Rebessi S, Tanori M, Scambia G, Di Majo V, Covelli V, Pazzaglia S, Saran A. Modulation of basal and squamous cell carcinoma by endogenous estrogen in mouse models of skin cancer. Carcinogenesis. 2009;30(2):340–347. doi: 10.1093/carcin/bgn243. [DOI] [PubMed] [Google Scholar]
- 104.Leslie KK, Espey E. Oral contraceptives and skin cancer: Is there a link? Am J Clin Dermat. 2005;6(6):349–355. doi: 10.2165/00128071-200506060-00002. [DOI] [PubMed] [Google Scholar]
- 105.Bosland MC, Prinsen MK, Rivenson A, Weisburger JH. Induction of skin and thyroid tumors in male rats by N-methyl-N-nitrosourea after sequential treatment with cyproterone acetate and testosterone propionate: effects of castration, rat strain and time of carcinogen injection. Carcinogenesis. 1992;13(4):669–674. doi: 10.1093/carcin/13.4.669. [DOI] [PubMed] [Google Scholar]
- 106.Ohata C, Tadokoro T, Itami S. Expression of estrogen receptor beta in normal skin, melanocytic nevi and malignant melanomas. J Dermatol. 2008;35(4):215–221. doi: 10.1111/j.1346-8138.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- 107.Nading MA, Nanney LB, Boyd AS, Ellis DL. Estrogen receptor beta expression in nevi during pregnancy. Exp Dermatol. 2008;17(6):489–497. doi: 10.1111/j.1600-0625.2007.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Giorgi V, Mavilia C, Massi D, Gozzini A, Aragona P, Tanini A, Sestini S, Paglierani M, Boddi V, Brandi ML, Lotti T. Estrogen receptor expression in cutaneous melanoma: A real-time reverse transcriptase-polymerase chain reaction and immunohistochemical study. Arch Dermatol. 2009;145(1):30–36. doi: 10.1001/archdermatol.2008.537. [DOI] [PubMed] [Google Scholar]
- 109.Tanemura A, van Hoesel AQ, Mori T, Yu T, Hoon DS. The role of estrogen receptor in melanoma. Expert Opin Ther Targets. 2007;11(12):1639–1648. doi: 10.1517/14728222.11.12.1639. [DOI] [PubMed] [Google Scholar]
- 110.Hsueh EC, Gupta RK, Lefor A, Reyzin G, Ye W, Morton DL. Androgen blockade enhances response to melanoma vaccine. J Surg Res. 2003;110(2):393–398. doi: 10.1016/s0022-4804(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 111.Morvillo V, Luthy IA, Bravo AI, Capurro MI, Portela P, Calandra RS, Mordoh J. Androgen receptors in human melanoma cell lines IIB-MEL-LES and IIB-MEL-IAN and in human melanoma metastases. Melanoma Res. 2002;12(6):529–538. doi: 10.1097/00008390-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 112.Kanda N, Watanabe S. 17beta-estradiol, progesterone, and dihydrotestosterone suppress the growth of human melanoma by inhibiting interleukin-8 production. J Invest Dermatol. 2001;117(2):274–283. doi: 10.1046/j.1523-1747.2001.01422.x. [DOI] [PubMed] [Google Scholar]
- 113.Richardson B, Price A, Wagner M, Williams V, Lorigan P, Browne S, Miller JG, Mac Neil S. Investigation of female survival benefit in metastatic melanoma. Brit J Cancer. 1999;80(12):2025–2033. doi: 10.1038/sj.bjc.6690637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rampen FH, Mulder JH. Malignant melanoma: an androgen-dependent tumour? Lancet. 1980;1(8168 Pt 1):562–564. doi: 10.1016/s0140-6736(80)91055-7. [DOI] [PubMed] [Google Scholar]
- 115.Slominski A, Wortsman J, Paus R, Elias PM, Tobin DJ, Feingold KR. Skin as an endocrine organ: Implications for its function. Drug Discov Today. 2008;5(2):137–144. doi: 10.1016/j.ddmec.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996;81(7):2746–2749. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- 117.Slominski A, Gomez-Sanchez CE, Foecking MF, Wortsman J. Metabolism of progesterone to DOC, corticosterone and 18OHDOC in cultured human melanoma cells. FEBS Lett. 1999;455(3):364–366. doi: 10.1016/s0014-5793(99)00889-3. [DOI] [PubMed] [Google Scholar]
- 118.Slominski A, Worstman J, Foecking MF, Shackleton CH, Gomez-Sanchez CE, Szczesniewski A. Gas chromatography/mass spectrometry characterization of corticosteroid metabolism in human immortalized keratinocytes. J Invest Dermatol. 2002;118:310–315. doi: 10.1046/j.0022-202x.2001.01648.x. [DOI] [PubMed] [Google Scholar]
- 119.Rogoff D, Gomez-Sanchez CE, Foecking MF, Wortsman J, Slominski A. Steroidogenesis in the human skin: 21-hydroxylation in cultured keratinocytes. J Steroid BiochMol Biol. 2001;78(1):77–81. doi: 10.1016/s0960-0760(01)00076-0. [DOI] [PubMed] [Google Scholar]
- 120.Thiboutot D, Jabara S, McAllister JM, Sivarajah A, Gilliland K, Cong Z, Clawson G. Human skin is a steroidogenic tissue: Steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB-1) J Invest Dermatol. 2003;120(6):905–914. doi: 10.1046/j.1523-1747.2003.12244.x. [DOI] [PubMed] [Google Scholar]
- 121.Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol. 2005;162(1–2):97–102. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 122.Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, Wortsman J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. American journal of physiology Endocr Metab. 2005;288:E701–E706. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 123.Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19(10):1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 124.Sharpley CF, Kauter KG, McFarlane JR. An initial exploration of in vivo hair cortisol responses to a brief pain stressor: latency, localization and independence effects. Physiol Res. 2009;58(5):757–761. doi: 10.33549/physiolres.931544. [DOI] [PubMed] [Google Scholar]
