Abstract
Objective
Synthetic glucocorticoid (sGC) administration to women threatening preterm delivery increases neonatal survival. Evidence shows that fetal exposure to glucocorticoid levels higher than appropriate for current maturation programs offspring development. We examined fetal sGC multigenerational effects on F1 and F2 female offspring hypothalamo-pituitary-adrenal-axis (HPAA) function.
Study Design
At 0.7 gestation, pregnant F0 ewes received four dexamethasone injections (2mg, approx. 60 ug.kg-1. day-1 12 h apart) or saline (control, C). F1 female offspring were bred to produce F2 female offspring. Post-pubertal HPAA function was tested in F1 and F2 ewes.
Results
F1 and F2 ewe lambs showed reduced birth-weight and morphometrics. Dexamethasone increased baseline but reduced stimulated HPAA activity in F1 and F2 female offspring.
Conclusions
This is the first demonstration that sGC doses in the clinical range have multigenerational effects on HPA activity in a precocial species, indicating the need for study of long-term effects of fetal sGC exposure.
Keywords: antenatal synthetic glucocorticoids, hypothalamus, pituitary, sheep, mutigenerational developmental programming
Introduction
The ground-breaking work of Liggins showed that glucocorticoids accelerate fetal lung development in sheep. 1 A subsequent clinical trial demonstrated significant decreases in morbidity and mortality in premature babies whose mothers received synthetic glucocorticoids (sGC) when threatening preterm delivery. 2 It has now become routine clinical practice to treat women who threaten preterm delivery before 34 weeks of gestation with sGC to improve outcomes in premature babies. 3 In the developing world the incidence of preterm delivery is rising with over 10% of pregnancies resulting in preterm delivery and these preterm deliveries account for 75 % of all neonatal deaths. 3,4 In one study in which the majority of patients received four or more weekly courses of GC (glucocorticoids), delivery at less than 32 weeks occurred in only 24% of sGC treated pregnancies and at less than 37 weeks in 63 % of all treated pregnancies. 4 Therefore 76 % of all synthetic GC exposed fetuses remained in utero for a significant period after fetal exposure and over one third can be considered as delivering at term. There is mounting evidence that in addition to the major benefits of accelerated lung maturation, fetal exposure to exogenous GC levels higher than appropriate for the current stage of fetal maturation produces intrauterine growth restriction in multiple species including sheep, non-human primates, and from data from retrospective analyses, in human pregnancy. 5,6,7,8,9,10 Maternal administration of sGC alters the trajectory of development of many fetal organ systems and evidence is accumulating from animal studies that there are unwanted later-life developmental programming effects on offspring endocrine, renal, and metabolic function. 11,12,13,14,15 These similarities across multiple species suggest common underlying mechanisms that result in adverse outcomes that need to be better understood.
Dexamethasone treatment of F0 ewes in late gestation produces offspring (F1) who show fasting hyperglycemia and glucose intolerance, and an increased glucose-stimulated insulin secretion in rats and sheep. 14,16,17 Treatment of F0 pregnant ewes with sGC early in the second half of gestation increased plasma adrenocorticotropic hormone (ACTH) and cortisol in their late-gestation fetuses.12 At six months post natal age F1 offspring from sCG treated F0 mothers show no difference in hypothalamic-pituitary-adrenal-axis (HPAA) responsiveness to a combined challenge by corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP). However, at one year of age, the F1 sGC exposed offspring showed elevated basal and stimulated cortisol levels with no change in ACTH response.11 In 2-yr-old F1 offspring exposed to repeated maternal betamethasone injections, ACTH or cortisol levels were unaltered, but ACTH responses to CRH/AVP were increased.18 At 3 years of age, basal ACTH was elevated, and both basal and stimulated cortisol levels were suppressed while basal and stimulated ACTHcortisol ratios were suppressed following fetal exposure to repeated sGC injections to their mothers.18 Female F1 rats exposed to dexamethasone given to the F0 mother in her drinking water during day15 – 21 of their own fetal life, although not treated with any agents during their own pregnancy gave birth to male offspring who showed reduced birth weight, glucose intolerance, and elevated hepatic phosphoenolpyruvate carboxykinase (PEPCK) activity.14 Multigenerational programming of metabolism was observed in offspring of male rats exposed prenatally to dexamethasone mated with control females. 14 Translation of multigenerational findings of effects of sGC induced developmental programming to possible effects of human fetal exposure requires studies in precocial species that more closely match the fetal maturational window of exposure and shorter duration – in both absolute terms and relative to the longer course of development – that occur during human clinical management of preterm labor. We hypothesized that exposure of F1female fetal lambs to sGC during their development at days 103-104 of gestation, equivalent of 27-28 weeks human gestation, would have effects on both F1 and F2 female offspring basal HPAA and responses to challenges.
