Abstract
Background
Hemolysis causes anemia in falciparum malaria, but its contribution to microvascular pathology in severe malaria (SM) is not well characterized. In other hemolytic diseases, release of cell-free hemoglobin causes nitric oxide (NO) quenching, endothelial activation, and vascular complications. We examined the relationship of plasma hemoglobin and myoglobin to endothelial dysfunction and disease severity in malaria.
Methods
Cell-free hemoglobin (a potent NO quencher), reactive hyperemia peripheral arterial tonometry (RH-PAT) (a measure of endothelial NO bioavailability), and measures of perfusion and endothelial activation were quantified in adults with moderately severe (n = 78) or severe (n = 49) malaria and control subjects (n = 16) from Papua, Indonesia.
Results
Cell-free hemoglobin concentrations in patients with SM (median, 5.4 μmol/L; interquartile range [IQR], 3.2–7.4 μmol/L) were significantly higher than in those with moderately severe malaria (2.6 μmol/L; IQR, 1.3–4.5 μmol/L) or controls (1.2 μmol/L; IQR, 0.9–2.4 μmol/L; P < .001). Multivariable regression analysis revealed that cell-free hemoglobin remained inversely associated with RH-PAT, and in patients with SM, there was a significant longitudinal association between improvement in RH-PAT index and decreasing levels of cell-free hemoglobin (P = .047). Cell-free hemoglobin levels were also independently associated with lactate, endothelial activation, and proinflammatory cytokinemia.
Conclusions
Hemolysis in falciparum malaria results in NO quenching by cell-free hemoglobin, and may exacerbate endothelial dysfunction, adhesion receptor expression and impaired tissue perfusion. Treatments that increase NO bioavailability may have potential as adjunctive therapies in SM.
Hemolysis of infected and uninfected red blood cells is an important cause of anemia in falciparum malaria [1], but its contribution to other pathophysiological pathways in severe malaria (SM) is less well characterized. A central process in the pathogenesis of severe falciparum malaria is microvascular obstruction resulting from cytoadherence of parasitized erythrocytes to activated endothelial cells, associated with impaired bioavailability of endothelial nitric oxide (NO) [2, 3]. Reduced NO bioavailability in malaria contributes to increased endothelial activation, increased cytoadherence of parasitized erythrocytes, and impaired vasomotor regulation [3, 4], and it is associated with increased mortality in murine malaria [5] and disease severity in both adults and children [3, 6]. The etiology of impaired NO bioavailability in malaria appears to be multifactorial [3, 6]. Decreased l-arginine (the substrate for NO synthesis) is noted in SM. This hypoargininemia is considered a major contributor to the low NO bioavailability in both adults and children [3, 7, 8], but recent data suggest that hemolysis may also be important.
Intravascular hemolysis plays a central role in the outcome and pathogenesis of hemolytic diseases, such as sickle cell disease (SCD) and paroxysmal nocturnal hemoglobinuria [9, 10]. The proposed mechanism is a reduction in endothelial NO bioavailability due to stoichiometric inactivation of NO by the cell-free hemoglobin released with erythrocyte rupture [9, 11]. In SCD, cell-free hemoglobin is elevated and strongly correlated with measurements of NO quenching, decreased NO-mediated vascular flow, and increased endothelial activation [11]. Hemolysis also releases erythrocyte arginase, an enzyme that metabolizes l-arginine. This reduces the amount of l-arginine available for conversion to NO, further contributing to endothelial dysfunction [7, 12]. In SCD, these mechanisms contribute to complications, including pulmonary hypertension and mortality [13].
Although most erythrocyte destruction in falciparum malaria occurs extravascularly [14], a significant proportion of hemolysis occurs in the intravascular compartment, with plasma haptoglobin concentrations markedly decreased during active disease [3, 14]. The clinical consequences of increased cell-free hemoglobin levels in malaria and other infections causing intravascular hemolysis have not been well characterized. Muscle breakdown during falciparum malaria increases plasma myoglobin [15], which, like hemoglobin, can quench NO [16]. We have described elsewhere an inverse association between endothelial function and plasma lactate dehydrogenase (LDH) levels in falciparum malaria [3]. However, LDH may not be a specific measure of hemolysis in malaria, with extraerythrocytic sources of LDH likely to be significant.
The association between cell-free hemoglobin, myoglobin, and endothelial NO bioavailability is not known in malaria. In a prospective observational study, we tested the following hypotheses in adult falciparum malaria: (1) levels of cell-free hemoglobin and myoglobin (mediators of NO quenching) are increased in proportion to disease severity; (2) these levels correlate with impairment of both endothelial function and tissue perfusion; and (3) these levels correlate with increases in endothelial activation, proinflammatory cytokine levels, and parasite biomass.
Patients, Materials, and Methods
Study site
The study was performed at Mitra Masyarakat Hospital in Timika, Papua, Indonesia, an area with unstable transmission of multidrug-resistant malaria [17, 18]. Written, informed consent was obtained from all patients or relatives; ethical approval was obtained from the institutional review boards of the National Institute of Health Research and Development and Menzies School of Health Research.
