Abstract
Understanding the functional impacts of pollinator species losses on plant populations is critical given ongoing pollinator declines. Simulation models of pollination networks suggest that plant communities will be resilient to losing many or even most of the pollinator species in an ecosystem. These predictions, however, have not been tested empirically and implicitly assume that pollination efficacy is unaffected by interactions with interspecific competitors. By contrast, ecological theory and data from a wide range of ecosystems show that interspecific competition can drive variation in ecological specialization over short timescales via behavioral or morphological plasticity, although the potential implications of such changes in specialization for ecosystem functioning remain unexplored. We conducted manipulative field experiments in which we temporarily removed single pollinator species from study plots in subalpine meadows, to test the hypothesis that interactions between pollinator species can shape individual species’ functional roles via changes in foraging specialization. We show that loss of a single pollinator species reduces floral fidelity (short-term specialization) in the remaining pollinators, with significant implications for ecosystem functioning in terms of reduced plant reproduction, even when potentially effective pollinators remained in the system. Our results suggest that ongoing pollinator declines may have more serious negative implications for plant communities than is currently assumed. More broadly, we show that the individual functional contributions of species can be dynamic and shaped by the community of interspecific competitors, thereby documenting a distinct mechanism for how biodiversity can drive ecosystem functioning, with potential relevance to a wide range of taxa and systems.
Keywords: ecosystem function, ecosystem services, phenotypic plasticity, foraging biology
Global pollinator declines are ongoing (1, 2), leading to concerns about the functional impacts of pollinator species losses on both pollination-dependent native plants (3) and crop production (4). Network-based simulation models of pollinator species losses, however, predict that major functional impacts on plant species persistence will not typically result until many, or even most, pollinator species are lost from a system (5–7), but these results have not been tested empirically. These predictions are based in part on redundancies in pollination networks: most plant species are visited by several pollinator species, contributing to the robustness of such networks (5). This robustness, however, is dependent on the implicit assumption that the functional roles of pollinator species are static. If interspecific interactions can dynamically alter species’ functional contributions—such as the efficacy of a particular plant–pollinator relationship—pollinator species losses may have greater cascading impacts on plant communities than predicted by current models (5–7).
Such dynamic changes in species functional roles are predicted by the ecological literature on interspecific competition and specialization but have not been studied in the context of pollination or other ecosystem functions and services. Competition between species has long been known to affect specialization in a wide range of taxa and ecosystems over both evolutionary and ecological timescales (8–12). When interspecific competition increases, each species in a system tends to specialize ecologically. Such competition-dependent specialization has been shown in a number of ecological attributes, including elevational range, dietary breadth, and timing of foraging (8, 9). By contrast, when intraspecific competition increases, individuals within that species tend to become more ecologically generalized. When considered over evolutionary time, this process can lead to niche differentiation (11), resulting in static ecological complementarity among species, a well-documented mechanism for positive biodiversity–ecosystem functioning relationships (13, 14). Over ecological timescales, however, interspecific competition can lead to dynamic specialization (8–10) via phenotypic plasticity, either behavioral or morphological.
In the case of pollination, short-term pollinator foraging specialization on particular plant species, or “floral fidelity,” is a dimension of dynamic ecological specialization with important implications for both plants and pollinators. For plants, floral fidelity is critical because transfer of conspecific pollen must occur in order for fertilization to take place. Reductions in floral fidelity can have deleterious effects on plant reproduction (15–17). For pollinators, more-specialized foraging behavior can be associated with higher energy returns relative to less-specialized foraging (18). At the same time, most pollinator species are generalists, with labile floral preferences over the course of their lifetime, and can include more-rewarding flowers in their foraging repertoire over relatively rapid timescales if given the opportunity (18–20). Thus, pollinator floral fidelity may be responsive to the changes in the floral reward landscape as competing species are lost from or added to a system. Despite the potential importance of dynamic specialization in pollination and other systems, its contributions to ecosystem functioning and the delivery of ecosystem services have not been considered to date.
