Abstract
We conducted randomized clinical trials to examine the impact of direct-to-consumer advertisements on the efficacy of a branded drug. We compared the objectively measured, physiological effect of Claritin (Merck & Co.), a leading antihistamine medication, across subjects randomized to watch a movie spliced with advertisements for Claritin or advertisements for Zyrtec (McNeil), a competitor antihistamine. Among subjects who test negative for common allergies, exposure to Claritin advertisements rather than Zyrtec advertisements increases the efficacy of Claritin. We conclude that branded drugs can interact with exposure to television advertisements.
Keywords: advertising, placebo effect
The medical literature on placebo effects suggests that beliefs about the quality of a drug can impact the drug’s efficacy. Pharmaceutical companies spend $4.8 billion a year on direct-to-consumer advertisements in United States alone (1). Might these advertisements impact consumers’ beliefs and thereby alter the efficacy of drugs?
We conducted two randomized clinical trials to measure the impact of direct-to-consumer advertising on the objective, physiological effect of Claritin (Merck & Co.), a leading antihistamine drug. A pilot study assessed the efficacy of Claritin across subjects exposed to advertisements for Claritin, advertisements for Zyrtec (McNeil), or control advertisements. At the outset of each experiment, we gave each subject a skin test for common allergens, namely, grass, trees, mold, dust mites, ragweed, and cats. Throughout the paper we refer to the subjects who had at least one positive skin test as “subjects with allergies” and to the others as “subjects without allergies.” Among subjects with allergies, the efficacy was the same across the three advertisement conditions, but among subjects without allergies, efficacy was significantly greater in the Claritin advertisements condition than in the Zyrtec advertisements condition.
The heterogeneity of the treatment effect based on the allergy status was discovered only ex post facto, so we conducted a follow-up trial to replicate these initial findings. To maximize statistical power, the follow-up trial used a larger sample, assigned subjects only to Claritin advertisements or Zyrtec advertisements, and block-randomized subjects based on their allergy status. In addition, we elicited subjects’ beliefs about the efficacy of Claritin to examine whether any difference in impact of the advertisements across the two subpopulations is driven by the relative malleability of their beliefs.
We use the pilot study primarily as a source of a directional, ex ante null hypothesis about the impact of the advertisements within the two subpopulations. Our statistical analysis focuses on the results from the follow-up trial. (Pooling the data from the two trials only strengthens our main result.)
The follow-up trial proceeded as follows. A subject was given a skin allergy test for common allergens. A research technician administered a histamine challenge on the subject’s forearm, and a baseline measurement of the wheal reaction was taken. The wheal reaction is the slightly reddened, elevated area at the site of the challenge and is a well-established measure of histamine response (2). All normal individuals, whether they have allergies or not, develop a wheal reaction to a histamine challenge. The subject reported her belief about the efficacy of Claritin. She was given 10 mg Claritin and was made aware that it was Claritin. She was shown a movie spliced with naturally timed advertisement breaks. In one condition (Claritin advertisements), one advertisement in each break was an advertisement for Claritin. In the other condition (Zyrtec advertisements), one advertisement in each break was an advertisement for Zyrtec. The Zyrtec advertisement stated that Zyrtec “starts working two hours faster than Claritin.” The histamine challenge and wheal measurement were repeated during the movie, 60 and 120 min after Claritin was administered. At the end of the experiment, the subject was again asked her belief about the efficacy of Claritin.
We define the efficacy of Claritin (at 60 and 120 min) as the percentage decrease in the size of the wheal reaction relative to the baseline. We thus have a subject-specific measure of efficacy. By comparing the efficacy across the subjects in the two advertisement conditions, we identify the impact of advertisements on the efficacy of Claritin.
