Abstract
In animal communication research, vocal labeling refers to incidents in which an animal consistently uses a specific acoustic signal when presented with a specific object or class of objects. Labeling with learned signals is a foundation of human language but is notably rare in nonhuman communication systems. In natural animal systems, labeling often occurs with signals that are not influenced by learning, such as in alarm and food calling. There is a suggestion, however, that some species use learned signals to label conspecific individuals in their own communication system when mimicking individually distinctive calls. Bottlenose dolphins (Tursiops truncatus) are a promising animal for exploration in this area because they are capable of vocal production learning and can learn to use arbitrary signals to report the presence or absence of objects. Bottlenose dolphins develop their own unique identity signal, the signature whistle. This whistle encodes individual identity independently of voice features. The copying of signature whistles may therefore allow animals to label or address one another. Here, we show that wild bottlenose dolphins respond to hearing a copy of their own signature whistle by calling back. Animals did not respond to whistles that were not their own signature. This study provides compelling evidence that a dolphin’s learned identity signal is used as a label when addressing conspecifics. Bottlenose dolphins therefore appear to be unique as nonhuman mammals to use learned signals as individually specific labels for different social companions in their own natural communication system.
Keywords: vocal matching, animal cognition, playback experiment, marine mammals, individual recognition
Labeling or naming is one of the describing features of human language (1). Although the widespread use of alarm and food signals in animals gives the impression of labeling, the structure of these signals is usually predetermined from birth (2). A cognitively more complex use is when labels are acquired through learning (3). Vocal production learning (3), which enables animals to copy novel sounds in their environment and develop their own individually distinctive repertoire of calls, has been observed in a select number of animals, namely songbirds, hummingbirds, parrots, bats, pinnipeds, cetaceans (4), and elephants (5). Among these animals, only parrots (6) and dolphins (7) have been found capable of using arbitrary, learned signals to label objects in experimental studies. For both groups there are data that suggest this ability is also present in their natural communication system (8–12). Thus, both dolphins and parrots present interesting avenues of research for understanding labeling or naming in the animal kingdom.
Bottlenose dolphins are particularly interesting in this respect because they develop individually distinctive signature signals, termed “signature whistles” (13–15). A signature whistle is a learned, individually distinctive whistle type in a dolphin’s repertoire that broadcasts the identity of the whistle owner (16). Instead of relying on morphological differences in the vocal tract for identity signaling, as found across the mammalian kingdom (17), dolphin identity is encoded in the frequency modulation pattern of their signature whistles (15). Conspecifics react to a synthetic version of the modulation pattern of an animal’s signature whistle as if it was the original whistle (18). Each individual develops its own modulation pattern early in life. This development is influenced by vocal learning (11), with animals often using calls heard in the environment and modifying them to create a novel and unique pattern (19, 20). In isolated dolphins, the signature whistle accounts for close to 100% of all whistles produced (13, 15). In wild groups, however, only about 38–70% of whistles are signature whistles; the rest are other shared whistle types (21–23). Animals that meet at sea tend to exchange signature whistles before they join each other (24). The dolphin’s fission fusion society, coupled with their restricted vision underwater, was likely responsible for the selection of these individually distinctive signature whistles (25).
Signature whistles form an important and stable component of an individual’s vocal repertoire (26), but dolphins are capable of vocal learning throughout their lives and individuals can copy the signature whistles of others (7, 9, 10). This means that the signature whistle of one animal may be found as a minor part of the vocal repertoire of other individuals, evident as occasional events of whistle copying or matching (9, 10, 27). This copying of signature whistles is relatively rare but may allow animals to label and address social companions (9–11). All other whistles produced by dolphins, often called nonsignature whistles, clearly also have communicative value but should be less suitable to address individuals because their frequency modulation patterns are not individually distinctive (9). To test whether whistles can be used to address individuals and, if so, what whistles can be used for addressing, we need to know how a receiver reacts to playbacks of whistles.
We investigated this in experiments on wild, free-ranging bottlenose dolphins off the east coast of Scotland. We performed focal follows and recorded the signature whistles of the animals in situ. To identify signature whistles of wild animals, we used the SIGID (SIGnature IDentification) method (28). We played back either a synthetic version of the animal’s signature whistle that we had just recorded, thereby producing a copy to address the animal that had the animal’s voice features removed, or we played control whistles of either an unfamiliar animal from a different population or a familiar animal from the same population (see Materials and Methods for details).
Results
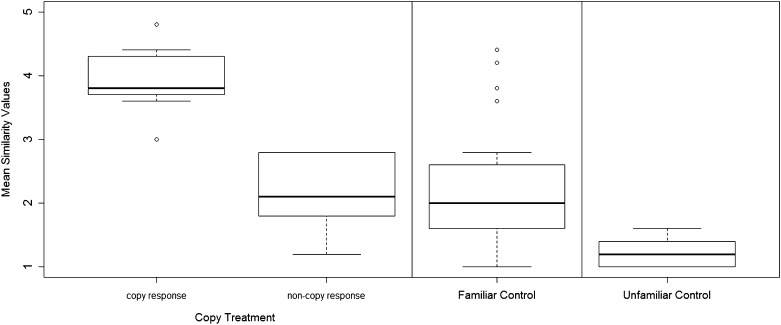
The vocal responses given by the animals in the 1 min following a playback were classified as either the same whistle type as the stimulus (a reply to being addressed) or a different whistle type (no reply), as decided by human visual classification where observers were blind to context (Materials and Methods). Only whistles that reached an average similarity score of >3 (27, 29) were deemed to be the same whistle type as the stimulus, indicating high whistle similarity (Fig. 1).
