Abstract
Objective
Detrimental effects of lean muscle loss have been hypothesized to explain J-shaped relationships of body mass index (BMI) with cardiovascular disease (CVD), yet associations of muscle mass with CVD are largely unknown. We hypothesized that low abdominal lean muscle area would be associated with greater calcified atherosclerosis, independent of other CVD risk factors.
Materials/Methods
We investigated 1020 participants from the Multi-Ethnic Study of Atherosclerosis who were free of clinical CVD. Computed tomography (CT) scans at the 4th and 5th lumbar disk space were used to estimate abdominal lean muscle area. Chest and abdominal CT scans were used to assess coronary artery calcification(CAC), thoracic aortic calcification (TAC), and abdominal aortic calcification (AAC).
Results
The mean age was 64±10 years, 48% were female, and mean BMI was 28±5 kg/m2. In models adjusted for demographics, physical activity, caloric intake, and traditional CVD risk factors, there was no inverse association of abdominal muscle mass with CAC(Prevalence Ratio [PR] 1.02 [95% CI 0.95,1.10]), TAC (PR 1.13 [95%CI 0.92, 1.39]) or AAC (PR 0.99 [95%CI 0.94, 1.04]) prevalence. Similarly, there was no significant inverse relationship between abdominal lean muscle area and CAC, TAC, and AAC severity.
Conclusion
In community-living individuals without clinical CVD, greater abdominal lean muscle area is not associated with less calcified atherosclerosis.
Keywords: Cardiovascular Disease, atherosclerosis, lean muscle
Introduction
Greater adiposity is a well-established cardiovascular disease (CVD) risk factor. However, the relation of body mass index (BMI) with CVD is complex, and many prior studies have reported the association to be J-shaped.[1–3] While high BMI is associated with higher CVD risk, those with BMI <18.5 are also at higher risk.[1] The mechanisms linking low BMI with CVD risk are unclear. Because BMI does not discriminate between mass from adiposity or muscle, and because of the well established atherosclerotic properties of adiposity, some have hypothesized that low muscle mass may account for the association of low BMI with CVD.[4]
Few studies have examined the associations between muscle mass and CVD.[5] We recently reported that lower urine creatinine excretion rate, an indirect measure of muscle mass, was strongly associated with all-cause mortality in patients with prevalent clinical CVD, independent of body composition (BMI), kidney function, and traditional CVD risk factors.[6] However, our prior study did not directly assess the relationship of muscle mass with CVD burden, but rather all-cause mortality; an association that might be driven by non-CVD pathways. Moreover, our prior study was carried out in individuals with prevalent CVD, so whether or not similar associations may extend to individuals in the general population who are as yet free of clinical CVD is uncertain.
The objective of the present analysis was to evaluate the association of abdominal lean muscle area with the prevalence and extent of calcified atherosclerosis in several vascular beds among a well characterized community-living population without clinically apparent CVD. We hypothesized that a lesser amount of abdominal lean muscle area would be associated with prevalence and severity of calcified atherosclerosis in the coronary, thoracic aortic, and abdominal aortic beds.
METHODS
Study Participants
Details regarding objectives, design, and recruitment of the Multi-Ethnic Study of Atherosclerosis (MESA) have been published previously.[7] Briefly, eligible participants were aged 45–84 years, and self-reported African-American, Chinese, Non-Hispanic White, or Hispanic race/ethnicity. Exclusion criteria included clinically recognized CVD (defined as history of heart attack, angina, CVD procedures, heart failure, cerebrovascular disease), active treatment for non-skin cancers, or pregnancy. In total, 6,814 participants enrolled from 6 centers across the US and underwent a baseline study visit (exam 1; 2000–2002) that included a chest computer tomography (CT) scan. Participants returned for 2 sequential follow-up visits approximately 18 months and 36 months after baseline, and were randomly assigned to undergo follow-up chest CT at one or the other of these follow-up visit. Participants were invited to participate in an ancillary study that extended the CT scan to include the abdominal region, concurrent with the randomly assigned timing of their follow-up chest CT. 1,974 individuals participated in the abdominal CT sub-study. Among these, abdominal scans from a subset of 1,215 individuals selected at random had been analyzed for abdominal body composition at the time we initiated this study. Participants who were missing covariates (n=195) were excluded from the analyses, resulting in a final analytic sample of 1,020 participants for this analysis.
Abdominal Muscle Area
Muscle area in the abdomen was determined using Medical Imaging Processing Analysis and Visualization (MIPAV) software, version 4.1.2. For each participant, one image slice at the L4/L5 disc space was analyzed to determine the muscle area of the bilateral psoas, oblique, rectus abdominus, and paraspinus muscles. Area within the fascial planes for each muscle group were categorized into one of 3 tissue types based on Hounsfield Units (HU); 0 to 100 were considered lean muscle, −190 to −30 were considered fat, and intervening HU were considered mixed connective tissue such as fascia. Abdominal lean muscle area was calculated by multiplying the number of pixels of the appropriate HU range within the fascial plane of the given muscle by the pixel area, and then summed across the 4 muscles of interest. Similar calculations were made for intramuscular fat and mixed connective tissue.
Abdominal, Coronary, and Thoracic Calcification
To measure abdominal artery calcification (AAC), electron-beam CT scanners were used at Northwestern University and University of California, Los Angeles (Imatron C-150). Multi-detector CT mode scanners were used at the remaining 3 field centers (Columbia University, Wake Forest University, and University of Minnesota field center). Images were reconstructed in a 35-cm field of view with 5-mm slice thickness. All scan scores were brightness adjusted with a standard phantom.