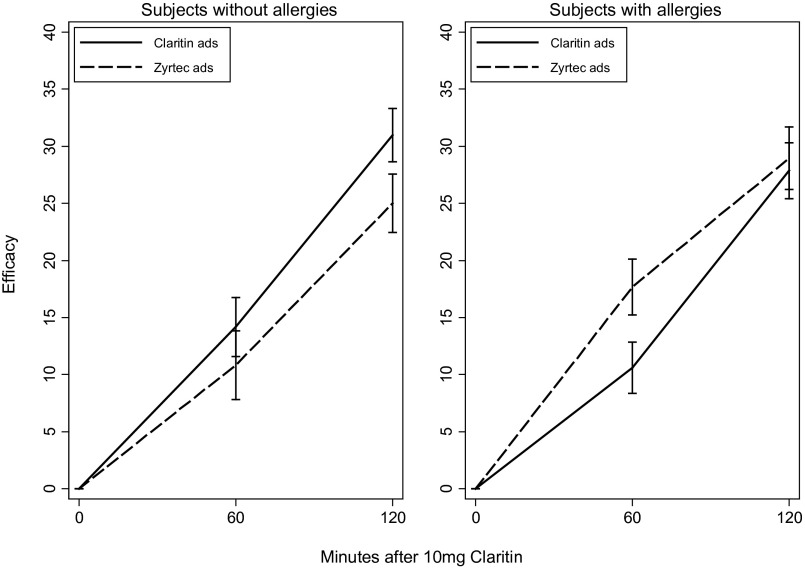
In the subpopulation without allergies, we find that the efficacy of Claritin at 120 min is substantially higher for subjects who were exposed to Claritin advertisements. Claritin advertisements have no significant impact on efficacy 60 min after the drug is taken. This pattern is consistent with the observed changes in the subjects’ beliefs. Exposure to Claritin advertisements in this subpopulation greatly increases the belief in the efficacy of Claritin. At the same time, the realized efficacy of Claritin at 120 min (but not at 60 min) is strongly correlated with the change in beliefs.
In the subpopulation with allergies, we find no relationship between exposure to Claritin advertisements and the change in beliefs. Moreover, the advertisements have no impact on the efficacy of Claritin at 120 min. We do find a curious negative impact of Claritin advertisements on Claritin’s efficacy at 60 min in this subpopulation, but this effect cannot be mediated by the (nonexistent) impact of advertisements on beliefs.
Overall, the results of the follow-up experiment support the view that television advertisements can impact the physiological efficacy of a branded drug, at least in subpopulations of consumers whose beliefs about the drug’s efficacy are sufficiently malleable.
Beliefs about active drugs can influence their efficacy (3, 4). Moreover, placebo treatments disguised as pharmacologically active treatments can have measurable physiological effects (5, 6). Hróbjartsson and Gøtzsche (7) examined 114 trials that had both a placebo and a no-treatment arm and found that placebos had a statistically significant, positive effect on health outcomes only in trials with continuous subjective outcomes, but their 95% confidence intervals admit the possibility of substantial positive effects from placebos for all types of outcomes.
A small existing literature examines the impact of commercial features of drugs on their efficacy. Previous experiments show that the color (8), the packaging (9), and the price (10) of drugs affect their perceived efficacy. Shiv et al. (11) examine the effects of price and advertisements on puzzle-solving performance following consumption of an energy drink. However, they rely on a behavioral outcome and use descriptive text rather than actual advertisements for the drink. Studies that rely on self-reported or behavioral outcomes might reflect only experimenter demand effects. Other studies show that the price (12) and the brand (13, 14) of a beverage can affect brain activity during its consumption. Our experiment used the short-term, objectively measured physiological efficacy of a drug to test placebo effects.
Economists have offered a variety of rationales for the enormous resources spent on advertising. Bagwell (15) categorizes three basic views on why consumers respond to advertising. One is that advertisements induce artificial product differentiation and raise firm profits at the expense of consumer welfare (16, 17). The second view is that advertising is informative (18–20). Our inquiry relates most closely to the third view, which holds that advertising is complementary to the consumption of a product (21, 22). Becker and Murphy (22) consider a model in which exposure to advertising for product 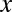 (denoted by
(denoted by 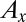 ) enters a stable utility function
) enters a stable utility function 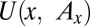 and increases the marginal utility of
and increases the marginal utility of 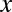 , i.e.,
, i.e., 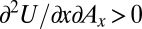 . If drug advertisements generate placebo effects, this fact would provide one mechanism through which advertising and consumption are complements.