Patients
Patients were ≥ 18 years old with moderately severe or severe Plasmodium falciparum malaria without Plasmodium vivax infection and with a hemoglobin level of >60 g/L; they had been prospectively enrolled in a study of endothelial dysfunction, as reported elsewhere [3]. In brief, SM was defined as P. falciparum parasitemia and ≥ 1 modified World Health Organization (WHO) criterion of severity (excluding severe anemia) [3]; moderately severe malaria (MSM) was defined as fever within the preceding 48 h, >1000 asexual P. falciparum parasites/μL, no WHO warning signs or SM criteria, and a requirement for inpatient parenteral therapy because of inability to tolerate oral treatment. Healthy controls were unrelated hospital visitors with no history of fever in the last 48 h and no parasites seen at microscopy, negative results of histidine-rich protein 2 (HRP2) antigen testing, no evidence of intercurrent illness, and no history of smoking in the preceding 12 h [3]. On recruitment, a standardized history was obtained, physical examination was performed, and endothelial function was measured. Heparinized blood was collected daily and centrifuged within 30 min of collection, and plasma was stored at −70°C. Patients with MSM were treated with intravenous quinine, and those with SM received either intravenous quinine or artesunate, with both groups also receiving either doxycycline or clindamycin. Parasite counts were determined from microscopy of Giemsa-stained thick and thin films. Hemoglobin, biochemistry, acid-base parameters, and lactate level (as a measure of tissue perfusion) were measured with a bedside analyzer (i-STAT), and LDH, creatine kinase, and creatinine levels were measured with a COBAS analyzer (Roche).
Cell-free hemoglobin, cytokines, endothelial activation, arginase, and l-arginine
Plasma concentrations of cell-free hemoglobin were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Bethyl Laboratories). Plasma concentrations of the endothelial activation markers, intercellular adhesion molecule 1 (ICAM-1), E-selectin and angiopoietin 2 were measured by ELISA (R&D), as described elsewhere [4]. Total parasite biomass was quantified by measuring plasma HRP2 with ELISA, as described elsewhere [3]. Tumor necrosis factor (TNF) and interleukin (IL)–6 concentrations were measured by flow cytometry (BD Cytometric Bead Array). Plasma arginase activity was measured using a radiometric assay [3, 12], and plasma l-arginine was measured by high-performance liquid chromatography (Shimadzu), using methods described elsewhere [3].
Endothelial function
Endothelial function was measured noninvasively by using peripheral arterial tonometry (Endo-PAT) to determine the change in digital pulse wave amplitude in response to reactive hyperemia, giving a reactive hyperemia peripheral arterial tonometry (RH-PAT) index. With this technique, the arterial pulsatile volume of the index finger at rest is compared with that after an increase in shear stress induced by 5 min of forearm ischemia (sphygmomanometer inflated to 200 mm Hg). Systemic influences were reduced by simultaneously performing peripheral arterial tonometry in the contralateral index finger without reactive hyperemia. Patients were assessed in a quiet, temperature-controlled environment, with arms relaxed and supported by cushions, according to the manufacturer's instructions [3, 19]. The RH-PAT index is at least 50% dependent on endothelial NO production [20]. Endothelial function was measured daily until death or discharge or until the RH-PAT index was above an a priori cutoff value (1.67) for 2 consecutive days [7].
Statistical methods
Statistical analysis was performed with Stata software, version 9.2 (Stata). Intergroup differences were compared by analysis of variance or Kruskal-Wallis test, where appropriate. Pearson's or Spearman's correlation coefficients were determined depending on normality of distributions. Multiple stepwise linear regression was used to adjust for confounding variables. Mixed-effects modeling using generalized estimating equations was used to assess longitudinal associations.
Results
Patients
Of the 177 subjects prospectively enrolled in the original study, plasma was available for cell-free hemoglobin assay in 49 (96%) of 51 patients with SM, all 78 with MSM (100%), and 16 (33%) of 48 healthy control subjects. Baseline characteristics of these 143 patients were not significantly different from those in the original group (Table 1). Of the patients with SM, 26 (53%) had coma, 17 (35%) had acute renal failure, 23 (47%) had hyperbilirubinemia with either renal impairment or high parasitemia (parasite load, >100,000/μL), and 30 (61%) had ≥2 severity criteria. In total, 35 patients with SM (71%) were treated with intravenous artesunate and 14 (29%) with intravenous quinine, whereas 77 of the 78 patients with MSM were treated with quinine (the other received artesunate). Eight patients (16%) died in the SM group, and none in the MSM group. Repeated measurements were possible in only 1 of the 8 fatal cases.
Table 1. Baseline Characteristics of Patients, According to Clinical Status.
| Characteristic | Healthy control subjects (n= 16) | Patients with malaria | |
|---|---|---|---|
|
| |||
| Moderately severe (n= 78) | Severe (n= 49) | ||
| Age, mean years (range) | 25 (19–42) | 28 (18–56) | 29 (18–56) |
|
| |||
| No. (%) of male subjects | 12 (75) | 32 (41) | 36 (74) |
|
| |||
| Weight, mean kg (range) | 58 (45–85) | 58 (43–77) | 57 (45–70) |
|
| |||
| Ethnicity, no. (%) of Papuan highlandersa | 15 (94) | 59 (76) | 27 (55) |
|
| |||
| No. (%) of current smokers | 8 (50) | 31 (40) | 22 (45) |
|
| |||
| Duration fever before presentation, median days (IQR)b | … | 2 (1–5) | 4 (1–7) |
|
| |||
| Systolic blood pressure, mean mm Hg (range)b | 131 (116–145) | 110 (80–134) | 105 (60–154) |
|
| |||
| Pulse rate, mean beats/min (range)b | 65 (44–91) | 81 (54–118) | 98 (61–138) |
|
| |||
| Respiratory rate, mean breaths/min (range)b | 20 (18–24) | 23 (16–32) | 30 (16–60) |
|
| |||
| Temperature, mean °C (range) | 35.6 (35–36.7) | 36.5 (34.8–40.2) | 37.2 (34.8–40.3) |
NOTE. IQR, interquartile range.
