Introduction
Eicosanoids are oxidation products of C20 polyunsaturated fatty acids (e.g. arachidonic acid) that include prostaglandins, thromboxanes, leukotrienes and hydroperoxy fatty acids. They have important biological roles in vivo, including regulation of renal, cardiovascular and gastrointestinal function. Historically, eicosanoids were thought to mediate their signaling actions exclusively as free acids, however evidence is now emerging that they may also be formed attached to other functional groups including phospholipids and glycerol, and that these more complex forms may also be pathophysiological signaling mediators in their own right. Early studies showed that exogenously added eicosanoids could become esterified into membrane phospholipids of cells, while more recently, it was uncovered that such esterified eicosanoids were formed endogenously. This review summarizes our current knowledge of this area, starting with the early discoveries documenting what is known about eicosanoid generation and their esterification, and moving on to discuss the discovery that esterified eicosanoids are generated endogenously by a number of different cell types. These discussions will include recent research that is highlighting new structures and functions of these important lipid mediators.
1. Generation of free eicosanoids
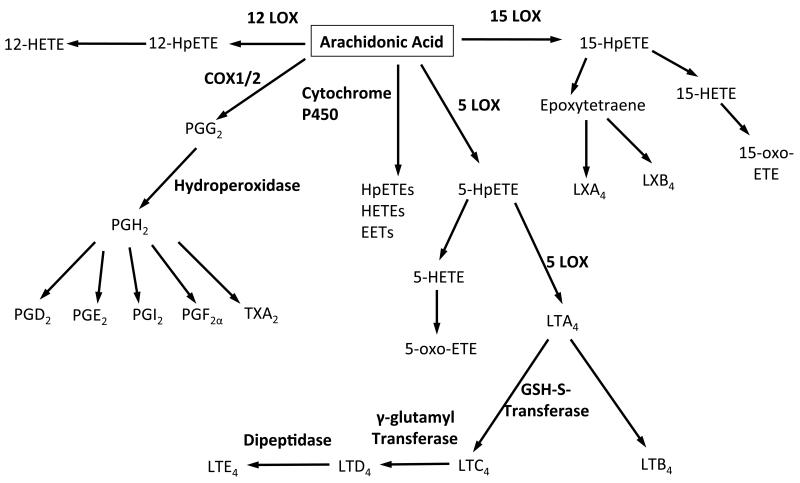
Eicosanoids are generated by three separate enzyme families, lipoxygenases (LOX), cyclooxygenases (COX) and cytochrome P450 (CYP), which catalyze lipid peroxidation in a stereo- and regio-specific manner (Figure 1). All display strict cell and tissue expression patterns, and their activities are controlled by both transcriptional and post-transcriptional regulation. In terms of esterified eicosanoids, all three enzyme families have been characterized in vitro and in vivo as sources.
Figure 1. Summary of eicosanoid generating pathways including enzymes and metabolites.
1.1 Lipoxygenases
Lipoxygenases catalyze the stereo selective oxygenation of polyunsaturated fatty acids containing at least one (1Z,4Z)-penta-1,4-dienoic system to the corresponding hydroperoxy derivatives. The radical mechanism of lipoxygenase occurs in three steps, (i) hydrogen abstraction, the position of which depends on the active site of the LOX isoform, (ii) radical rearrangement and (iii) oxygen insertion. LOXs are classified based on their position of oxygen insertion in the arachidonate substrate. 12/15-LOXs have been well characterized in human and mouse platelets, which posses the platelet 12-LOX isoform, human monocytes (15-LOX), human neutrophils (5-LOX) and murine peritoneal macrophages (12/15-LOX). There are also a number of skin isoforms, including 12R-LOX and 8-LOXs. The primary product of LOX oxidation is the unstable hydroperoxide, which is rapidly reduced to its corresponding hydroxide in a reaction catalyzed by glutathione peroxidases (GPX). For example, in the case of platelet 12-LOX, the hydroperoxide, 12S-hydroperoxyeicosatetraenoic acid (12S-HpETE), is reduced to 12-hydroxyeicosatetraenoic acid (12S-HETE). Alternatively, further LOX metabolism of the hydroperoxides forms lipoxins (e.g. LXA4) and hepoxilins. Since LXA4 generation requires the action of 15- and 5-LOX, or 5- and 12-LOX, it is believed that their formation requires sequential transcellular metabolism. HETEs can also be oxidized to form their keto derivatives, ketoeicosatetraenoic acid (KETEs), usually via the action of either 5-hydroxyeicosanoid dehydrogenase (HEDH) or 15-prostaglandin dehydrogenase (PGDH). For a detailed review of LOX enzymes and their products, the reader is directed to [1-5].
1.2 Cyclooxygenases
Cyclooxygenases, also known as prostaglandin endoperoxide H synthases, catalyze the conversion of arachidonic acid and oxygen to prostaglandin H2 (PGH2). The mechanism includes a cyclooxygenase reaction in which arachidonate is converted to prostaglandin G2 (PGG2), and a peroxidase reaction in which PGG2 undergoes a two-electron reduction to PGH2. It is initiated by oxidation of the heme peroxidase to an oxoferryl porphyrin cation radical, which then oxidizes a tyrosine to a tyrosyl radical. This then mediates hydrogen abstraction from arachidonate to yield an arachidonyl radical and is followed by rearrangements and sequential additions of oxygen at C-11 and C-15 to yield PGG2. Finally, the peroxidase activity reduces the 15-hyroperoxide group of PGG2 to its corresponding alcohol yielding PGH2. There are two isoforms of COX. COX-1, which is constitutively expressed by platelets and the gastrointestinal tract and is of particular importance for gastrointestinal protection. COX-2 is the inducible form of the enzyme, the expression of which is enhanced by various cytokines and growth factors [6, 7]. Primary products of COX often undergo further enzymatic metabolism to form secondary eicosanoids and their metabolites such as prostaglandin E2 (PGE2), D2 and thromboxaneA2 (TXA2). These possess potent biological activities at nM concentrations, including regulating inflammation, pain and thrombosis.
1.3 Cytochrome P450
Cytochrome P450s comprise a family of membrane bound hemeproteins that catalyze the redox-coupled activation of molecular oxygen and the delivery of an active form of atomic oxygen to a ground state substrate carbon acceptor. These enzymes are widely distributed in plants, insects, and animal tissues. P450s behave as classic monooxygenases with the enzymatic cleavage of molecular oxygen directly followed by the insertion of a single atom of oxygen into the substrate, while the remainder is released as water. Catalytic turnover requires electron transfer from NADPH to the P450 heme iron, a reaction catalyzed by a membrane bound flavoprotein, NADPH-cytochrome P450 reductase [8-10]. Eicosanoid products of this pathway include epoxy-lipids (EETs) and omega hydroxylated fatty acids, such as 20-HETE.
2. Generation of esterified eicosanoids by incorporation of exogenous HETEs into cellular lipids
Until recently, it was widely believed that most eicosanoids are both generated and then signal as free carboxylic acids. Specifically, arachidonate is hydrolyzed from membrane phospholipids (PL) by phospholipases, in particular cytosolic PLA2, which acts on primarily phosphatidylcholine (PC). Following this, eicosanoids diffuse out of the cell and activate G-protein coupled receptors on neighboring cells to trigger tightly regulated signaling events. In contrast to this, a number of groups in the 1980’s-90’s showed that primary and cultured cells could take up radio-labeled HETEs and incorporate them into several classes of phospholipids. The phenomenon was reported in a variety of cell types and using several different HETE positional isomers. This is discussed below and summarized in Table 1.