. If drug advertisements generate placebo effects, this fact would provide one mechanism through which advertising and consumption are complements.
Results
Balancing Tests.
The follow-up study enrolled 340 subjects. Table 1 shows the demographics of the subjects. By design, half of the subjects had a positive skin test to at least one common allergen. In both subpopulations (based on the allergy status), there are no significant differences across the advertisement conditions in the age, sex, race, baseline wheal reaction, or initial belief about the efficacy of Claritin.
Table 1.
Baseline characteristics of subjects by allergy status and advertisement condition
| Subpopulation characteristics | Claritin advertisements | Zyrtec advertisements | P value |
| Subjects without allergies | |||
| Number of subjects | 85 | 85 | |
| Age, y | 27.25 | 26.40 | 0.65 |
| Male, % | 31 | 31 | 1.00 |
| Black, % | 28 | 26 | 0.73 |
| Baseline wheal, mm2 | 21.72 | 21.61 | 0.96 |
| Belief in efficacy | 5.55 | 5.42 | 0.26 |
| Subjects with allergies | |||
| Number of subjects | 83 | 87 | |
| Age, y | 27.83 | 29.30 | 0.11 |
| Male, % | 48 | 41 | 0.37 |
| Black, % | 35 | 40 | 0.48 |
| Baseline wheal, mm2 | 21.18 | 21.50 | 0.58 |
| Belief in efficacy | 5.43 | 5.14 | 0.28 |
Variable baseline wheal is the size of the wheal reaction to the baseline histamine challenge, administered before Claritin. Belief in efficacy is the subject’s initial answer to the question “On a scale of 1 to 7, where higher numbers mean more effective, how effective do you think Claritin is in eliminating allergy symptoms?” P values are based on a Wilcoxon rank-sum test comparing the subjects in the Claritin advertisements condition with the subjects in the Zyrtec advertisements condition.
Overall Efficacy of Claritin.
Our main interest is not in the effect of Claritin per se but rather in the way that this effect varies across the advertisement conditions. Nonetheless, we report the overall effect of Claritin to provide some context for our main results.
Claritin is quite effective in reducing the wheal reaction to a histamine challenge. Recall that efficacy (at 60 and 120 min) is defined as the percentage decrease in the size of the wheal reaction to the histamine challenge relative to the baseline. For subjects without allergies, the average efficacy is 12.51% at 60 min and 27.99% at 120 min. For subjects with allergies, the average efficacy is 14.21% at 60 min and 28.43% at 120 min. Based on a t test, at both 60 and 120 min there is no significant difference in efficacy across the two subpopulations (60 min: P = 0.514; 120 min: P = 0.863).
Impact of Advertisements on the Efficacy of Claritin.
Fig. 1 depicts the efficacy of Claritin at 60 and 120 min across the two advertisement conditions and the two subpopulations. Among subjects without allergies, the efficacy at 60 min was 10.83% in the Zyrtec advertisements condition and 14.18% in the Claritin advertisements condition. This difference is not statistically significant. At 120 min, however, the efficacy in the Zyrtec advertisements condition was 25.00% compared with 30.97% in the Claritin advertisements condition. This difference of 5.97 percentage points is statistically significant (t test; one-sided P = 0.043). (Based on our pilot study, we had a directional hypothesis for the follow-up experiment. Accordingly, we use a one-sided P value.) In the subpopulation with allergies, the efficacy at 60 min is significantly smaller in the Claritin advertisements condition than in the Zyrtec advertisements condition; the difference at 120 min is not significant.
Fig. 1.
Impact of advertising on the efficacy of Claritin by allergy status. Solid and dotted lines indicate mean efficacy. Whiskers indicate the SE of mean efficacy.
Mediation Through Change in Beliefs.