- 125.Slominski A, Zbytek B, Szczesniewski A, Wortsman J. Cultured human dermal fibroblasts do produce cortisol. J Invest Dermatol. 2006;126(5):1177–1178. doi: 10.1038/sj.jid.5700204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, Davis SC, Resnik S, Brem H, Tomic-Canic M. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem. 2011;286(12):10265–10275. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cirillo N, Prime SS. Keratinocytes synthesize and activate cortisol. J Cell Biochem. 2011;112(6):1499–1505. doi: 10.1002/jcb.23081. [DOI] [PubMed] [Google Scholar]
- 128.Hannen RF, Michael AE, Jaulim A, Bhogal R, Burrin JM, Philpott MP. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochem Biophys Res Comm. 2011;404(1):62–67. doi: 10.1016/j.bbrc.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 129.Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski AT. Cutaneous hypothalamic pituitary adrenal (HPA) axis homologue - regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab. 2011;301(3):E484–93. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tiganescu A, Walker EA, Hardy RS, Mayes AE, Stewart PM. Localization, age- and site-dependent expression, and regulation of 11beta-hydroxysteroid dehydrogenase type 1 in skin. J Invest Dermatol. 2011;131(1):30–36. doi: 10.1038/jid.2010.257. [DOI] [PubMed] [Google Scholar]
- 131.Karagas MR, Cushing GL, Jr, Greenberg ER, Mott LA, Spencer SK, Nierenberg DW. Non-melanoma skin cancers and glucocorticoid therapy. BritJ Cancer. 2001;85(5):683–686. doi: 10.1054/bjoc.2001.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chakravarty EF, Michaud K, Wolfe F. Skin cancer, rheumatoid arthritis, and tumor necrosis factor inhibitors. J Rheumatol. 2005;32(11):2130–2135. [PubMed] [Google Scholar]
- 133.Vogt A, Hebert J, Hwang J, Lu Y, Epstein EH. Anti-rejection drug treatment increases basal cell carcinoma burden in Ptch1+/− mice. J Invest Dermatol. 2005;124(1):263–267. doi: 10.1111/j.0022-202X.2004.23573.x. [DOI] [PubMed] [Google Scholar]
- 134.Glover MT, Deeks JJ, Raftery MJ, Cunningham J, Leigh IM. Immunosuppression and risk of non-melanoma skin cancer in renal transplant recipients. Lancet. 1997;349(9049):398. doi: 10.1016/S0140-6736(97)80015-3. [DOI] [PubMed] [Google Scholar]
- 135.Gjersvik P, Hansen S, Moller B, Leivestad T, Geiran O, Simonsen S, Pfeffer P, Fauchald P. Are heart transplant recipients more likely to develop skin cancer than kidney transplant recipients? Transpl Int. 2000;13 (Suppl 1):S380–381. doi: 10.1007/s001470050366. [DOI] [PubMed] [Google Scholar]
- 136.Patel AS, Karagas MR, Perry AE, Spencer SK, Nelson HH. Gene-drug interaction at the glucocorticoid receptor increases risk of squamous cell skin cancer. J Invest Dermatol. 2007;127(8):1868–1870. doi: 10.1038/sj.jid.5700798. [DOI] [PubMed] [Google Scholar]
- 137.Slominski A, Janjetovic Z, Kim TK, Wright AC, Greese LN, Riney SJ, Nguyen MN, Tuckey RC. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012;32:3733–3742. [PMC free article] [PubMed] [Google Scholar]
- 138.Hawkins EF, Horn D, Markland FS., Jr Receptor for glucocorticoids in RPMI 3460 melanoma cells. Cancer Res. 1980;40(7):2174–2178. [PubMed] [Google Scholar]
- 139.Neifeld JP, Lippman ME. Steroid hormone receptors and melanoma. J Invest Dermatol. 1980;74(6):379–381. doi: 10.1111/1523-1747.ep12544454. [DOI] [PubMed] [Google Scholar]
- 140.Banciu M, Fens MH, Storm G, Schiffelers RM. Antitumor activity and tumor localization of liposomal glucocorticoids in B16 melanoma-bearing mice. J Contr Release. 2008;127(2):131–136. doi: 10.1016/j.jconrel.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 141.Banciu M, Metselaar JM, Schiffelers RM, Storm G. Liposomal glucocorticoids as tumor-targeted anti-angiogenic nanomedicine in B16 melanoma-bearing mice. J Steroid Biochem Mol Biol. 2008;111(1–2):101–110. doi: 10.1016/j.jsbmb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 142.Slominski A, Wortsman J, Mazurkiewicz JE, Matsuoka L, Dietrich J, Lawrence K, Gorbani A, Paus R. Detection of proopiomelanocortin-derived antigens in normal and pathologic human skin. J Lab Clin Med. 1993;122(6):658–666. [PubMed] [Google Scholar]
- 143.Kim MH, Cho D, Kim HJ, Chong SJ, Lee KH, Yu DS, Park CJ, Lee JY, Cho BK, Park HJ. Investigation of the corticotropin-releasing hormone-proopiomelanocortin axis in various skin tumours. Brit J Dermatol. 2006;155(5):910–915. doi: 10.1111/j.1365-2133.2006.07442.x. [DOI] [PubMed] [Google Scholar]
- 144.Slominski A. POMC gene expression in mouse and hamster melanoma cells. FEBS Lett. 1991;291(2):165–168. doi: 10.1016/0014-5793(91)81274-c. [DOI] [PubMed] [Google Scholar]
- 145.Nagahama M, Funasaka Y, Fernandez-Frez ML, Ohashi A, Chakraborty AK, Ueda M, Ichihashi M. Immunoreactivity of alpha-melanocyte-stimulating hormone, adrenocorticotrophic hormone and beta-endorphin in cutaneous malignant melanoma and benign melanocytic naevi. Brit J Dermatol. 1998;138(6):981–985. doi: 10.1046/j.1365-2133.1998.02263.x. [DOI] [PubMed] [Google Scholar]