Non-contrast CT images were analyzed centrally using a standard protocol by the MESA CT Reading Center. Calcium in the wall of the distal abdominal aorta in the 8-cm segment proximal to the aortic bifurcation was measured. Atherosclerotic calcification was defined as a plaque of ≥1mm2 with a density of >130 Hounsfield units and quantified using the Agatston scoring method. Details regarding protocol, acquisition, and interpretation of AAC scans, coronary artery calcification (CAC), and thoracic aortic calcification (TAC) in the MESA study have been reported previously. [8, 9],[10, 11]
At the same scanning examination, CAC was measured using either electron-beam tomography (3 sites) or multi-detector CT (2 sites). Participants were scanned twice consecutively, and each scan was read by a trained physician-reader independently at a centralized reading center (Harbor-University of California, Los Angeles, Medical Center/ Los Angeles Biomedical Research Institute, Torrance, California). The results from the 2 scans were averaged to provide a more accurate point estimate of the amount of calcium present.[11] Further details of the scanning methodology have been published previously.[8]
An ancillary study was performed to retrospectively measure TAC on the chest scans obtained for the purposes of CAC scoring. TAC was measured from the lower edge of the pulmonary artery to the cardiac apex and was quantified by using the same methods described for CAC previously.
Other Measurements
Standardized questionnaires were used to obtain data on demographics, tobacco, and alcohol use. Participants brought their medications to the study appointments which were recorded by study personnel using standardized protocols. Weight was measured with participants wearing light clothing and no shoes with a digital scale and height was measured using stadiometers. BMI was calculated (weight [kg] / height [meters] squared). Total dietary kilocalories (kcals) per day were estimated using a 66-item interviewer-administered semi-quantitative food-frequency questionnaire (FFQ). The questionnaire was a modified version of the 61-item instrument developed by Willett et al[12] that accommodated food preferences of Hispanic and Chinese participants. Physical activity (METS-min/week) was estimated using the MESA Typical Week Physical Activity Survey (TWPAS), adapted from the Cross-Cultural Activity Participation Study.[13, 14] After 5 minutes of rest, seated blood pressure was measured 3 times using a Dinamap automated oscillometric sphygmomanometer (model Pro 100; Critikon, Tampa, Florida); the last 2 measurements were averaged and used in analysis. Hypertension was defined as use of antihypertensive medications, systolic blood pressure ≥140, or diastolic blood pressure ≥90 mm Hg. Fasting (8 hour) blood samples were drawn and measured of glucose and lipids using standard clinical chemistry analyzers.[15] Participants were considered to have diabetes if they used hypoglycemic medications or insulin, or if their fasting blood glucose was ≥ 126 mg/dL.
Statistical Analysis
Participants were stratified by gender and then categorized into tertiles of abdominal lean muscle area. We evaluated the distribution of major demographics and other covariates by sex in the study sample. Skewed variables are presented by median and interquartile ranges. For the primary analysis, CAC, TAC, and AAC, scores were dichotomized as either present (score > 0) or absent. Since the prevalence of each of these was > 20% in our study sample, the outcomes may not meet the rare disease assumption, and odds ratios may overestimate estimates of the relative risks. Therefore, we calculated prevalence rate ratio (PRR) estimates using general linear models, where y=exp(βTX) with a log link and binomial distribution. When models did not converge, we used a Gaussian distribution with robust standard errors. Among the subset of persons with prevalent calcification, we natural log-transformed CAC, TAC, and AAC scores, and used linear regression to determine the association of abdominal lean muscle area with severity of calcification.
All analyses were evaluated using a similar sequence of models. An initial model adjusted for age, gender, and race/ethnicity. Model 2 added height, weight, intramuscular fat area, and mixed connective tissue area. Model 3 included model 2 variables plus smoking (never, current, former), alcohol use (yes or no), hypertension (yes or no), diabetes (yes or no), HDL, total cholesterol, CRP, physical activity and caloric intake. Last, we created interaction terms to evaluate whether the observed relationships differed by strata of age (45–54, 55–64, 65–74, 74–84yrs), sex, and BMI (<25, 25–30, >30). P values < 0.05 were considered statistically significant for all analyses including interaction terms. All analyses were conducted using STATA (Version 11.0; StataCorp, College Station, TX, USA).
RESULTS
The mean age of the 1,020 participants was 64 ± 10 years, 48% were female, and the mean BMI was 27.8 ± 4.9 kg/m2. Thirty-three percent were Caucasian, 15% were African-American, 36% were Hispanic, and 16% were Chinese. The mean abdominal lean muscle area was 99.2 ± 27.7 cm2, and differed significantly by sex (116.5 ± 24.3 cm2 in men and 80.6 ± 16.9 cm2 in women; P< 0.001). Thus, we divided participants into tertiles based on the distribution of abdominal lean muscle area within each sex. Table 1 shows the distribution of demographics, life-style factors, and body composition measures by sex-specific abdominal muscle tertiles. In both genders, compared to the lowest tertile, participants with greater abdominal muscle area were younger, more frequently African-American or Hispanic, had greater height and weight, and reported greater physical activity and caloric intake. When evaluated with correlation coefficients, abdominal lean muscle area was moderately correlated with weight (r=0.45) and more modestly correlated with BMI (r=0.17), and waist to hip ratio (r=0.18).
Table 1.