P < .01, by χ2 test.
P < .01, by analysis of variance or 2-sided t test.
Cell-free hemoglobin, myoglobin, and clinical disease
Plasma concentrations of cell-free hemoglobin were significantly elevated in patients with SM, compared with those with MSM and control subjects (overall, P < .001) (Table 2 and Figure 1). Among patients with SM, the median cell-free hemoglobin level in the 14 patients with isolated cerebral malaria was 5.4 μmol/L (interquartile range [IQR], 1.9–8.4 μmol/L). These values were not significantly different from those in patients with dysfunction of 2–4 organ systems. A patient with blackwater fever and acute renal failure had a cell-free hemoglobin concentration of 17 μmol/L. In patients with SM, there was no significant difference in cell-free hemoglobin concentration between survivors and those who died. Plasma myoglobin was significantly increased in patients with SM compared with those with MSM and controls (P < .001). In longitudinal analysis, there was a significant decrease in plasma cell-free hemoglobin concentration with clinical recovery, with a median decrease of 1.50 μmol/L per day during the first 5 days of hospitalization (P < .001) (Figure 2).
Table 2. Baseline Laboratory and Physiological Measurements, According to Clinical Status.
| Measurement | Healthy control subjects (n= 16) | Patients with malaria | |
|---|---|---|---|
|
| |||
| Moderately severe (n= 78) | Severe (n= 49) | ||
| White blood cell count, × 103 cells/μL | ND | 5.9 (5.4–6.9) | 9.5 (8.5–10.5) |
|
| |||
| Hemoglobin, mean g/L (range) | ND | 121 (70–170) | 109 (60–163) |
| Cell-free hemoglobin, median μmol/L (IQR) | 1.2 (0.9–2.4) | 2.6 (1.3–4.5) | 5.4 (3.2–7.4) |
|
| |||
| Plasma myoglobin, median μmol/L (IQR) | 0.002 (0.001–0.0025) | 0.002 (0.001–0.003) | 0.015 (0.005–0.03) |
| RH-PAT index | 1.77 (1.5–2.0) | 1.82 (1.71–1.93) | 1.37 (1.32–1.42) |
|
| |||
| Plasma creatinine, μmol/L | ND | 88 (82–94) | 286 (207–365) |
|
| |||
| Lactate, mmol/L | ND | 1.4 (1.2–1.6) | 2.93 (2.3–3.5) |
|
| |||
| Parasite density, geometric meanμL (range) | ND | 14,900 (850–127,000) | 35,100 (125–725,000) |
|
| |||
| HRP2, mean loge ng/mL (range) | ND | 5.75 (1.34–8.79) | 8.08 (1–10.98) |
|
| |||
| Soluble ICAM-1, pg/mL | ND | 569 (516–623) | 938 (792–1084) |
|
| |||
| Soluble E-selectin, pg/mL | ND | 106 (95–118) | 153 (113–193) |
|
| |||
| Plasma angiopoietin 2, pg/mL | 2700 (2000–3300) | 6500 (5000–8000) | 17,000 (14,000–22,000) |
|
| |||
| Plasma arginase activity, μmol/mL/h | 0.13 (0.07–0.16) | 0.20 (0.14–0.24) | 0.26 (0.22–0.31) |
|
| |||
| Plasmal-arginine, μmol/L | 78 (67–89) | 41 (37–44) | 49 (43–56) |
NOTE. Data are means (95% confidence intervals), unless otherwise indicated. P < .01 for all variables, by analysis of variance (overall) or 2-sided t test. HRP2, histidine-rich protein 2; ICAM-1, intercellular adhesion molecule 1; IQR, interquartile range; ND, not determined; RH-PAT, reactive hyperemia peripheral arterial tonometry.
Figure 1.
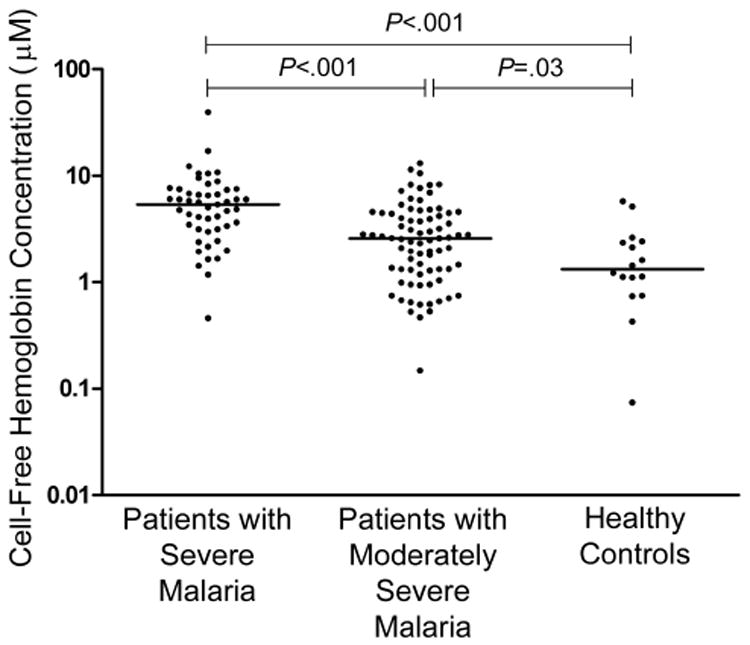
Cell-free hemoglobin concentrations in patients with moderately severe and severe malaria and healthy controls (P < .001, by Kruskal-Wallis test). Horizontal lines indicate medians for each group. Differences among groups were compared using the Kruskal-Wallis test, and the Mann-Whitney U test was used for post hoc pairwise comparisons.