Table 1. Summary of esterified eicosanoids generated by mammalian cells, and their actions, where known.
| EXOGENOUS: | ||
| Cell Type | Species generated | Function |
| Epithelial cells | 16-, 17-, 18-, 19-, 20- HETE- PE/PS/PI/PC 15-HETE-PI/PE 12-HETE-PI/PE/PC/PS 5-HETE-PE/PC |
|
| Endothelial cells | 5-HETE-PC 12-HETE-PC 15-HETE-PI 16-, 17-, 18-, 19-, 20- HETE- PE/PS/PI/PC |
Inhibits histamine- and arachidonate- stimulated production of PGE2 and 6- ketoPGF1α in human endothelial cells. |
| Neutrophils | 15-HETE-PC/PI | |
| Murine macrophages | 15-HETE-PC/PI | |
| Human peripheral blood mononuclear cells |
12-HETE-PI | |
| ENDOGENOUS: | ||
| Platelets | 12-HETE-PE/PC | Enhances coagulation of platelets |
| Murine macrophages | 12-HETE-PE PGE2-G 2-AG PGH2-G, which is then converted to PGD2-G, PGE2- D, PGF2α-G and PGI2-G. PGE2-AEA |
Calcium mobilization and activation of the ERK/MAPK pathway. Inhibits LPS/IFNγ-induced IL-12 and IL- 23 expression in microglia and macrophages by reducing expression of IL-12p40. |
| Human peripheral blood monocytes |
15-HETE-PE | Inhibition of LPS-induced G-CSF and TNFα generation my human monocytes. Inhibition of NET formation by neutrophils. Promotes MUC5AC formation by |
| Neutrophils | 5-HETE-PE/PC | Inhibition of NET formation. Enhances IL-8 and superoxide generation by neutrophils. |
| Leprosy lesions, low density lipoproteins |
PC-esterified isoprostanes | Exacerbate leprosy infection Increase MCP-1, IL-8 generation via PPARa in endothelial cells, increase monocyte binding to endothelium. |
2.1 Epithelial cells
In kidney epithelial cells, exogenous 5-HETE was shown to be incorporated primarily into PC and PE, within both microsomal and plasma membranes [11]. 12-HETE was incorporated into PE, PC, PS and PI of the same cell line, and into PC and PE of human bronchial epithelial cells [11, 12]. In contrast, 15-HETE was incorporated primarily into PI in a lung epithelial cell line and also kidney epithelial cells [13, 14]. Finally a study in tracheal epithelial cells showed 15-HETE being incorporated into PI, whilst 5- and 12-HETE were incorporated into PC and PE [15]. Overall, epithelial cell studies show that exogenous 5- and 12-HETEs are incorporated mainly into the most abundant phospholipids of these cells (e.g. PE and PC), however uptake of 15-HETE into the far less abundant PI appears to be more selective. Mechanisms of HETE incorporation have not been characterized but are likely to involve fatty acyl CoA ligases, such as members of the MBOAT or LPCAT families that esterify free fatty acids into phospholipids. However, their potential roles in HETE esterification have not yet been studied. Selective incorporation of 15-HETE into different phospholipids as opposed to 5- or 12-HETE indicates that distinct pathways may exist for esterification of different eicosanoid positional isomers.
2.2 Endothelial and kidney cells
Similar studies were performed in aortic endothelial cells, with 12-HETE incorporated into PC within microsomal membranes, 15-HETE into PI, and 20-HETE into various phospholipids of aortic endothelial cells [16-18]. Separate studies showed that 5-HETE is incorporated into PC in human endothelium[19]. In kidney cells, 5-HETE is incorporated into di- and mono-glycerides of adrenal glomerulosa cells, and 12-HETE is incorporated into PC, while cortex and medulla tissues of the kidney also contain esterified cytochrome P450-derived HETEs (16-, 17-, 18-, 19-, 20-HETE) [20, 21]. As for epithelial cells, 15-HETE is again preferentially incorporated into PI but the mechanistic basis for this is unknown.
2.3. Immune cells
Several studies showed that exogenous HETEs could be incorporated into phospholipids of various immune cells. Brezinski and Serhan demonstrated 15-HETE uptake by neutrophils and incorporation into PI (20%) while another 4% was incorporated into other PL classes including PE and PC. In contrast, 5-HETE was predominantly incorporated into PC or triglycerides [22]. Similarly, 15-HETE has been shown to be taken up by murine resident macrophages and incorporated into PI, PC and PE phospholipids [23]. Finally 12-HETE is esterified into PC and PI phospholipids in human peripheral blood mononuclear cells, and this incorporation remains stable with 50% of the HETE-PL pool still present after 18 hours [24]. In all these studies, incorporation of free HETEs takes place over long periods of several hours. A role for esterified HETEs acting as a pool of “stored” eicosanoid which can be released on further agonist activation was proposed, and in support, fMLP, phorbol or calcium ionophore (A23187) were shown to mediate deacylation of PI and release of 15-HETE which subsequently acts as a second signal to inhibit LTB4 generation by the neutrophils themselves [22].
While these studies concentrated on the fate of exogenously added HETEs, the incorporation of endogenously generated eicosanoids was not explored. Importantly, in many experiments, HETE positional isomers were added that would not be expected to be generated by the cells themselves, and at variable concentrations. For example, neutrophils generate 5- but not 12- or 15-HETEs while endothelial cells do not generally express LOX isoforms under basal conditions. Thus, these experiments defined patterns of HETE incorporation that might be expected to require transcellular HETE movement during inflammation or cell activation. Whether such HETE incorporation into phospholipids occurs in vivo is not known.
3. Acute generation of esterified eicosanoids using endogenous substrate
While previous studies demonstrated incorporation of exogenous HETEs into cellular complex lipids, the specific molecular species of PL involved were not defined. More recently, the development of new generation high sensitivity bench top mass spectrometers has enabled identification of specific molecular species of esterified eicosanoids present in complex biological samples. Specifically, it has allowed identification of the fatty acid composition of these lipids without extensive purification or derivatisation. The most widely used method for the detection of free and esterified eicosanoids is electrospray ionization (ESI) combined with reverse phase liquid chromatography (RP-HPLC) tandem mass spectrometry (MS/MS). Phospholipids and eicosanoids ionize readily using ESI and are separated well based on their fatty acid composition using RP-HPLC. Once ionized, parent ions can be fragmented on collision induced dissociation (CID) with inert gas giving characteristic daughter ions that can identify either esterified or free fatty acid eicosanoids. These methods are described in detail in the several reviews [25-27]. Although this has not yet been applied to the study of the fate of exogenously-added HETEs, it has been used uncover several new families of bioactive esterified eicosanoids, including esterified isoprostanes, isofurans and isolevuglandins, and specific LOX-derived esterified HETEs, that are generated acutely by activated human and murine platelets, neutrophils and monocytes/macrophages [28-33]. These will be described in detail in the following sections and are summarized in Table 1.
3.1 Monocytes and macrophages
3.1.1 Esterified LOX products
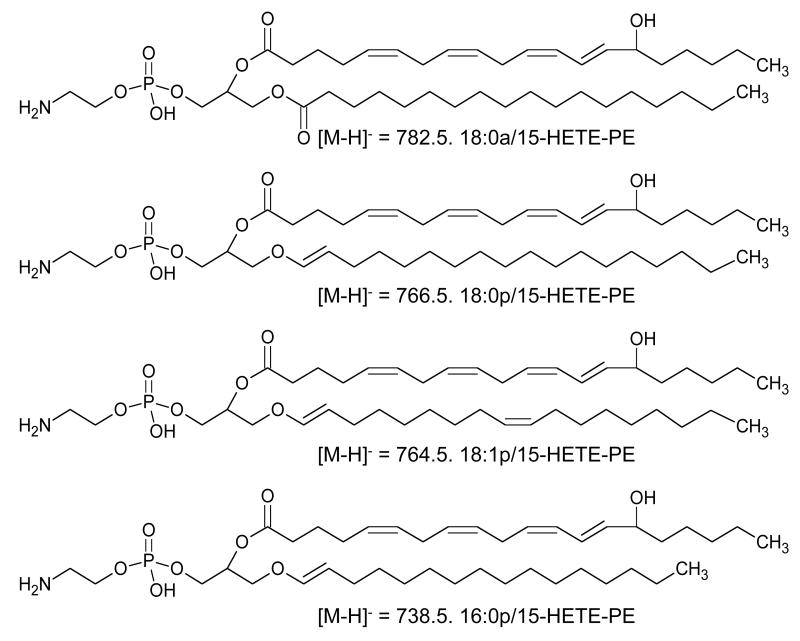
12/15-LOX is expressed in monocytes/macrophages in response to interleukin-4 and -13 and oxidizes arachidonate at either C12 (mouse, rat, pig) or C15 (human, rabbit) [34, 35]. The human isoform is referred to as 15-LOX or 15-LOX1. In 2005, we reported that human monocytes induced with IL-4 acutely generate a family of four esterified 15-HETEs following activation with ionophore (A23187). These comprise one diacyl and three plasmalogen PE species (i.e. 18:0a, 18:0p, 18:1p, 16:0p/15-HETE-PE) [32] (Figure 2). Similarly, murine peritoneal macrophages generate the equivalent 12-HETE-PEs, since they express the functional equivalent, 12/15 LOX [31]. In both cases, HETE-PEs appear within 5 minutes of activation, and remain fairly stable over time, up to 3 hrs. To date the only known agonist to stimulate the generation of these lipids is ionophore (A23187), which promotes calcium mobilization and subsequent translocation of the enzyme to the inner membrane surface. Various physiological activators have been tested, including LPS, zymosan, β-glucan, flagelin, PAM3CSK4 and a cell free supernatant derived from a clinical isolate of S. epidermidis, but all were inactive. Monocytes and macrophages already contain basal levels of HETE-PEs that generally increase only 2-3-fold on ionophore (A23187) activation [31, 32]. This suggests that the enzyme maybe already constitutively active, generating HETE-PEs without inflammatory stimulation and that the lipids are performing homeostatic functions. This is different to other LOXs (e.g. 5-LOX and platelet 12-LOX) that are inactive in resting cells and switched on by pathophysiological agonists (see later).