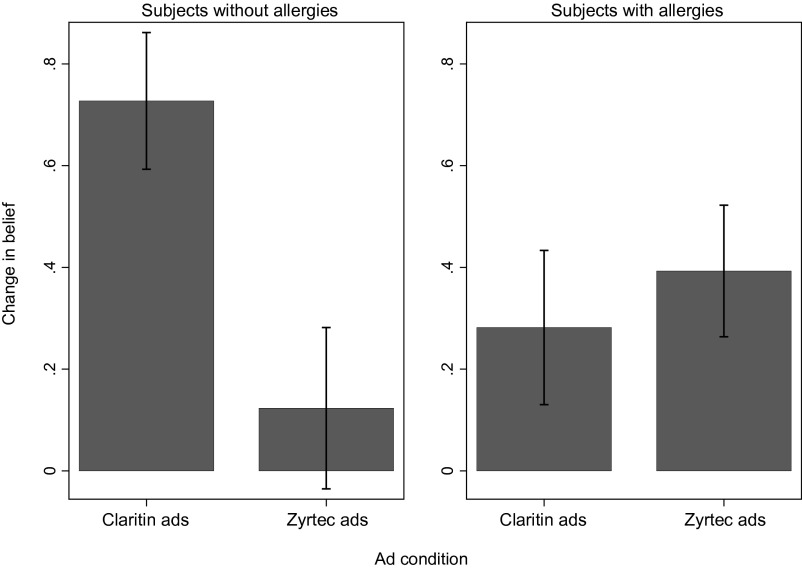
The results in Fig. 1 are broadly consistent with the observed changes in beliefs. Fig. 2 depicts changes in beliefs across the two advertisement conditions and the two subpopulations. Among subjects without allergies, those who were exposed to Claritin advertisements increased their belief in its efficacy substantially more than those were exposed to Zyrtec advertisements (t test; two-sided P = 0.004). Among subjects with allergies, there were no significant differences in belief change across the two advertisement conditions (t test; two-sided P = 0.577). This pattern buttresses the view that the heterogeneous impact of the advertisements across the two subpopulations is driven by the differential malleability of their beliefs.
Fig. 2.
Impact of advertisements on change in beliefs about Claritin by allergy status. Bars indicate mean change in belief. Whiskers indicate the SE of mean change in belief.
In Table 2 we report the correlation between belief change and the realized efficacy of Claritin. In both subpopulations, efficacy at 120 min (but not at 60 min) is significantly correlated with belief change. Thus, the curious negative impact of Claritin advertisements on efficacy at 60 min among subjects with allergies cannot be mediated by belief change. (Advertisement conditions did not change the beliefs in that subpopulation, and belief change is uncorrelated with efficacy at 60 min.) However, the observed positive impact of Claritin advertisements on efficacy at 120 min among subjects with allergies might be mediated by the advertisements’ impact on the subjects’ beliefs.
Table 2.
Regression of Claritin efficacy on self-reported beliefs about efficacy by allergy status
| Efficacy at 60 min | Efficacy at 120 min | |
| Subjects without allergies | ||
| Constant (SE) | 11.24 (2.23) | 25.86 (1.95) |
| Change in belief (SE) | 2.10 (1.63) | 3.76 (1.43) |
| Observations | 150 | 150 |
| R2 | 0.19 | 0.61 |
| Subjects with allergies | ||
| Constant (SE) | 14.05 (1.82) | 27.00 (1.95) |
| Change in belief (SE) | 1.41 (1.40) | 3.86 (1.50) |
| Observations | 161 | 162 |
| R2 | 0.31 | 0.59 |
The table reports coefficients from an ordinary least squares regression. Twenty nonallergic subjects and eight allergic subjects said they did not have beliefs about Claritin at baseline; they are coded as missing observations. There also is one missing measurement of wheal reaction at 60 min, accounting for the difference in the number of observations at 60 and 120 min among subjects with allergies.