Here, we assessed the impacts of single-pollinator species losses on dynamic specialization (floral fidelity) and pollination functioning using field manipulations in subalpine meadows in the Rocky Mountains of Colorado, United States. Our manipulations temporarily and nondestructively removed the most abundant bumble bee (Hymenoptera: Apidae: Bombus) species from field plots using targeted aerial netting. The identity of the target species varied between plots, and we manipulated 6 Bombus species, or more than half of the 11 species in the system. We assessed each individual plot in both a control and a manipulated state, allowing us to hold the plant community constant within our comparisons. We assessed how (a) dynamic specialization (floral fidelity) of bumble bees changed between the control state and the manipulated state of each plot, and followed the linked changes through the steps of the pollination process in terms of (b) bee pollen carriage, (c) deposition of pollen on floral stigmas, and finally (d) plant reproductive function, i.e., seed output (Fig. 1). We tracked the pollination process in a larkspur, Delphinium barbeyi (Ranunculaceae), an abundant wildflower that is visited by at least 10 of the 11 bumble bee species in the system.
Fig. 1.
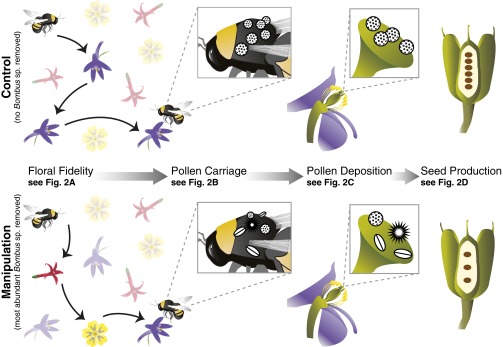
Floral fidelity and pollination function. Displays the steps of the process by which the single-species removal experiments lead to changes in plant reproductive functioning in the manipulated (Lower) versus the control (Upper) state in each plot. Species removals lead to reductions in floral fidelity in bee foraging, a lower proportion of conspecific pollen carried by bees and transferred to floral stigmas, and ultimately reduced seed set.
Results
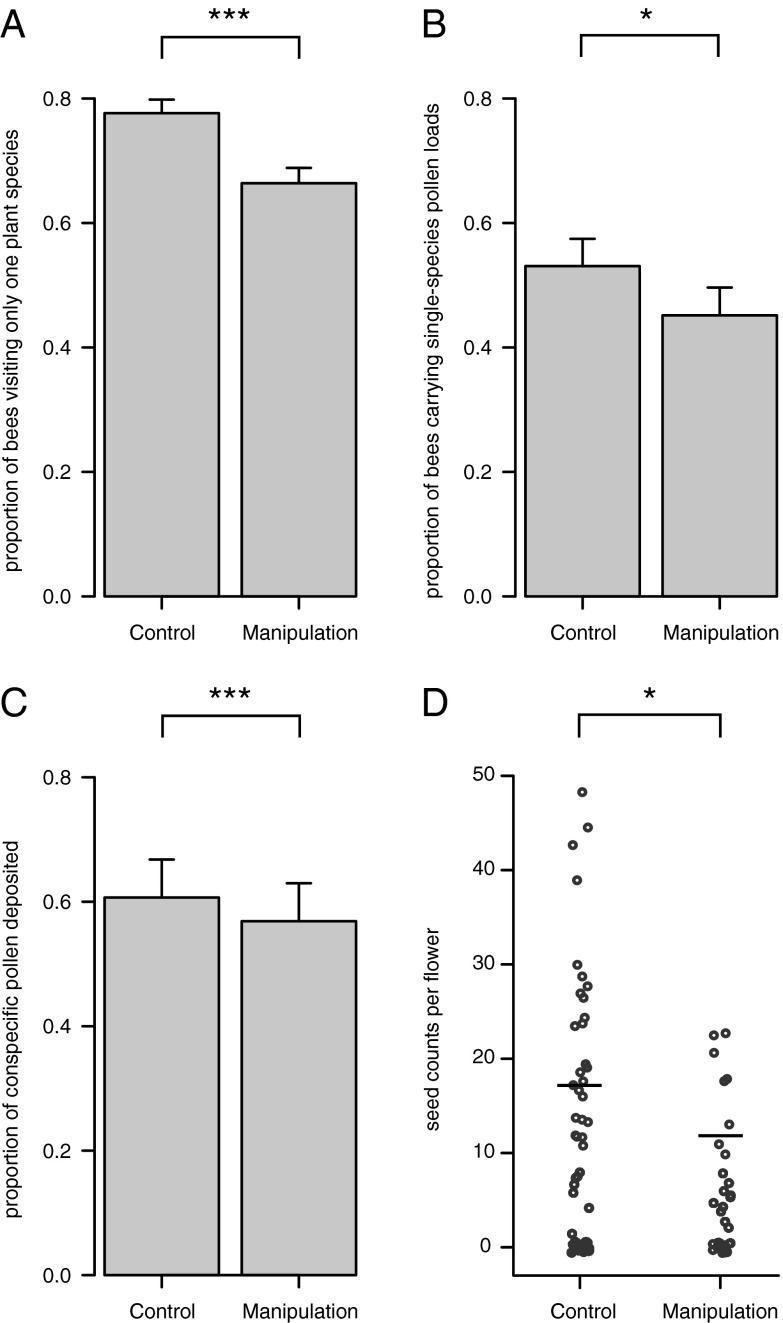