Figure 2.
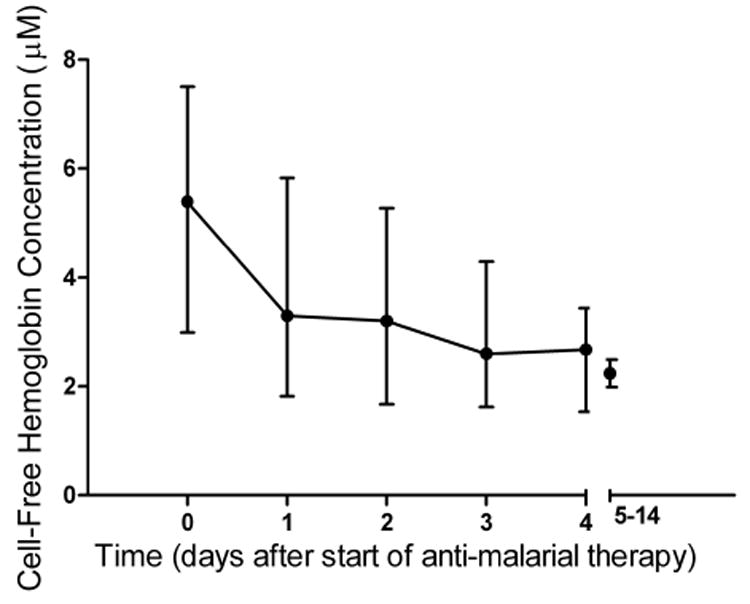
Longitudinal course of cell-free hemoglobin concentrations in patients with severe malaria. Median values (circles) and interquartile range (bars) are displayed for all time points. The x-axis values represent time from the start of antimalarial therapy (day 0, 0–12 h; day 1, 13–36 h; day 2, 37–60 h; day 3, 61–84 h; day 4, 85–109 h; and days 5–14, >110 h).
Cell-free hemoglobin, myoglobin, and the RH-PAT index
Patients with SM had significantly lower RH-PAT indexes at enrollment than those with MSM and control subjects (P < .001) (Table 2). Cell-free hemoglobin levels were inversely associated with RH-PAT indexes at univariate regression (Figure 2 and Table 3) in all patients with malaria (r = −0.36; P < .001) and in the subgroup with SM (r = −0.29; P = .06). There was an inverse association between myoglobin and the RH-PAT index in all patients with malaria (rs = −0.32; P < .001) but not in those with SM (Table 3). In the bivariate model that included plasma myoglobin and cell-free hemoglobin, myoglobin was no longer significantly associated with RH-PAT. For this reason and because concentrations of myoglobin were >100-fold lower than those of cell-free hemoglobin, myoglobin was excluded from subsequent regression analyses. After adjustment for confounders (including plasma HRP2, angiopoietin 2, plasma arginase, and disease severity), plasma arginase and cell-free hemoglobin remained significantly inversely associated with RH-PAT both in all patients with malaria (P = .02 and P < .001, respectively) and in those with SM (P = .046 and P = .02, respectively). In a longitudinal multivariable, mixed-effects model in patients with SM who survived >24 h after enrollment, there were significant associations between improvement in RH-PAT index and both decreasing cell-free hemoglobin (P = .047) and increasing l-arginine (P = .001) concentrations.
Table 3. Correlation between Cell-Free Hemoglobin Concentration and Physiological Measures or Biomarkers of Severity.
| Measurement | All patients with malaria | Patients with severe malaria | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Correlation, rsa | P | df | Correlation, rsa | P | df | |
|
| ||||||
| RH-PAT index | −0.36 | <.001 | 136 | −0.29 | .06 | 48 |
|
| ||||||
| Lactate | 0.38 | <.001 | 128 | 0.29 | .04 | 47 |
|
| ||||||
| HRP2 | 0.50 | <.001 | 109 | 0.25 | .10 | 45 |
|
| ||||||
| ICAM-1 | 0.40 | <.001 | 109 | 0.29 | .04 | 47 |
|
| ||||||
| E-selectin | 0.34 | <.001 | 117 | 0.19 | .20 | 47 |
|
| ||||||
| Angiopoietin 2 | 0.34 | <.001 | 143 | 0.18 | .20 | 48 |
|
| ||||||
| TNFb | … | … | … | 0.33 | .04 | 39 |
|
| ||||||
| IL-6b | … | … | … | 0.41 | .01 | 39 |
NOTE. df, degrees of freedom; HRP2, histidine-rich protein 2; ICAM-1, intercellular adhesion molecule 1; IL, interleukin; RH-PAT, reactive hyperemia peripheral arterial tonometry; TNF, tumor necrosis factor.