Baseline Characteristics by Sex Specific Abdominal Muscle Area Tertiles
| Abdominal Muscle Tertiles | |||
|---|---|---|---|
| I | II | III | |
| Range (cm2) | |||
| Men | < 104.7 | 104.7–126.7 | > 126.7 |
| Women | < 72.8 | 72.8–88.4 | > 88.4 |
| Age, years (± SD) | |||
| Men | 69 ± 9 | 63 ± 9 | 59 ± 8 |
| Women | 70 ± 8 | 64 ± 9 | 60 ± 9 |
| Race/Ethnicity, N(%) | |||
| Men | |||
| Caucasian | 55 (31%) | 69 (39%) | 54 (31%) |
| Chinese | 49 (28%) | 29 (16%) | 11 (6%) |
| African-American | 15 (9%) | 19 (11%) | 32 (18%) |
| Hispanic | 57 (32%) | 59 (34%) | 78 (45%) |
| Women | |||
| Caucasian | 59 (36%) | 56 (34%) | 44 (27%) |
| Chinese | 32 (19%) | 28 (17%) | 14 (9%) |
| African-American | 15 (9%) | 17 (10%) | 50 (30%) |
| Hispanic | 59 (36%) | 63 (38%) | 56 (34%) |
| Height, cm ± SD | |||
| Men | 169 ± 8 | 172 ± 7 | 173 ± 7 |
| Women | 157 ± 7 | 159 ± 7 | 160 ± 7 |
| Weight, lbs. ± SD | |||
| Men | 163 ± 30 | 179 ± 32 | 194 ± 27 |
| Women | 152 ± 35 | 156 ± 34 | 164 ± 30 |
| Diabetes, N (%) | |||
| Men | 33 (19%) | 30 (17%) | 29 (17%) |
| Women | 22 (13%) | 21 (13%) | 27 (16%) |
| Hypertension, N (%) | |||
| Men | 82 (46%) | 68 (39%) | 70 (41%) |
| Women | 92 (56%) | 79 (48%) | 71 (44%) |
| Smoking, N (%) | |||
| Men | |||
| Never | 62 (35%) | 74 (42%) | 63 (36%) |
| Former | 96 (55%) | 81 (46%) | 81 (47%) |
| Current | 17 (10%) | 20 (11%) | 29 (17%) |
| Women | |||
| Never | 111 (67%) | 105 (64%) | 100 (61%) |
| Former | 51 (31%) | 47 (29%) | 46 (28%) |
| Current | 3 (2%) | 12 (7%) | 18 (11%) |
| Total cholesterol, mg/dL ± SD | |||
| Men | 183 ± 33 | 180 ± 35 | 184 ± 37 |
| Women | 195 ± 32 | 200 ± 38 | 199 ± 34 |
| HDL cholesterol, mg/dL ± SD | |||
| Men | 49 ± 14 | 46 ± 11 | 44 ± 10 |
| Women | 57 ± 16 | 54 ± 13 | 54 ± 15 |
| C-reactive protein, mg/L* | |||
| Men | 1.1 (0.6, 2.3) | 1.1 (0.6, 2.2) | 1.3 (0.6, 2.5) |
| Women | 1.6 (0.8, 3.6) | 1.8 (0.8, 4.5) | 2.2 (0.8, 4.2) |
| Alcohol use, N (%) | |||
| Men | 101 (57%) | 113 (64%) | 100 (57%) |
| Women | 64 (39%) | 64 (39%) | 65 (40%) |
| Physical activity, Met-min/week* | |||
| Men | 735 (105, 1691) | 1050 (158, 1988) | 1050 (323, 2625) |
| Women | 630 (0, 1650) | 630 (180, 1454) | 818 (79, 2205) |
| Caloric intake, kilocalories* | |||
| Men | 1547 (1150, 2107) | 1728 (1295, 2242) | 1660 (1295, 2432) |
| Women | 1177 (861, 1595) | 1264 (925, 1713) | 1395 (1025, 1880) |
Median (interquartile range)
In age, sex, and race/ethnicity adjusted models, we observed no statistically significant associations between abdominal lean muscle area with CAC, TAC, or AAC prevalence, both when abdominal lean muscle area was evaluated by tertiles within each sex, and when evaluated as a continuous predictor variable (Table 2). Results were similar in the fully adjusted models. In addition, we observed that the relationships of abdominal lean muscle area with CAC, TAC, or AAC prevalence was similar irrespective of the quantity of abdominal fat (p-interactions all > 0.17).
Table 2.