Rates of floral fidelity, proportions of conspecific pollen carriage and deposition, and seed production significantly decreased in the manipulated state—with a single pollinator species removed—relative to the control state. We assessed floral fidelity on a per-plant-visit basis (proportion of individual bee foraging movements that were within-plant-species vs. between-plant-species) and on a per-bee basis (proportion of bees that visited only a single plant species, versus those that foraged on more than one plant species). On a per-plant-visit basis, foraging movements between individual plants of different species increased by an average of 156% in manipulated plots relative to controls, based on observation of >23,500 between-plant foraging movements in 736 individual bumble bees [P = 6.35 × 10−6, generalized linear mixed-effects model (GLMM); see Materials and Methods; N = 20 sites with control/manipulation pairs; Table 1]. On a per-bee basis, the percentage of individual bees visiting only one species of plant within a single foraging bout decreased from 77.7% to 66.4% in the control relative to the manipulated state of each plot (Fig. 2A, Table 1) in the same 736 individual bees (P = 0.000748, GLMM; N = 20 sites). Patterns of pollen carriage also reflected decreased floral fidelity: bumble bees in the manipulated state carried 17.5% more mixed-species pollen loads relative to controls (P = 0.040, GLMM; pollen loads from 254 bees; N = 15 sites; Fig. 2B, Table 1). The proportion of conspecific pollen deposited on D. barbeyi stigmas concurrently decreased from 61% to 56% in control vs. the manipulated state (P = 2.67 × 10−7, GLMM; counts of >47,000 pollen grains from 129 plants in N = 5 sites; Fig. 2C, Table 1). These changes in specialization behavior, pollen carriage, and stigmatic deposition were ultimately reflected in decreased ecosystem function, i.e., a significant reduction in seed production in D. barbeyi in the manipulated relative to control state of each plot (P = 0.0331, GLMM; counts of 1,599 developed seeds in 192 plants in N = 5 sites; Fig. 2D, Table 1). Based on GLMM model coefficients and mean Bombus species richness and abundance, single-pollinator species removals reduced mean seed count per flower by 32.0%.
Table 1.