Figure 2. Structures of HETE-PEs generated by ionophore (A23187)-activated IL-4-treated human monocytes.
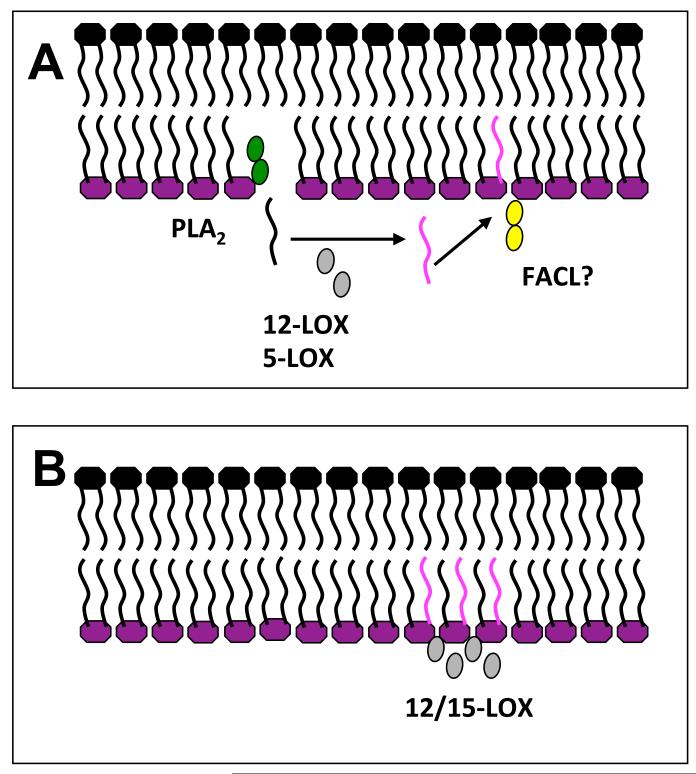
Unique among LOX isoforms, the 15-LOX can directly oxidize esterified arachidonate in phospholipids [36]. This has been shown both using purified rabbit 15-LOX and also in human eosinophils activated with ionophore (A23187). Oxidation of membranes by 15-LOX is mediated by a combination of hydrophobic interactions between surface-exposed apolar amino acid side chains and membrane lipids, and calcium, which potentially forms salt bridges between the negatively charged head groups of the phospholipids, and the acidic surface amino acids of the membrane contact plane of the enzyme thereby supporting the whole complex [37]. Thus, in intact cells, this LOX isoform could potentially generate esterified HETEs either through direct oxidation of phospholipids, or through oxidation of free arachidonate, which would then be re-esterified (Figure 3). In monocytes/macrophages, using stable isotope techniques, we found that HETE-PEs are generated by direct attack on membrane phospholipids by the enzyme, with no requirement for hydrolysis of arachidonate by phospholipases [31, 32]. It is unknown however, whether lipoxygenases show any specificity for particular phospholipid substrates in terms of their head group.
Figure 3. Two potential mechanisms exist to explain acute generation of HETE-PLs by LOX oxidation.
Structures in pink represent oxidized fatty acid chains. Panel A. Hydrolysis-dependent formation. PLA2 hydrolysis followed by 12-LOX oxidation, then re-esterification using Fatty acid CoA ligase (FACL), e.g. in neutrophils and platelets. Panel B. Direct membrane oxidation. Direct oxidation of membrane PL by 12/15-LOX, e.g. monocytes/macrophages.
In addition to HETE-PEs, human IL-4 stimulated monocytes and murine peritoneal macrophages generate analogous hydroperoxyeicosatetraenoic acid-PEs (HpETE-PEs) and ketoeicosatetraenoic acid-PEs (KETE-PEs), with positional isomers matching cellular LOX isoform expression, and the same molecular species of PE as for HETE-PEs. In general, amounts of these are lower than for HETE-PEs, but their generation is also stimulated by ionophore (A23187) and the lipids are absent in macrophages from 12/15-LOX−/− mice (Hammond et al unpublished). It is believed that HpETE-PEs are generated by LOX, and then reduced to HETE-PEs by cellular glutathione peroxidases. Approximately 50% of the HpETE-PE is reduced to HETE-PE in monocytes and macrophages, with the rest decomposing over 3 hrs following its generation to unknown lipid products (Hammond et al, unpublished).
As well as in vitro generation, 12/15-LOX-derived HETE-PEs have also been reported in vivo. In mice, 12-HETE-PEs are found in the peritoneal cavity, peritoneal membrane, lymph nodes, and intestines, similar to the distribution of 12/15-LOX derived free 12-HETE. In the peritoneal cavity, 12-HETE-PEs are associated with the resident peritoneal macrophage population present in the cavity of naïve animals. Following the induction of peritonitis (Toll receptor dependent) by either live bacteria or bacterial products, 12-HETE-PEs and 12/15-LOX expressing cells are rapidly cleared, and then reappear during inflammatory resolution. This further supports the idea that HETE-PEs play a role in immune regulation and/or inflammation resolution [31, 38]. In contrast, levels of 12-HETE-PEs were elevated in a Th2 dependent model of lung inflammation with the highest levels coinciding with the peak of IL-4 and IL-13 generation [31]. This observation is consistent with the ability of Th2 cytokines to strongly induce the enzyme. Thus, 12/15-LOX-derived HETE-PEs could be associated with eosinophils or lung macrophages that are recruited and differentiate during airway inflammation. The human analogs, 15-HETE-PEs, are also detected in cultured human bronchial airway epithelia and their generation can be stimulated in culture using IL-4 [39].
3.1.2. Esterified COX products
Zymosan-activated peritoneal macrophages and murine macrophage cell lines generate esterified eicosanoids via the action of COX. Naïve peritoneal cells express mainly COX-1 and little COX-2, however the latter is strongly induced using microbial products including zymosan and LPS. Marnett and co-workers have conducted several elegant studies that show generation of esterified COX products in these cells, in response to inflammatory activation. Specifically, COX-2 can oxidize endocannabinoid substrates 2-arachidonylglycerol (2-AG) or arachidonyl ethanolamine (AEA) to form novel lipids, for example, prostaglandin H2 glycerol ester (PGH2-G), which is then converted to PGD2-G, PGE2-D, PGF2α-G and PGI2-G, by additional enzymes [40, 41]. Due to the lack of a side pocket near the base of the active site, COX-1 is only weakly able to oxidize 2-AG and is not thought to be a significant source of these lipids. COX-2 exhibits comparable kcat/Km for 2-AG as AA, and both 2-AG and AEA are present at appreciable concentrations in mammalian tissues. The pattern of esterified prostaglandins generated from endogenous 2-AG oxidation by COX-2 matches that of free eicosanoids, although these are present at 500-1000 fold lower concentrations than their free acid forms [42]. Generation of PGD2-G is calcium dependent and thought to be mediated by sequential action of diacylglycerol (DAG) lipase (hydrolysis of DAG to 2-AG), followed by COX-2 [43]. Related to this, metabolism of 2-AG has also been shown by 15-LOX (human), both in vitro and in cells to form 15-HETE-G, a PPARγ ligand [44]
3.2 Platelets
Platelets express a platelet-specific 12-LOX isoform, and their ability to high levels of free eicosanoids such as 12-HETE (40 – 60 ng/4 × 107 platelets) on agonist activation (thrombin, collagen) has long been known. The function of this isoform is unclear however since 12-HETE is relatively inactive in terms of regulating platelet function directly. We recently found that human platelets generate four PE and two PC-esterified 12-HETEs including both plasmalogen and acyl forms (5.85 ± 1.42 (PE) and 18.35 ± 4.61 (PC) ng/4 × 107 cells). The six molecular species of esterified HETEs generated by human platelets include a mixture of diacyl and plasmalogen forms: 18:0a, 18:0p, 18:1p, and 16:0p/12-HETE-PE and 18:0a and 16:0a/12-HETE-PC. Their generation is stimulated by either collagen, thrombin, convulxin or ionophore (A23187) within 5 min, and they continue to elevate up to 3 hrs post activation [29, 32]. In response to thrombin, they are formed via activation of PAR-1/4 receptors, utilizing several intracellular signaling intermediates including Ca2+, protein kinase C, sPLA2, and src tyrosine kinases. Using PLA2 inhibitors and stable isotope incorporation, it was found that esterified 12-HETEs are generated by incorporation of pre-formed 12-HETE, rather than direct oxidation of phospholipids [29]. The rapid timescale of HETE-PL formation, which parallels that of free 12-HETE, indicates that the enzymes needed for this process (including PLA2, LOX and a fatty acyl CoA ligase) must be tightly coupled, perhaps in an enzyme complex at or near the plasma membrane of the cell. Interestingly, exogenously added 12-HETE-d8 was not incorporated into phospholipids during the short timescale of acute platelet activation. This indicates that exogenous HETEs are not equivalent to endogenous, further supporting the idea that generation of endogenous free HETE is tightly coupled to its esterification into phospholipids.