Cross-sectional Association of Abdominal Lean Muscle Area with Coronary, Abdominal, and Thoracic Aortic Calcification Prevalence
| Tertiles in Men | Tertiles in Women | Continuous | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | I | II | III | |||
| Range (cm2) | < 104.7 | 104.7–126.7 | > 126.7 | < 72.8 | 72.8–88.4 | > 88.4 | Per SD (26.7 cm2) | |
| N | 176 | 176 | 175 | 165 | 164 | 164 | ||
| CAC Prevalence | ||||||||
| Model 1; PR (95% CI) | 1.00 (Ref) | 1.03 (0.91, 1.16) | 1.04 (0.90, 1.20) | 1.00 (Ref) | 0.99 (0.82, 1.18) | 0.94 (0.73, 1.20) | 1.06 (0.99, 1.14) | 0.05 |
| Model 2; PR (95% CI) | 1.00 (Ref) | 0.99 (0.88, 1.12) | 0.94 (0.81, 1.10) | 1.00 (Ref) | 0.98 (0.81, 1.17) | 0.92 (0.71, 1.20) | 1.05 (0.97, 1.12) | 0.25 |
| Model 3; PR (95% CI) | 1.00 (Ref) | 0.97 (0.86, 1.09) | 0.91 (0.79, 1.06) | 1.00 (Ref) | 0.91 (0.7, 1.10) | 0.89 (0.68, 1.16) | 1.02 (0.95, 1.10) | 0.53 |
| TAC Prevalence | ||||||||
| Model 1; PR (95% CI) | 1.00 (Ref) | 0.84 (0.59, 1.19) | 0.98 (0.66, 1.46) | 1.00 (Ref) | 1.07 (0.75, 1.53) | 1.16 (0.77, 1.73) | 1.02 (0.86, 1.20) | 0.83 |
| Model 2; PR (95% CI) | 1.00 (Ref) | 0.83 (0.57, 1.21) | 0.99 (0.61, 1.60) | 1.00 (Ref) | 1.14 (0.77, 1.71) | 1.37 (0.86, 2.18) | 1.09 (0.88, 1.34) | 0.42 |
| Model 3; PR (95% CI) | 1.00 (Ref) | 0.81 (0.55, 1.20) | 0.99 (0.60, 1.65) | 1.00 (Ref) | 1.11 (0.71, 1.72) | 1.41 (0.90, 2.22) | 1.13 (0.92, 1.39) | 0.25 |
| AAC Prevalence | ||||||||
| Model 1; PR (95% CI) | 1.00 (Ref) | 1.03 (0.94, 1.12) | 1.01 (0.90, 1.15) | 1.00 (Ref) | 1.10 (0.99, 1.23) | 1.03 (0.90, 1.18) | 1.04 (0.99, 1.09) | 0.13 |
| Model 2; PR (95% CI) | 1.00 (Ref) | 0.98 (0.90, 1.08) | 0.94 (0.82, 1.07) | 1.00 (Ref) | 1.08 (0.97, 1.21) | 0.98 (0.85, 1.13) | 0.99 (0.95, 1.05) | 0.93 |
| Model 3; PR (95% CI) | 1.00 (Ref) | 0.99 (0.91, 1.10) | 0.94 (0.83, 1.07) | 1.00 (Ref) | 1.02 (0.92, 1.14) | 0.91 (0.80, 1.03) | 0.99 (0.94, 1.04) | 0.62 |
Abbreviations: CAC=coronary artery calcification, TAC=thoracic aorta calcification, AAC=abdominal aortic calcification, PR=prevalence ratio from relative risk regression, CI=confidence interval, Ref=reference category.
Model 1: Adjusted for age, sex, race/ethnicity
Model 2: Adjusted for model 1 variables plus height, weight, intramuscular mixed connective tissue area, and total intramuscular fat area
Model 3: Adjusted for model 2 variables plus diabetes, hypertension, smoking, total cholesterol, HDL cholesterol, C-reactive protein, alcohol, physical activity, and caloric intake.
Next, we evaluated the association of abdominal lean muscle area with severity of CAC, AAC, and TAC among the subset of individuals with CAC, TAC, and AAC scores > 0 (n=577, 218, and 716, respectively). We observed no significant association of abdominal lean muscle area with severity of any of these calcification measures in the demographic or fully adjusted models in either men or women (Table 3). As with calcification prevalence, associations of abdominal muscle area with calcification severity was similar irrespective of abdominal fat content (p interactions all > 0.27).
Table 3.
Cross-sectional Association of Abdominal Lean Muscle Area with Severity of Coronary, Thoracic, and Abdominal Aortic Calcification
| Tertiles in Men | Tertiles in Women | Continuous | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | I | II | III | |||
| Range (cm2) | < 104.7 | 104.7–126.7 | > 126.7 | < 72.8 | 72.8–88.4 | > 88.4 | Per SD (26.7 cm2) | |
| CAC Severity* | ||||||||
| Model 1; β (95% CI) | 1.00 (Ref) | 0.79 (0.53, 1.40) | 1.35 (0.78, 2.32) | 1.00 (Ref) | 1.13 (0.68, 1.88) | 1.19 (0.66, 2.12) | 1.16 (0.94, 1.42) | 0.16 |
| Model 2; β (95% CI) | 1.00 (Ref) | 0.78 (0.47, 1.28) | 1.16 (0.64, 2.12) | 1.00 (Ref) | 1.22 (0.73, 2.05) | 1.43 (0.76, 2.72) | 1.19 (0.94, 1.49) | 0.14 |
| Model 3; β (95% CI) | 1.00 (Ref) | 0.81 (0.49, 1.34) | 1.27 (0.70, 2.32) | 1.00 (Ref) | 0.15 (−0.37, 0.67) | 1.45 (0.75, 2.80) | 1.21 (0.97, 1.54) | 0.09 |
| TAC Severity* | ||||||||
| Model 1; β (95% CI) | 1.00 (Ref) | 0.91 (0.42, 2.01) | 0.87 (0.38, 1.99) | 1.00 (Ref) | 1.13 (0.54, 2.36) | 0.73 (0.33, 1.62) | 0.89 (0.64, 1.21) | 0.44 |
| Model 2; β (95% CI) | 1.00 (Ref) | 0.94 (0.41, 2.14) | 0.86 (0.34, 2.26) | 1.00 (Ref) | 1.03 (0.49, 2.18) | 0.83 (0.36, 1.99) | 0.91 (0.64, 1.28) | 0.60 |
| Model 3; β (95% CI) | 1.00 (Ref) | 0.97 (0.42, 2.23) | 0.93 (0.34, 2.16) | 1.00 (Ref) | 1.04 (0.46, 2.39) | 0.72 (0.27, 1.93) | 0.95 (0.66, 1.36) | 0.79 |
| AAC Severity* | ||||||||
| Model 1; β (95% CI) | 1.00 (Ref) | 0.77 (0.52, 1.14) | 0.87 (0.56, 1.36) | 1.00 (Ref) | 1.16 (0.69, 1.95) | 1.04 (0.62, 1.75) | 1.05 (0.88, 1.26) | 0.58 |
| Model 2; β (95% CI) | 1.00 (Ref) | 0.71 (0.47, 1.06) | 0.79 (0.49, 1.31) | 1.00 (Ref) | 1.01 (0.63, 1.63) | 1.25 (0.70, 2.20) | 1.08 (0.89, 1.32) | 0.43 |
| Model 3; β (95% CI) | 1.00 (Ref) | 0.82 (0.54, 1.23) | 0.90 (0.54, 1.48) | 1.00 (Ref) | 0.97 (0.61, 1.54) | 0.97 (0.55, 1.70) | 1.07 (0.88, 1.31) | 0.49 |
Evaluated among 557 participants with CAC>0, 716 participants with AAC>0, and 280 participants with TAC>0.