Statistical results
| Floral fidelity | Stigmatic pollen deposition | ||||||||||||||
| Fixed effects | Per plant visit | Per bee individual | Pollen carriage | Seed set | |||||||||||
| Coeff. | SE | P value | Coeff. | SE | P value | Coeff. | SE | P value | Coeff. | SE | P value | Coeff. | SE | P value | |
| (Intercept) | 5.416 | 0.647 | < 2e−16*** | 1.715 | 0.580 | 0.003** | −1.226 | 0.791 | 0.121 | −2.860 | 0.576 | 6.77e−07*** | 1.352 | 0.619 | 0.029* |
| Manipulation | −0.978 | 0.223 | 1.12e−05*** | −0.689 | 0.204 | 0.001*** | −0.598 | 0.291 | 0.040* | −1.062 | 0.206 | 2.67e−07*** | −0.386 | 0.181 | 0.033* |
| Bombus abundance | −0.008 | 0.007 | 0.253 | −0.021 | 0.124 | 0.863 | −0.011 | 0.010 | 0.307 | −0.046 | 0.007 | 5.95e−12*** | −0.011 | 0.005 | 0.017* |
| Bombus richness | 0.055 | 0.132 | 0.675 | −0.004 | 0.006 | 0.489 | 0.379 | 0.187 | 0.043* | 1.068 | 0.118 | < 2e−16*** | 0.396 | 0.122 | 0.001** |
Results of generalized linear mixed-effects models with binomial errors (floral fidelity and pollen deposition results) and negative binomial errors with zero-inflation (seed set results). For the “Manipulation” line, the model coefficient reports the difference moving from control to manipulated plots. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 2.
Floral fidelity, pollen carriage, stigmatic pollen deposition, and seed production. (A) Per-bee floral fidelity based on foraging observations, i.e., the relative proportions of bees displaying fidelity (visiting only one species of plant) in control vs. manipulated plots. Error bars show means + SEM, based on observations of 736 individual bees in 20 sites; GLMM P = 0.000748. (B) Floral fidelity based on pollen carriage, i.e., the relative proportions of bees displaying fidelity (carrying pollen from only one species of plant) in control vs. manipulated plots. Error bars show means + SEM, based on observations of 254 individual bees in 15 sites; GLMM P = 0.040. (C) Stigmatic pollen deposition expressed as the proportion of conspecific pollen in bee-carried pollen loads. Error bars show means + SEM, based on counts of >47,000 pollen grains from 129 plants in N = 5 sites, GLMM P = 2.67 × 10−7. (D) Seed counts per flower, for sites where Bombus species richness = 5. This value for species richness allows for comparison between the control and manipulated state, as the only value with more than one site represented in each state. It is also the median and modal value of species richness. Raw seed production values per flower are shown and are jittered to allow for display of overlapping points; horizontal black bars represent mean values predicted by the GLMM, assuming mean values of Bombus abundance. GLMM P = 0.033, based on counts of 1,599 developed seeds in 192 plants in 5 sites.
Our results also provide support that the effects of the manipulations on plant reproductive functioning were driven by differences in Bombus species richness, rather than changes in Bombus abundance. The single pollinator species removal manipulations reduced Bombus relative abundance (not just species richness) on average although the difference was marginally nonsignificant (P = 0.059, paired t test, N = 20 site pairs). Individuals of nontarget Bombus species could freely enter the plots, and abundance effects were highly variable (mean paired abundance changes in manipulated relative to control plots = −11.4%; range: −88.6% to +225%). In 7 of the 20 sites, Bombus abundance was greater in the manipulated state relative to the control (despite the removal of the target species), allowing us to statistically control for changes in Bombus abundance due to the manipulations. Bumble bee abundance was not significantly related to either of the two floral fidelity outcomes or to pollen carriage (Table 1). The relationships between Bombus abundance and both stigmatic pollen deposition and seed set, although statistically significant, were negative with small model coefficients (Table 1), i.e., indicating that increased bumble bee abundance was associated with slightly lower conspecific pollen deposition and seed production. Thus, the pattern of reduced ecosystem functioning in the manipulations is not likely driven by abundance changes.