Unlike free eicosanoids, phospholipid-esterified HETES remain cell associated with a proportion of the HETE-PEs trafficking to the outside of the plasma membrane. It is assumed that HETE-PC is already on the outer surface, consistent with the localization of unoxidized PC. Studies using reductants indicate that the primary hydroperoxide lipids generated by 12-LOX are rapidly reduced endogenously by glutathione peroxidases to their corresponding HETEs [29]. HpETE- or KETE-PLs analogous to the monocyte/macrophage products of 12/15-LOXs were not detected in these cells.
Platelets also generate a second family of esterified eicosanoids via the 12-LOX pathway, where the 12-HETE is substituted by 12-LOX-oxidized docosahexanoic acid (DHA) [30] The products comprise phospholipid-esterified hydroxydocosahexaenoic acid (HDOHE), specifically two diacyl (16:0a, 18:0a) and two plasmalogen (16:0p, 18:0p) PEs containing predominantly the 14-HDOHE positional isomer. Studies using recombinant human 12-LOX showed the formation of predominantly free 14-HDOHE, and this, combined with its inability to directly oxidize esterified DHA suggests that the formation of HDOHE-PEs occurs via PLA2 hydrolysis of DHA, followed by 12-LOX oxidation, then re-esterification of free 14-HDOHE. This was further supported by the observation that inhibition of cPLA2 blocks their generation [30]. 14-HDOHE-PEs form acutely upon agonist activation by thrombin in a calcium dependent manner, at significant rates (2 - 4 ng/4 × 107 cells) [30].
3.3 Neutrophils
Neutrophils express 5-LOX which has long been known to generate the free 5-HpETE on agonist activation by bacterial peptides, ionophore (A23187) and phorbol esters. 5-HpETE can then be further metabolized to 5-HETE, leukotrienes (LTB4 and cysteinyl leukotrienes) and 5-oxo-ETE. We recently observed that that human neutrophils activated with bacterial products (fMLP) or serum opsonised Staphylococcus epidermidis acutely generate four 5-LOX derived lipids comprising plasmalogen and diacyl phospholipid esterified 5-HETEs (specifically 16:0a/5-HETE-PC, 18:0p/5-HETE-PE, 18:1p/5-HETE-PE and 16:0p/5-HETE-PE) [33]. They form in a calcium, sPLA2, cPLA2 and PLC dependent manner via esterification of newly generated 5-HETE and remain cell associated, localizing to both nuclear and extra-nuclear membranes. Their acute generation occurs within 2 min, and they are stable over a time period of 3 hours, with total 5-HETE-PEs reaching up to 0.37 ng/106 neutrophils. In contrast, the 5-HETE-PC is generated within 2 minutes of activation, but rapidly metabolized to unknown products. These 5-HETE-PEs and PC were detected in lavages from a murine model of bacterial peritonitis, as well as lavages from patients with bacterial peritonitis, showing that they are formed in human disease. In murine infection, formation of 5-HETE-PEs coincided with the neutrophil recruitment to the peritoneal cavity, and their levels decreased as the cells were cleared, suggesting neutrophil 5-LOX as their enzymatic source. In human lavages, whilst 5-HETE-PEs were detected, it was not possible to determine their source, however neutrophils are again most likely, as levels broadly correlated with cell count. [33].
3.4 Low-density lipoprotein and diseased tissue
A number of additional esterified eicosanoids have been detected in biological samples, including low-density lipoprotein and diseased tissue. Esterified isoprostanes and isofurans have been shown as free acids using GC/MS, following hydrolysis of PLs [28, 45-47]. In addition, intact epoxyisoprostane and epoxycyclopentenone PLs were described using LC/MS/MS. These were purified from air oxidized PC as a bioactive fraction, using NP-HPLC, then structurally characterized using RP-HPLC/MS/MS. [48, 49]. Subsequent studies have shown that PC-esterified epoxyisoprostane and epoxycyclopentenone can regulate endothelial cell PPAR-α activity thereby inducing MCP-1 and IL-8 expression by these cells, both of which are involved in the recruitment of monocytic cells to the vessel wall [50]. In support of this, other studies have shown that 1-palmitoyl-2-(5,6-epoxyisoprostane E2)-sn-glycero-3-phosphocholine can induce monocyte binding to endothelial cells [49]. This implicates such oxidized lipids in the onset of atherogenesis. Epoxycyclopentenone-containing phospholipids have also been implicated in the restoration of the endothelial barrier after acute periods of inflammation or injury by mediating cytoskeletal rearrangements [51]. Finally, it has been observed that host derived PC-esterified epoxyisoprostanes accumulate in human leprosy lesions where they are thought to inhibit innate immune responses thereby exacerbating the pathogenesis of microbial infections [52].
4. Biological roles of esterified eicosanoids
While the roles of many oxidized phospholipids generated by non-enzymatic pathways are quite well studied, less is known about the roles of esterified eicosanoids generated in a specific manner by cellular enzymes. Nonetheless, recent studies have begun to identify specific biological actions in isolated immune cells in vitro, particularly in the context of immune regulation and coagulation.
4.1 Regulation of Coagulation
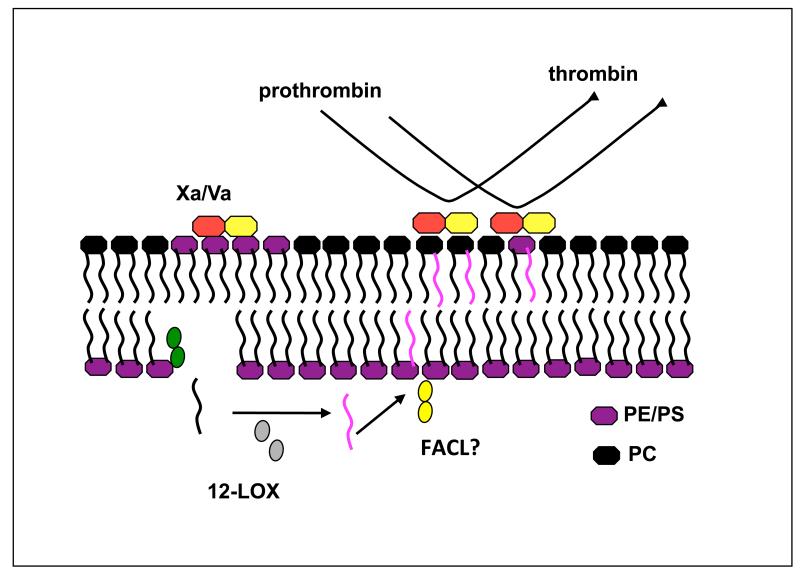
Coagulation involves the co-ordinated activities of plasma-derived clotting factors (proteases), which sequentially activate leading to formation of the prothrombinase complex (Xa/Va). This associates with negatively charged phospholipids on the platelet plasma membrane surface, where it converts prothrombin to thrombin, ultimately leading to clot formation. Negatively charged phospholipids known to be important in this process include PS and PE. Since HETE-PLs are present on the outside of the platelet, we wondered what effect they might have on coagulation. In vitro experiments using liposomes supplemented with HETE-PCs demonstrated that these lipids could dose-dependently enhance tissue factor-dependent coagulation in vitro [29] (Figure 4). PE and PS facilitate coagulation factor interactions with the membrane via calcium binding to their negatively charged phosphate. Although native PC is unable to mediate this interaction, 12-HETE-PCs possess a hydroxyl group at the C12 position, which might become accessible at the cell surface as a result of fatty acid chain bending due to the incorporation of a polar group. This may expose the –OH group leaving it accessible to interaction with calcium. Alternatively, the presence of the –OH may push the head group further out of the membrane leading to increased accessibility of the phosphate for calcium interactions.