- 146.Funasaka Y, Sato H, Chakraborty AK, Ohashi A, Chrousos GP, Ichihashi M. Expression of proopiomelanocortin, corticotropin-releasing hormone (CRH): and CRH receptor in melanoma cells, nevus cells, and normal human melanocytes. J Investig Dermatol Symp Proc. 1999;4(2):105–109. doi: 10.1038/sj.jidsp.5640192. [DOI] [PubMed] [Google Scholar]
- 147.Abdel-Malek Z, Scott MC, Suzuki I, Tada A, Im S, Lamoreux L, Ito S, Barsh G, Hearing VJ. The melanocortin-1 receptor is a key regulator of human cutaneous pigmentation. Pigment Cell Res. 2000;13 (Suppl 8):156–162. doi: 10.1034/j.1600-0749.13.s8.28.x. [DOI] [PubMed] [Google Scholar]
- 148.Bohm M, Luger TA, Tobin DJ, Garcia-Borron JC. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J Investig Dermatol. 2006;126(9):1966–1975. doi: 10.1038/sj.jid.5700421. [DOI] [PubMed] [Google Scholar]
- 149.Lathe R. Steroid and sterol 7-hydroxylation: Ancient pathways. Steroids. 2002;67(12):967–977. doi: 10.1016/s0039-128x(02)00044-2. [DOI] [PubMed] [Google Scholar]
- 150.Hill M, Havlikova H, Vrbikova J, Kancheva R, Kancheva L, Pouzar V, Cerny I, Starka L. The identification and simultaneous quantification of 7-hydroxylated metabolites of pregnenolone, dehydroepiandrosterone, 3beta,17beta-androstenediol, and testosterone in human serum using gas chromatography-mass spectrometry. J Steroid Biochem Mol Biol. 2005;96(2):187–200. doi: 10.1016/j.jsbmb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 151.Matsuzaki Y, Yoshida S, Honda A, Miyazaki T, Tanaka N, Takagiwa A, Fujimoto Y, Miyazaki H. Simultaneous determination of dehydroepiandrosterone and its 7-oxygenated metabolites in human serum by high-resolution gas chromatography--mass spectrometry. Steroids. 2004;69(13–14):817–824. doi: 10.1016/j.steroids.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 152.Tiemann M, Han Z, Soccio R, Bollineni J, Shefer S, Sehayek E, Breslow JL. Cholesterol feeding of mice expressing cholesterol 7alpha-hydroxylase increases bile acid pool size despite decreased enzyme activity. Proc Natl Acad Sci USA. 2004;101(7):1846–1851. doi: 10.1073/pnas.0308426100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shinkyo R, Xu L, Tallman KA, Cheng Q, Porter NA, Guengerich FP. Conversion of 7-dehydrocholesterol to 7-ketocholesterol is catalyzed by human cytochrome P450 7A1 and occurs by direct oxidation without an epoxide intermediate. J Biol Chem. 2011;286(38):33021–33028. doi: 10.1074/jbc.M111.282434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Starck L, Lövgren-Sandblom A, Björkhem I. Cholesterol treatment forever? The first Scandinavian trial of cholesterol supplementation in the cholesterol-synthesis defect Smith-Lemli-Opitz syndrome. J Intern Med. 2002;252(4):314–321. doi: 10.1046/j.1365-2796.2002.01037.x. [DOI] [PubMed] [Google Scholar]
- 155.Honda A, Salen G, Shefer S, Batta AK, Honda M, Xu G, Tint GS, Matsuzaki Y, Shoda J, Tanaka N. Bile acid synthesis in the Smith-Lemli-Opitz syndrome: effects of dehydrocholesterols on cholesterol 7alpha-hydroxylase and 27-hydroxylase activities in rat liver. J Lipid Res. 1999;40(8):1520–1528. [PubMed] [Google Scholar]
- 156.Larsson H, Bottiger Y, Iuliano L, Diczfalusy U. In vivo interconversion of 7beta-hydroxycholesterol and 7-ketocholesterol, potential surrogate markers for oxidative stress. Free Radical Biol Med. 2007;43(5):695–701. doi: 10.1016/j.freeradbiomed.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 157.Pettersson H, Lundqvist J, Oliw E, Norlin M. CYP7B1-mediated metabolism of 5alpha-androstane-3alpha,17beta-diol (3alpha-Adiol): a novel pathway for potential regulation of the cellular levels of androgens and neurosteroids. Biochim Biophys Acta. 2009;1791(12):1206–1215. doi: 10.1016/j.bbalip.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 158.Trap C, Nato F, Chalbot S, Kim SB, Lafaye P, Morfin R. Immunohistochemical detection of the human cytochrome P4507B1: Production of a monoclonal antibody after cDNA immunization. J Neuroimmunol. 2005;159(1–2):41–47. doi: 10.1016/j.jneuroim.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 159.Tang W, Pettersson H, Norlin M. Involvement of the PI3K/Akt pathway in estrogen-mediated regulation of human CYP7B1: identification of CYP7B1 as a novel target for PI3K/Akt and MAPK signalling. J Steroid Biochem Mol Biol. 2008;112(1–3):63–73. doi: 10.1016/j.jsbmb.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 160.Pettersson H, Holmberg L, Axelson M, Norlin M. CYP7B1-mediated metabolism of dehydroepiandrosterone and 5alpha-androstane-3beta,17beta-diol--potential role(s) for estrogen signaling. FEBS J. 2008;275(8):1778–1789. doi: 10.1111/j.1742-4658.2008.06336.x. [DOI] [PubMed] [Google Scholar]