Materials and Methods
Care and Use of animals
All procedures were approved by the University of Wyoming Animal Care and Use Committee. F0 nulliparous Rambouillet X Columbia crossbred ewes (approximately 16 months of age) were used to produce the first filial generation (F1) ewe lambs studied. Founder generation (F0) ewes were bred to a single ram in one of two consecutive years. After natural mating, ewes were fed in accordance with National research council (NRC) requirements for maintenance.19 On d 103 and 104 of gestation ewes averaged 73.6 ± 4.0 kg (Mean ± SEM). Dexamethasone (DEX) ewes (n= 10) received four injections of 2 mg of dexamethasone (im; Vedco, St, Joseph, MO) 12 h apart. Control (C) ewes (n= 12) received equal volumes of saline. Ewes were allowed to lamb naturally and the flock that these ewes originated from has an approximate gestation length of 150 days. There were 8 C twin (2 sets were both females) and 4 C singles. In the DEX group there were 7 twin (3 female twin sets) and 3 singles. After lambing, all F0 ewes were given free choice access to alfalfa hay. Prior to two weeks of age, F1 female lambs were tail-docked as per Federation of Animal Science Societies recommendations.20 F1 ewe lambs were given free access to a standard commercially available creep feed (Lamb Creep B30 w/Bovatec; Ranch-way Feeds, Ft. Collins, CO) from birth to weaning at four months of age and placed in an outdoor housing facility with shelter and ad libitum water. These F1 ewe lambs were maintained in accordance with NRC requirements (20) for replacement ewes. Diet consisted of alfalfa and corn to meet NRC with ad libitum access to a trace mineral salt block. Diets were adjusted up for weight gain every month.
F1 ewe-lambs received maintenance requirements until either 16 or 28 months of age depending on the year in which they were born. F1 ewes were then naturally mated to a single ram with mating date noted using a marking ram harness. F1 ewes were allowed to lamb naturally. Second filial generation (F2) ewe lambs (n = 9 DEX and 8 C) were fed in a similar fashion as their F1 mothers and postnatal body weights were recorded at the same times. There were 3 F2 C twin (all male female twin sets) and 5 F2 C singles. In the F2 DEX lambs there were 5 twin (1 female twin sets and 3 male female twin sets) and 4 singles.
To determine the responsiveness of different components of the HPAA, a series of challenges were conducted in 6 randomly subsampled female F1 and F2 offspring all from different mothers at 2.5 or 3.5 year of age (n = 3 per age and birth type in each treatment group) for F1 and ∼ 6 months of age for F2 females (3 per birth type per treatment). F1 ewes were seasonally anestrous and F2 lambs had not obtained puberty when selected for challenges. A catheter (Abbocath, 16ga, Abbott Laboratories, North Chicago, IL) was placed aseptically into the jugular vein 48 hour prior to blood sampling. Catheters were sutured in to the skin to secure them and a 124.5 cm extension set (Seneca Medical, Tiffin, OH) was attached to the catheter to allow for infusion and sampling without disturbing the ewe. The neck and shoulder area of each animal were covered with netting material (Surgilast Tubular elastic dressing retainer, Derma Science Inc, Princeton NJ) to prevent the catheters from being damaged. Ewes and lambs were maintained in neighboring individual pens with free access to water. Bovine CRH, human AVP, and human ACTH were all purchased from Sigma Chemicals (Sigma-Aldridge, St Louis, MO; catalog numbers C2671, V9879, and 02275 respectively). Two days after catheter placement, an ACTH challenge was conducted using similar procedures to Turner et al. with slight modifications as previously published.21,22 Jugular blood samples were obtained into chilled tubes (heparin plus NaF; 2.5 mg/mL; Sigma, St. Louis, MO) at -30, -15, and 0 min relative to a 0.2 μg/kg intravenous bolus injection of ACTH in saline (2.0 to 3.0 mL) over 2 seconds. Blood samples were collected at 2, 5, 10, 15, 20, 30, 45, 60, 80, 100, 120, and 150 min after ACTH administration. All blood samples were immediately placed on ice, centrifuged at 2,500 × g and plasma collected and stored at -80° C. On the following day, a CRH/AVP challenge was conducted as previously reported by Bloomfield et al. with the following modifications we previously reported.22,23 Jugular blood samples were collected at - 30, - 15 and 0 min relative to injection of 0.5 μg/kg of CRH and 0.1 μg/kg of AVP in saline as an intravenous bolus (2.0 to 3.0 mL) over 2 seconds. Jugular blood samples were obtained at 2, 5, 10, 15, 20, 30, 45, 60, 90, 120, 180, and 240 min after CRH/AVP infusion. Blood samples were processed as for the ACTH challenge. Following the ACTH challenge, blood from the catheter in one F2 C lamb was not accessible.