Figure 4. Potential role for HETE-PLs in enhancing thrombin generation.
Following their generation, some HETE-PE translocates to the outside of the plasma membrane. The presence of –OH groups on phospholipids result in faster rates of tissue factor-dependent thrombin generation in vitro.
Scott syndrome is a rare bleeding disorder associated with impaired coagulation, and patients with this defect display an attenuated externalization of their platelet aminophospholipids, due to a genetic defect in a lipid transporter protein [53]. We found that in Scott syndrome platelets, PS and 12-HETE-PE externalization is inhibited. However, they still generate a normal amount of HETE-PC, which may partially explain why they are able to support a low rate of tissue factor-dependent thrombin generation [29].
4.2 Toll-like receptor signaling inhibition
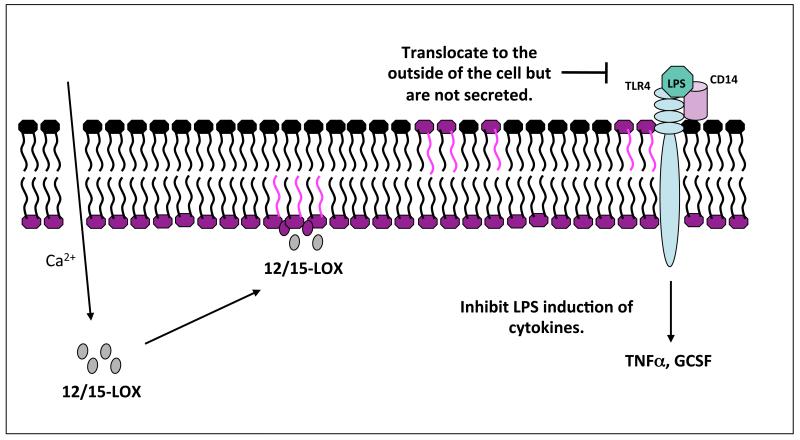
Studies on lipopolysaccharide (LPS)-stimulated human monocytes found that 18:0a/15-HETE-PE inhibits TNF-α and G-CSF generation, thereby acting in an anti-inflammatory manner [31]. Based on previous studies on non-enzymatically-generated truncated products of PC oxidation, we speculate that the mechanism may involve inhibition of the interaction of LPS with either LPS-binding protein (LBP) or CD14 [54]. In this regard HETE-PE may be acting in a protective manner, to dampen immune responses and aid in the resolution of inflammation and infection (Figure 5).
Figure 5. HETE-PE generation and action in human monocytes.
Monocytes are These are generated by 15-LOX through direct oxidation of the membrane, some translocate to the outside of the cell, and known biological actions include regulation of LPS generation of cytokines.
4.3 Regulation of anti-microbial actions in human neutrophils
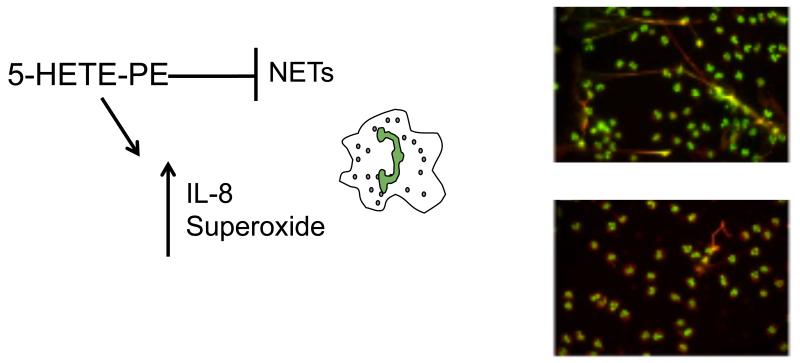
Neutrophil Extracellular Traps (NETs) are weblike structures comprising an extracellular scaffold of DNA, which contain neutrophil antimicrobial proteins of granular and nuclear origin. These enhance the neutrophils ability to trap bacteria within the circulation thereby limiting microbial dissemination, and the associated damage from neutrophil granular contents [55, 56]. We found that both 5- and 15-HETE-PEs are able to inhibit neutrophil extracellular trap (NET) formation in vitro, whilst inhibitors of 5-LOX enhanced their formation [33]. In order for NETs to be generated, the nuclear membrane dissolves, and the plasma membrane ruptures. It is not currently known how this disintegration of the membranes occurs, however we can speculate that the presence of HETE-PEs in both the nuclear and plasma membranes, may make them well placed to potentially play a role in the release of DNA through regulating membrane fluidity or permeability.
5-HETE-PE selectively increases neutrophil generation of IL-8, but not TNFα [33]. This is in contrast to free 5-HETE, which abolishes IL-8 and TNFα generation by neutrophils, indicating that the free vs. esterified forms of these LOX products work via different mechanisms. In contrast, both 5-HETE-PE and 5-HETE independently enhanced superoxide generation in neutrophils, so in this case, the 5-HETE functional group is required [33]. In terms of regulation of neutrophil function, it is currently not clear whether 5-HETE-PEs are pro- or anti-inflammatory. Increasing IL-8 and superoxide generation would be expected to promote bacterial killing, but could also increase host damage, while inhibition of NET generation could be either damaging or beneficial depending on the context (Figure 6). Thus, further work will be required to unravel the in vivo consequences of neutrophil HETE-PE generation during inflammatory disease or infection.
Figure 6. HETE-PE action in human neutrophils.
5-HETE-PE inhibits NET formation by neutrophils but enhances IL-8 and superoxide generation. Adherent neutrophils were activated with phorbol with (lower panel) or without (upper panel) 10 μM HETE-PE. Histone H1: green; myeloperoxidase: red. Part of this figure was originally published in [24].
4.4 Formation of covalent adducts
A number of oxidized phospholipids and eicosanoids have been reported to form protein adducts due to their electrophillic nature. The presence of an α,β-unsaturated carbonyl group with the carbon chain of such lipids affords them the ability to form both Schiff base and Michael additions with certain amino acids such as cysteine. Protein-lipid adducts are currently of great interest and diverse biological actions for such structures have been reported. Most of these studies focus on free eicosanoid-protein adducts which are known to have a number of cell signaling behaviors, however the formation of esterified lipid-protein adducts are less well characterized. Nonetheless, studies by one group have shown that a biotinylated form of oxidized PC readily adducts to plasma proteins, in particular apolipoprotein A1 (ApoA1), a core component of high-density lipoprotein (HDL) [57, 58]. Other groups have shown that isoketal containing oxidized phospholipids formed adducts which profoundly altered protein function, inhibiting potassium channels in a dose dependent manner [59]. Finally it has been seen that phospholipid esterified lipid hydroperoxides may be generated in vivo and be involved in the pathogenesis of atherosclerosis related oxidative stress [60].
4.5 Roles in Asthma
In animal models and human studies, increases in IL-4 and IL-13 have been shown to cause differentiation of ciliated epithelial cells into mucus producing goblet cells resulting in mucus hypersecretion, and contributing to severity of asthma and lung allergy. IL-4 and IL-13 induce 15-LOX, and high levels of this enzyme along with MUC5AC (a secreted mucin, which is associated with asthma) are expressed in human asthmatic epithelial cells [61-64]. It has been recently demonstrated that IL-13-stimulated 15-LOX expression is associated with generation of two species of 15-HETE-PE by human bronchial epithelial cells. This generation of HETE-PEs enhanced expression of MUC5AC, suggesting a role for esterified HETEs in contributing to the pathophysiology of asthma [39]. Associated with these studies is the recent finding that both 15-LOX and 15-HETE-PE can regulate MAPK/ERK activation, which is also known to be elevated in asthma. Specifically, 15-LOX and 15-HETE-PE induce the dissociation of phosphatidylethanolamine-binding protein 1 (PEBP1) from Raf-1, allowing the activation of ERK. Thus, this pathway appears to be of central importance for enhancing critical inflammatory pathways integral to asthma pathogenesis [65].