Correlation with cell-free hemoglobin level.
TNF and IL-6 levels were measured only in patients with severe malaria.
Cell-free hemoglobin and endothelial activation
Relative to patients with MSM, patients with SM had significantly higher plasma concentrations of soluble ICAM-1, E-selectin, and angiopoietin 2 (Table 2). Cell-free hemoglobin was correlated with all 3 parameters among all patients with malaria: ICAM-1 (r = 0.4; P < .001), E-selectin (r = 0.33; P < .001), and angiooietin 2 (r = 0.42; P < .001) (Table 3). After adjustment for plasma HRP2 and disease severity, cell-free hemoglobin remained significantly associated with ICAM-1.
Cell-free hemoglobin and biomarkers of severity
Compared with patients with MSM, those with SM had higher concentrations of lactate (P < .001) and plasma HRP2 (P < .001) (Table 2). Blood lactate was correlated with cell-free hemoglobin, both in all patients with malaria (rs = 0.38; P < .001) and those with SM (rs = 0.29; P = .04) (Table 3). Although plasma HRP2 was correlated with cell-free hemoglobin (r = 0.5; P < .001), this association was not significant in the subgroup with SM. In multivariable analysis including all patients with malaria and controlling for disease severity, lactate was associated with plasma HRP2 (P < .001) and cell-free hemoglobin (P = .05). In a longitudinal mixed-effects model, the fall in lactate during clinical recovery from SM was significantly associated with the decrease in cell-free hemoglobin concentration (r = −0.5; P < .001).
TNF and IL-6 levels were measured only in patients with SM, with median plasma concentrations of 2.4 pg/mL (IQR, 1.5–4.2 pg/mL) for TNF and 84 pg/mL (IQR, 15–501 pg/mL) for IL-6. In patients with SM, cell-free hemoglobin level was correlated significantly with both TNF level (r = 0.33; P = .04) and IL-6 (r = 0.41; P = .01) (Table 3).
Cell-free hemoglobin, LDH, and markers of organ dysfunction
The LDH levels were correlated significantly with levels of cell-free hemoglobin (rs = 0.65; P < .001), creatinine (rs = 0.68; P < .001), and creatine kinase (rs = 0.48; P < .001). These associations remained significant after stratification by disease severity.
Cell-free hemoglobin, plasma arginase activity, and markers of hepatic function
Plasma arginase activity levels were increased in patients with SM (P < .001) (Table 3). Among all patients with malaria, plasma arginase activity was correlated with levels of cell-free hemoglobin (r = 0.29; P = .005) and alanine transaminase (r = 0.29; P = .01), suggesting potential contributions to circulating arginase from both erythrocytic and hepatic sources.
Discussion
The plasma cell-free hemoglobin concentration increases with malaria disease severity, and levels are associated with impaired endothelial NO bioavailability, endothelial activation, increased parasite biomass, and impaired tissue perfusion, as measured by blood lactate concentrations. Quenching of NO by cell-free hemoglobin probably contributes to impaired endothelial homeostasis with microvascular dysfunction, increased adhesion receptor expression, increased microvascular sequestration of parasitized erythrocytes, and tissue hypoxia. Endothelial dysfunction was independently associated with increases in plasma cell-free hemoglobin and plasma arginase activity. This suggests that, as in SCD, both of these consequences of hemolysis contribute to the impaired bioavailability of endothelial NO found in malaria.
Similar to the findings in SCD, we found a significant association between LDH and cell-free hemoglobin [21]. LDH levels are much higher in malaria than in SCD, and they are associated with creatinine and creatine kinase levels—measures of renal and muscle damage [3]. Thus, unlike the mechanism in SCD, nonerythrocytic sources probably contribute significantly to the elevated LDH concentrations. Cell-free hemoglobin has been clearly shown to be a direct measure of NO quenching in SCD [11] and therefore accurately reflects both hemolysis and NO quenching capacity in malaria. The rate constants for the NO dioxygenation reactions of myoglobin and hemoglobin are similar. However, plasma myoglobin concentrations were ∼160-fold lower than cell-free hemoglobin concentrations, indicating that the contribution of myoglobin to NO scavenging in malaria is much less significant than that of cell-free hemoglobin.
NO reacts with oxyhemoglobin, producing methemoglobin and nitrate. The rapid rate of this reaction, together with the large amount of erythrocytic hemoglobin, should theoretically reduce NO to levels too low to function physiologically [22, 23]. In explaining this paradox, NO diffusion is reduced by up to 600-fold by an erythrocyte-free zone at the edge of the endothelium and erythrocyte submembrane barriers [24, 25]. However, intravascular hemolysis with hemoglobin release into plasma can reduce endothelial NO bioavailability dramatically [9, 26]. Furthermore, cytoadherence of parasitized erythrocytes to the endothelium with loss of the erythrocyte-free zone may be an additional mechanism increasing NO quenching in malaria.