Hormone quantification
Concentrations of ACTH were determined as previously described by Gilbert et al. using an Immulite 1000 immunoassay analyzer (Siemens Medical Solutions, Malvern, PA) in a single assay with a inter-assay CVs of < 5 %.24 Concentrations of cortisol were determined as previously described by Dong et al. using Coat-A Count Cortisol RIA (Siemens Medical Solutions Diagnostics, Los Angeles, CA) with an intra-assay and inter-assay CV < 7 %. 25
Statistical analysis
Data are presented as means ± least square SEM, and differences considered significant at P ≤ 0.05, with a tendency at P ≤ 0.10. Graphpad Prism (GraphPad Software Inc, La Jolla, CA) was used to calculate the area under the curve (AUC) for plasma cortisol and ACTH responses following CRH/AVP infusion and plasma cortisol response following the ACTH challenge. Baseline concentrations of ACTH and cortisol before infusion were obtained by averaging the three samples before both challenges. Plasma cortisol and ACTH response following CRH/AVP administration and plasma cortisol response following ACTH challenge were analyzed as repeated measures using MIXED procedure of SAS (SAS Inst. Inc., Cary, NC) with treatment and time and their interaction in the model statement. Areas under the curves for ACTH and cortisol during CRH/AVP and cortisol release during ACTH challenge, along with baseline concentrations of cortisol and ACTH were analyzed using the GLM procedure of SAS with treatment in the model. A cortisol to ACTH ratio was calculated for baseline and for AUC during the CRH/AVP challenge and the ratio was analyzed using the GLM procedure of SAS with treatment in the model. F1 ewe maternal age was initially included in all F1 ewe models initially but was found to be non-significant (P > 0.52) and birth type was also included in both F1 and F2 animals but was found to be non-significant (P > 0.20) and both were therefore removed from the final models.
Results
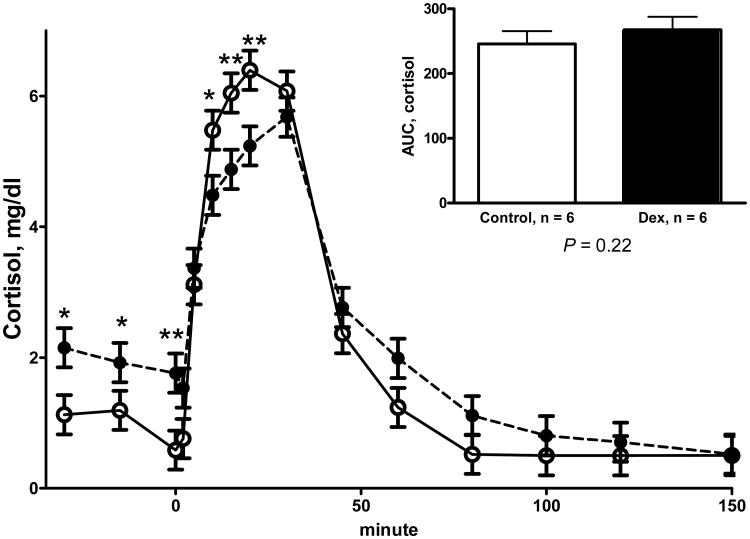
In the F1 generation the gestation length was shorter for DEX ewes compared to C ewes (P < 0.05; 152.3 ± 0.5 vs 155.4 ± 0.5) and tended to be shorted in the F2 generation (P < 0.06; 147.6 ± 0.7 vs 149.6 ± 0.6). 26 Birth weight was also reduced in F1 lambs (P = 0.04) from DEX treated mothers compared to C treated mothers (5.03 ± 0.18 vs 5.59 ± 0.17) and this birth weight reduction was maintained into the F2 generation (P = 0.03) with lambs born to ewes whose mothers were treated with DEX has a smaller lambs compared to ewes grandmothers were C (5.72 ± 0.26 vs 6.62 ± 0.29). 26 Body weights of F1 DEX ewes at catheterization were less (P < 0.05) than F1 C ewes (F1 65.1 ± 2.5 vs. 72.2 ± 2.5 kg, respectively). Baseline plasma ACTH was similar in F1 DEX and C ewes (P = 0.26; 30.1 ± 9.1 vs 36.8 ± 9.1 pg/ml respectively) but cortisol was greater (P < 0.05) in F1 DEX ewes compared to C F1 ewes (1.8 ± 0.1 vs 0.8 ± 0.1 mg/dl, respectively). In the F1 ewes there was a treatment * time interaction (P = 0.04) for the cortisol response (Figure 1) during the ACTH challenge. F1 ewes from mothers treated with dexamethasone had reduced (P < 0.05) cortisol release at 2, 20, and 30 min after intravenous ACTH compared to ewes from C mothers. However, there was no difference (P = 0.37, Figure 1) in the area under the curve for cortisol. There was a significant (P ≤ 0.04) treatment * time interaction for plasma ACTH and cortisol during the CRH/AVP challenge (Figure 2), with F1 DEX offspring showing reduced (P < 0.01) plasma ACTH and cortisol at 5 to 15 and 10 to 30 minutes post infusion respectively. The decreased plasma ACTH and cortisol response to the CRH/AVP challenge was confirmed by reduced (P = 0.04) area under the curve for both ACTH and cortisol. The cortisol to ACTH ratio was not different between DEX and C F1ewes during baseline sampling prior to CRH/AVP challenge (P = 0.06; 0.035 ± 0.007 vs 0.056 ± 0.007, respectively). During the CRH/AVP challenge the AUC cortisol to ACTH ratio was similar between DEX and C F1 ewes (P = 0.09; 0.164 ± 0.014 vs 0.186 ± 0.014, respectively).