4.6 Regulation of intracellular pathways by glyceryl prostaglandins
Glyceryl prostaglandins generated by the action of COX-2 in macrophages have distinct and in some cases, more potent biological actions than their free acid analogs. Initial reports found that PGE2-G triggers calcium mobilization, transient increases in inositol 1,4,5-triphosphate (IP3), and membrane association and activation of PKC at nM concentrations, implicating this eicosanoid ester in the activation of a number of signaling cascades, including the ERK/MAPK pathway [66]. More recently, it was also found to modulate inhibitory synaptic transmission in mouse hippocampal neurons [67]. This is in contrast to the unoxidized 2-AG, which has opposing effects at similar concentrations (1 μM). The AEA oxidation product, PGE2-AEA inhibits LPS/IFNγ-induced IL-12 and IL-23 expression in microglia and macrophages by reducing expression of IL-12p40 [42]. This is similar to reported effects of AEA itself, and pharmacological studies suggest that AEA acts following COX-2 oxidation and formation of an EP2 receptor agonist [42]. Thus, it is clear that glyceryl-PGs play important roles in cell signaling that are distinct to the well-described actions of their free acid analogs.
4.7 Biological actions resulting from esterification of exogenous HETEs and non-enzymatically-generated esterified eicosanoids
Earlier sections focused on biological actions of endogenously generated esterified eicosanoids generated by COXs or LOXs. However, those formed either by esterification of exogenous HETEs or through non-enzymatic mechanisms have also been found to possess potent signaling properties.
In this regard, uptake of exogenous 5-HETE into endothelial cells inhibits histamine- and arachidonate-stimulated production of PGE2 and 6-ketoPGF1α [19]. Also, 5-HETE incorporation by adrenal glomerulosa cells decreases aldosterone release, thereby playing a potential role in steroidogenesis [20]. In previous studies, 12-HETE uptake into the cellular lipids of mouse thyroid glands caused inhibition of cyclic AMP production [68]. Whether this signaling was mediated by the esterified HETE itself, or following later cleavage to regenerate the free eicosanoid is not clear. In support, a role for esterified HETEs acting as a pool of “stored” eicosanoid, which can be released on further agonist activation, has been proposed (described earlier). Specifically, fMLP, phorbol or calcium ionophore (A23187) were shown to mediate deacylation of PI and release of 15-HETE which subsequently acts as a second signal to inhibit LTB4 generation by the neutrophils themselves [22].
A large number of in vivo studies have investigated the generation and multitude of biological actions mediated by non-enzymatically generated oxidized phospholipids in disease and signaling processes, and for this the reader is directed to a recent comprehensive review of this field [69]. Many of these demonstrate functions exerted by crude preparations of PLs that comprise a plethora of 100’s of different lipid species, likely including the families of lipids described in this review. More recently, fractionation studies have isolated particular bioactive species from these mixtures and many highlight in particular the potent signaling actions of lipids formed through truncation of the oxidized arachidonate at sn2. These would not be considered eicosanoids however since the C20 hydrocarbon chain is no longer intact. Of relevance, lysoPC-containing 15-hydroxyeicosapentaenoyl (1-(15-hydroxyeicosapentaenoyl)-lysoPC (15-HEPE-lysoPC)) has been shown to play a number of anti-inflammatory roles in a murine model of peritonitis. Specifically, i.p. administration of this lipid inhibited plasma leakage, and decreased leukocyte infiltration. In addition, it reduced formation of the 5-LOX products, LTC4 and LTB4, as well as levels of pro-inflammatory cytokines. Additionally, 15-HEPE-lysoPC caused a partial suppression of LTC4-induced plasma leakage and LTB4-induced leukocyte infiltration [70]. A number of other studies have shown that Ox-PAPC and PS are ligands for CD36 expressed by macrophages, and their binding enhances foam cell formation, and uptake of apoptotic cells, both key elements in the pathology of atherosclerosis [71-73]. As before, most of these activities appear to be mediated primarily by truncated products rather than esterified intact eicosanoids.
5. Conclusion
Eicosanoids were traditionally considered to signal as free acid mediators via specific G protein-coupled receptors, following their generation and secretion from cells. More recently, a new paradigm has emerged whereby they are generated by primary immune cells on acute activation. This suggests that esterified eicosanoids are families of signaling intermediates in their own right, mediating distinct and sometimes more potent biological actions to their un-esterified analogs. The recent advances in high sensitivity bench top mass spectrometry instrumentation has also been a significant factor in enabling this research, allowing detailed characterization of molecular structure (e.g. sn1 fatty acids, as well as head group) of novel lipid families for the first time. Current efforts in this area are focused on increasing our understanding of the detailed biological actions of these lipids in vivo, and delineating their role in disease processes.
References
- [1].Kuhn H, O’Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Prog Lipid Res. 2006;45:334–356. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- [2].Kuhn H. Biosynthesis, metabolization and biological importance of the primary 15-lipoxygenase metabolites 15-hydro(pero)XY-5Z,8Z,11Z,13E-eicosatetraenoic acid and 13-hydro(pero)XY-9Z,11E-octadecadienoic acid. Prog Lipid Res. 1996;35:203–226. doi: 10.1016/s0163-7827(96)00008-2. [DOI] [PubMed] [Google Scholar]
- [3].Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem Sci. 2007;32:332–341. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- [4].Dailey LA, Imming P. 12-Lipoxygenase: classification, possible therapeutic benefits from inhibition, and inhibitors. Curr Med Chem. 1999;6:389–398. [PubMed] [Google Scholar]
- [5].Yoshimoto T, Takahashi Y. Arachidonate 12-lipoxygenases. Prostaglandins Other Lipid Mediat. 2002;68-69:245–262. doi: 10.1016/s0090-6980(02)00034-5. [DOI] [PubMed] [Google Scholar]
- [6].Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- [7].Morita I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002;68-69:165–175. doi: 10.1016/s0090-6980(02)00029-1. [DOI] [PubMed] [Google Scholar]
- [8].Ortiz de Montellano PR. The 1994 Bernard B. Brodie Award Lecture. Structure, mechanism, and inhibition of cytochrome P450. Drug Metab Dispos. 1995;23:1181–1187. [PubMed] [Google Scholar]
- [9].Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50(Suppl):S52–56. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xu X, Zhang XA, Wang DW. The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases. Adv Drug Deliv Rev. 63:597–609. doi: 10.1016/j.addr.2011.03.006. [DOI] [PubMed] [Google Scholar]
- [11].Gordon JA, Spector AA. Effects of 12-HETE on renal tubular epithelial cells. Am J Physiol. 1987;253:C277–285. doi: 10.1152/ajpcell.1987.253.2.C277. [DOI] [PubMed] [Google Scholar]
- [12].Gormand F, Chabannes B, Moliere P, Perrin-Fayolle M, Lagarde M, Pacheco Y. Uptake of 12-HETE by human bronchial epithelial cells (HBEC): effects on HBEC cytokine production. Prostaglandins. 1996;51:263–273. doi: 10.1016/0090-6980(96)00021-4. [DOI] [PubMed] [Google Scholar]
- [13].Profita M, Vignola AM, Sala A, Mirabella A, Siena L, Pace E, Folco G, Bonsignore G. Interleukin-4 enhances 15-lipoxygenase activity and incorporation of 15(S)-HETE into cellular phospholipids in cultured pulmonary epithelial cells. Am J Respir Cell Mol Biol. 1999;20:61–68. doi: 10.1165/ajrcmb.20.1.3151. [DOI] [PubMed] [Google Scholar]