In vitro, NO decreases endothelial adhesion receptor expression [27] and cytoadherence of P. falciparum–parasitized erythrocytes [28]. The association between plasma ICAM-1 concentrations and cell-free hemoglobin suggests that quenching of endothelial NO in malaria contributes to increased adhesion receptor expression in vivo. Endothelial activation increases parasite sequestration, which is suggested by the independent associations observed between cell-free hemoglobin and both ICAM-1 and HRP2. Increased blood lactate, a prognostic marker in SM, is considered to be a result of reduced tissue perfusion and oxygen delivery. In our study, both HRP2 and cell-free hemoglobin were independently associated with increased lactate concentrations, suggesting that both parasite sequestration and NO quenching contribute to impaired perfusion [29].
In both malaria and SCD, most erythrocyte destruction occurs extravascularly through phagocytosis of erythrocytes by macrophages, with a lesser contribution from intravascular hemolysis [14, 30]. Nevertheless, hemolysis in the intravascular compartment is sufficient to result in increased plasma hemoglobin, with the concentrations we describe in malaria being comparable to those reported from other studies of acute malaria in both children and adults [14, 31, 32]. Although the plasma hemoglobin concentrations are not markedly elevated, the physiological effects on endothelial NO bioavailability, even at these concentrations, have been shown to be highly significant in vitro [23].
The clinical consequences of hemolysis-related NO quenching have been best studied in SCD, a disorder in which increased plasma cell-free hemoglobin is associated with elevated plasma NO-quenching capacity, decreased blood flow in response to NO donors, and increased endothelial adhesion receptor expression [10, 11]. NO quenching is also hypothesized to explain the increases in blood pressure and mortality seen with use of artificial hemoglobin solutions [33].
Although there are differences in the clinical features and pathogenic mechanisms between SCD and severe falciparum malaria, there may be similarities in the microvascular consequences of increased plasma hemoglobin. Concentrations of cell-free hemoglobin in SM were comparable to those found in SCD [11], which are sufficient to result in increased ICAM-1 and E-selectin levels and attenuate the blood flow response to NO donors [11]. In vitro, 6 μmol/L hemoglobin, a level similar to the median values found in the patients with SM, inhibits the vasodilator response to NO [23]. Furthermore, quantitative models show that the micromolar amounts of cell-free hemoglobin found in SCD (and now shown in SM) can reduce NO-mediated vasodilation in arterioles [34]. Cell-free hemoglobin–related NO quenching will therefore impair arteriolar vasodilatory regulation, the microcirculatory mechanism in which NO is most effective in regulating organ perfusion [23]. In malaria, this may cause decreased functional capillary density [35], adding to the impaired microvascular flow and tissue hypoxia [36] already occurring from microvascular sequestration of parasitized erythrocytes [29]. Decreased functional capillary density is associated with increased mortality in rodent malaria models [37] and human sepsis [38]. Deleterious effects of decompartmentalized hemoglobin are minimized by binding to haptoglobin, but this system is usually overwhelmed in malaria, with >90% of adults with SM having undetectable plasma haptoglobin [3].
Increased levels of proinflammatory cytokines, including TNF, are associated with a poor clinical outcome in African children and Asian adults with falciparum malaria [39, 40]. In vitro, hemoglobin impairs NO-mediated cytotoxic activity in macrophages [41]. NO attenuates proinflammatory cytokinemia by inhibiting activation of nuclear transcription factor κB [42]. The association between cell-free hemoglobin and TNF and IL-6 in malaria suggests that NO quenching may increase nuclear transcription factor κB–mediated production of proinflammatory cytokines, endothelial activation, cytoadherence, and consequent cellular damage.
Our study has several limitations. RH-PAT is at least 50% NO dependent [20]. We cannot exclude a contribution to RH-PAT in malaria from other vasodilators, such as prostacyclin and endothelium-derived hyperpolarizing factor [20]. Assessment of digital arteriolar function may not fully reflect microcirculatory NO bioavailability in malaria-affected organs. It is possible that increased cell-free hemoglobin may be an epiphenomenon, with the pathological associations arising from the effects of pathogenic parasite products released during erythrocyte rupture. This is unlikely, however, because most hemolysis in malaria arises from the destruction of nonparasitized erythrocytes [1]. Furthermore, cell-free hemoglobin remained inversely correlated with endothelial NO bioavailability and perfusion even after adjustment for potential covariates, including parasite biomass. Based on the strong relationship between cell-free hemoglobin and NO quenching in SCD, we postulate that this mechanism underlies the clear relationship between cell-free hemoglobin and impaired endothelial NO bioavailability and endothelial activation in malaria. Increased cell-free hemoglobin also results in increased plasma heme, which may also contribute to endothelial dysfunction and activation. Heme has additional proinflammatory properties and deleterious effects on endothelial cells, including breakdown of endothelial integrity, with microvascular thrombus formation and obstruction [43]. Because we excluded individuals with hemoglobin concentrations of <60 g/L, we are unable to extrapolate our results to severe malarial anemia. Although we cannot exclude a degree of artifactual hemolysis during blood sampling, contributing to the variability in cell-free hemoglobin concentrations, the same method of collection was used in all subjects and would not account for the increased levels in those with severe disease nor for the associations with endothelial NO. Furthermore, the plasma hemoglobin levels we observed are comparable to findings of other studies [14, 31, 32]. Finally, our study population was confined to adults, and additional studies are required to determine whether similar pathogenic processes occur in children with malaria. Because NO bioavailability is also impaired in African children with cerebral malaria [6] and cell-free hemoglobin levels are similarly increased in children with malaria [14, 32], it is likely that NO quenching also contributes to endothelial dysfunction in children with SM.