Figure 1.
Plasma cortisol responses to an ACTH challenge in 2.5 or 3.5 year old F1 female offspring of mothers who received four, injections of 2 mg of dexamethasone 12 hours apart starting on d 103 and 104 of gestation (DEX; closed symbol, n = 6) and F1 offspring whose mothers received similar timed injections of saline (C; open symbol, n = 6). Area under the curve (AUC) is depicted in the inset. Values are means ± SEM. Trt P = 0.93, time P < 0.0001, Trt* time P = 0.04.
Figure 2.
Plasma A) ACTH and B) cortisol responses to a CRH/AVP challenge in 2.5 or 3.5 year old F1 female offspring of mothers who received four, injections of 2 mg of dexamethasone 12 hours apart starting on d 103 and 104 of gestation (DEX; closed symbol, n = 6) and F1 offspring whose mothers received similar timed injections of saline (C; open symbol, n = 6). Area under the curve (AUC) is depicted in the inset. Values are means ± SEM. Trt P = 0.01, time P < 0.0001, Trt* time P = 0.04; and Trt P = 0.01, time P < 0.0001, Trt* time P < 0.0001; ACTH and cortisol respectively.
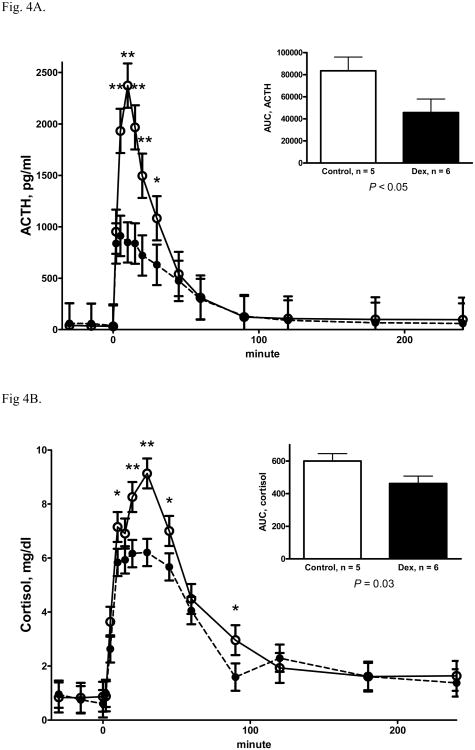
Body weights of F2 DEX lambs at catheterization were less (P < 0.05) than F2 C lambs (44.8 ± 2.4 vs. 51.2 ± 2.4 kg, respectively). Baseline ACTH were higher (P < 0.05) in F2 DEX lambs than F2 C lambs (56.2 ± 6.4 vs. 35.4 ± 6.4 pg/ml, respectively) and cortisol was also higher (P < 0.05) in F2 DEX lambs compared to F2 C lambs(1.8 ± 0.1 vs. 0.9 ± 0.1 mg/dl, respectively). In the F2 lambs at 6 months of age during the ACTH challenge, there was a significant treatment * time interaction (P < 0.001, Figure 3) with F2 DEX lambs having increased (P < 0.05) plasma cortisol before the ACTH administration and reduced (P < 0.05) plasma cortisol at 10, 15 and 20 minute post ACTH administration compared to F2 C lambs. There was no difference (P = 0.22, figure 3) in the area under the curve for plasma cortisol during the ACTH challenge. During the CRH/AVP challenge, F2 offspring from DEX treated grandmothers had a significant treatment * time interaction for ACTH response with DEX treated F2 lambs having reduced (P < 0.05, Figure 4a) plasma ACTH at 5 to 30 minutes post infusion compared to C F2 lambs. Plasma cortisol was reduced (P < 0.01, Figure 4b) in F2 DEX lambs compared to F2 C lambs during the CRH/AVP challenge. There was significantly reduced (P < 0.05) plasma ACTH and cortisol area under the curve (Figure 4) in the F2 DEX lambs compared to the F2 C lambs. The cortisol to ACTH ratio during baseline sampling prior to CRH/AVP challenge was not different between DEX and C F2 lambs (P = 0.48; 0.024 ± 0.004 vs 0.023 ± 0.004, respectively). During the CRH/AVP challenge the AUC cortisol to ACTH ratio was similar between DEX and C F2 lambs (P = 0.16; 0.009 ± 0.001 vs 0.010 ± 0.001, respectively).
Figure 3.
Plasma cortisol responses to a ACTH challenge in 6 mo old F2 offspring whose grandmothers received four, injections of 2 mg of dexamethasone 12 hours apart starting on d 103 and 104 of gestation (DEX; closed symbol, n = 6) and F2 offspring whose grandmothers received similar timed injections of saline (C; open symbol, n = 6). Area under the curve (AUC) is depicted in the inset. Values are means ± SEM. Trt P = 0.29, time P < 0.0001, Trt* time P < 0.001.