- [14].Girton RA, Spector AA, Gordon JA. 15-HETE: selective incorporation into inositol phospholipids of MDCK cells. Kidney Int. 1994;45:972–980. doi: 10.1038/ki.1994.131. [DOI] [PubMed] [Google Scholar]
- [15].Alpert SE, Walenga RW. Human tracheal epithelial cells selectively incorporate 15-hydroxyeicosatetraenoic acid into phosphatidylinositol. Am J Respir Cell Mol Biol. 1993;8:273–281. doi: 10.1165/ajrcmb/8.3.273. [DOI] [PubMed] [Google Scholar]
- [16].Wang LX, Kaduce TL, Spector AA. Localization of 12-hydroxyeicosatetraenoic acid in endothelial cells. J Lipid Res. 1990;31:2265–2276. [PubMed] [Google Scholar]
- [17].Shen XY, Figard PH, Kaduce TL, Spector AA. Conversion of 15-hydroxyeicosatetraenoic acid to 11-hydroxyhexadecatrienoic acid by endothelial cells. Biochemistry. 1988;27:996–1004. doi: 10.1021/bi00403a024. [DOI] [PubMed] [Google Scholar]
- [18].Kaduce TL, Fang X, Harmon SD, Oltman CL, Dellsperger KC, Teesch LM, Gopal VR, Falck JR, Campbell WB, Weintraub NL, Spector AA. 20-hydroxyeicosatetraenoic acid (20-HETE) metabolism in coronary endothelial cells. J Biol Chem. 2004;279:2648–2656. doi: 10.1074/jbc.M306849200. [DOI] [PubMed] [Google Scholar]
- [19].Richards CF, Johnson AR, Campbell WB. Specific incorporation of 5-hydroxy-6,8,11,14-eicosatetraenoic acid into phosphatidylcholine in human endothelial cells. Biochim Biophys Acta. 1986;875:569–581. doi: 10.1016/0005-2760(86)90079-2. [DOI] [PubMed] [Google Scholar]
- [20].Richards CF, Campbell WB. Incorporation of hydroxyeicosatetraenoic acids into cellular lipids of adrenal glomerulosa cells: inhibition of aldosterone release by 5-HETE. Prostaglandins. 1989;38:565–580. doi: 10.1016/0090-6980(89)90150-0. [DOI] [PubMed] [Google Scholar]
- [21].Carroll MA, McGiff JC. Renal cytochrome P450-dependent eicosanoids. Adv Exp Med Biol. 1997;407:255–260. doi: 10.1007/978-1-4899-1813-0_38. [DOI] [PubMed] [Google Scholar]
- [22].Brezinski ME, Serhan CN. Selective incorporation of (15S)-hydroxyeicosatetraenoic acid in phosphatidylinositol of human neutrophils: agonist-induced deacylation and transformation of stored hydroxyeicosanoids. Proc Natl Acad Sci U S A. 1990;87:6248–6252. doi: 10.1073/pnas.87.16.6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pawlowski NA, Scott WA, Andreach M, Cohn ZA. Uptake and metabolism of monohydroxy-eicosatetraenoic acids by macrophages. J Exp Med. 1982;155:1653–1664. doi: 10.1084/jem.155.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Joulain C, Meskini N, Anker G, Lagarde M, Prigent AF. Esterification of 12(S)-hydroxy-5,8,10,14-eicosatetraenoic acid into the phospholipids of human peripheral blood mononuclear cells: inhibition of the proliferative response. J Cell Physiol. 1995;164:154–163. doi: 10.1002/jcp.1041640120. [DOI] [PubMed] [Google Scholar]
- [25].Morgan AH, Hammond VJ, Morgan L, Thomas CP, Tallman KA, Garcia-Diaz YR, McGuigan C, Serpi M, Porter NA, Murphy RC, O’Donnell VB. Quantitative assays for esterified oxylipins generated by immune cells. Nat Protoc. 5:1919–1931. doi: 10.1038/nprot.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Murphy RC, Barkley RM, Zemski Berry K, Hankin J, Harrison K, Johnson C, Krank J, McAnoy A, Uhlson C, Zarini S. Electrospray ionization and tandem mass spectrometry of eicosanoids. Anal Biochem. 2005;346:1–42. doi: 10.1016/j.ab.2005.04.042. [DOI] [PubMed] [Google Scholar]
- [27].O’Donnell VB. Mass spectrometry analysis of oxidized phosphatidylcholine and phosphatidylethanolamine. Biochimica et biophysica acta. 1811:818–826. doi: 10.1016/j.bbalip.2011.07.018. [DOI] [PubMed] [Google Scholar]
- [28].Chen Y, Morrow JD, Roberts LJ., 2nd Formation of reactive cyclopentenone compounds in vivo as products of the isoprostane pathway. J Biol Chem. 1999;274:10863–10868. doi: 10.1074/jbc.274.16.10863. [DOI] [PubMed] [Google Scholar]
- [29].Thomas CP, Morgan LT, Maskrey BH, Murphy RC, Kuhn H, Hazen SL, Goodall AH, Hamali HA, Collins PW, O’Donnell VB. Phospholipid-esterified eicosanoids are generated in agonist-activated human platelets and enhance tissue factor-dependent thrombin generation. J Biol Chem. 2010;285:6891–6903. doi: 10.1074/jbc.M109.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Morgan LT, Thomas CP, Kuhn H, O’Donnell VB. Thrombin-activated human platelets acutely generate oxidized docosahexaenoic-acid-containing phospholipids via 12-lipoxygenase. Biochem J. 2010;431:141–148. doi: 10.1042/BJ20100415. [DOI] [PubMed] [Google Scholar]
- [31].Morgan AH, Dioszeghy V, Maskrey BH, Thomas CP, Clark SR, Mathie SA, Lloyd CM, Kuhn H, Topley N, Coles BC, Taylor PR, Jones SA, O’Donnell VB. Phosphatidylethanolamine-esterified eicosanoids in the mouse: tissue localization and inflammation-dependent formation in Th-2 disease. J Biol Chem. 2009;284:21185–21191. doi: 10.1074/jbc.M109.021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Maskrey BH, Bermudez-Fajardo A, Morgan AH, Stewart-Jones E, Dioszeghy V, Taylor GW, Baker PR, Coles B, Coffey MJ, Kuhn H, O’Donnell VB. Activated platelets and monocytes generate four hydroxyphosphatidylethanolamines via lipoxygenase. J Biol Chem. 2007;282:20151–20163. doi: 10.1074/jbc.M611776200. [DOI] [PubMed] [Google Scholar]
- [33].Clark SR, Guy CJ, Scurr MJ, Taylor PR, Kift-Morgan AP, Hammond VJ, Thomas CP, Coles B, Roberts GW, Eberl M, Jones SA, Topley N, Kotecha S, O’Donnell VB. Esterified eicosanoids are acutely generated by 5-lipoxygenase in primary human neutrophils and in human and murine infection. Blood. 2011;117:2033–2043. doi: 10.1182/blood-2010-04-278887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kuhn H, Thiele BJ. The diversity of the lipoxygenase family. Many sequence data but little information on biological significance. FEBS Lett. 1999;449:7–11. doi: 10.1016/s0014-5793(99)00396-8. [DOI] [PubMed] [Google Scholar]
- [35].Schewe T, Halangk W, Hiebsch C, Rapoport SM. A lipoxygenase in rabbit reticulocytes which attacks phospholipids and intact mitochondria. FEBS Lett. 1975;60:149–152. doi: 10.1016/0014-5793(75)80439-x. [DOI] [PubMed] [Google Scholar]
- [36].Brinckmann R, Schnurr K, Heydeck D, Rosenbach T, Kolde G, Kuhn H. Membrane translocation of 15-lipoxygenase in hematopoietic cells is calcium-dependent and activates the oxygenase activity of the enzyme. Blood. 1998;91:64–74. [PubMed] [Google Scholar]
- [37].Walther M, Wiesner R, Kuhn H. Investigations into calcium-dependent membrane association of 15-lipoxygenase-1. Mechanistic roles of surface-exposed hydrophobic amino acids and calcium. J Biol Chem. 2004;279:3717–3725. doi: 10.1074/jbc.M309564200. [DOI] [PubMed] [Google Scholar]
- [38].Dioszeghy V, Rosas M, Maskrey BH, Colmont C, Topley N, Chaitidis P, Kuhn H, Jones SA, Taylor PR, O’Donnell VB. 12/15-Lipoxygenase regulates the inflammatory response to bacterial products in vivo. J Immunol. 2008;181:6514–6524. doi: 10.4049/jimmunol.181.9.6514. [DOI] [PubMed] [Google Scholar]
- [39].Zhao J, Maskrey B, Balzar S, Chibana K, Mustovich A, Hu H, Trudeau JB, O’Donnell V, Wenzel SE. Interleukin-13-induced MUC5AC is regulated by 15-lipoxygenase 1 pathway in human bronchial epithelial cells. Am J Respir Crit Care Med. 2009;179:782–790. doi: 10.1164/rccm.200811-1744OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kozak KR, Marnett LJ. Oxidative metabolism of endocannabinoids. Prostaglandins Leukot Essent Fatty Acids. 2002;66:211–220. doi: 10.1054/plef.2001.0359. [DOI] [PubMed] [Google Scholar]
- [41].Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, Jakobsson PJ, Marnett LJ. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- [42].Rouzer CA, Marnett LJ. Non-redundant functions of cyclooxygenases: oxygenation of endocannabinoids. J Biol Chem. 2008;283:8065–8069. doi: 10.1074/jbc.R800005200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- [44].Kozak KR, Gupta RA, Moody JS, Ji C, Boeglin WE, DuBois RN, Brash AR, Marnett LJ. 15-Lipoxygenase metabolism of 2-arachidonylglycerol. Generation of a peroxisome proliferator-activated receptor alpha agonist. J Biol Chem. 2002;277:23278–23286. doi: 10.1074/jbc.M201084200. [DOI] [PubMed] [Google Scholar]