Abbreviations: CAC=coronary artery calcification, TAC=thoracic aorta calcification, AAC=abdominal aortic calcification, CI=confidence interval, Ref=reference category.
Model 1: Adjusted for age, sex, race/ethnicity
Model 2: Adjusted for model 1 variables plus height, weight, intramuscular mixed connective tissue area, and total intramuscular fat area
Model 3: Adjusted for model 2 variables plus diabetes, hypertension, smoking, total cholesterol, HDL cholesterol, C-reactive protein, alcohol, physical activity, and caloric intake.
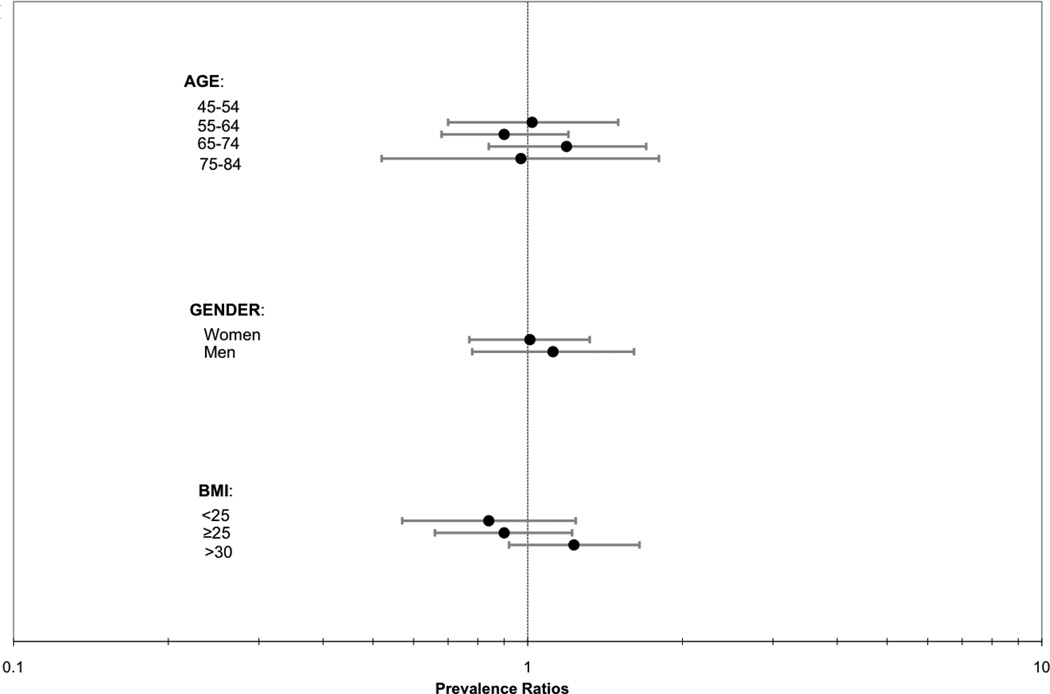
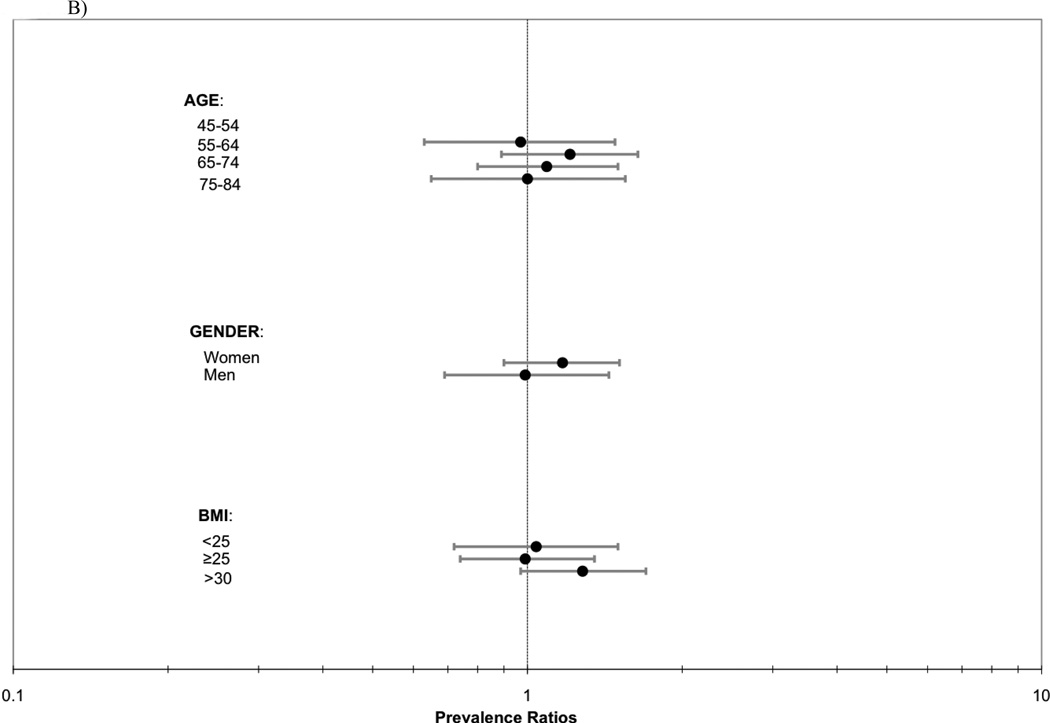
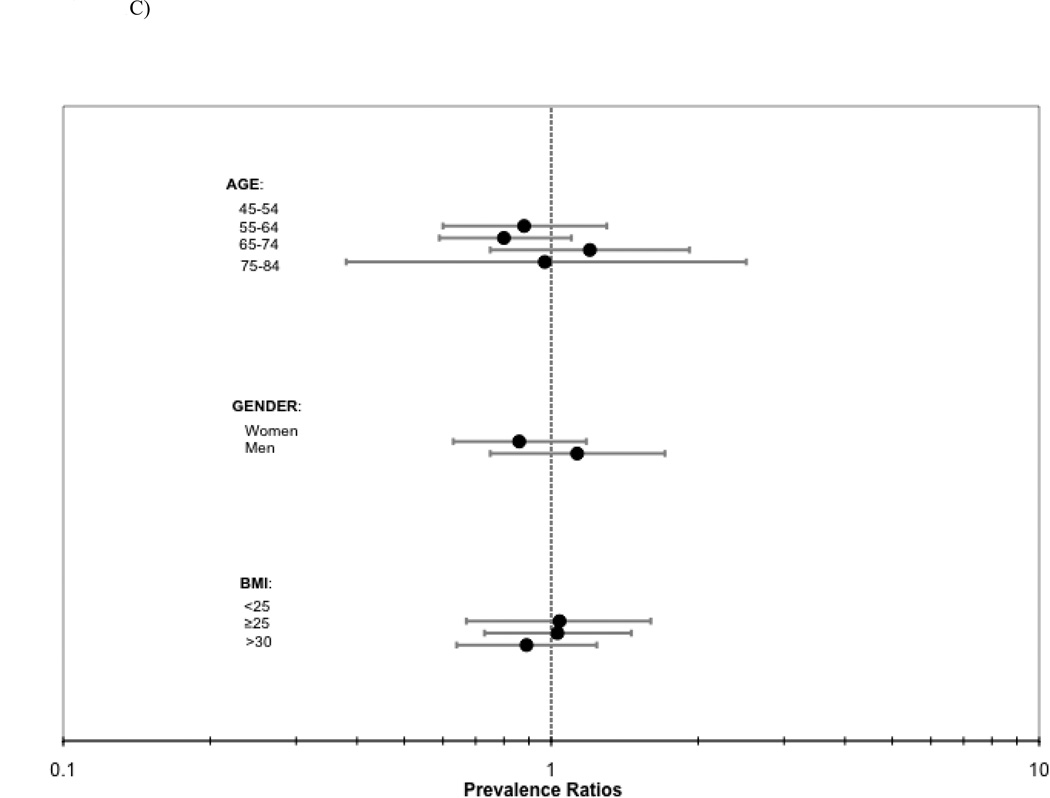
Last, we evaluated for heterogeneity in the association of abdominal lean muscle area with CAC, TAC, AAC prevalence by age, gender, and BMI strata. In each case, results were similar across groups (P interactions all > 0.14; Figure 1).
Figure 1.
ABC. Association of abdominal lean muscle area (per SD [27.6 cm2] greater) with a) CAC, b) TAC, and c) AAC prevalence by strata of age, gender and BMI.
Abbreviations: CAC=coronary artery calcification, TAC=thoracic aortic calcification, AAC=abdominal aortic calcificiation, BMI=body mass index.
DISCUSSION
In a multi-ethnic community-living population without clinically apparent CVD, we hypothesized that lower absolute amounts of abdominal lean muscle area would be associated with greater calcified atherosclerosis. However, we observed no associations of abdominal lean muscle area with calcification in 3 separate vascular beds. Our findings suggest that, contrary to our hypothesis, abdominal lean muscle area may not be associated with the extent and severity of calcified atherosclerosis in individuals without prevalent CVD.
In a prior study, we demonstrated that lower creatinine excretion – an indirect measure of muscle mass – was strongly associated with mortality in a cohort of individuals with prevalent CVD.[6] Therefore, it was surprising to us that we found no association of muscle mass with subclinical CVD in the present study. Several factors may be responsible for this seeming discrepancy. First, the spectrum of severity of CVD may have differed. On the one hand, evaluation of the relationship of muscle mass with subclinical CVD in a population free of prevalent CVD may be advantageous. Had we demonstrated the hypothesized relationship, it would have suggested that low muscle mass may have promoted early development of calcified atherosclerosis. Evaluation of the association in persons with prevalent CVD is more challenging because any observed association may have reflected either the effect of low muscle mass on CVD, or alternatively CVD may have led to sarcopenia and cardiac cachexia. On the other hand, exclusion of persons with prevalent CVD means we evaluated a population with a spectrum of less severe disease. If low muscle mass does promote CVD, it remains possible that a more severe spectrum of CVD is required for the association to become apparent.