Figure 4.
ACTH Plasma A) ACTH and B) cortisol responses to a CRH/AVP challenge in 6 mo old F2 offspring whose grandmothers received four, injections of 2 mg of dexamethasone 12 hours apart starting on d 103 and 104 of gestation (DEX; closed symbol, n = 6) and F2 offspring whose grandmothers received similar timed injections of saline (C; open symbol, n = 5). Area under the curve (AUC) is depicted in the inset. Values are means ± SEM. Trt P = 0.07, time P < 0.0001, Trt* time P < 0.0001; and Trt P < 0.01, time P < 0.0001, Trt* time P = 0.22; ACTH and cortisol respectively.
Discussion
We have previously shown in this model of maternal DEX treatment that both F1 and F2 female offspring have decreased birthweight and reduced postnatal growth.26 In addition to the altered fetal and postnatal growth, we found increased fasting plasma glucose and insulin and increased plasma glucose and decreased plasma insulin in response to an intravenous bolus glucose tolerance test in both F1 and F2 female offspring.26 It is possible that the altered HPAA activity reported here played some part in the changes in glucose and insulin metabolism that we have previously reported that are themselves likely to lead to other adult onset pathologies such as metabolic syndrome and hypertension.15,26,27,28 In the current study, maternal treatment with DEX results in increased basal cortisol in FI offspring and increased basal ACTH and cortisol in F2 offspring. During HPAA challenges both F1 and F2 DEX offspring have a marked reduction in hypothalamic and pituitary function.
In a study by Sloboda et al, maternal administration of sGC resulted in increased basal plasma ACTH, cortisol and cortisol binding capacity near term and had effects on GR and mineralocorticoid receptor expression in F1 fetuses and early in postnatal life.12,29 As these offspring age, they show increased HPAA responsiveness at one year of age.11 However at 3 years of age, basal ACTH was elevated while in contrast during CRH/AVP challenge ACTH and cortisol response was decreased in offspring from mothers that were given repeated sGC during late gestation, a finding which we confirm here.18 Our F1 DEX female offspring appear to have similar HPAA function and responses to those observed in this study by Sloboda et al following repeated maternal betamethasone administration.11 The difference is that we observed HPAA hyporesponsiveness at 6 months in F2 female DEX offspring a life-stage when Sloboda et al observed no difference in HPAA activity in F1 offspring.11 One potential explanation is the difference in timing, dose, and form of sGC given along with possible differences in the F1 and F2 generation since they were not tested at the same age. We administered a total of four injections of 2 mg of DEX every 12 hours on d 103 and 104 while Sloboda et al gave 0.5 mg/kg betamethasone on d 104 111,118 and 124 of gestation. We agree with the statement by these authors that “the effects of glucocorticoids on postnatal HPA responses may vary according to the time in gestation that the steroids was administered”.11 In humans infants it has been shown that intrauterine exposure to sGC reduces HPA activity in unstimulated conditions after pain but not pharmacological studies.30 However in humans not much is known about the potential persistent effects of sGC after infancy.
A possible target of sGC programming is the fetal brain. In fetal sheep treated with betamethasone, brain weight is reduced and hippocampal glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) show a transient decreased after administration of betamethasone and later increased mRNA expression associated with sGC administration.29 In addition, antenatal sCG have been shown to have adverse effects on cerebral myelination in late gestation.31 At 12 weeks of postnatal age 11β-hydroxysteroid dehydrogenase (11βHSD) 1 mRNA expression was increased in the hippocampus of lambs whose mothers were treated with sGC during late gestation.29 At 3.5 years of age offspring from mothers treated with sGC have increased MR and 11βHSD mRNA expression in the hippocampus compared to offspring of mothers given saline.29 In guinea pigs fetal sex influences the effects of sCG exposure. Male fetuses whose mother were treated with repeated courses of sGC have decreased CRH mRNA in the hypothalamic paraventricular nucleus and increased hippocampal MR and GR mRNA expression compared to male fetuses whose mother were given saline.32 However female fetuses from these same mothers, sCG exposed fetuses show similar decreased CRH mRNA in the hypothalamic paraventricular nucleus, but no differences in hippocampal GR and MR expression.32 These results in adult male offspring with reduced basal plasma ACTH and cortisol associated with decreased hypothalamic CRH mRNA expression and increased hippocampal MR mRNA expression.33 Therefore, it appears that the fetus is unable to protect structures of the brain that are sensitive to exposure to levels of GC higher than appropriate for the current stage of maturation and that this results in changes to the brain that persist into adulthood in multiple species.