Discussion
Our results suggest that a commercial phenomenon, television advertising, may be an important trigger for psychologically mediated physiological effects of a drug. Our findings also inform economic theories of advertising. They suggest that, at least in the context of new consumers of pharmaceutical products, advertisements can have a large impact on the efficacy of a drug.
Our study has several limitations. First, it does not establish whether, relative to a baseline of no advertisements, Claritin advertisements improve Claritin efficacy or Zyrtec advertisements reduce Claritin efficacy. We eliminated the control group (no drug advertisements) in the follow-up trial to increase statistical power, but we hope future research will shed light on whether positive or negative advertisements have a greater effect. (In our pilot study, among subjects without allergies, Zyrtec advertisements reduced and Claritin advertisements increased the efficacy of Claritin relative to the control condition, but neither effect was statistically significant on its own.)
Second, our measure of drug efficacy is difficult to translate into welfare. Consumers’ willingness to pay for antihistamines presumably depends on the ability of those drugs to alleviate allergy symptoms (such as a hay fever), but we have no way to gauge whether a given percent reduction in the symptoms corresponds to a substantial economic magnitude.
Finally, our study does not identify the specific physiological pathway through which advertisements modify the effect of Claritin. We demonstrate that, among subjects without allergies, advertisements alter subjects’ beliefs about the efficacy of Claritin (Fig. 2), and we present evidence suggesting that these altered beliefs influence the efficacy (Table 2). Our data, however, do not speak to the physiological link between beliefs and efficacy. One possible pathway is that pessimistic beliefs induced by Zyrtec advertisements trigger stress in the subjects and that this stress has a proinflammatory effect mediated by the production of glucocorticoids by the hypothalamic–pituitary–adrenal axis or catecholamines by the sympathetic nervous system (23). Identifying the pathway between beliefs and efficacy would provide guidance on whether our results would generalize to other types of medications.
Our focus on the subjects without allergies somewhat diminishes the policy relevance of our findings. That said, our results are likely to apply also to patients who were recently diagnosed with allergies or to patients who are particularly susceptible to advertisements. A recent study (24) reports that new users account for as many as 15% of all patients using antihistamines.
Although additional studies would be necessary to reach any firm policy conclusions, our study raises a possibility that would have important implications for regulation of drug advertisements. Pharmaceutical companies’ expenditure on direct-to-consumer advertising has grown more than 330% since 1996 and has more than doubled as a percentage of total drug sales (1). Government policy toward such advertising is heavily debated (25). The United States and New Zealand are the only developed countries in the world that permit direct-to-consumer advertising of prescription drugs, and the European Commission is considering changes to its regulation of pharmaceutical advertising (26). A recent survey identified more than 2,800 articles on the benefits and costs of direct-to-consumer advertising of drugs (27). Some of these studies claim that direct-to-consumer advertising elevates demand for prescription drugs by inducing patients to request advertised medications from their physicians (28). Our study suggests that advertising may have other important effects on public health. Specifically, television advertising may influence the efficacy of a branded drug once it is consumed.
Methods
Drug Choice.
We used three criteria to identify a drug for our studies. The drug had to be branded and advertised directly to consumers, so that real advertisements could be used as stimuli. The drug also had to have objectively measurable effects to rule out the possibility that advertisements affect only the perception of effectiveness or the willingness of subjects to report what they believe investigators want to hear. Finally, the drug had to be fast acting, so that subjects’ behavior could be controlled for the duration of the experiment; otherwise, it is possible that advertisements might affect efficacy only indirectly, by modifying compliance or other behavior rather than by altering the physiological consequences of the drug.
Antihistamine drugs fit all these criteria. Histamine is an organic compound that is generated by mast cells when those cells are exposed to appropriate allergens. Histamines released by mast cells attach to H1 receptors on the walls of blood vessels and thereby trigger vasodilation and increased vascular permeability to allow transudation of fluids. A consequence of this vasodilation is local edema. Antihistamine drugs bind to the same receptors as histamines, antagonizing their action on blood vessels. Therefore, antihistamine drugs reduce the histamine-induced edema of the skin.