Fig. 1.
Mean similarity values of whistles produced in response to playback stimuli. We used a high similarity value (>3) as an indication that the animal replied to the playback with the same whistle type as the playback stimuli (a copy response). Similarities were rated by five blind observers who had significant agreement in their judgement (Cohen’s κ = 0.46, z = 22.5, P < 0.0001). Note that all outliers in the familiar control condition were in response to natural and not synthetic whistles.
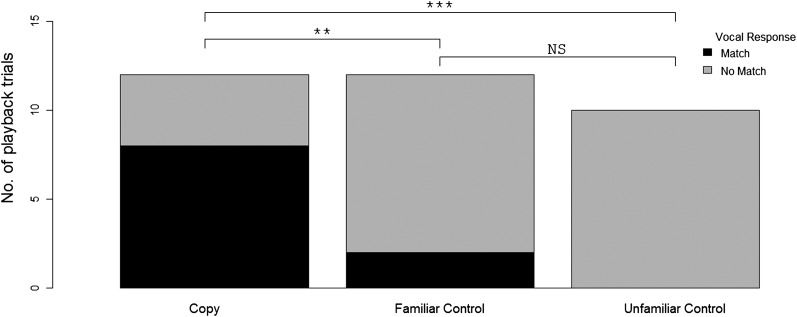
The dolphins’ responses differed significantly between the copy treatment and both the familiar controls (Barnard’s exact test, Wald statistic = 1.8, P = 0.04) and the unfamiliar controls (Barnard’s exact test, Wald statistic = 3.2, P = 0.001) (Fig. 2). Animals responded to hearing their own signature whistle by calling back with the same whistle type (Fig. 3), which occurred only twice with the familiar controls and did not occur with the unfamiliar controls. This result supports the hypothesis that signature whistle copies can be used to label or address specific individuals. Although both the copy treatment and unfamiliar control whistles were predominantly synthetic to remove voice features, we used natural whistles for the familiar control stimuli to preserve familiarity cues that might be present in voice features (Materials and Methods). This method resulted in a conservative test of addressing because familiarity cues in controls could have favored a reply to these whistles. However, to include all playbacks in our analysis, we also conducted an additional test comparing copy treatments and familiar whistles in which we included synthetic whistles as familiar control stimuli that were deemed to be signature whistles in the field by listening to their delivery pattern (28), but were not confirmed to be signatures later on when analyzed with a post hoc SIGID method (28). The dolphin’s responses remained significantly different when comparing the copy treatment playbacks with familiar controls consisting of the natural and the synthetic stimuli (Barnard’s exact test, Wald statistic = 2.4, P = 0.007). All results remained significant when applying Holm’s sequential Bonferroni adjustment (30). Because the result did not change when pooling natural and synthetic familiar whistles, we used the pooled sample for further analyses.
Fig. 2.
Response of wild bottlenose dolphins to whistle playbacks. The playbacks were either their own signature whistle (copy, n = 12), an unfamiliar signature whistle (unfamiliar control, n = 10), or a familiar whistle (familiar control, n = 12). The response may either be an animal replying with the same whistle as the playback stimulus (black) or not replying to the playback stimulus with the same whistle type (gray). The asterisks indicate a significant difference (**P = 0.007; ***P = 0.001). NS, not significant.
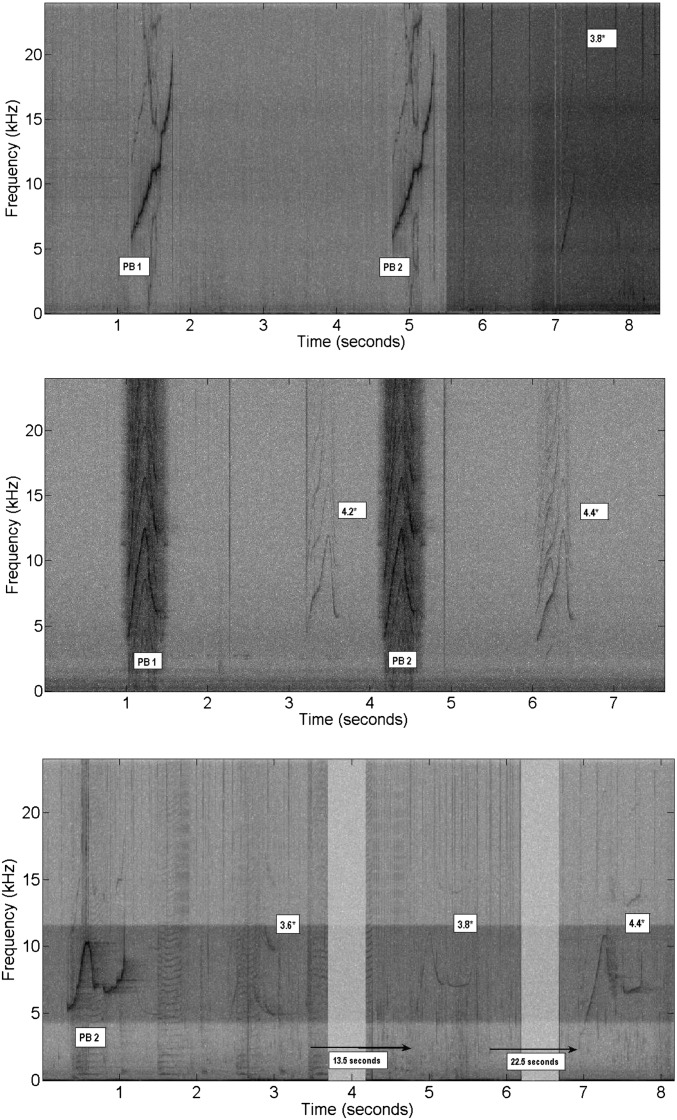
Fig. 3.