Discussion
Our results demonstrate that interspecific interactions can dynamically change the ecosystem functional roles of particular species. Specifically, we show that pollination functioning can be reduced with the removal of a single pollinator species, despite the fact that other potentially effective pollinator species remained in the system. This empirical finding contrasts with prevailing predictions of network robustness to pollinator species losses, based on simulation models that do not account for changes in the efficacy of particular plant–pollinator relationships. The reduction in plant reproductive functioning that we observed is associated with dynamic changes in pollinator behavior, through the steps shown in Fig. 1. When we removed an interspecific competitor, remaining individual pollinators broadened their foraging strategies and thus showed less fidelity to particular plant species. Reduced floral fidelity translates functionally into less conspecific pollen carried by bees and transferred between individual plants of the same species, which was ultimately reflected in decreased plant seed production in our manipulated sites. Thus, our results show that the ecosystem functional contributions of bee species in our system are not fixed, but instead are dynamic and dependent on interactions with competing species.
These findings highlight a distinct mechanism for how biodiversity can shape ecosystem services and functions. Complementarity—in which different species perform distinct fixed roles in different components of ecosystem function—is the primary accepted mechanism for biodiversity–ecosystem functioning (BDEF) patterns (13, 14). In contrast, we show a role for biodiversity per se in ecosystem function, in shaping dynamic specialization and its functional consequences over short (ecological or behavioral) timescales. Although we focused on specialization and did not assess dynamic complementarity in our experiments, complementarity and specialization are often ecologically intertwined (21), and there is evidence for interspecific competition shaping specialization in ways that likely increase dynamic complementarity (20). The mechanism of interspecific competition driving dynamic specialization is widespread both taxonomically and geographically (8–10), supporting the idea that our results likely extend to other BDEF relationships more generally.
If dynamic specialization and/or complementarity drive some ecosystem functions, however, why has this result not been uncovered in the hundreds of studies on BDEF relationships? Three factors might contribute. First, the vast majority of BDEF studies have focused solely on plants and other sessile autotrophs (14, 22) whereas much of the work on competition and dynamic specialization has focused on animals (8, 9, 12). Given the rapid behavioral plasticity of animals in response to interspecific competition (10), dynamic specialization and/or complementarity may be especially important for animal-driven ecosystem functions. Still, there is recent evidence of dynamic specialization in plant communities driven by morphological phenotypic plasticity (23, 24). Second, most BDEF studies cover relatively short timescales; but longer-timescale experiments, especially for plants, may allow for more dynamic specialization to occur and may explain in part the result that greater biomass “overyielding” in species-rich treatments relative to monocultures often occurs only after months or years in long-term BDEF experiments (25–27). Third, the bulk of BDEF experiments are designed such that species identities and abundances are tightly controlled. Although such studies are undoubtedly of great value for mechanistically untangling BDEF relationships, manipulative experiments under natural field conditions (28), especially in terms of interspecific competition regimes, may allow for stronger dynamic responses and thus greater impacts on ecosystem function.
As we have demonstrated, losses of a single pollinator species lead to reductions in dynamic specialization, driven by interspecific interactions, that in turn drive significant negative effects on ecosystem functioning in terms of plant reproduction. Our work thus suggests that ongoing pollinator declines could already be causing negative impacts on plant populations, in contrast to the network-based simulation models that predict plant communities will be robust to pollinator species losses. To prevent disruptions of pollination and other critical ecosystem functions and services, we must move beyond assuming that the functional roles of species are static and work to understand how and under what conditions phenotypic plasticity and interspecific competition can interact to drive dynamic changes in ecosystem services and functions.
Materials and Methods
Study Area and Site Selection.
We worked in 20 plots in subalpine meadows in the landscape surrounding the Rocky Mountain Biological Laboratory (38°57.5′N, 106°59.3′W, 2,900 m above sea level), in the Gunnison National Forest, western Colorado, United States. We selected 20 × 20-m plots in sites with comparable densities of bumble bees and floral resources. Sites were separated by a minimum distance of 1 km. We collected data over two summer growing seasons, in 2010 and 2011.
Manipulations.