The multi-generational effects of maternal DEX administration on the female offspring and the consistency of the changes between F1 and F2 offspring are an important finding of this study that used only one course of sGC given to the F0 mother. In the guinea pig, multiple courses of sGC have been shown to lead to alterations in HPAA function and behavioral changes in both F1 and F2 offspring.34 In another rodent study that evaluated multi- generational effects of synthetic GC, glucose intolerance and elevated phosphoenolpyruvate carboxykinase (PEPCK) in the liver were observed in the F1 generation exposed during fetal life to sGC and these changes persisted in both male and female F2 offspring.14 Other programming insults such as maternal nutrient intake have been shown to lead to multigenerational offspring effects. Prenatal nutrient restriction in the rat results in decreased growth in female F1 and in male and female F2 offspring along with insulin resistance.35 However, this report also shows that both the timing of restriction either during pregnancy or lactation and also sex of the F2 generation has effects on the responses. In a similar study, prenatal protein restriction in the guinea pig results in alteration in the heart morphology in both F1 and F2 offspring, and also alteration in basal cortisol and HPA responses during challenges.36 In mice, maternal high fat feeding during pregnancy results in increased F1 and F2 offspring body length and insulin resistance.37 There is very limited opportunity to study multi-generational effects in humans – in part because of the time frame and the difficulty of tracking of generations. One study showed that female offspring from mothers who experienced the Dutch Winter famine during the first trimester of pregnancy had children with reduced size at birth independent of maternal weight.38 In another multi-generational study children of holocaust survivors that experienced post-traumatic stress disorder (PTSD) had lower urinary cortisol excretion compared to children of holocaust survivors without PTSD.39 There is much need for further study of the mechanisms that underlie multi-generational passage of phenotype as a result of fetal programming. The mechanism that programs phenotypic outcomes resulting from nutritional or sGC challenges across multiple generations are currently unknown. However, in a recent review paper, Matthews and Phillips propose two possible pathways: 1) an altered maternal endocrine environment and adaptations during pregnancy and 2) transmission of inappropriate epigenetic modifications.40 The alterations in the DEX F1 in both glucose and insulin regulation and HPAA axis function presented by ourselves here and others in rodents as described above could have resulted in the altered maternal endocrine environment that could have resulted in the observed F2 offspring phenotype.14,26,34 Several recent studies have shown that fetal and early life environments can alter DNA methylation along with histone acetylation of specific gene promoters.41,42,43,44 In addition a very recent paper reports that maternal administration of sGC results in DNA methylation and also several genes that modulate the epigenetic state in organs of F1 and F2 male guinea-pig offspring. 45 It is likely that a combination of both maternal environment and direct changes to epigenetic modifications lead to the multi-generational effects we report on DEX F1 and F2 phenotype. It is important that the dose we have used is approximately one-third the dose used clinically and that the sGC were administered as a single course further enhancing the need for more information on this important and efficacious obstetric clinical management. 46
Acknowledgments
This project was supported by the University of Wyoming National Institute of Health Grant INBRE P20- RR-16474-04 and HD 21350
Footnotes
Disclosure: None of the authors have a conflict of interest
Some of the findings were presented at the Developmental Origins of Health and Disease 7th world Congress, Portland, OR; September 18-21, 2011.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liggins GC. Preterm delivery of foetal lambs infused with glucocorticoids. J Endocrinol. 1969;45:515–523. doi: 10.1677/joe.0.0450515. [DOI] [PubMed] [Google Scholar]
- 2.Liggins GC, Howie RN. A controlled trail of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in preterm infants. Pediatrics. 1972;50:515–525. [PubMed] [Google Scholar]
- 3.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Wapner RJ, Sorokin Y, Thom EA, et al. National Institute of Child Health and Human Development Maternal Fetal Medicine Units Network. Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am J Obstet Gynecol. 2006;195:633–642. doi: 10.1016/j.ajog.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 5.Jobe AH, Wada N, Berry LM, Ikegami M, Ervin MG. Single and repetitive maternal glucocorticoid exposures reduce fetal growth in sheep. Am J Obstet Gynecol. 1998;178:880–885. doi: 10.1016/s0002-9378(98)70518-6. [DOI] [PubMed] [Google Scholar]