Spectrograms of three examples of copy treatments where animals called back with the same whistle; sampling rate: 48,000 Hz, FFT length: 1,024, Hanning window function. Playback stimuli are labeled (PB) and the average similarities of the whistles produced by the animals to the playback are given; high similarity whistles (vocal reply) are highlighted (*). If the time between the playback and the response is greater than a few seconds, arrows have been inserted indicating the actual time.
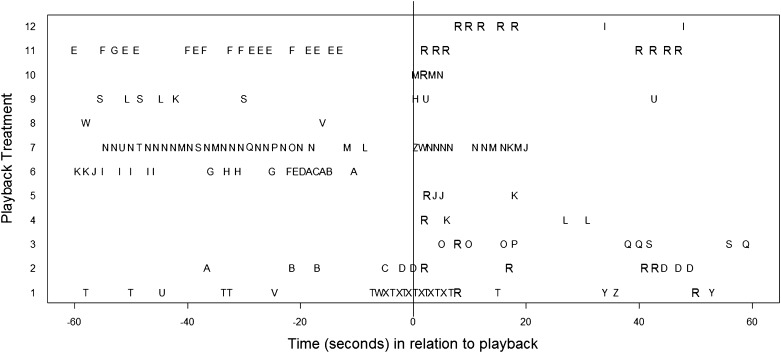
Animals responded to hearing their signature whistle by calling back for 8 of the 12 copy playback treatments. None of these eight playbacks had a whistle of the same type as the stimulus produced in the 1-min period preceding the playback (Fig. 4). The number of same type vocal responses (replies) to the copy playbacks varied with the mean number of replies being 2.75 whistles (range: 1–7) (Fig. 4), and for the two cases in which a familiar control playback was followed by a matching response, the number of replies was one and three whistles, respectively.
Fig. 4.
Whistle sequence and composition of each of the twelve copy playbacks. Each letter represents a different whistle type, which is positioned to when it was produced in relation to the playback (start at time of playback = 0) is shown. The “R” shows where animals replied with the same whistle as the playback stimuli (replies) as decided by human observers. The animals never produced the same whistle as the playback stimulus (labeled R) in the 1 min preceding a playback.
This result could be explained in two ways. First, the owner of the signature whistle replied when he was “addressed” or, second, another animal heard the signature whistle of an animal it knew and called back with a copy of that signature whistle. We were unable to identify which animal in the group replied to the playback but we can use the rate of signature whistle copying in wild animals to assess who replied to the playback. Rates of copying are low in wild animals. Published rates of copying shortly after hearing a whistle as in our experiment lie at 0.2 copies per minute (10, 27). Given this natural rate of copying, it is unlikely that the responses given here to the copy playbacks were signature whistle copies, because this would result in a rate of 1.83 copies per minute (22 copies in 12 min). The small number of matching responses given to two of the familiar control playbacks may in fact also have been replies to signature whistles because the signature whistle identification method we used (SIGID) does not always successfully identify every signature whistle (28). There was, however, a significant difference in the timing of the vocal response to the copy playbacks and the familiar controls.
The mean latency to the first whistle of any type produced after the playback did not differ between the copy treatments and the unfamiliar controls (Wilcoxon paired test: W = 35.5, df = 17, P = 1), but did differ significantly between the copy treatments and the familiar controls (W = 10, df = 16, P = 0.01). The mean latency to first whistle produced after the copy treatments was 2 s (range: 0.0001–8), and after the familiar controls it was 14 s (range: 0.97–41). The latency to first whistle produced after the playback also differed between the two control treatments (W = 9, df = 14, P = 0.05). The latency to replies (i.e., to whistles of the same type as the stimulus) also varied, with a mean initial time to the first reply of 3.8 s (range: 1.7–8) for the copy playbacks (Fig. 4), and 16.9 s (range: 1.7–34.7) for the two cases where the familiar control sound was followed by a whistle of the stimulus type. These data and the composition of whistle types following a playback of a signature whistle of a group member (Fig. 4) clearly showed that the reply was specific to the stimulus, and that dolphins did not simply all react with their own signature whistle when hearing the signature whistle of a group member.