People who have allergies release endogenous histamines when they are exposed to an allergen. In our study, we injected people with histamines. Individuals both with and without allergies develop a skin edema from these exogenous histamines. Hence, we are able to study the effect of antihistamines in reducing the histamine-induced edema for all subjects and can identify the impact of advertisements on efficacy for subjects with and without allergies.
We chose Claritin (loratadine), a leading antihistamine, as the drug for our experiments. Claritin has been one of the most widely consumed drugs in the United States, with annual sales reaching as high as $3 billion (29). It also is one of the most widely advertised drugs in history; in 2000 alone, its maker, Schering-Plough, spent $111 million advertising Claritin (30). Previous studies have shown that the number of prescriptions written for Claritin is correlated with advertising expenditure on Claritin (31).
Subjects.
Our pilot study was conducted from July to October of 2008, and the follow-up experiment was conducted from October 2011 to July 2012, both at the University of Chicago Nasal Physiology Laboratory. The pilot study enrolled 150 subjects, and the follow-up study enrolled 340 subjects. In each study, subjects were recruited through signs posted throughout the University. We enrolled healthy individuals of either sex between the ages of 18 and 65 y. We excluded individuals if they had consumed an antihistamine in the previous 4 d; were pregnant or lactating; were of childbearing age and were not using specific contraception; had had an upper respiratory infection in the previous 14 d; had used any investigational agent in the last 30 d; were using any medications that might affect skin testing for allergens; or had any serious medical condition. We paid subjects $100 for participating in the pilot study and $80 for participating in the follow-up experiment.
During the informed consent process, we told the subjects that the purpose of the study was to examine the effect of Claritin. After subjects completed all study procedures, we informed them that the study also examines the impact of direct-to-consumer advertisements on the efficacy of Claritin.
Protocol.
The detailed procedure following the skin test differed between the pilot study and the follow-up study. Because we focus on the results of the follow-up study, we describe that protocol first. Both studies were approved by the Biological Science Division Institutional Review Board at the University of Chicago.
Follow-up study.
After the consent process, we gave each subject a skin test for common allergens, namely, grass, trees, mold, dust mites, ragweed, and cats. After the skin test, subjects were administered a histamine challenge. The histamine challenge was a skin puncture that administered a percutaneous dose of 6 mg/mL histamine base solution (10 mg/mL histamine dihydrochloride; Hollister-Stier Laboratories) to the forearm. Each subject had the same arm used in every challenge. For some subjects it was the right arm and for others the left arm. After each histamine challenge, a research technician blinded to the subject’s condition measured the extent of the skin inflammation by tracing the wheal reaction. (In the pilot study, we also took an additional measure of skin inflammation, the flare. The flare is a halo of red, flushed skin which surrounds the wheal. Measurement of flare turned out to be very noisy, especially in patients with a dark skin tone. Accordingly, we took only wheal measurements in the follow-up study.) One research technician traced the outline of the wheal on a transparent tape which was affixed to the subject’s file. The subjects were told not to scratch the site of the challenge. To ensure compliance, we measured their wheal reaction shortly after each challenge and had the research technician monitor them in the meantime. Of the 1,020 intended measurements, one measurement was missed because the subject accidentally rubbed the tape and erased the tracing. At the end of the study, another research technician, also blinded to the subject’s condition, used ImageJ software to measure the traced area of the wheal.
After the baseline challenge, subjects were asked whether they had previously taken Claritin. After answering, subjects were given a 10-mg tablet of Claritin to consume in front of the research technician. We presented the tablet in its commercial packaging, so subjects were aware it was Claritin. Subjects then were asked how effective they thought Claritin is at eliminating allergy symptoms, on a scale of 1–7, with 7 meaning highly effective.