- 6.Newnham JP, Evans SF, Godfrey M, Huang W, Ikegami M, Jobe A. Maternal, but not fetal, administration of corticosteroids restricts fetal growth. J Matern Fetal Med. 1997;8:81–87. doi: 10.1002/(SICI)1520-6661(199905/06)8:3<81::AID-MFM3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 7.Johnson JWC, Mitzner W, Beck JC, et al. Long-term effects of betamethasone on fetal development. Am J Obstet Gynecol. 1981;141:1053–1064. doi: 10.1016/s0002-9378(16)32697-7. [DOI] [PubMed] [Google Scholar]
- 8.Banks BA, Cnaan A, Morgan MA, et al. Multiple courses of antenatal corticosteroids and outcome of premature neonates. North American Thyrotropin-Releasing Hormone Study Group. Am J Obstet Gynecol. 1999;181:709–17. doi: 10.1016/s0002-9378(99)70517-x. [DOI] [PubMed] [Google Scholar]
- 9.French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol. 1999;180:114–21. doi: 10.1016/s0002-9378(99)70160-2. [DOI] [PubMed] [Google Scholar]
- 10.Bloom SL, Sheffield JS, McIntire DD, Leveno KJ. Antenatal dexamethasone and decreased birth weight. Obstet Gynecol. 2001;97:485–90. doi: 10.1016/s0029-7844(00)01206-0. [DOI] [PubMed] [Google Scholar]
- 11.Sloboda DM, Moss TJ, Gurrin LC, Newnham J, Challis JRG. The effect of prenatal betamethasone administration on postnatal ovine hypothalamic-pituitary-adrenal function. J Endocrinol. 2002;172:71–81. doi: 10.1677/joe.0.1720071. [DOI] [PubMed] [Google Scholar]
- 12.Sloboda DM, Newnham JP, Challis JRG. Effects of repeated maternal betamethasone administration on growth and hypothalamic-pituitary-adrenal function of the ovine fetus at term. J Endocrinol. 2000;165:79–91. doi: 10.1677/joe.0.1650079. [DOI] [PubMed] [Google Scholar]
- 13.Tang L, Bi J, Valego N, et al. Prenatal betamethasone exposure alters renal function in immature sheep: sex differences in effects. Am J Physiol Regul Integr Comp Physiol. 2010;299:R793–R803. doi: 10.1152/ajpregu.00590.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp, Physiol. 2005;228:R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- 15.Nyirenda MJ, Welberg LA, Seck JR. Programming hyperglycaemia in the rat through prenatal exposure to glucocorticoids-fetal effect or maternal influence? J Endocrinol. 2001;170:653–60. doi: 10.1677/joe.0.1700653. [DOI] [PubMed] [Google Scholar]
- 16.Moss TJM, Sloboda DM, Gurrin LC, Harding R, Challis JRG, Newnham JP. Programming effects in sheep of prenatal growth restriction and glucocorticoid exposure. Am J Physiol Regul Integr Comp Physiol. 2001;281:R960–R970. doi: 10.1152/ajpregu.2001.281.3.R960. [DOI] [PubMed] [Google Scholar]
- 17.Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate caroxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998;101:2174–2181. doi: 10.1172/JCI1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sloboda DM, Moss TJ, Li S, Doherty D, Nitsos I, Challis JRG, Newnham JP. Prenatal betamethasone exposure results in pituitary-adrenal hyporesponsiveness in adult sheep. Am J Physiol Endocrinol Metab. 2007;292:E61–E70. doi: 10.1152/ajpendo.00270.2006. [DOI] [PubMed] [Google Scholar]
- 19.National Research Council. Nutrient Requirements of Sheep. Washington, DC: National Academy Press; 1985. [Google Scholar]
- 20.Federation of Animal Science Societies. Fed Anim Sci Soc. Third. Savoy, IL: 2010. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. [Google Scholar]
- 21.Turner AI, Canny BJ, Hobbs RJ, Bond JD, Clarje IJ, Tilbrook AJ. Influence of sex and gonadal status of sheep on cortisol secretion in response to ACTH and on cortisol and LH secretion in response to stress: important of different stressors. J Endocrinol. 2002;173:113–122. doi: 10.1677/joe.0.1730113. [DOI] [PubMed] [Google Scholar]
- 22.Long NM, Nijland MJ, Nathanielsz PW, Ford SP. The effect of early to mid-gestational nutrient restriction on female offspring fertility and hypothalamic-pituitary-adrenal axis response to stress. J Anim Sci. 2010;88:2029–2037. doi: 10.2527/jas.2009-2568. [DOI] [PubMed] [Google Scholar]
- 23.Bloomfield FH, Oliver MH, Giannoulias CD, Gluckman PD, Harding JE, Challis JR. Brief undernutrition in late-gestation sheep programs the hypothalamic-pituitary-adrenal axis in adult offspring. Endocrinol. 2003;144:2933–2940. doi: 10.1210/en.2003-0189. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert JS, Lang AL, Grant AR, Nijland MJ. Maternal nutrient restriction in sheep: hypertension and decreased nephron number in offspring at 9 months of age. J Physiol. 2005;565:137–147. doi: 10.1113/jphysiol.2005.084202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong F, Ford SP, Nijland MJ, Nathanielsz PW, Ren J. Influence of maternal undernutrition and overfeeding on cardiac ciliary neurotrophic factor receptor and ventricular size in fetal sheep. J Nutr Biochem. 2008;19:409–14. doi: 10.1016/j.jnutbio.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long NM, Shasa DR, Ford SP, Nathanielsz PW. Growth and insulin dynamics in two generations of female offspring of mothers receiving a single course of synthetic glucocorticoids. Am J Obstet Gynecol. 2012 doi: 10.1016/j.ajog.2012.06.024. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds RM, Walker BR, Syddall HE, Whorwood CB, Wood PJ, Phillips DI. Elevated plasma cortisol in glucose-intolerant men: differences in responses to glucose and habituation to venipuncture. J Clin Endocrinol Metab. 2001;86:1149–1153. doi: 10.1210/jcem.86.3.7300. [DOI] [PubMed] [Google Scholar]