- 161.Hennebert O, Montes M, Favre-Reguillon A, Chermette H, Ferroud C, Morfin R. Epimerase activity of the human 11beta-hydroxysteroid dehydrogenase type 1 on 7-hydroxylated C19-steroids. J Steroid Biochem Mol Biol. 2009;114(1–2):57–63. doi: 10.1016/j.jsbmb.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 162.Muller C, Pompon D, Urban P, Morfin R. Inter-conversion of 7alpha- and 7beta-hydroxy-dehydroepiandrosterone by the human 11beta-hydroxysteroid dehydrogenase type 1. J Steroid Biochem Mol Biol. 2006;99(4–5):215–222. doi: 10.1016/j.jsbmb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 163.Schweizer RA, Zurcher M, Balazs Z, Dick B, Odermatt A. Rapid hepatic metabolism of 7-ketocholesterol by 11beta-hydroxysteroid dehydrogenase type 1: Species-specific differences between the rat, human, and hamster enzyme. J Biol Chem. 2004;279(18):18415–18424. doi: 10.1074/jbc.M313615200. [DOI] [PubMed] [Google Scholar]
- 164.Nashev LG, Chandsawangbhuwana C, Balazs Z, Atanasov AG, Dick B, Frey FJ, Baker ME, Odermatt A. Hexose-6-phosphate dehydrogenase modulates 11beta-hydroxysteroid dehydrogenase type 1-dependent metabolism of 7-keto- and 7beta-hydroxy-neurosteroids. PloS one. 2007;2(6):e561. doi: 10.1371/journal.pone.0000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jellinck PH, Croft G, McEwen BS, Gottfried-Blackmore A, Jones G, Byford V, Bulloch K. Metabolism of dehydro- epiandrosterone by rodent brain cell lines: Relationship between 7-hydroxylation and aromatization. J Steroid Biochem Mol Biol. 2005;93(1):81–86. doi: 10.1016/j.jsbmb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 166.Chalbot S, Morfin R. Neurosteroids: metabolism in human intestine microsomes. Steroids. 2005;70(4):319–326. doi: 10.1016/j.steroids.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 167.Li A, Bigelow JC. The 7-hydroxylation of dehydroepiandrosterone in rat brain. Steroids. 2010;75(6):404–410. doi: 10.1016/j.steroids.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 168.Hampl R, Sulcova J, Bilek R, Hill M. How short-term transdermal treatment of men with 7-oxo-dehydroepiandrosterone influence thyroid function. Physiol Res / Acad Scientiarum Bohemoslovaca. 2006;55(1):49–54. doi: 10.33549/physiolres.930759. [DOI] [PubMed] [Google Scholar]
- 169.Tang W, Eggertsen G, Chiang JY, Norlin M. Estrogen-mediated regulation of CYP7B1: A possible role for controlling DHEA levels in human tissues. J Steroid Biochem Mol Biol. 2006;100(1–3):42–51. doi: 10.1016/j.jsbmb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 170.Tang W, Norlin M. Regulation of steroid hydroxylase CYP7B1 by androgens and estrogens in prostate cancer LNCaP cells. Biochem Biophys Res Comm. 2006;344(2):540–546. doi: 10.1016/j.bbrc.2006.03.175. [DOI] [PubMed] [Google Scholar]
- 171.Morfin R, Starka L. Neurosteroid 7-hydroxylation products in the brain. Int Rev Neurobiol. 2001;46:79–95. doi: 10.1016/s0074-7742(01)46059-4. [DOI] [PubMed] [Google Scholar]
- 172.Sterzl I, Hampl R, Hill M, Hrda P, Matucha P. Immuno- modulatory cytokines in human seminal plasma correlate with immunomodulatory steroids. Steroids. 2003;68(9):725–731. doi: 10.1016/s0039-128x(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 173.Morfin R, Courchay G. Pregnenolone and dehydroepiandrosterone as precursors of native 7-hydroxylated metabolites which increase the immune response in mice. J Steroid Biochem Mol Biol. 1994;50(1–2):91–100. doi: 10.1016/0960-0760(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 174.Auci DL, Reading CL, Frincke JM. 7-Hydroxy androstene steroids and a novel synthetic analogue with reduced side effects as a potential agent to treat autoimmune diseases. Autoimmu Rev. 2009;8(5):369–372. doi: 10.1016/j.autrev.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 175.Matsunaga M, Ukena K, Baulieu EE, Tsutsui K. 7alpha-Hydroxypregnenolone acts as a neuronal activator to stimulate locomotor activity of breeding newts by means of the dopaminergic system. Proc Natl Acad Sci USA. 2004;101(49):17282–17287. doi: 10.1073/pnas.0407176101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Roussi S, Winter A, Gosse F, Werner D, Zhang X, Marchioni E, Geoffroy P, Miesch M, Raul F. Different apoptotic mechanisms are involved in the antiproliferative effects of 7beta-hydroxysitosterol and 7beta-hydroxycholesterol in human colon cancer cells. Cell Death Differ. 2005;12(2):128–135. doi: 10.1038/sj.cdd.4401530. [DOI] [PubMed] [Google Scholar]
- 177.Wang Y, Kumar N, Solt LA, Richardson TI, Helvering LM, Crumbley C, Garcia-Ordonez RD, Stayrook KR, Zhang X, Novick S, Chalmers MJ, Griffin PR, Burris TP. Modulation of retinoic acid receptor-related orphan receptor alpha and gamma activity by 7-oxygenated sterol ligands. J Biol Chem. 2010;285(7):5013–5025. doi: 10.1074/jbc.M109.080614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fischer TW, Zmijewski MA, Zbytek B, Sweatman TW, Slominski RM, Wortsman J, Slominski A. Oncostatic effects of the indole melatonin and expression of its cytosolic and nuclear receptors in cultured human melanoma cell lines. Int J Oncol. 2006;29(3):665–672. doi: 10.3892/ijo.29.3.665. [DOI] [PubMed] [Google Scholar]
- 179.Kobayashi H, Kromminga A, Dunlop TW, Tychsen B, Conrad F, Suzuki N, Memezawa A, Bettermann A, Aiba S, Carlberg C, Paus R. A role of melatonin in neuroectodermal-mesodermal interactions: The hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB J. 2005;19(12):1710–1712. doi: 10.1096/fj.04-2293fje. [DOI] [PubMed] [Google Scholar]