No significant differences could be found in whistle rates between the copy treatment and unfamiliar control playbacks in the 1 min following playbacks (Wilcoxon paired test: W = 54.5, df = 20, P = 0.74) or for the copy treatment and familiar controls in the 1 min following playbacks (W = 98.5, df = 22, P = 0.1). There was also no significant increase or decrease in whistle rate from the 1 min before to the 1 min after playback (copy treatment: W = 28.5, df = 11, P = 0.7; unfamiliar controls: W = 15, df = 9, P = 0.72; familiar controls: W = 12, df= 11, P = 0.4).
Mean movement responses toward the boat were positive (6 m) for copy treatments and negative for both the control playbacks (unfamiliar = −18 m; familiar = −17 m). This difference was, however, not significant between either the copy and unfamiliar control playbacks (t test: t = −0.75, df = 18, P value = 0.4) or the copy and familiar control playbacks (Wilcoxon paired test: W = 54, df = 21, P = 0.47), which may be because of small sample size and large variance (Table 1). There was also no significant difference between the mean group sizes for the different treatment types (Wilcoxon paired test: W = 130, df = 33, P > 0.95). The mean group size for all control playbacks was 13 animals (range: 2–25) and 12 animals (range: 3–23) for the copy treatment.
Table 1.
Animal response to playback stimuli
| Treatment | Group size | Whistle rate before (no./min/individual) | Whistle rate after (no./min/individual) | Movement response (m) | High similarity whistles (reply) |
| (U) Control 1Sy. | 8 | 0.187 | 0.5 | −40 | 0 |
| (U) Control 2Sy. | 5 | 0.8 | 0.6 | −55 | 0 |
| (U) Control 3Sy. | 10 | 0.3 | 0.3 | +30 | 0 |
| (U) Control 4Sy. | 24 | 0.0 | 0.0 | —* | 0 |
| (U) Control 5Sy. | 13† | 0.16 | 0.07 | −30 | 0 |
| (U) Control 6Sy. | 11 | 0.18 | 1.72 | 0 | 0 |
| (U) Control 7Sy. | 25 | 0.32 | 0.04 | −30 | 0 |
| (U) Control 8Sy. | 25 | 0.28 | 0.0 | 0 | 0 |
| (U) Control 9Sy. | 8 | 0.625 | 0.5 | +140 | 0 |
| (U) Control 10Sy. | 3 | 0.0 | 2.33 | −180 | 0 |
| (F) Control 1N. | 13† | 0.0 | 0.0 | −20 | 0 |
| (F) Control 2N. | 13† | 0.0 | 0.0 | 0 | 0 |
| (F) Control 3N. | 13† | 0.0 | 0.0 | 0 | 0 |
| (F) Control 4N. | 10 | 0.7 | 0.2 | +20 | 0 |
| (F) Control 5N. | 10 | 0.0 | 0.6 | +5 | 1 |
| (F) Control 6N. | 2 | 0.5 | 0.0 | 0 | 0 |
| (F) Control 7N. | 2 | 0.0 | 0.0 | 0 | 0 |
| (F) Control 8N. | 6 | 0.0 | 0.83 | −80 | 3 |
| (F) Control 9Sy. | 22 | 0.54 | 0.14 | −70 | 0 |
| (F) Control 10Sy. | 25 | 0.0 | 0.12 | 0 | 0 |
| (F) Control 11Sy. | 3 | 0.67 | 1.3 | +10 | 0 |
| (F) Control 12Sy. | 30 | 0.03 | 0.26 | −20 | 0 |
| Treatment 1Sy. | 10 | 1.1 | 1.1 | −70 | 2 |
| Treatment 2Sy. | 3 | 1.67 | 3.3 | +150 | 4 |
| Treatment 3Sy. | 9 | 0.0 | 0.4 | +30 | 1 |
| Treatment 4Sy. | 4 | 0.0 | 1.0 | +20 | 1 |
| Treatment 5Sy. | 17 | 0.0 | 0.23 | −30 | 1 |
| Treatment 6Sy. | 21 | 0.9 | 0.0 | +35 | 0 |
| Treatment 7Sy. | 22 | 1.22 | 0.59 | +30 | 0 |
| Treatment 8Sy. | 5 | 0.4 | 0.0 | −10 | 0 |
| Treatment 9Sy. | 23 | 0.26 | 0.13 | 0 | 0 |
| Treatment 10Sy. | 11 | 11 | 0.45 | −70 | 1 |
| Treatment 11N. | 15 | 1.06 | 0.4 | −20 | 7 |
| Treatment 12N. | 12† | 0.0 | 0.58 | —* | 5 |
For each playback type the group size is shown along with whistle rates (number of whistles divided by group size) before and after playback, the movement response and whether animals replied to the playback. Superscript “Sy.” denotes synthetic stimuli and “N.” denotes natural stimuli. F, familiar control; Treatment, copy playbacks; U, unfamiliar control).
Equipment failure meant movement response was not available for these playbacks.
Group sizes were estimated as photo-identification was not available for these playbacks.
Discussion
These results present evidence that signature whistles can be used to address bottlenose dolphins. The significance of this finding lies in the kind of signal that is used for addressing. Birds have complex learned communication signals and engage in copying and matching of sounds in which they address each other. Songbirds have been shown to respond to songs that are in their repertoire by singing back with the same song, called “type matching” (31). Songbirds are more likely to respond to song if sung by an unfamiliar stranger than a familiar neighbor (31). However, this response is not universal because some species respond to the most similar song irrespective of caller (32). These responses can be strongest to playbacks of “self-song” recorded from the focal bird (32), but as these recordings were not synthesized it is unclear whether the birds reacted to their own voice features or the song type they shared with the playback.