We assessed each plot in a control and then a manipulated state, with one day in between; we kept the interval between control and manipulated states short because of the rapid turnover in flowering phenology in our high-altitude system, allowing us to keep the plant community constant in our control–manipulation comparisons. We conducted experiments in each plot only once per year. We used targeted aerial netting to remove the most abundant Bombus species in each plot. [The Bombus species in our system are as follows (manipulated species designated with an asterisk): appositus*, balteatus*, bifarius*, californicus, flavifrons*, frigidus, mixtus, nevadensis*, occidentalis, rufocinctus, and sylvicola*.] Manipulated bees were kept alive in individual vials in a shaded, cool container until the end of the study period (several hours) and then released. After removing all visible individuals of the target species, we waited 1 h before collecting data. One member of the field team patrolled the site boundaries to prevent bees of the target species from moving into the plot.
Foraging Observations.
We directly followed the foraging sequences of Bombus individuals, recording the identity of each plant species visited in a sequence. We followed individual bees in the order in which they were first seen. We discontinued an observation when the bee was lost from sight, when it ventured more than 5 m outside of the plot, when it had been observed for 10 full minutes, or when we had tallied 100 individual plants visited. We discarded observations of fewer than five plants visited.
Bombus Inventory.
We inventoried Bombus species richness and abundance using nondestructive aerial netting, with two field team members netting for a 20-min period, not including handling time (the time from when a bee was in the net until it was in a closed vial). To avoid double-counting, each bee was kept in an individual glass vial, identified to species, and kept in a cool, dark location until the inventory time period was over, at which point bees were released.
Bee Pollen Load Removal and Analysis.
We removed pollen loads from 15 bumble bees in each site, selected haphazardly from those sampled during the Bombus inventory, in both control and manipulated states. We induced cold anesthesia by placing the glass vial containing the bee on ice, and removed pollen nondestructively under a stereoscope set up in the field, using cubes of fuchsin jelly (29). We mounted the pollen-containing jelly on microscope slides and later assessed floral fidelity in each pollen load by identifying the pollen types contained within. We counted pollen loads as monospecific if >95% of pollen grains represented a single species, and as heterospecific otherwise.
Measuring Stigmatic Pollen Deposition and Seed Set.
We assessed the effects of our manipulations on stigmatic pollen deposition and seed production in D. barbeyi (Ranunculaceae), a common, long-lived perennial herbaceous wildflower in our plots that is pollinated by several species of bumble bees. We prebagged racemes containing immature floral buds of D. barbeyi in each plot 48–72 h before experiments. We opened 15 separate bags containing mature, virgin flowers in the control and the manipulation experimental periods, and reclosed the bags at the end of a standardized 4-h period. We returned to the site 3–4 d after the treatment to harvest stigmas (allowing sufficient time for pollen tube growth), and 7–15 d later to harvest fruits after they had matured. Stigmas were removed from the flower and mounted on a slide with fuschin jelly in the field to avoid any loss of pollen in transit. We counted both total number of pollen grains as well as proportion of conspecific (D. barbeyi) and heterospecific pollen grains on the stigmas. We dissected mature fruits and counted developed and undeveloped seeds in the laboratory.
Data Analysis.
Data collected within a study site are not independent: bees and plants are likely to be closely related genetically, and environmental conditions, floral resources, and competitive community context are all similar. To address this lack of independence and prevent pseudoreplication, we used generalized linear mixed-effects models (GLMMs) with site as random effect. Thus, the site represents the level of experimental replication across all of our statistical models. We included three fixed effects in all models: experimental state (manipulated vs. control), Bombus species richness, and Bombus abundance, allowing us to statistically control for the changes in abundance in our manipulations, as well as the differing levels of initial Bombus species richness in each plot. We assessed full models to maintain consistency and comparability among the different analyses.
Our data on floral fidelity, pollen carriage, and stigmatic pollen deposition were measured in a binomial fashion: individual bees displayed either fidelity (i.e., visited only one species) or infidelity; foraging transitions were either to a conspecific or to a heterospecific plant; pollen loads were either “pure” (monospecific, and thus displaying fidelity) or else “mixed” (heterospecific, and thus displaying infidelity); and pollen grains on stigmas were identified as either conspecific (i.e., pollen of D. barbeyi) or heterospecific. We used GLMMs with binomial errors to model these response variables. For all of these outcomes, the data were overdispersed (i.e., greater variance relative to the expectation in a binomial distribution), and so we included an individual-level random effect in the model to compensate for the overdispersion (30). We used the lme4 library (31) for the R statistical programming language (32) to conduct binomial-errors GLMMs.