- 180.Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nuc Receptor Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88(2):296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 182.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 183.Zhu J, DeLuca HF. Vitamin D 25-hydroxylase - Four decades of searching, are we there yet? Arch Biochem Biophys. 2012;523(1):30–36. doi: 10.1016/j.abb.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 184.Gupta RP, He YA, Patrick KS, Halpert JR, Bell NH. CYP3A4 is a vitamin D-24- and 25-hydroxylase: analysis of structure function by site-directed mutagenesis. J Clin Endocrinol Metab. 2005;90(2):1210–1219. doi: 10.1210/jc.2004-0966. [DOI] [PubMed] [Google Scholar]
- 185.Tieu EW, Li W, Chen J, Baldisseri DM, Slominski AT, Tuckey RC. Metabolism of cholesterol, vitamin D3 and 20-hydroxyvitamin D3 incorporated into phospholipid vesicles by human CYP27A1. J Steroid Biochem Mol Biol. 2012;129(3–5):163–171. doi: 10.1016/j.jsbmb.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sakaki T, Sugimoto H, Hayashi K, Yasuda K, Munetsuna E, Kamakura M, Ikushiro S, Shiro Y. Bioconversion of vitamin D to its active form by bacterial or mammalian cytochrome P450. Biochim Biophys Acta. 2011;1814(1):249–256. doi: 10.1016/j.bbapap.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 187.Bikle DD. Vitamin D: An ancient hormone. Exp Dermatol. 2011;20(1):7–13. doi: 10.1111/j.1600-0625.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 188.Holick MF. Sunlight, UV-radiation, vitamin D and skin cancer: How much sunlight do we need? Adv Exp Med Biol. 2008;624:1–15. doi: 10.1007/978-0-387-77574-6_1. [DOI] [PubMed] [Google Scholar]
- 189.Schuessler M, Astecker N, Herzig G, Vorisek G, Schuster I. Skin is an autonomous organ in synthesis, two-step activation and degradation of vitamin D(3): CYP27 in epidermis completes the set of essential vitamin D(3)-hydroxylases. Steroids. 2001;66(3–5):399–408. doi: 10.1016/s0039-128x(00)00229-4. [DOI] [PubMed] [Google Scholar]
- 190.Lehmann B, Genehr T, Knuschke P, Pietzsch J, Meurer M. UVB-induced conversion of 7-dehydrocholesterol to 1alpha,25-dihydroxyvitamin D3 in an in vitro human skin equivalent model. J Invest Dermatol. 2001;117(5):1179–1185. doi: 10.1046/j.0022-202x.2001.01538.x. [DOI] [PubMed] [Google Scholar]
- 191.Lehmann B, Tiebel O, Meurer M. Expression of vitamin D3 25-hydroxylase (CYP27) mRNA after induction by vitamin D3 or UVB radiation in keratinocytes of human skin equivalents--a preliminary study. Arch Dermatol Res. 1999;291(9):507–510. doi: 10.1007/s004030050445. [DOI] [PubMed] [Google Scholar]
- 192.Krämer C, Seltmann H, Seifert M, Tilgen W, Zouboulis CC, Reichrath J. Characterization of the vitamin D endocrine system in human sebocytes in vitro. J Steroid Biochem Mol Biol. 2009;113(1–2):9–16. doi: 10.1016/j.jsbmb.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 193.Bikle DD, Nemanic MK, Gee E, Elias P. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J Clin Invest. 1986;78(2):557–566. doi: 10.1172/JCI112609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pillai S, Bikle DD, Elias PM. 1,25-Dihydroxyvitamin D production and receptor binding in human keratinocytes varies with differentiation. J Biol Chem. 1988;263(11):5390–5395. [PubMed] [Google Scholar]
- 195.Vanhooke JL, Prahl JM, Kimmel-Jehan C, Mendelsohn M, Danielson EW, Healy KD, DeLuca HF. CYP27B1 null mice with LacZreporter gene display no 25-hydroxyvitamin D3-1alpha-hydroxylase promoter activity in the skin. Proc Nat Acad Sci USA. 2006;103(1):75–80. doi: 10.1073/pnas.0509734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ellfolk M, Norlin M, Gyllensten K, Wikvall K. Regulation of human vitamin D(3) 25-hydroxylases in dermal fibroblasts and prostate cancer LNCaP cells. Mol Pharmacol. 2009;75(6):1392–1399. doi: 10.1124/mol.108.053660. [DOI] [PubMed] [Google Scholar]
- 197.Seifert M, Tilgen W, Reichrath J. Expression of 25-hydroxyvitamin D-1alpha-hydroxylase (1alphaOHase, CYP27B1) splice variants in HaCaT keratinocytes and other skin cells: Modulation by culture conditions and UV-B treatment in vitro. Anticancer Res. 2009;29(9):3659–3667. [PubMed] [Google Scholar]
- 198.Mitschele T, Diesel B, Friedrich M, Meineke V, Maas RM, Gärtner BC, Kamradt J, Meese E, Tilgen W, Reichrath J. Analysis of the vitamin D system in basal cell carcinomas (BCCs) Lab Invest. 2004;84(6):693–702. doi: 10.1038/labinvest.3700096. [DOI] [PubMed] [Google Scholar]
- 199.Reichrath J, Rafi L, Rech M, Mitschele T, Meineke V, Gärtner BC, Tilgen W, Holick MF. Analysis of the vitamin D system in cutaneous squamous cell carcinomas. J Cutan Pathol. 2004;31(3):224–231. doi: 10.1111/j.0303-6987.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 200.Reichrath J, Rech M, Moeini M, Meese E, Tilgen W, Seifert M. In vitro comparison of the vitamin D endocrine system in 1,25(OH)2D3-responsive and -resistant melanoma cells. Cancer Biol Ther. 2007;6(1):48–55. doi: 10.4161/cbt.6.1.3493. [DOI] [PubMed] [Google Scholar]