We then provided each subject with a portable DVD player with headphones and had subjects watch a movie (Shakespeare in Love). All subjects’ personal belongings, such as cell phones, iPods, and reading material, were confiscated for the duration of the study (under the premise that we needed to ensure a “controlled environment”). Consequently, all subjects did watch the movie for the duration of the experiment. The movie was spliced with naturally timed advertisement breaks (at roughly 6.5-min intervals) as if edited for broadcast television. Each break was 90 s long and contained multiple advertisements for automobiles and breakfast cereals.
We stratified the sample into subjects with and without a positive skin test and block-randomized each subpopulation to two advertisement conditions: Claritin advertisements and Zyrtec advertisements. One advertisement in each commercial break was replaced with an advertisement for the designated product (Claritin or Zyrtec). Subjects saw the same version of the designated product’s advertisement in each advertisement break. The advertisement used in the Zyrtec advertisements condition claimed, “Zyrtec’s a lot faster. It starts working two hours faster than Claritin.”
Sixty and 120 min after subjects consumed Claritin, we repeated the histamine challenge and the measurement of the wheal reaction. After the final histamine challenge, we again asked subjects to rate the efficacy of Claritin on a 1–7 scale.
Pilot study.
The protocol for the pilot study differed from the follow-up experiment in several respects. First, the pilot study did not block-randomize subjects based on their allergy status. Second, the pilot study assigned subjects to three conditions rather than two. In addition to the Claritin advertisements condition and Zyrtec advertisements condition, it included a control condition in which advertisements for neither drug were shown. Moreover, in the Claritin advertisements and Zyrtec advertisements conditions, subjects watched three versions of the designated product’s advertisements over the different breaks. Only one of the three Zyrtec advertisements mentioned Claritin. Third, in the pilot study we conducted the baseline histamine challenge after the subject had begun watching the movie. Finally, the pilot study did not elicit subjects’ beliefs about the efficacy of Claritin.
Acknowledgments
We thank Marcella De Tineo and James Lane for administering the study. The study was funded by the Stigler Center for the Study of the Economy and the State at the University of Chicago and by the University of Chicago Law School.
Footnotes
Conflict of interest statement: R.N. has received grants from McNeil, Teva Pharmaceutical Industries, Nasaleze, and Kalypsis; is or has been a speaker for Teva Pharmaceutical Industries and Sunovion; and is or has been a consultant for Teva Pharmaceutical Industries, Kalypsys, Sanofi, Meda, Sunovion, and Merck.
This article is a PNAS Direct Submission.
1E.K. and A.M. contributed equally to this work.
References
- 1. PhRMA (2008) The facts about pharmaceutical marketing and promotion. Available at http://phrma.org/sites/default/files/pdf/marketing_and_promotion_facts_071108_final.pdf. Accessed June 24, 2013.
- 2.Nelson HS. Variables in allergy skin testing. Immunol Allergy Clin North Am. 2001;21(2):281–290. [Google Scholar]
- 3.Malani A. Identifying Placebo Effects with Data from Clinical Trials. J Polit Econ. 2006;114(2):236–256. [Google Scholar]
- 4. Malani A, Houser D (2008) Expectations mediate objective physiological placebo effects. Advances in Health Economics and Health Services Research, pp 311–327. [PubMed]
- 5.Hashish I, Hai HK, Harvey W, Feinmann C, Harris M. Reduction of postoperative pain and swelling by ultrasound treatment: A placebo effect. Pain. 1988;33(3):303–311. doi: 10.1016/0304-3959(88)90289-8. [DOI] [PubMed] [Google Scholar]
- 6.Wager TD, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 7.Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344(21):1594–1602. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- 8.de Craen AJM, Roos PJ, Leonard de Vries A, Kleijnen J. Effect of colour of drugs: Systematic review of perceived effect of drugs and of their effectiveness. BMJ. 1996;313(7072):1624–1626. doi: 10.1136/bmj.313.7072.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branthwaite A, Cooper P. Analgesic effects of branding in treatment of headaches. Br Med J (Clin Res Ed) 1981;282(6276):1576–1578. doi: 10.1136/bmj.282.6276.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waber RL, Shiv B, Carmon Z, Ariely D. Commercial features of placebo and therapeutic efficacy. JAMA. 2008;299(9):1016–1017. doi: 10.1001/jama.299.9.1016. [DOI] [PubMed] [Google Scholar]
- 11.Shiv B, Carmon Z, Ariely D. Placebo effects of marketing actions: Consumers may get what they pay For. J Mark Res. 2005;42(4):383–393. [Google Scholar]
- 12.Plassmann H, O’Doherty J, Shiv B, Rangel A. Marketing actions can modulate neural representations of experienced pleasantness. Proc Natl Acad Sci USA. 2008;105(3):1050–1054. doi: 10.1073/pnas.0706929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClure SM, et al. Neural correlates of behavioral preference for culturally familiar drinks. Neuron. 2004;44(2):379–387. doi: 10.1016/j.neuron.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Lucchiari C, Pravettoni G. The effect of brand on EEG modulation: A study on mineral water. Swiss J Psychol. 2012;71(4):199–204. [Google Scholar]
- 15. Bagwell K (2007) The economic analysis of advertising. Handbook of Industrial Organization, eds Armstrong M, Porter R H (Elsevier, Amsterdam), Vol 3 pp 1701–1844.