In summary, hemolysis causes more than just anemia in falciparum malaria. Cell-free hemoglobin increases in proportion to disease severity in malaria and is associated with decreased endothelial NO bioavailability, increased endothelial adhesion receptor expression, proinflammatory cytokinemia, impaired tissue perfusion, and parasite sequestration. Therapies considered for use in SCD to decrease the deleterious effects of cell-free hemoglobin [44], such as haptoglobin infusion, may have potential efficacy in SM. Treatments that increase NO bioavailability (eg, l-arginine [3, 45] or nitrite infusion or inhaled NO) may also have potential as adjunctive therapies in SM.
Acknowledgments
We thank Govert Waramori, Marlini Malisan, Margaretha Ferre, Ferryanto Chalfein, Prayoga, Roesmini, and Yoshi Elvi for nursing, technical, and logistical assistance; Mitra Masyarakat Hospital staff for clinical care; and Jeanne Rini, Paulus Sugiarto, Mauritz Okeseray, and Lembaga Pengembangan Masyarakat Amungme Kamoro for support. Purified P. falciparum HRP2 protein was kindly supplied by David Sullivan.
Financial support: National Health and Medical Research Council (International Collaborative Research Grant [ICRG] 283321 and practitioner fellowship to N.M.A.), Wellcome Trust (ICRG GR071614MA and career development award 074637 to R.N.P.), VA Research Service, National Institutes of Health (grants AI55982 and AI041764), and the Tudor Foundation.
Footnotes
Potential conflicts of interest: N.M.A., D.L.G., and J.B.W. are named as inventors in a US patent for the use of l-arginine as treatment for severe malaria but have transferred all their rights to their respective institutional malaria research collaborations. This patent is issued for US rights only, and no rights are being sought in other countries. All other authors report no other conflicting interests.
Presented in part: Annual Scientific Meeting of the American Society of Tropical Medicine and Hygiene, New Orleans, December 2008 (abstract 1194).
References
- 1.Jakeman GN, Saul A, Hogarth WL, Collins WE. Anaemia of acute malaria infections in non-immune patients primarily results from destruction of uninfected erythrocytes. Parasitology. 1999;119(Pt 2):127–33. doi: 10.1017/s0031182099004564. [DOI] [PubMed] [Google Scholar]
- 2.Dondorp AM, Pongponratn E, White NJ. Reduced microcirculatory flow in severe falciparum malaria: pathophysiology and electron-microscopic pathology. Acta Trop. 2004;89:309–17. doi: 10.1016/j.actatropica.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Yeo TW, Lampah DA, Gitawati R, et al. Impaired nitric oxide bioavailability and l-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007;204:2693–704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeo TW, Lampah DA, Gitawati R, et al. Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc Natl Acad Sci USA. 2008;105:17097–102. doi: 10.1073/pnas.0805782105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gramaglia I, Sobolewski P, Meays D, et al. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat Med. 2006;12:1417–22. doi: 10.1038/nm1499. [DOI] [PubMed] [Google Scholar]
- 6.Anstey NM, Weinberg JB, Hassanali MY, et al. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184:557–67. doi: 10.1084/jem.184.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeo TW, Lampah DA, Gitawati R, et al. Recovery of endothelial function in severe falciparum malaria: relationship with improvement in plasma l-arginine and blood lactate concentrations. J Infect Dis. 2008;198:602–8. doi: 10.1086/590209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopansri BK, Anstey NM, Weinberg JB, et al. Low plasma arginine concentrations in children with cerebral malaria and decreased nitric oxide production. Lancet. 2003;361:676–8. doi: 10.1016/S0140-6736(03)12564-0. [DOI] [PubMed] [Google Scholar]
- 9.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–62. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 10.Wood KC, Hsu LL, Gladwin MT. Sickle cell disease vasculopathy: a state of nitric oxide resistance. Free Radic Biol Med. 2008;44:1506–28. doi: 10.1016/j.freeradbiomed.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–9. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 12.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–95. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 14.Ekvall H, Arese P, Turrini F, et al. Acute haemolysis in childhood falciparum malaria. Trans R Soc Trop Med Hyg. 2001;95:611–7. doi: 10.1016/s0035-9203(01)90095-1. [DOI] [PubMed] [Google Scholar]
- 15.Davis TM, Pongponratn E, Supanaranond W, et al. Skeletal muscle involvement in falciparum malaria: biochemical and ultrastructural study. Clin Infect Dis. 1999;29:831–5. doi: 10.1086/520444. [DOI] [PubMed] [Google Scholar]
- 16.Flögel U, Merx MW, Godecke A, Decking UK, Schrader J. Myoglobin: a scavenger of bioactive NO. Proc Natl Acad Sci USA. 2001;98:735–40. doi: 10.1073/pnas.011460298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratcliff A, Siswantoro H, Kenangalem E, et al. Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine-pyrimethamine in southern Papua, Indonesia. Trans R Soc Trop Med Hyg. 2007;101:351–9. doi: 10.1016/j.trstmh.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karyana M, Burdarm L, Yeung S, et al. Malaria morbidity in Papua Indonesia, an area with multidrug resistant Plasmodium vivax and Plasmodium falciparum. Malar J. 2008;7:148. doi: 10.1186/1475-2875-7-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Celermajer DS. Reliable endothelial function testing: at our fingertips? Circulation. 2008;117:2428–30. doi: 10.1161/CIRCULATIONAHA.108.775155. [DOI] [PubMed] [Google Scholar]