- 201.Seifert M, Rech M, Meineke V, Tilgen W, Reichrath J. Differential biological effects of 1,25-dihydroxyVitamin D3 on melanoma cell lines in vitro. J Steroid Biochem Mol Biol. 2004;89–90:375–379. doi: 10.1016/j.jsbmb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 202.Diesel B, Seifert M, Radermacher J, Fischer U, Tilgen W, Reichrath J, Meese E. Towards a complete picture of splice variants of the gene for 25-hydroxyvitamin D31alpha-hydroxylase in brain and skin cancer. J Steroid Biochem Mol Biol. 2004;89–90(1–5):527–532. doi: 10.1016/j.jsbmb.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 203.Brozyna AA, Jozwicki W, Janjetovic Z, Slominski AT. Expression of vitamin D activating enzyme 1-alpha-hydroxylase (CYP27B1) decreases during melanoma progression. Hum Pathol. 2013;44(3):374–87. doi: 10.1016/j.humpath.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bikle DD. The vitamin D receptor: a tumor suppressor in skin. Discov Med. 2011;11(56):7–17. [PMC free article] [PubMed] [Google Scholar]
- 205.Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010;9(12):941–955. doi: 10.1038/nrd3318. [DOI] [PubMed] [Google Scholar]
- 206.Teichert AE, Elalieh H, Elias PM, Welsh J, Bikle DD. Overexpression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. J Invest Dermatol. 2011;131(11):2289–2297. doi: 10.1038/jid.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Szyszka P, Zmijewski MA, Slominski AT. New vitamin D analogs as potential therapeutics in melanoma. Exp Rev Anticancer Ther. 2012;12(5):585–599. doi: 10.1586/era.12.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Halsall JA, Osborne JE, Epstein MP, Pringle JH, Hutchinson PE. The unfavorable effect of the A allele of the vitamin D receptor promoter polymorphism A-1012G has different mechanisms related to susceptibility and outcome of malignant melanoma. Dermatoendocrinol. 2009;1(1):54–57. doi: 10.4161/derm.1.1.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Halsall JA, Osborne JE, Potter L, Pringle JH, Hutchinson PE. A novel polymorphism in the 1A promoter region of the vitamin D receptor is associated with altered susceptibilty and prognosis in malignant melanoma. Brit J Cancer. 2004;91(4):765–770. doi: 10.1038/sj.bjc.6602006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Santonocito C, Capizzi R, Concolino P, Lavieri MM, Paradisi A, Gentileschi S, Torti E, Rutella S, Rocchetti S, Carlo AD, Stasio ED, Ameglio F, Zuppi C, Capoluongo E. Association between cutaneous melanoma, Breslow thickness and vitamin D receptor BsmI polymorphism. BritJ Dermatol. 2007;156(2):277–282. doi: 10.1111/j.1365-2133.2006.07620.x. [DOI] [PubMed] [Google Scholar]
- 211.Li C, Liu Z, Zhang Z, Strom SS, Gershenwald JE, Prieto VG, Lee JE, Ross MI, Mansfield PF, Cormier JN, Duvic M, Grimm EA, Wei Q. Genetic variants of the vitamin D receptor gene alter risk of cutaneous melanoma. J Invest Dermatol. 2007;127(2):276–280. doi: 10.1038/sj.jid.5700544. [DOI] [PubMed] [Google Scholar]
- 212.Lesiak A, Norval M, Wodz-Naskiewicz K, Pawliczak R, Rogowski-Tylman M, Sysa-Jedrzejowska A, Sobjanek M, Wlodarkiewicz A, Narbutt J. An enhanced risk of basal cell carcinoma is associated with particular polymorphisms in the VDR and MTHFR genes. Exp Dermatol. 2011;20(10):800–804. doi: 10.1111/j.1600-0625.2011.01328.x. [DOI] [PubMed] [Google Scholar]
- 213.Schäfer A, Emmert S, Kruppa J, Schubert S, Tzvetkov M, Mössner R, Reich K, Berking C, Volkenandt M, Pföhler C, Schön MP, Vogt T, König IR, Reichrath J. No association of vitamin D metabolism-related polymorphisms and melanoma risk as well as melanoma prognosis: a case-control study. Arch Dermatol Res. 2012;304(5):353–361. doi: 10.1007/s00403-012-1243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zeljic K, Supic G, Stamenkovic Radak M, Jovic N, Kozomara R, Magic Z. Vitamin D receptor, CYP27B1 and CYP24A1 genes polymorphisms association with oral cancer risk and survival. J Oral Pathol Med. 2012 doi: 10.1111/j.1600-0714.2012.01164.x. [DOI] [PubMed] [Google Scholar]
- 215.Pillai DK, Iqbal SF, Benton AS, Lerner J, Wiles A, Foerster M, Ozedirne T, Holbrook HP, Payne PW, Gordish-Dressman H, Teach SJ, Freishtat RJ. Associations between genetic variants in vitamin D metabolism and asthma characteristics in young African Americans: a pilot study. J Investig Med. 2011;59(6):938–946. doi: 10.231/JIM.0b013e318220df41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sundqvist E, Bäärnhielm M, Alfredsson L, Hillert J, Olsson T, Kockum I. Confirmation of association between multiple sclerosis and CYP27B1. Eur J Hum Genet. 2010;18(12):1349–1352. doi: 10.1038/ejhg.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shui IM, Mucci LA, Kraft P, Tamimi RM, Lindstrom S, Penney KL, Nimptsch K, Hollis BW, Dupre N, Platz EA, Stampfer MJ, Giovannucci E. Vitamin D-related genetic variation, plasma vitamin D, and risk of lethal prostate cancer: A prospective nested case-control study. J Natl Cancer Inst. 2012;104(9):690–699. doi: 10.1093/jnci/djs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cross HS, Nittke T, Kallay E. Colonic vitamin D metabolism: Implications for the pathogenesis of inflammatory bowel disease and colorectal cancer. Mol Cell Endocrinol. 