There are, however, two main differences between bird song and dolphin whistles. First, most bird song is produced in the context of mate attraction and territory defense (33). Dolphins do not produce song but use single whistles as social sounds in affiliative contexts (9, 10, 24–26); neither are they territorial (34). Second, bird song types used in matching are rarely exclusive to an individual but repertoires tend to be shared (33). In bottlenose dolphins, on the other hand, the signature whistle is almost only used by one individual. The signature whistles of others can also form a minor part of an animal’s vocal repertoire as a result of copying. However, such copies are only used very rarely (10, 27). The fact that a signature whistle is primarily used by the whistle owner allows it to serve as a label for that particular individual when copied.
The learning of identity signals, as seen in bottlenose dolphins, is rare but has also been found in some bird species, such as green-rumped parrotlets (35). Although birds can discriminate individuals based on their contact calls (36), it is unclear what influences the development of the parameters used for individual recognition. An interesting exception might be the song sparrow, in which animals seem to modify learned calls after learning a perfect copy, thus introducing individual uniqueness (37). It is unknown whether these identity-encoding aspects are copied by conspecifics when engaging in song matching. Contact call learning in birds, however, tends to lead to a high similarity in contact calls between chicks and model adults. It is therefore important to distinguish between general vocal convergence in calls over time and the copying of signals to address specific individuals. If two or more animals converge in their calls, these calls can only be used for addressing the group collectively rather than individuals. Some bird species use vocal imitation to converge on shared call types between pairs or groups of animals (38–41). It is unclear whether the production of these shared calls functions in addressing the group. In bottlenose dolphins, the selective use of a signature whistle by one animal allows for the occasional copying of that whistle by another animal to be an effective way of addressing an individual. A parallel to the dolphin signature whistle may exist, however, in some species of parrot that can use calls to label (12) or address (42) conspecifics in captivity and use call matching in the wild (8).
It remains to be seen what the underlying mechanism for addressing or labeling is. At a basic level, an animal may learn that producing a particular call leads to a desirable result, such as the approach of an associated animal without an understanding of the link between the call and the approaching individual. Alternatively, an animal may have a modality-independent representation of an individual and displays goal-directed behavior to make contact. Results on cross-modal representation (43), the understanding of the link between a whistle and an individual (44), and goal-directed behavior in dolphins (45, 46) suggest this more complex mechanism.
It is clear that signature whistles have meaning (1) in that they are labels for individuals (18), and may be induced by an intention to contact a specific individual. Given that bottlenose dolphins in captivity are able to learn novel signals to label artificial objects and use these labels to report the presence or absence of objects (45), it is hard to see why these skills would not be used in the wild when animals are trying to make contact with specific individuals. Such a representational use of learned identity labels represents an interesting parallel to humans and the apparent necessity for these vocal labels in maintaining group cohesion may lie at the root of the evolution of complex communication and cognition systems.
Materials and Methods
Playbacks.
Group follows of wild bottlenose dolphins were conducted off the east coast of Scotland in the Moray Firth and in St. Andrews Bay from June to August 2001 and May to September 2010. The study was approved by the Animal Welfare and Ethics Committee of the University of St Andrews. Follows were conducted upon a small 6-m boat at sea state three or less. Photographs were used to ensure that playbacks were conducted on different groups. Acoustic recordings were taken with either two or four HTI-96 MIN or HTI-94 SSQ hydrophones (frequency response: 0.002–30 kHz ±1 dB) towed at 2-m depth. Recordings were made using either a Tascam DAP1 DAT recorder sampling at 48 kHz (frequency response: 0.02–22 kHz ± 0.5 dB) or directly onto a Toshiba laptop computer using either an Edirol UA-25 (sampling rate 96 kHz, 16 bit) or an Avisoft 416 Ultrasoundgate sound card (sampling rate 100 kHz, 8 bit).
Recordings were observed on the boat using real-time spectrogram displays in Adobe Audition v2.0 (Adobe Systems). This process enabled signature whistles produced by the focal group to be identified in situ using the SIGID method (28). The SIGID method uses the stereotypy and temporal patterning, which are unique to signature whistles, to identify them in wild free-ranging groups of animals. The SIGID analysis was performed by a human observer in real time on the boat and later checked by repeating the analysis in the laboratory using the sound recordings. Once a signature whistle sequence had been identified during a follow, a synthesized version of the identified signature whistle was prepared using SIGNAL software following the methods described in ref. 18. In copy treatment playbacks we used synthetic signature whistles, with the exception of two playbacks where natural whistles had to be used. Animals responded to both natural and synthetic playback stimuli (Table 1). Because it is difficult to perform the SIGID analysis in real time, it was rerun in the laboratory and only those playbacks in which the playback stimuli (whistles that had been recorded in situ) were confirmed as signature whistles were used in the analysis as treatment (signature whistle copy) playbacks. Only four of the synthetic whistles were not confirmed to be signature whistles in the post hoc SIGID analysis. In the familiar controls, we played one of six whistles that we recorded locally from other groups with very low background noise (recorded close to the animals with no boat noise and no biological background noise). Given the high level of local connectedness between animals in this population (47), these calls were classed as familiar to our target animals. The SIGID method did not classify these as signature whistles. Four of these whistles were only used once in a playback and two of them had to be used twice. Familiar control whistles were left unaltered to preserve any possible familiarity cues in voice features. However, to include all our data and to minimize pseudoreplication, we conducted two tests for familiar playbacks, one only with these unaltered whistles, and one in which we included the four synthetic whistles that were played back but that were not found to be signatures. Results did not differ between these tests (Results). Unfamiliar control stimuli consisted of six synthetic signature whistles modeled after signature whistles of captive dolphins from Zoo Duisburg, Germany (two captive born/two wild-caught in the Gulf of Mexico) and The Seas, Epcot, Florida (one captive born/one wild-caught in the Gulf of Mexico). Four of these recordings were used in two playbacks and two were used only once. All playback stimuli are shown in Fig. S1.