- [45].Fessel JP, Hulette C, Powell S, Roberts LJ, 2nd, Zhang J. Isofurans, but not F2-isoprostanes, are increased in the substantia nigra of patients with Parkinson’s disease and with dementia with Lewy body disease. J Neurochem. 2003;85:645–650. doi: 10.1046/j.1471-4159.2003.01709.x. [DOI] [PubMed] [Google Scholar]
- [46].Fessel JP, Porter NA, Moore KP, Sheller JR, Roberts LJ., 2nd Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc Natl Acad Sci U S A. 2002;99:16713–16718. doi: 10.1073/pnas.252649099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kayganich KA, Murphy RC. Fast atom bombardment tandem mass spectrometric identification of diacyl, alkylacyl, and alk-1-enylacyl molecular species of glycerophosphoethanolamine in human polymorphonuclear leukocytes. Anal Chem. 1992;64:2965–2971. doi: 10.1021/ac00047a015. [DOI] [PubMed] [Google Scholar]
- [48].Subbanagounder G, Deng Y, Borromeo C, Dooley AN, Berliner JA, Salomon RG. Hydroxy alkenal phospholipids regulate inflammatory functions of endothelial cells. Vascul Pharmacol. 2002;38:201–209. doi: 10.1016/s1537-1891(02)00170-2. [DOI] [PubMed] [Google Scholar]
- [49].Watson AD, Subbanagounder G, Welsbie DS, Faull KF, Navab M, Jung ME, Fogelman AM, Berliner JA. Structural identification of a novel pro-inflammatory epoxyisoprostane phospholipid in mildly oxidized low density lipoprotein. The Journal of biological chemistry. 1999;274:24787–24798. doi: 10.1074/jbc.274.35.24787. [DOI] [PubMed] [Google Scholar]
- [50].Subbanagounder G, Wong JW, Lee H, Faull KF, Miller E, Witztum JL, Berliner JA. Epoxyisoprostane and epoxycyclopentenone phospholipids regulate monocyte chemotactic protein-1 and interleukin-8 synthesis. Formation of these oxidized phospholipids in response to interleukin-1beta. The Journal of biological chemistry. 2002;277:7271–7281. doi: 10.1074/jbc.M107602200. [DOI] [PubMed] [Google Scholar]
- [51].Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res. 2004;95:892–901. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- [52].Cruz D, Watson AD, Miller CS, Montoya D, Ochoa MT, Sieling PA, Gutierrez MA, Navab M, Reddy ST, Witztum JL, Fogelman AM, Rea TH, Eisenberg D, Berliner J, Modlin RL. Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J Clin Invest. 2008;118:2917–2928. doi: 10.1172/JCI34189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–838. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- [54].von Schlieffen E, Oskolkova OV, Schabbauer G, Gruber F, Bluml S, Genest M, Kadl A, Marsik C, Knapp S, Chow J, Leitinger N, Binder BR, Bochkov VN. Multi-hit inhibition of circulating and cell-associated components of the toll-like receptor 4 pathway by oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2009;29:356–362. doi: 10.1161/ATVBAHA.108.173799. [DOI] [PubMed] [Google Scholar]
- [55].Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- [56].Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol. 2009;30:513–521. doi: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- [57].Szapacs ME, Kim HY, Porter NA, Liebler DC. Identification of proteins adducted by lipid peroxidation products in plasma and modifications of apolipoprotein A1 with a novel biotinylated phospholipid probe. J Proteome Res. 2008;7:4237–4246. doi: 10.1021/pr8001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tallman KA, Kim HY, Ji JX, Szapacs ME, Yin H, McIntosh TJ, Liebler DC, Porter NA. Phospholipid-protein adducts of lipid peroxidation: synthesis and study of new biotinylated phosphatidylcholines. Chem Res Toxicol. 2007;20:227–234. doi: 10.1021/tx600331s. [DOI] [PubMed] [Google Scholar]
- [59].Brame CJ, Boutaud O, Davies SS, Yang T, Oates JA, Roden D, Roberts LJ., 2nd Modification of proteins by isoketal-containing oxidized phospholipids. The Journal of biological chemistry. 2004;279:13447–13451. doi: 10.1074/jbc.M313349200. [DOI] [PubMed] [Google Scholar]
- [60].Kawai Y, Kato Y, Fujii H, Makino Y, Mori Y, Naito M, Osawa T. Immunochemical detection of a novel lysine adduct using an antibody to linoleic acid hydroperoxide-modified protein. J Lipid Res. 2003;44:1124–1131. doi: 10.1194/jlr.M200442-JLR200. [DOI] [PubMed] [Google Scholar]
- [61].Kondo M, Tamaoki J, Takeyama K, Nakata J, Nagai A. Interleukin-13 induces goblet cell differentiation in primary cell culture from Guinea pig tracheal epithelium. Am J Respir Cell Mol Biol. 2002;27:536–541. doi: 10.1165/rcmb.4682. [DOI] [PubMed] [Google Scholar]
- [62].Kondo M, Tamaoki J, Takeyama K, Isono K, Kawatani K, Izumo T, Nagai A. Elimination of IL-13 reverses established goblet cell metaplasia into ciliated epithelia in airway epithelial cell culture. Allergol Int. 2006;55:329–336. doi: 10.2332/allergolint.55.329. [DOI] [PubMed] [Google Scholar]
- [63].Morcillo EJ, Cortijo J. Mucus and MUC in asthma. Curr Opin Pulm Med. 2006;12:1–6. doi: 10.1097/01.mcp.0000198064.27586.37. [DOI] [PubMed] [Google Scholar]
- [64].Brown CD, Kilty I, Yeadon M, Jenkinson S. Regulation of 15-lipoxygenase isozymes and mucin secretion by cytokines in cultured normal human bronchial epithelial cells. Inflamm Res. 2001;50:321–326. doi: 10.1007/PL00000251. [DOI] [PubMed] [Google Scholar]
- [65].Zhao J, O’Donnell VB, Balzar S, Croix C.M. St, Trudeau JB, Wenzel SE. 15-Lipoxygenase 1 interacts with phosphatidylethanolamine-binding protein to regulate MAPK signaling in human airway epithelial cells. Proc Natl Acad Sci U S A. 2011;108:14246–14251. doi: 10.1073/pnas.1018075108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Nirodi CS, Crews BC, Kozak KR, Morrow JD, Marnett LJ. The glyceryl ester of prostaglandin E2 mobilizes calcium and activates signal transduction in RAW264.7 cells. Proc Natl Acad Sci U S A. 2004;101:1840–1845. doi: 10.1073/pnas.0303950101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sang N, Zhang J, Chen C. PGE2 glycerol ester, a COX-2 oxidative metabolite of 2-arachidonoyl glycerol, modulates inhibitory synaptic transmission in mouse hippocampal neurons. J Physiol. 2006;572:735–745. doi: 10.1113/jphysiol.2006.105569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Levasseur S, Sun F, Friedman Y, Burke G. Esterification of 12-hydroxy-5,8,10,14-eicosatetraenoate into mouse thyroid lipids: possible physiological significance. Adv Prostaglandin Thromboxane Leukot Res. 1983;12:247–251. [PubMed] [Google Scholar]
- [69].Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stockl J. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2009;12:1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hung ND, Kim MR, Sok DE. Mechanisms for anti-inflammatory effects of 1-[15(S)-hydroxyeicosapentaenoyl] lysophosphatidylcholine, administered intraperitoneally, in zymosan A-induced peritonitis. Br J Pharmacol. 2011;162:1119–1135. doi: 10.1111/j.1476-5381.2010.01117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Groeneweg M, Vergouwe MN, Scheffer PG, Vermue HP, Sollewijn Gelpke MD, Sijbers AM, Leitinger N, Hofker MH, de Winther MP. Modification of LDL with oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (oxPAPC) results in a novel form of minimally modified LDL that modulates gene expression in macrophages. Biochim Biophys Acta. 2008;1781:336–343. doi: 10.1016/j.bbalip.2008.04.016. [DOI] [PubMed] [Google Scholar]
- [72].Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006;203:2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chou MY, Hartvigsen K, Hansen LF, Fogelstrand L, Shaw PX, Boullier A, Binder CJ, Witztum JL. Oxidation-specific epitopes are important targets of innate immunity. J Intern Med. 2008;263:479–488. doi: 10.1111/j.1365-2796.2008.01968.x. [DOI] [PubMed] [Google Scholar]