2011;347(1–2):70–79. doi: 10.1016/j.mce.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 219.Dong LM, Ulrich CM, Hsu L, Duggan DJ, Benitez DS, White E, Slattery ML, Farin FM, Makar KW, Carlson CS, Caan BJ, Potter JD, Peters U. Vitamin D related genes, CYP24A1 and CYP27B1, and colon cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(9):2540–2548. doi: 10.1158/1055-9965.EPI-09-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Reichrath J, Rafi L, Rech M, Meineke V, Tilgen W, Seifert M. No evidence for amplification of 25-hydroxyvitamin D-1alpha-OHase (1alpha-OHase) or 1,25-dihydroxyvitamin D-24-OHase (24-OHase) genes in malignant melanoma (MM) J Steroid Biochem Mol Biol. 2004;89–90(1–5):163–166. doi: 10.1016/j.jsbmb.2004.03.096. [DOI] [PubMed] [Google Scholar]
- 221.Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol. 2011;347(1–2):80–89. doi: 10.1016/j.mce.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch Biochem Biophys. 2011;523(1):9–18. doi: 10.1016/j.abb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 223.Chiellini G, Rapposelli S, Zhu J, Massarelli I, Saraceno M, Bianucci AM, Plum LA, Clagett-Dame M, DeLuca HF. Synthesis and biological activities of vitamin D-like inhibitors of CYP24 hydroxylase. Steroids. 2012;77(3):212–223. doi: 10.1016/j.steroids.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Schuster I, Egger H, Herzig G, Reddy GS, Schmid JA, Schüssler M, Vorisek G. Selective inhibitors of vitamin D metabolism--new concepts and perspectives. Anticancer Res. 2006;26(4A):2653–2668. [PubMed] [Google Scholar]
- 225.Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29(12):664–673. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 226.Zhou S, Yung Chan S, Cher Goh B, Chan E, Duan W, Huang M, McLeod HL. Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin Pharmacokinet. 2005;44(3):279–304. doi: 10.2165/00003088-200544030-00005. [DOI] [PubMed] [Google Scholar]
- 227.Zmijewski MA, Li W, Chen J, Kim TK, Zjawiony JK, Sweatman TW, Miller DD, Slominski AT. Synthesis and photochemical transformation of 3 beta,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids. 2011;76(1–2):193–203. doi: 10.1016/j.steroids.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Slominski A, Kim T, Zmijewski MA, Janjetovic Z, Li W, Chen J, Kusniatsova EI, Semak I, Postlethwaite R, Miller D, Zjawiony J, Tuckey RC. Novel vitamin D photoproducts and their precursors in the skin. Dermato-Endocrinol. 2013;5(1) doi: 10.4161/derm.23938. in press, http://dx.doi.org/10.4161/derm.23938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, Slominski AT. Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3 beta, 17 alpha, 20-triol and their bioactivity in melanoma cells. Steroids. 2009;74(2):218–228. doi: 10.1016/j.steroids.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Komagata S, Nakajima M, Takagi S, Mohri T, Taniya T, Yokoi T. Human CYP24 catalyzing the inactivation of calcitriol is post-transcriptionally regulated by miR-125b. Mol Pharmacol. 2009;76(4):702–709. doi: 10.1124/mol.109.056986. [DOI] [PubMed] [Google Scholar]
- 231.Essa S, Reichrath S, Mahlknecht U, Montenarh M, Vogt T, Reichrath J. Signature of VDR miRNAs and epigenetic modulation of vitamin D signaling in melanoma cell lines. Anticancer Res. 2012;32(1):383–389. [PubMed] [Google Scholar]
- 232.Liu PT, Wheelwright M, Teles R, Komisopoulou E, Edfeldt K, Ferguson B, Mehta MD, Vazirnia A, Rea TH, Sarno EN, Graeber TG, Modlin RL. MicroRNA-21 targets the vitamin D-dependent antimicrobial pathway in leprosy. Nat Med. 2012;18(2):267–273. doi: 10.1038/nm.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yang CH, Yue J, Pfeffer SR, Handorf CR, Pfeffer LM. MicroRNA miR-21 regulates the metastatic behavior of B16 melanoma cells. J Biolog Chem. 2011;286(45):39172–39178. doi: 10.1074/jbc.M111.285098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Satzger I, Mattern A, Kuettler U, Weinspach D, Niebuhr M, Kapp A, Gutzmer R. microRNA-21 is upregulated in malignant melanoma and influences apoptosis of melanocytic cells. Exp Dermatol. 2012;21(7):509–514. doi: 10.1111/j.1600-0625.2012.01510.x. [DOI] [PubMed] [Google Scholar]
- 235.Tang EK, Li W, Janjetovic Z, Nguyen MN, Wang Z, Slominski A, Tuckey RC. Purified mouse CYP27B1 can hydroxylate 20,23-dihydroxyvitamin D3, producing 1alpha,20,23-trihydroxyvitamin D3, which has altered biological activity. Drug Metab Disposit. 2010;38(9):1553–1559. doi: 10.1124/dmd.110.034389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tang EK, Voo KJ, Nguyen MN, Tuckey RC. Metabolism of substrates incorporated into phospholipid vesicles by mouse 25-hydroxyvitamin D3 1alpha-hydroxylase (CYP27B1) J Steroid Biochem Mol Biol. 2010;119(3–5):171–179. doi: 10.1016/j.jsbmb.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 237.Tuckey RC, Janjetovic Z, Li W, Nguyen MN, Zmijewski MA, Zjawiony J, Slominski A. Metabolism of 1alpha-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1alpha,20-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 2008;112:213–219. doi: 10.1016/j.jsbmb.