Each dolphin group was only exposed to one playback consisting of two whistles of the same type separated by a 3-s interwhistle interval. All playbacks were conducted with the boat engine switched off. In three playbacks only one whistle was played because of technical difficulties (two of the copy treatments and one of the familiar controls). To make sure that the animal that emitted the playback whistle remained with the focal group, the playback was aborted if any animals were deemed to have left the group while the playback signal was prepared on the computer. Playbacks were performed by playing sound files using either a Lubell LL916 underwater speaker (Lubell Labs: 0.6–21 kHz ± 8 dB) at 2-m depth and a Magnat classic 1000 XL car amplifier (frequency response: 0.005–100 kHz ± 3 dB) or a J-9 speaker and a Phonic MAR2 amplifier. The playback source level was set to 150 dB ± 3 dB re 1 μPa at 1 m (rms) measured with a calibrated B&K 8103 hydrophone.
Playbacks were randomized in their sequence and were conducted when the focal group was participating in nonpolarized behavior (animals exhibiting nondirectional movements with surfacings facing different directions) or were socializing (animals interacting with each other in close proximity). We noted the distance of all members of the focal group for a minimum of six surfacings immediately before and after the playback. The distances of the animals from the boat were estimated by eye and, when possible, corroborated with a laser range finder (Bushnell Scout 1000: ± 1-m accuracy). The error of estimates by eye was ± 10 m. The distance of the closest animal to the boat before and after the playback was used to determine a directional movement response (±) of the animals to or from the boat.
Analysis.
The acoustic recordings were analyzed by inspecting the spectrograms (FFT length 1024, 87.5% overlap, Hanning window) in Adobe Audition v2.0. All statistical procedures were conducted in R (R project for statistical computing; GNU project). Visual classification allowed the similarity of the whistle response given by the dolphins to the playbacks to be quantified using human observers. Visual classification is widely used in animal communication studies (15, 33) and has been shown to be more reliable than computer-based classification when analyzing dolphin call types (15, 48). Five human observers, all experienced in sound analysis and blind to context, rated similarity of extracted whistle contours (frequency modulation pattern) using a similarity index ranging from 1 (low similarity) to 5 (high similarity). Only whistles that reached an average score of >3 (27, 29) were deemed to be the same whistle type as the stimuli, indicating high whistle similarity. The κ-statistic was used to ascertain observer agreement (49).
A Barnard’s exact test was used to compare the animals’ vocal responses to the playback treatments (50). Barnard’s test was used as an alternative to Fisher’s exact test because the discrete nature of Fisher’s exact test means it produces highly conservative P values for small sample sizes. Whistle rates of the group of animals (rate per individual per minute) were compared before and after playback for each treatment. A Lilliefors (Kolmogorov–Smirnov) test was used to test for normality followed by a paired t test or Wilcoxon paired test with a Bonferroni-adjusted significance level of P < 0.016. Whistle rates were compared between playback treatments post playback. A Lilliefors (Kolmogorov–Smirnov) test was used to test for normality followed by a t test or a Wilcoxon test with a Bonferroni-adjusted significance level of P < 0.025. The same tests were also performed on the movement response using a significance level of P < 0.025.
Supplementary Material
Acknowledgments
We thank Thomas Götz, Luke Rendell, Paul Thompson, all our field assistants, and our human judges for their help during this study; Peter Tyack and Peter McGregor for comments on previous drafts of this work; and Heidi Harley and Kerstin Jurczynski for their support recording captive dolphins at the Seas and at Zoo Duisburg. The project was funded by a Biotechnology and Biological Sciences Research Council Studentship, a Marie Curie Fellowship of the European Community programme “Improving Human Research Potential and the Socio-economic Knowledge Base” under contract HPMF-CT-2000-00510, a Royal Society University Research Fellowship, and a Fellowship of the Wissenschaftskolleg Berlin. The study was carried out under Scottish Natural Heritage Research License numbers 2791 and 10778.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1304459110/-/DCSupplemental.
References
- 1.Hurford JR. The Origins of Meaning: Language in the Light of Evolution. Oxford, UK: Oxford Univ Press; 2007. [Google Scholar]
- 2. Seyfarth RM, Cheney DL (1997) in Social Influences on Vocal Development, eds Snowdon CT, Hausberger M (Cambridge Univ Press, Cambridge, UK), pp 249–273.