A second possible explanation for the apparent discrepancy in results is that the prior study evaluated all-cause mortality. It is possible that low muscle mass is a marker of frailty, poor overall health status, or diseases other than CVD such as malignancy or malnutrition. If so, such associations may have driven its relationship with all-cause mortality in our prior study, rather than relationships with the burden of CVD per se. , Last, the prior study evaluated creatinine excretion rate as a surrogate marker of muscle mass,[6] whereas abdominal CT scans were used here. It is possible that muscle quality and function may influence creatinine excretion rate, above and beyond the amount of muscle mass observed on abdominal CT.
There are few studies that have evaluated the association of muscle mass with calcified atherosclerosis in community-living populations. In a prior study of patients who were referred for total body CT scanning for preventive medicine, we recently observed that abdominal lean muscle area was inversely associated with thoracic aortic calcification, but we did not observe associations with coronary, iliac, or abdominal aortic calcification.[16] Thus, our findings in the present study were similar in that we identified no statistically significant associations of abdominal lean muscle area with coronary and abdominal aortic calcification but differ in regards to thoracic aortic calcification. The prior study evaluated a primarily Caucasian population, who self-referred and the sample size was smaller (n=394). It is possible that these differences in study samples may have resulted in the disparate findings.
To our knowledge, except for our prior studies summarized above, no other study has evaluated the association of abdominal lean muscle area by CT with calcified atherosclerosis. A few studies have evaluated similar associations using other measures of muscle mass and subclinical CVD. Alexandersen and colleagues reported an inverse association between peripheral fat free mass by dual energy absorptiometry (DEXA) and aortic calcium severity measured using lateral abdominal radiographs in a predominantly Caucasian male cohort. The authors reported an inverse association that was independent of age and BMI.[5] Investigators in the Hoorn Study evaluated the association of regional body composition using DEXA with peripheral and central arterial stiffness.[17] Greater leg lean mass was associated with lower peripheral and central arterial stiffness after adjusting for age, sex, height, mean arterial pressure, trunk fat, and leg fat mass.[17] However, this paper did not report on the association of central muscle mass with arterial stiffness, precluding direct comparisons by anatomic region. Last, in a Japanese cohort free of CVD, Ochi and colleagues reported that low thigh muscle mass by CT was associated with greater carotid intima-media thickness and brachial ankle pulse wave velocity in men, but not women.[18] Thus, existing data suggest that lower limb muscle mass is inversely associated with several measures of subclinical CVD,[17],[18] whereas results are much less consistent for central muscle mass. As peripheral arterial disease represents a peripheral manifestation of atherosclerosis and is strongly associated with lower limb sarcopenia,[19–22] the local burden of peripheral atherosclerosis may be responsible for the association of low leg muscle mass with CVD; an association that may not extend to muscle mass in other anatomic regions. Future studies with concurrent measures of regional muscle mass are required to confirm or refute this hypothesis.
We also considered whether use of CT scans to quantify muscle area may have contributed to the null findings of our study. We observed that muscle area was correlated with other measures of body mass and size, as expected. Moreover, prior studies have demonstrated that skeletal muscle from MRI images at the L4-L5 vertebrae are strongly correlated with whole body skeletal muscle in Caucasians (r2=0.60).[23] The correlation of skeletal muscle measurement by CT and MRI is well established.[24] Some non-skeletal muscle tissues may be included in fat free mass by DEXA[25] and thus DEXA and CT-measured muscle may not be identical. Overall, we believe that the use of the abdominal CT measurements as an index of muscle mass is unlikely to have led to the null findings in our study.
Strengths of this study included the relatively large study sample, multi-ethnic and non-clinic based population, measures of calcification in multiple vascular beds, and availability of information on important confounding factors including caloric intake and physical activity. The study also has important limitations. Despite being the largest study sample to our knowledge to evaluate the association of abdominal lean muscle area with calcified atherosclerosis, we cannot exclude that an association may have been missed due to chance. Results should be interpreted within the confines of the 95% confidence intervals, which suggest that any missed association is likely to be modest, at best. The cross-sectional study design does not allow evaluation of temporal direction of associations, thus it remains possible that low abdominal lean muscle area may be associated with future development or progression of CVD, or vice versa. Whether or not results generalize to persons with prevalent CVD is uncertain.
In summary, we observed no statistically significant associations between abdominal lean muscle area with calcified atherosclerosis in the coronary, thoracic aortic, or abdominal aortic arteries in a multi-ethnic community-living population without clinically apparent CVD. As prior studies have demonstrated that lower muscle mass is associated with greater all-cause mortality in the general population,[26] and in populations with CVD[6], it is possible that low abdominal lean muscle area may not influence atherosclerotic disease burden per se, but rather that the associations of low muscle mass with mortality may reflect greater burden of non-atherosclerotic diseases linked with muscle wasting and mortality.