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EKY, Nguyen MN, Benson HAE, Korik E, Janjetovic Z, Chen J, Yates CR, Postlethwaite A, Li W, Tuckey RC. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26:3901–3915. doi: 10.1096/fj.12-208975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li W, Chen J, Janjetovic Z, Kim TK, Sweatman T, Lu Y, Zjawiony J, Tuckey RC, Miller D, Slominski A. Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity. Steroids. 2010;75(12):926–935. doi: 10.1016/j.steroids.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lu Y, Chen J, Janjetovic Z, Michaels P, Tang EK, Wang J, Tuckey RC, Slominski AT, Li W, Miller DD. Design, synthesis, and biological action of 20R-hydroxyvitamin D3. J Med Chem. 2012;55(7):3573–3577. doi: 10.1021/jm201478e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Slominski AT, Kim TK, Chen J, Nguyen MN, Li W, Yates CR, Sweatman T, Janjetovic Z, Tuckey RC. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int J Biochem Cell Biol. 2012;44(11):2003–2018. doi: 10.1016/j.biocel.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, Slominski AT. Synthesis and photo-conversion of androsta- and pregna-5,7-dienes to vitamin D3-like derivatives. Photochem Photobiol Sci. 2008;7(12):1570–1576. doi: 10.1039/b809005j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kim TK, Chen J, Li W, Zjawiony J, Miller D, Janjetovic Z, Tuckey RC, Slominski A. A new steroidal 5,7-diene derivative, 3beta-hydroxyandrosta-5,7-diene-17beta-carboxylic acid, shows potent anti-proliferative activity. Steroids. 2010;75(3):230–239. doi: 10.1016/j.steroids.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zbytek B, Janjetovic Z, Tuckey RC, Zmijewski MA, Sweatman TW, Jones E, Nguyen MN, Slominski AT. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J Invest Dermatol. 2008;128(9):2271–2280. doi: 10.1038/jid.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, Slominski AT. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PloS one. 2009;4(6):e5988. doi: 10.1371/journal.pone.0005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, Slominski AT. Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3beta, 17alpha, 20-triol and their bioactivity in melanoma cells. Steroids. 2009;74(2):218–228. doi: 10.1016/j.steroids.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM, Jr, Slominski AT. 20, 23-dihydroxyvitamin D3, novel P450scc product stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J Cell Physiol. 2010;223(1):36–48. doi: 10.1002/jcp.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, Sweatman T, Li W, Zjawiony J, Miller D, Chen TC, Lozanski G, Holick MF. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PloS one. 2010;5(3):e9907. doi: 10.1371/journal.pone.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Janjetovic Z, Brozyna AA, Tuckey RC, Kim TK, Nguyen MN, Jozwicki W, Pfeffer SR, Pfeffer LM, Slominski AT. High basal NF-kappaB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Brit J Cancer. 2011;105(12):1874–1884. doi: 10.1038/bjc.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Slominski AT, Kim TK, Janjetovic Z, Tuckey RC, Bieniek R, Yue J, Li W, Chen J, Nguyen MN, Tang EK, Miller D, Chen TC, Holick M. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol. 2011;300(3):C526–541. doi: 10.1152/ajpcell.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Slominski AT, Li W, Bhattacharya SK, Smith RA, Johnson PL, Chen J, Nelson KE, Tuckey RC, Miller D, Jiao Y, Gu W, Postlethwaite AE. Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity. J Invest Dermatol. 2011;131(5):1167–1169. doi: 10.1038/jid.2010.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zmijewski MA, Li W, Chen J, Kim TK, Zjawiony JK, Sweatman TW, Miller DD, Slominski AT. Synthesis and photochemical transformation of 3beta,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids. 2011;76(1–2):193–203. doi: 10.1016/j.steroids.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Wang J, Slominski A, Tuckey RC, Janjetovic Z, Kulkarni A, Chen J, Postlethwaite AE, Miller D, Li W. 20-hydroxyvitamin D(3) inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012;32(3):739–746. [PMC free article] [PubMed] [Google Scholar]
- 254.Kim TK, Wang J, Janjetovic Z, Chen J, Tuckey RC, Nguyen MN, Tang EK, Miller D, Li W, Slominski AT. Correlation between secosteroid-induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength. Mol Cell Endocrinol. 2012;361(1–2):143–152. doi: 10.1016/j.mce.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Slominski A, Janjetovic Z, Tuckey RC, Nguyen MN, Bhattacharya KG, Wang J, Li W, Jiao Y, Gu W, Brown M, Postlethwaite AE. 20S-Hydroxyvitamin D3, Noncalcemic Product of CYP11A1 Action on Vitamin D3, Exhibits Potent Antifibrogenic Activity in vivo. J Clin Endocrinol Metab. 2013;98(2):E298–303. doi: 10.1210/jc.2012-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) Nomenclature of vitamin D. Recommendations 1981. Eur J Biochem. 1982;124(2):223–227. [PubMed] [Google Scholar]
- 257.Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J. 2001;15(14):2754–2756. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]