- 3.Janik VM, Slater PJB. The different roles of social learning in vocal communication. Anim Behav. 2000;60(1):1–11. doi: 10.1006/anbe.2000.1410. [DOI] [PubMed] [Google Scholar]
- 4.Janik VM, Slater PJ. Vocal learning in mammals. Adv Stud Behav. 1997;26:59–99. [Google Scholar]
- 5.Poole JH, Tyack PL, Stoeger-Horwath AS, Watwood S. Animal behaviour: Elephants are capable of vocal learning. Nature. 2005;434(7032):455–456. doi: 10.1038/434455a. [DOI] [PubMed] [Google Scholar]
- 6.Pepperberg IM. Functional vocalisations by an African grey parrot (Psittacus erithacus) Z Tierpsychol. 1981;55(2):139–160. [Google Scholar]
- 7.Richards DG, Wolz JP, Herman LM. Vocal mimicry of computer-generated sounds and vocal labeling of objects by a bottlenosed dolphin, Tursiops truncatus. J Comp Psychol. 1984;98(1):10–28. [PubMed] [Google Scholar]
- 8.Balsby TJ, Bradbury JW. Vocal matching by orange-fronted conures (Aratinga canicularis) Behav Processes. 2009;82(2):133–139. doi: 10.1016/j.beproc.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Janik VM, Slater PJB. Context-specific use suggests that bottlenose dolphin signature whistles are cohesion calls. Anim Behav. 1998;56(4):829–838. doi: 10.1006/anbe.1998.0881. [DOI] [PubMed] [Google Scholar]
- 10.King SL, Sayigh LS, Wells RS, Fellner W, Janik VM. Vocal copying of individually distinctive signature whistles in bottlenose dolphins. Proc Roy Soc B. 2013;280(1757):20130053. doi: 10.1098/rspb.2013.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tyack PL, Sayigh LS (1997) in Social Influences on Vocal Development, eds Snowdon CT, Hausberger M (Cambridge Univ Press, Cambridge, UK), pp 208–233.
- 12.Wanker R, Sugama Y, Prinage S. Vocal labelling of family members in spectacled parrotlets, Forpus conspicillatus. Anim Behav. 2005;70(1):111–118. [Google Scholar]
- 13. Caldwell MC, Caldwell DK, Tyack PL (1990) in The Bottlenose Dolphin, eds Leatherwood S, Reeves R (Academic, New York), pp 199–234.
- 14.Janik VM. Cognitive skills in bottlenose dolphin communication. Trends Cogn Sci. 2013;17(4):157–159. doi: 10.1016/j.tics.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Sayigh LS, Esch HC, Wells RS, Janik VM. Facts about signature whistles of bottlenose dolphins, Tursiops truncatus. Anim Behav. 2007;74(6):1631–1642. [Google Scholar]
- 16.Janik VM, Sayigh LS. Communication in bottlenose dolphins: 50 years of signature whistle research. J Comp Physiol A. 2013;199(6):479–489. doi: 10.1007/s00359-013-0817-7. [DOI] [PubMed] [Google Scholar]
- 17. Boughman JW, Moss CF (2003) in Acoustic Communication, eds Simmons AM, Popper AN, Fay RR (Springer, New York), pp 138–224.
- 18.Janik VM, Sayigh LS, Wells RS. Signature whistle shape conveys identity information to bottlenose dolphins. Proc Natl Acad Sci USA. 2006;103(21):8293–8297. doi: 10.1073/pnas.0509918103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fripp D, et al. Bottlenose dolphin (Tursiops truncatus) calves appear to model their signature whistles on the signature whistles of community members. Anim Cogn. 2005;8(1):17–26. doi: 10.1007/s10071-004-0225-z. [DOI] [PubMed] [Google Scholar]
- 20.Miksis JL, Tyack PL, Buck JR. Captive dolphins, Tursiops truncatus, develop signature whistles that match acoustic features of human-made model sounds. J Acoust Soc Am. 2002;112(2):728–739. doi: 10.1121/1.1496079. [DOI] [PubMed] [Google Scholar]
- 21.Buckstaff KC. Effects of watercraft noise on the acoustic behavior of bottlenose dolphins, Tursiops truncatus, in Sarasota Bay, Florida. Mar Mamm Sci. 2004;20:709–725. [Google Scholar]
- 22.Cook MLH, Sayigh LS, Blum JE, Wells RS. Signature-whistle production in undisturbed free-ranging bottlenose dolphins (Tursiops truncatus) Proc Roy Soc B. 2004;271(1543):1043–1049. doi: 10.1098/rspb.2003.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watwood SL, Owen ECG, Tyack PL, Wells RS. Signature whistle use by temporarily restrained and free-swimming bottlenose dolphins, Tursiops truncatus. Anim Behav. 2005;69(6):1373–1386. [Google Scholar]
- 24.Quick NJ, Janik VM. Bottlenose dolphins exchange signature whistles when meeting at sea. Proc Roy Soc B. 2012;279(1738):2539–2545. doi: 10.1098/rspb.2011.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janik VM. Acoustic communication in delphinids. Adv Stud Behav. 2009;40:123–157. [Google Scholar]
- 26.Sayigh LS, Tyack PL, Wells RS, Scott MD. Signature whistles of free-ranging bottlenose dolphins (Tursiops truncatus): Stability and mother-offspring comparisons. Behav Ecol Sociobiol. 1990;26(4):247–260. [Google Scholar]