Seed production is a count variable, and flowers with insufficient pollination do not produce seeds, resulting in zero counts. Seed count data were both highly overdispersed and zero-inflated relative to a Poisson distribution, so we modeled seed production with a zero-inflated negative binomial GLMM using the glmmADMB library (33) for R.
Acknowledgments
We thank N. Waser and M. Price for helpful discussion throughout the project, and P. Armsworth, P. Humphrey, K. Levy, S. Philpott, N. Waser, and two anonymous reviewers for constructive comments on the manuscript. T. Dynes and the Rocky Mountain Biological Laboratory staff, especially J. Reithel and I. Billick, provided key research and logistical support. L. Anderson, J. Brokaw, T. Lamperty, F. Oviedo, R. Perenyi, L. Thomas, and K. Webster provided field assistance. M. Arford identified pollen and processed bee-carried pollen samples; T. Dynes, L. Atalla, and A. Delva processed stigmatic pollen samples. S. Sultan provided long-term inspiration on the broad implications of phenotypic plasticity. This work was funded by US National Science Foundation Grants DEB-1120572 (to B.J.B.) and DBI-1034780, OIA-0963529, and DBI-0753774 (to I. Billick), the Rocky Mountain Biological Laboratory (B.J.B. and H.M.B.), Emory University (B.J.B.), and University of California, Santa Cruz (H.M.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Biesmeijer JC, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313(5785):351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- 2.Potts SG, et al. Global pollinator declines: Trends, impacts and drivers. Trends Ecol Evol. 2010;25(6):345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Ollerton J, Winfree R, Tarrant S. How many flowering plants are pollinated by animals? Oikos. 2011;120:321–326. [Google Scholar]
- 4.Klein AM, et al. Importance of pollinators in changing landscapes for world crops. Proc Biol Sci. 2007;274(1608):303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Memmott J, Waser NM, Price MV. Tolerance of pollination networks to species extinctions. Proc Biol Sci. 2004;271(1557):2605–2611. doi: 10.1098/rspb.2004.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Memmott J, Craze PG, Waser NM, Price MV. Global warming and the disruption of plant-pollinator interactions. Ecol Lett. 2007;10(8):710–717. doi: 10.1111/j.1461-0248.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser-Bunbury CN, Muff S, Memmott J, Müller CB, Caflisch A. The robustness of pollination networks to the loss of species and interactions: A quantitative approach incorporating pollinator behaviour. Ecol Lett. 2010;13(4):442–452. doi: 10.1111/j.1461-0248.2009.01437.x. [DOI] [PubMed] [Google Scholar]
- 8.Rosenzweig M. Habitat selection and population interactions: The search for mechanism. Am Nat. 1991;137:S5–S28. [Google Scholar]
- 9.Pimm S, Rosenzweig M, Mitchell W. Competition and food selection: Field tests of a theory. Ecology. 1985;66:798–807. [Google Scholar]
- 10.Bolnick DI, et al. Ecological release from interspecific competition leads to decoupled changes in population and individual niche width. Proc Biol Sci. 2010;277(1689):1789–1797. doi: 10.1098/rspb.2010.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Futuyma DJ, Moreno G. The evolution of ecological specialization. Annu Rev Ecol Syst. 1988;19:207–233. [Google Scholar]
- 12.Fretwell SD, Lucas HL. On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheor. 1969;19:16–36. [Google Scholar]
- 13.Cardinale BJ. Biodiversity improves water quality through niche partitioning. Nature. 2011;472(7341):86–89. doi: 10.1038/nature09904. [DOI] [PubMed] [Google Scholar]