- 20.Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101:545–8. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 21.Kato GJ, McGowan V, Machado RF, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107:2279–85. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lancaster JR., Jr A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide. 1997;1:18–30. doi: 10.1006/niox.1996.0112. [DOI] [PubMed] [Google Scholar]
- 23.Vaughn MW, Kuo L, Liao JC. Effective diffusion distance of nitric oxide in the microcirculation. Am J Physiol. 1998;274:H1705–14. doi: 10.1152/ajpheart.1998.274.5.H1705. [DOI] [PubMed] [Google Scholar]
- 24.Liao JC, Hein TW, Vaughn MW, Huang KT, Kuo L. Intravascular flow decreases erythrocyte consumption of nitric oxide. Proc Natl Acad Sci USA. 1999;96:8757–61. doi: 10.1073/pnas.96.15.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. J Biol Chem. 2000;275:2342–8. doi: 10.1074/jbc.275.4.2342. [DOI] [PubMed] [Google Scholar]
- 26.Minneci PC, Deans KJ, Zhi H, et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–17. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Caterina R, Libby P, Peng HB, et al. Nitric oxide decreases cytokineinduced endothelial activation: nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–8. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serirom S, Raharjo WH, Chotivanich K, Loareesuwan S, Kubes P, Ho M. Anti-adhesive effect of nitric oxide on Plasmodium falciparum cytoadherence under flow. Am J Pathol. 2003;162:1651–60. doi: 10.1016/S0002-9440(10)64299-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dondorp AM, Ince C, Charunwatthana P, et al. Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis. 2008;197:79–84. doi: 10.1086/523762. [DOI] [PubMed] [Google Scholar]
- 30.Naumann HN, Diggs LW, Barreras L, Williams BJ. Plasma hemoglobin and hemoglobin fractions in sickle cell crisis. Am J Clin Pathol. 1971;56:137–47. doi: 10.1093/ajcp/56.2.137. [DOI] [PubMed] [Google Scholar]
- 31.Jakobsen PH, Bygbjerg IC, Theander TG, et al. Soluble haemoglobin is a marker of recent Plasmodium falciparum infections. Immunol Lett. 1997;59:35–42. doi: 10.1016/s0165-2478(97)00098-9. [DOI] [PubMed] [Google Scholar]
- 32.O'Donnell A, Weatherall DJ, Taylor AM, Reeder JC, Allen SJ. Muscle cell injury, haemolysis and dark urine in children with falciparum malaria in Papua New Guinea. Trans R Soc Trop Med Hyg. 2006;100:817–25. doi: 10.1016/j.trstmh.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death. JAMA. 2008;299:2304–12. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeffers A, Gladwin MT, Kim-Shapiro DB. Computation of plasma hemoglobin nitric oxide scavenging in hemolytic anemias. Free Radic Biol Med. 2006;41:1557–65. doi: 10.1016/j.freeradbiomed.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellis C, Bateman R, Sharpe M, Sibbald W, Gill R. Effect of a maldistribution of microvascular blood flow on capillary O2 extraction in sepsis. Am J Physiol Heart Circ Physiol. 2002;282:H156–64. doi: 10.1152/ajpheart.2002.282.1.H156. [DOI] [PubMed] [Google Scholar]
- 36.Krogh A. The number and the distribution of capillaries in muscle with the calculation of the oxygen pressure necessary for supplying tissue. J Physiol (Lond) 1919;52:409–515. doi: 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martini J, Gramaglia I, Intaglietta M, van der Heyde HC. Impairment of functional capillary density but not oxygen delivery in the hamster window chamber during severe experimental malaria. Am J Pathol. 2007;170:505–17. doi: 10.2353/ajpath.2007.060433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet. 2002;360:1395–6. doi: 10.1016/s0140-6736(02)11393-6. [DOI] [PubMed] [Google Scholar]
- 39.Grau GE, Taylor TE, Molyneux ME, et al. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–91. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 40.Day NP, Hien TT, Schollaardt T, et al. The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J Infect Dis. 1999;180:1288–97. doi: 10.1086/315016. [DOI] [PubMed] [Google Scholar]
- 41.Weinberg JB, Hibbs JB., Jr Endocytosis of red blood cells or haemoglobin by activated macrophages inhibits their tumoricidal effect. Nature. 1977;269:245–7. doi: 10.1038/269245a0. [DOI] [PubMed] [Google Scholar]
- 42.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–16. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 43.Ferreira A, Balla A, Jeney V, Balla G, Soares MP. A central role for free heme in the pathogenesis of severe malaria: the missing link? J Mol Med. 2008;86:1097–111. doi: 10.1007/s00109-008-0368-5. [DOI] [PubMed] [Google Scholar]
- 44.Morris CR, Gladwin MT, Kato GJ. Nitric oxide and arginine dysregulation: a novel pathway to pulmonary hypertension in hemolytic disorders. Curr Mol Med. 2008;8:620–32. doi: 10.2174/156652408786241447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeo TW, Rooslamiati I, Gitawati R, et al. Pharmacokinetics of l-arginine in adults with moderately severe malaria. Antimicrob Agents Chemother. 2008;52:4381–7. doi: 10.1128/AAC.00421-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