- 27.Janik VM. Whistle matching in wild bottlenose dolphins (Tursiops truncatus) Science. 2000;289(5483):1355–1357. doi: 10.1126/science.289.5483.1355. [DOI] [PubMed] [Google Scholar]
- 28.Janik VM, King SL, Sayigh LS, Wells RS. Identifying signature whistles from recordings of groups of unrestrained bottlenose dolphins (Tursiops truncatus) Mar Mamm Sci. 2013;29(1):109–122. [Google Scholar]
- 29.Watwood SL, Tyack PL, Wells RS. Whistle sharing in paired male bottlenose dolphins, Tursiops truncatus. Behav Ecol Sociobiol. 2004;55(6):531–543. [Google Scholar]
- 30.Holm S. A simple sequential rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70. [Google Scholar]
- 31.Searcy WA, Beecher MD. Song as an aggressive signal in songbirds. Anim Behav. 2009;78(6):1281–1292. [Google Scholar]
- 32.Falls JB, Krebs JR, McGregor PK. Song matching in the great tit (Parus major): The effect of similarity and familiarity. Anim Behav. 1982;30(4):997–1009. [Google Scholar]
- 33.Catchpole CK, Slater PJB. Bird Song: Biological Themes and Variations. 2nd Ed. Cambridge, UK: Cambridge Univ Press; 2008. [Google Scholar]
- 34. Connor RC, Wells RS, Mann J, Read AJ (2000) in Cetacean Societies: Field Studies of Dolphins and Whales, eds Mann J, Connor RC, Tyack PL, Whitehead H (Univ of Chicago Press, Chicago), pp 91–126.
- 35.Berg K, Delgado S, Cortopassi KA, Beissinger SR, Bradbury JW. Vertical transmission of vocal signatures in a wild parrot. Proc Roy Soc B. 2011;279(1728):585–591. doi: 10.1098/rspb.2011.0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vignal C, Mathevon N, Mottin S. Audience drives male songbird response to partner’s voice. Nature. 2004;430(6998):448–451. doi: 10.1038/nature02645. [DOI] [PubMed] [Google Scholar]
- 37.Nordby JC, Campbell SE, Beecher MD. Selective attrition and individual song repertoire development in song sparrows. Anim Behav. 2007;74(5):1413–1418. [Google Scholar]
- 38. Brown ED, Farabaugh SM (1997) in Social Influences on Vocal Development, eds Snowdon CT, Hausberger M (Cambridge Univ Press, Cambridge, UK), pp 98–127.
- 39.Mammen DL, Nowicki S. Individual differences and within-flock convergence in chickadee calls. Behav Ecol Sociobiol. 1981;9(3):179–186. [Google Scholar]
- 40.Hile AG, Plummer TK, Striedter GF. Male vocal imitation produces call convergence during pair bonding in budgerigars, Melopsittacus undulatus. Anim Behav. 2000;59(6):1209–1218. doi: 10.1006/anbe.1999.1438. [DOI] [PubMed] [Google Scholar]
- 41.Mundinger PC. Vocal imitation and individual recognition of finch calls. Science. 1970;168(3930):480–482. doi: 10.1126/science.168.3930.480. [DOI] [PubMed] [Google Scholar]
- 42.Balsby TJS, Momberg JV, Dabelsteen T. Vocal imitation in parrots allows addressing of specific individuals in a dynamic communication network. PLoS ONE. 2012;7(11):e49747. doi: 10.1371/journal.pone.0049747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harley HE, Putman EA, Roitblat HL. Bottlenose dolphins perceive object features through echolocation. Nature. 2003;424(6949):667–669. doi: 10.1038/nature01846. [DOI] [PubMed] [Google Scholar]
- 44.Harley HE. Whistle discrimination and categorization by the Atlantic bottlenose dolphin (Tursiops truncatus): A review of the signature whistle framework and a perceptual test. Behav Processes. 2008;77(2):243–268. doi: 10.1016/j.beproc.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 45. Herman LM (2006) in Rational Animals? eds Hurley S, Nudds M (Oxford Univ Press, Oxford, UK), pp 439–467.
- 46. Kuczaj SA, II, Walker RT (2006) in Comparative Cognition: Experimental Explorations of Animal Intelligence, eds Wasserman EA, Zentall TR (Oxford Univ Press, Oxford, UK), pp 580–601.
- 47.Lusseau D, et al. Quantifying the influence of sociality on population structure in bottlenose dolphins. J Anim Ecol. 2006;75(1):14–24. doi: 10.1111/j.1365-2656.2005.01013.x. [DOI] [PubMed] [Google Scholar]
- 48.Janik VM. Pitfalls in the categorization of behaviour: A comparison of dolphin whistle classification methods. Anim Behav. 1999;57(1):133–143. doi: 10.1006/anbe.1998.0923. [DOI] [PubMed] [Google Scholar]
- 49.Siegel S, Castellan NJ., Jr . Nonparametric Statistics for the Behavioural Sciences. New York: McGraw-Hill; 1988. [Google Scholar]
- 50.Barnard GA. A new test for 2 × 2 tables. Nature. 1945;156(3954):177. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.