ACKNOWLEDGEMENTS
This study was supported by R21HL091217 (JHI) and associated ARRA supplement R21HL091217-01A2S1 (NJ and JHI), R01HL088451 (MAA), R01 HL071739 (MJB) and contracts N01-HC95159 through N01-HC-95165 and N01-HC-95169 all from the National Heart Lung and Blood Institute. The authors thank Ms. Clydene Nee for assistance with the manuscript, in addition to the other investigators and the staff of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. This material is the result of work supported with resources of the VA San Diego Healthcare System
Abbreviations
- BMI
body mass index
- CVD
cardiovascular disease
- CT
Computed tomography
- CAC
coronary artery calcification
- TAC
thoracic aortic calcification
- AAC
abdominal aortic calcification
- PR
Prevalence Ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
Author Contributions:
Nicole Jensky, Joachim Ix: 1) conception and design or analysis and interpretation of data, or both; 2) drafting of the manuscript or revising it critically for important intellectual content; and 3) final approval of the manuscript submitted.
Matthew Allison: 1) conception and design or analysis and interpretation of data, or both 2) drafting of the manuscript or revising it critically for important intellectual content; and 3) final approval of the manuscript submitted
Michael Criqui, Ian de Boer: 2) drafting of the manuscript or revising it critically for important intellectual content; and 3) final approval of the manuscript submitted.
Greg Burke, Mercedes Carnethon, Matthew Budoff 3) final approval of the manuscript submitted.
References
- 1.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368(9536):666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 2.Hanson RL, McCance DR, Jacobsson LT, et al. The U-shaped association between body mass index and mortality: relationship with weight gain in a Native American population. J Clin Epidemiol. 1995;48(7):903–916. doi: 10.1016/0895-4356(94)00217-e. [DOI] [PubMed] [Google Scholar]
- 3.Zheng W, McLerran DF, Rolland B, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364(8):719–729. doi: 10.1056/NEJMoa1010679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Donovan G, Owen A, Kearney EM, et al. Cardiovascular disease risk factors in habitual exercisers, lean sedentary men and abdominally obese sedentary men. Int J Obes (Lond) 2005;29(9):1063–1069. doi: 10.1038/sj.ijo.0803004. [DOI] [PubMed] [Google Scholar]
- 5.Alexandersen P, Tanko LB, Bagger YZ, et al. Associations between aortic calcification and components of body composition in elderly men. Obesity (Silver Spring) 2006;14(9):1571–1578. doi: 10.1038/oby.2006.181. [DOI] [PubMed] [Google Scholar]
- 6.Ix JH, de Boer IH, Wassel CL, et al. Urinary creatinine excretion rate and mortality in persons with coronary artery disease: the Heart and Soul Study. Circulation. 2010;121(11):1295–1303. doi: 10.1161/CIRCULATIONAHA.109.924266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 8.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 9.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 10.Budoff MJ, Nasir K, Katz R, et al. Thoracic aortic calcification and coronary heart disease events: the multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2011;215(1):196–202. doi: 10.1016/j.atherosclerosis.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Criqui MH, Kamineni A, Allison MA, et al. Risk factor differences for aortic versus coronary calcified atherosclerosis: the multiethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30(11):2289–2296. doi: 10.1161/ATVBAHA.110.208181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 13.Ainsworth BE, Irwin ML, Addy CL, et al. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999;8(6):805–813. doi: 10.1089/152460999319129. [DOI] [PubMed] [Google Scholar]
- 14.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9) Suppl:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 15.Cushman M, Cornell ES, Howard PR, et al. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41(2):264–270. [PubMed] [Google Scholar]
- 16.Jensky C, Wright, Wassel, Alcaraz, Allison The Association Between Abdominal Body Composition and Vascular Calcification. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2011.70. [DOI] [PubMed] [Google Scholar]
- 17.Snijder MB, Henry RM, Visser M, et al. Regional body composition as a determinant of arterial stiffness in the elderly: The Hoorn Study. J Hypertens. 2004;22(12):2339–2347. doi: 10.1097/00004872-200412000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Ochi M, Kohara K, Tabara Y, et al. Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis. 2010;212(1):327–332. doi: 10.1016/j.atherosclerosis.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 19.McDermott MM, Criqui MH, Greenland P, et al. Leg strength in peripheral arterial disease: associations with disease severity and lower-extremity performance. J Vasc Surg. 2004;39(3):523–530. doi: 10.1016/j.jvs.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 20.McDermott MM, Guralnik JM, Albay M, et al. Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: the InCHIANTI Study. J Am Geriatr Soc. 2004;52(3):405–410. doi: 10.1111/j.1532-5415.2004.52113.x. [DOI] [PubMed] [Google Scholar]
- 21.McDermott MM, Tian L, Ferrucci L, et al. Associations between lower extremity ischemia, upper and lower extremity strength, and functional impairment with peripheral arterial disease. J Am Geriatr Soc. 2008;56(4):724–729. doi: 10.1111/j.1532-5415.2008.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDermott MM, Hoff F, Ferrucci L, et al. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2007;55(3):400–406. doi: 10.1111/j.1532-5415.2007.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S. Relation between whole-body and regional measures of human skeletal muscle. Am J Clin Nutr. 2004;80(5):1215–1221. doi: 10.1093/ajcn/80.5.1215. [DOI] [PubMed] [Google Scholar]
- 24.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85(1):115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 25.Levine JA, Abboud L, Barry M, et al. Measuring leg muscle and fat mass in humans: comparison of CT and dual-energy X-ray absorptiometry. J Appl Physiol. 2000;88(2):452–456. doi: 10.1152/jappl.2000.88.2.452. [DOI] [PubMed] [Google Scholar]
- 26.Oterdoom L. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207(2):534–540. doi: 10.1016/j.atherosclerosis.2009.05.010. [DOI] [PubMed] [Google Scholar]