- 28.Phillips D. Endocrine programming and fetal origins of adult disease. Trends Endocrinol Metab. 2002;13:363. doi: 10.1016/s1043-2760(02)00696-3. [DOI] [PubMed] [Google Scholar]
- 29.Sloboda DM, Moss TJ, Li S, Matthews SG, Challis JRG, Newnham JP. Expression of glucocorticoid receptor, mineralocorticoid receptor, and 11β-hydroxysteriod dehydrogenase 1nad 2 in the fetal and postnatal ovine hippocampus; ontogeny and effects of prenatal glucocorticoid exposure. J Endocrinol. 2008;197:213–220. doi: 10.1677/JOE-07-0375. [DOI] [PubMed] [Google Scholar]
- 30.Tegethoff M, Pryce C, Mienlschmidt G. Effects of intrauterine exposure to synthetic glucocorticoids on fetal, newborn, and infant hypothalamic-pituitary-adrenal axis function in humans: s systematic review. Endocrine Rev. 2009;30:753–789. doi: 10.1210/er.2008-0014. [DOI] [PubMed] [Google Scholar]
- 31.Antonow-Schlorke I, Helgert A, Gey C, Coksaygan T, Schubert H, Nathanielsz PW, Witte OW, Schwab M. Adverse effects of antenatal glucocorticoids on cerebral myelination in sheep. Obstet Gynecol. 2009;113:143–151. doi: 10.1097/AOG.0b013e3181924d3b. [DOI] [PubMed] [Google Scholar]
- 32.McCabe L, Marash D, Li A, Matthews SG. Repeated antenatal glucocorticoid treatment decreases hypothalamic corticotropin releasing hormone mRNA but not corticosteroid receptor mRNA expression in the fetal guinea-pig brain. J Neuroendocrinol. 2001;13:425–431. doi: 10.1046/j.1365-2826.2001.00649.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Li A, Matthews SG. Maternal glucocorticoid treatment programs HPA regulation in adult offspring: sex-specific effects. Am J Physiol Endocrinol Metab. 2001;280:E729–E739. doi: 10.1152/ajpendo.2001.280.5.E729. [DOI] [PubMed] [Google Scholar]
- 34.Iqbal M, Moisiadid VG, Kostali A, Matthews SG. Transgenerational Effects of prenatal synthetic glucocorticoids on hypothalamic-pituitary-adrenal function. Endocrin. 2012 doi: 10.1210/en2010-1054. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodrigues-Gonzalez GL, Guzman C, Larrea F, Nathanielsz PW. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol. 2005;566:225–236. doi: 10.1113/jphysiol.2005.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertram C, Khan O, Ohri S, Phillips DI, Matthews SG, Hanson MA. Transgenerational effects of prenatal nutrient restriction on cardiovascular and hypothalamic-pituitary-adrenal function. J Physiol. 2008;586:2217–2229. doi: 10.1113/jphysiol.2007.147967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunn GA, Bale TL. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology. 2009;150:4999–5009. doi: 10.1210/en.2009-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Painter RC, Osmond C, Gluckman P, Hanson M, Phillips DI, Roseboom TJ. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG. 2008;115:1243–1249. doi: 10.1111/j.1471-0528.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 39.Yehuda R, Teicher MH, Seckl JR, Grossman RA, Morris A, Bierer L. Parental posttraumatic stress disorder as a vulnerability factor for low cortisol trait in offspring of holocaust survivors. Arch Gen Psychiatry. 2007;64:1040–1048. doi: 10.1001/archpsyc.64.9.1040. [DOI] [PubMed] [Google Scholar]
- 40.Mathews SG, Phillips DIW. Minireview: transgenerational inheritance of the stress response; a new frontier in stress research. Endocrinol. 2010;151:7–13. doi: 10.1210/en.2009-0916. [DOI] [PubMed] [Google Scholar]
- 41.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 42.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 43.Szyf M, McGowan P, Meaney MJ. The social environment and the epigenome. Environ Mol Mutagen. 2008;49:46–60. doi: 10.1002/em.20357. [DOI] [PubMed] [Google Scholar]
- 44.Nijland MJ, Mitsuya K, Li C, et al. Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability. J Physiol. 2010;588:1349–359. doi: 10.1113/jphysiol.2009.184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cruod A, Petropoulos S, Moisiadis VG, Iqbal M, Kostaki A, Machnes Z, Szyf M, Matthews SG. Prenatal synthetic glucocorticoid treatment changes DNA methylation states in male organs systems: multigenerational effects. Endocrinol. 2012 doi: 10.121/en.2011-2160. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Institutes of Health. Consensus development panel on the effects of corticosteroids for fetal maturation on perinatal outcomes. Effects of corticosteroids for fetal maturation on perinatal outcomes. JAMA. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]