- 16.Braithwaite D. The economic effects of advertisement. Econ J. 1928;38(149):16–37. [Google Scholar]
- 17. Galbraith JK (1958) The Affluent Society (Houghton Miflin, Boston); reprinted (1998) (Houghton Mifflin Harcourt)
- 18.Nelson P. Advertising as information. J Polit Econ. 1974;82(4):729–754. [Google Scholar]
- 19.Butters GR. Equilibrium distributions of sales and advertising prices. Rev Econ Stud. 1977;44(3):465–491. [Google Scholar]
- 20.Milgrom P, Roberts J. Price and advertising signals of product quality. J Polit Econ. 1986;94(4):796–821. [Google Scholar]
- 21.Stigler GJ, Becker GS. De gustibus non est disputandum. Am Econ Rev. 1977;67(2):76–90. [Google Scholar]
- 22.Becker GS, Murphy KM. A simple theory of advertising as a good or bad. Q J Econ. 1993;108(4):941–964. [Google Scholar]
- 23.McEwen BS, Underhill LH, McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 24. Nielsen/Wolters Kluwer Health (2008) Consumer behavior and managed care: Impact of the Zyrtec Rx-to-OTC switch. Available at http://download.lww.com/wolterskluwer_vitalstream_com/PermaLink/Allergy-whitepaperFinal.pdf. Accessed June 24, 2013.
- 25.Gellad ZF, Lyles KW. Direct-to-consumer advertising of pharmaceuticals. Am J Med. 2007;120(6):475–480. doi: 10.1016/j.amjmed.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Whalen J (December 11, 2008) Europe moves to loosen ban against drug ads. The Wall Street Journal, Section B, p 4.
- 27.Gilbody S, Wilson P, Watt I. Benefits and harms of direct to consumer advertising: A systematic review. Qual Saf Health Care. 2005;14(4):246–250. doi: 10.1136/qshc.2004.012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenthal MB, Berndt ER, Donohue JM, Epstein AM, Frank RG. Demand Effects of Recent Changes in Prescription Drug Promotion. 2003. Forum for Health Economics & Policy (Berkeley Electronic Press), Vol 6. [Google Scholar]
- 29.Clark MJ, Million RP. Allergic rhinitis: Market evolution. Nat Rev Drug Discov. 2009;8(4):271–272. doi: 10.1038/nrd2762. [DOI] [PubMed] [Google Scholar]
- 30. Harris G (March 22, 2002) As blockbuster Claritin goes generic, Schering-Plough pushes a close sibling. The Wall Street Journal, Section A, p 1.
- 31.Zachry WM, 3rd, et al. Relationship between direct-to-consumer advertising and physician diagnosing and prescribing. Am J Health Syst Pharm. 2002;59(1):42–49. doi: 10.1093/ajhp/59.1.42. [DOI] [PubMed] [Google Scholar]