- 14.Hooper DU, et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol Monogr. 2005;75:3–35. [Google Scholar]
- 15.Flanagan RJ, Mitchell RJ, Karron JD. Effects of multiple competitors for pollination on bumblebee foraging patterns and Mimulus ringens reproductive success. Oikos. 2010;120:200–207. [Google Scholar]
- 16.Arceo-Gómez G, Ashman T-L. Heterospecific pollen deposition: Does diversity alter the consequences? New Phytol. 2011;192(3):738–746. doi: 10.1111/j.1469-8137.2011.03831.x. [DOI] [PubMed] [Google Scholar]
- 17.Morales C, Traveset A. Interspecific pollen transfer: Magnitude, prevalence and consequences for plant fitness. Crit Rev Plant Sci. 2008;27:221–238. [Google Scholar]
- 18.Gegear RJ, Thomson JD. Does the flower constancy of bumble bees reflect foraging economics? Ethology. 2004;110:793–805. [Google Scholar]
- 19.Inouye DW. Resource partitioning in bumblebees: Experimental studies of foraging behavior. Ecology. 1978;59:672–678. [Google Scholar]
- 20.Morse DH. Resource partitioning in bumble bees: The role of behavioral factors. Science. 1977;197(4304):678–680. doi: 10.1126/science.197.4304.678. [DOI] [PubMed] [Google Scholar]
- 21.Blüthgen N, Klein A-M. Functional complementarity and specialisation: The role of biodiversity in plant-pollinator interactions. Basic Appl Ecol. 2011;12:282–291. [Google Scholar]
- 22.Hooper DU, et al. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature. 2012;486(7401):105–108. doi: 10.1038/nature11118. [DOI] [PubMed] [Google Scholar]
- 23.Ashton IW, Miller AE, Bowman WD, Suding KN. Niche complementarity due to plasticity in resource use: Plant partitioning of chemical N forms. Ecology. 2010;91(11):3252–3260. doi: 10.1890/09-1849.1. [DOI] [PubMed] [Google Scholar]
- 24.Burns JH, Strauss SY. Effects of competition on phylogenetic signal and phenotypic plasticity in plant functional traits. Ecology. 2012;93:126–137. [Google Scholar]
- 25.Hooper DU, Dukes JS. Overyielding among plant functional groups in a long-term experiment. Ecol Lett. 2003;7:95–105. [Google Scholar]
- 26.Tilman D, et al. Diversity and productivity in a long-term grassland experiment. Science. 2001;294(5543):843–845. doi: 10.1126/science.1060391. [DOI] [PubMed] [Google Scholar]
- 27.Cardinale BJ, et al. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc Natl Acad Sci USA. 2007;104(46):18123–18128. doi: 10.1073/pnas.0709069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selmants PC, Zavaleta ES, Pasari JR, Hernandez DL. Realistic plant species losses reduce invasion resistance in a California serpentine grassland. J Ecol. 2012;100:723–731. [Google Scholar]
- 29.Kearns CA, Inouye DW. Techniques for Pollination Biologists. Niwot, CO.: Univ Press of Colorado; 1993. [Google Scholar]
- 30.Elston DAD, Moss RR, Boulinier TT, Arrowsmith CC, Lambin XX. Analysis of aggregation, a worked example: Numbers of ticks on red grouse chicks. Parasitology. 2001;122(Pt 5):563–569. doi: 10.1017/s0031182001007740. [DOI] [PubMed] [Google Scholar]
- 31.Bates D, Maechler M, Bolker B. 2011. lme4: Linear Mixed-Effects Models Using S4 Classes. R Package, version 0.999375-42, http://CRAN.R-project.org/package=lme4.
- 32.R Development Core Team 2012. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria), http://www.R-project.org/
- 33.Skaug H, Fournier D, Nielsen A, Magnusson A, Bolker B. 2011. glmmADMB: Generalized Linear Mixed Models Using AD Model Builder. R Package, version 0.7, http://glmmadmb.r-forge.r-project.org, http://admb-project.org.